Abstract
Recent evidence indicates that extracellular nucleotides regulate plant growth. Exogenous ATP has been shown to block auxin transport and gravitropic growth in primary roots of Arabidopsis (Arabidopsis thaliana). Cells limit the concentration of extracellular ATP in part through the activity of ectoapyrases (ectonucleoside triphosphate diphosphohydrolases), and two nearly identical Arabidopsis apyrases, APY1 and APY2, appear to share this function. These findings, plus the fact that suppression of APY1 and APY2 blocks growth in Arabidopsis, suggested that the expression of these apyrases could influence auxin transport. This report tests that hypothesis. The polar movement of [3H]indole-3-acetic acid in both hypocotyl sections and primary roots of Arabidopsis seedlings was measured. In both tissues, polar auxin transport was significantly reduced in apy2 null mutants when they were induced by estradiol to suppress the expression of APY1 by RNA interference. In the hypocotyl assays, the basal halves of APY-suppressed hypocotyls contained considerably lower free indole-3-acetic acid levels when compared with wild-type plants, and disrupted auxin transport in the APY-suppressed roots was reflected by their significant morphological abnormalities. When a green fluorescent protein fluorescence signal encoded by a DR5:green fluorescent protein construct was measured in primary roots whose apyrase expression was suppressed either genetically or chemically, the roots showed no signal asymmetry following gravistimulation, and both their growth and gravitropic curvature were inhibited. Chemicals that suppress apyrase activity also inhibit gravitropic curvature and, to a lesser extent, growth. Taken together, these results indicate that a critical step connecting apyrase suppression to growth suppression is the inhibition of polar auxin transport.
In both animals and plants, cells release nucleotides into their extracellular matrix, where they function as signaling agents, inducing rapid increases in the concentration of cytosolic calcium that are transduced into downstream changes in cell physiology (Kim et al., 2006; Burnstock, 2007; Roux and Steinebrunner, 2007; Tanaka et al., 2010a, 2010b; Demidchik et al., 2011). Prominent among these downstream changes in plants are changes in the growth of cells, including the growth of pollen tubes (Steinebrunner et al., 2003), root hairs (Clark et al., 2010b), and cotton (Gossypium hirsutum) fibers (Clark et al., 2010a). These results suggest the possibility that the signaling changes induced by extracellular nucleotides intersect with signaling changes induced by one or more of the hormones that regulate plant cell growth. Consistent with this possibility, Tang et al. (2003) showed that a concentration of applied nucleotides that inhibited the gravitropic growth of roots could block the transport of the growth hormone auxin and that this effect could not be attributed to either pH changes or chelation of divalent cations. Correspondingly, Clark et al. (2010a) showed that when the application of nucleotides to cotton ovules growing in culture altered the rate of cotton fiber growth, it also induced the production of ethylene, a hormone known to regulate the growth of cotton fibers.
Given the potency of extracellular nucleotides to regulate cellular activities, it would be important for cells to control the concentration of these nucleotides. In both animals and plants, the principal enzymes that limit the buildup of extracellular ATP (eATP) and extracellular ADP are ectoapyrases (apyrase; EC 3.6.1.5). These enzymes, which are nucleoside triphosphate diphosphohydrolases, are characterized by apyrase-conserved regions whose peptide sequences are highly similar throughout the plant and animal kingdoms (Clark and Roux, 2009). Based on this structural criterion, there are seven apyrases in Arabidopsis (Arabidopsis thaliana; APY1–APY7), and two of these, APY1 and APY2, share 87% protein sequence identity but are less than 30% similar to the other five apyrases. These two apyrases partially complement each other’s function and play central roles in growth control in Arabidopsis, as judged both by genetic and biochemical criteria (Wolf et al., 2007; Wu et al., 2007). Polyclonal antibodies raised to APY1 (Steinebrunner et al., 2000) inhibit the apyrase activity released into the medium of growing pollen tubes, and when these antibodies were added to the culture medium of germinated pollen, they both blocked the growth of the pollen and raised the concentration of ATP in the medium (Wu et al., 2007). Similarly, treatment of cultured cotton ovules with antibodies that recognize cotton fiber apyrase both inhibits the growth of the fibers and increases the concentration of ATP in the medium, further establishing the link between apyrase activity and regulation of the extracellular ATP concentration ([eATP]) in growing tissues (Clark et al., 2010a).
Because wild-type pollen tubes expressing active APY1 or APY2 and cultured cotton fibers with wild-type apyrase activity grow at a normal rate, and because the antibodies inhibit apyrase activity (Wu et al., 2007), the growth inhibition induced by the antibodies further implicated apyrase activity as critical for the growth of these tissues. The antibodies were unlikely to enter the pollen tubes or cotton fibers, so these results also suggested that the pollen and cotton apyrases were ectoapyrases. However, these data do not rule out a possible Golgi function for APY1 and APY2 and for the cotton APY(s), as discussed by Wu et al. (2007) and Clark and Roux (2011). In fact, there is strong evidence that APY1 and APY2 are localized in the Golgi and may function there to regulate protein glycosylation and/or affect polysaccharide synthesis (Chiu et al., 2012; Schiller et al., 2012).
Although the suppression of APY1/APY2 or of apyrase activity has a dramatic effect on growth, overexpression of APY1 or APY2 has much less of an effect. Constitutive expression of APY1 induces a small but statistically significant increase in the growth of etiolated hypocotyls, while overexpressing APY2 has no effect on this growth (Wu et al., 2007). This is probably because the wild-type levels of apyrase expression are near optimal for growth (Roux and Steinebrunner, 2007).
The double knockout apy1apy2 is sterile, because the pollen of this mutant does not germinate (Steinebrunner et al., 2003). However, when APY1 is suppressed only approximately 60% by an inducible RNA interference (RNAi) construct in apy2 null mutants, pollen of these mutants will germinate, permitting fertilization and subsequent normal development, although the adult plants of these mutants are dwarf (Wu et al., 2007). Suppression of ectoapyrase activity would be expected to raise the equilibrium concentration of eATP (Wu et al., 2007), and since higher levels of eATP can inhibit auxin transport in roots (Tang et al., 2003), it was reasonable to hypothesize that the suppression of apyrase by RNAi could suppress auxin transport. The experiments described in this report test this hypothesis. The results indicate that suppression of APY1/APY2 expression in an inducible RNAi line, R2-4A (Wu et al., 2007), results in a significant inhibition of polar auxin transport in Arabidopsis hypocotyls and roots, with a concomitant altered distribution of endogenous auxin. Consistent with this result and with the results of Tang et al. (2003), suppression of APY1/APY2 also blocks the asymmetric distribution of a GFP reporter encoded by a DR5:GFP construct in gravistimulated primary roots of Arabidopsis seedlings and diminishes the extent of the elongation zone in these roots. These results are consistent with the novel conclusion that inhibition of auxin transport is a key step in the signaling pathway that links the inhibition of apyrase expression to growth inhibition.
RESULTS
APY1 and APY2 Play a Role in Polar Auxin Transport in Hypocotyls and Roots
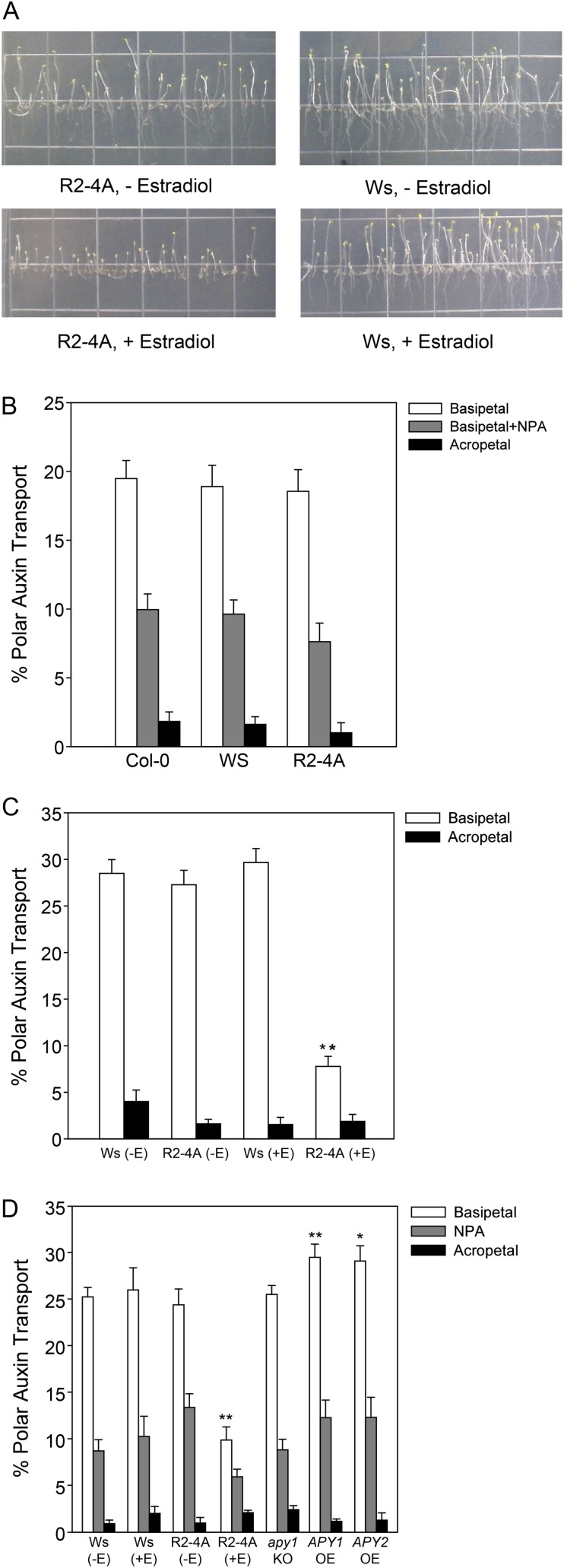
To determine if APY1 and APY2 play a role in auxin transport, we assayed polar auxin transport in hypocotyls of loss- and gain-of-function apyrase mutants, including the RNAi line R2-4A, in which the expression of APY1 in the apy2 null background can be suppressed by estradiol treatment. First, we tested whether the estradiol-treated R2-4A seedlings grown in the conditions used for the auxin transport assays (6 d of growth in the dark followed by 2 d of growth in the light) showed the inhibited growth phenotype reported previously (Wu et al., 2007). We observed an inhibition of hypocotyl and root growth under these conditions (Fig. 1A). In hypocotyls, indole-3-acetic acid (IAA) moves in a single basipetal or rootward polarity, with acropetal or shootward transport at background levels. We assayed basipetal and acropetal transport of [3H]IAA in hypocotyl sections from the wild type (Columbia [Col-0] and Wassilewskija [Ws] ecotypes) and the noninduced R2-4A seedlings, which, without estradiol treatment, is an apy2 single knockout. We found that basipetal auxin transport was not statistically different in all three genotypes, just as in the background of acropetal auxin movement (Fig. 1B). The auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) inhibited basipetal auxin transport to the same degree in each of these genotypes. However, we found that suppression of APY1 in induced R2-4A plants resulted in significant inhibition of basipetal auxin transport (Fig. 1C). Next, we tested polar auxin transport in the single apy1 and apy2 knockout lines as well as in the corresponding overexpressing lines for each of these apyrases. There was no difference observed in IAA transport in the hypocotyl assay between single knockout lines and the Ws wild type (Fig. 1D). In contrast, the APY1-OE (for overexpressor) line showed a statistically significant increase in polar auxin transport compared with the Ws wild type (P < 0.05), while the APY2-OE line showed a strong but marginally insignificant (P < 0.07) increase compared with the Ws wild type (Fig. 1D). The APY1-OE line was also more resistant than wild-type plants to the inhibitory effects of 600 µm ATPγS, a poorly hydrolyzable ATP nucleotide, on hypocotyl growth (Supplemental Fig. S1). The growth of R2-4A hypocotyls was also more resistant to treatment with 600 µm ATPγS, possibly because their growth is already inhibited. Basipetal polar auxin transport was inhibited in hypocotyls of the estradiol-induced R2-4A line to the same level that NPA inhibited transport in hypocotyls of the untreated and treated Ws wild-type seedlings.
Figure 1.
Polar auxin transport is inhibited in Arabidopsis hypocotyls after the induction of apyrase RNAi by estradiol and promoted in hypocotyls from seedlings overexpressing APY1 and APY2. A, Estradiol-treated RNAi R2-4A seedlings grown for 6 d in the dark and then transferred to light for 2 d show inhibited growth compared with Ws, noninduced RNAi R2-4A, and estradiol-treated Ws seedlings. B, Auxin transport is similar in noninduced genotypes, and there is no difference in the ability of NPA to inhibit basipetal auxin transport among noninduced genotypes. C, Basipetal auxin transport is greatly reduced after suppression of APY2 by the induction of mRNA interference with estradiol treatment. D, Basipetal auxin transport is promoted in APY1-OE and APY2-OE hypocotyls but is unaffected in the apy1 and apy2 single knockout (KO) lines. NPA inhibits basipetal auxin transport in hypocotyls of all genotypes but to a lesser degree in hypocotyls of the estradiol-treated RNAi R2-4A line. se values are marked by vertical bars. In B, n = 10 for all groups; in C and D, n = 10 for basipetal groups and n = 5 for acropetal and NPA groups. These results are representative of three or more biological repeats. Statistically evaluated differences between samples are indicated by asterisks (Student’s t test; **P < 0.05, *P < 0.07). [See online article for color version of this figure.]
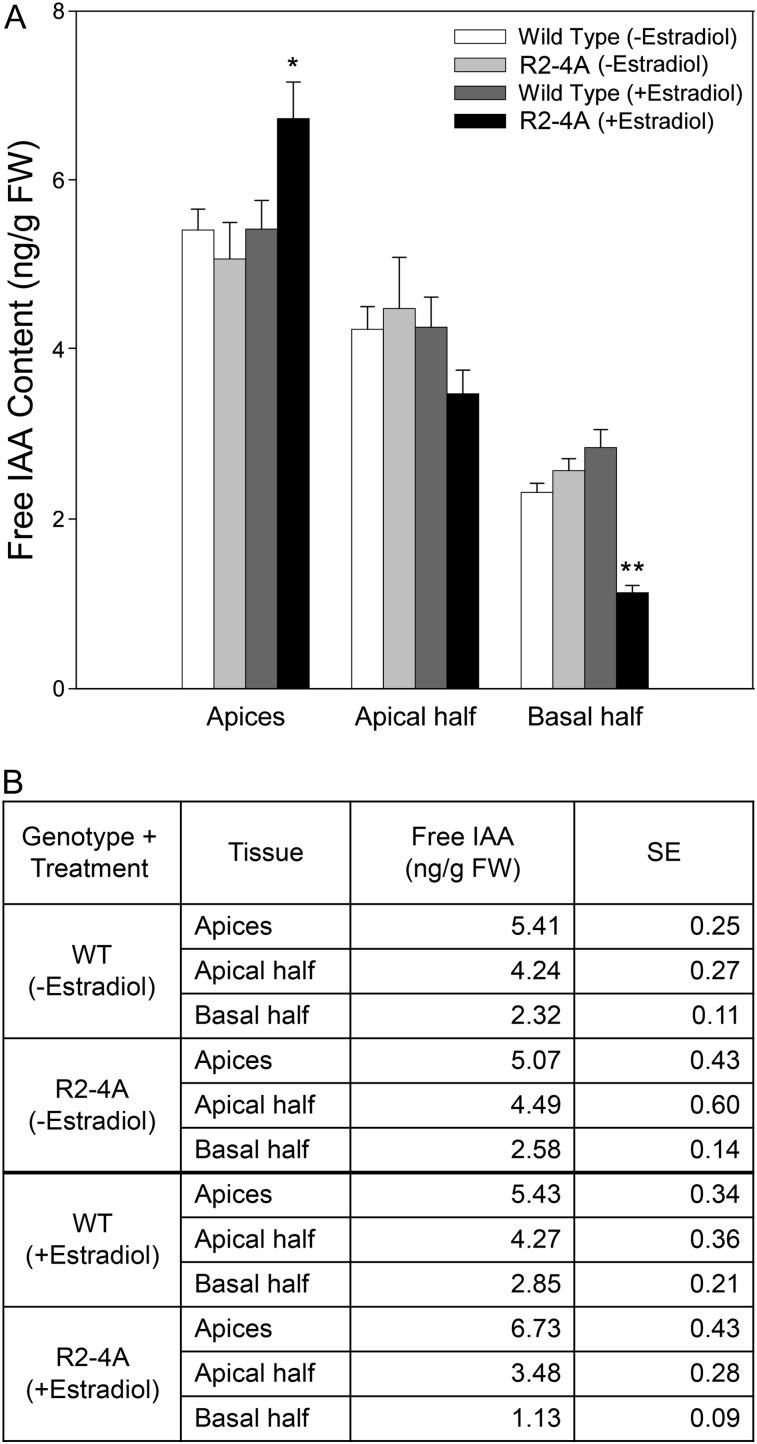
In order to determine whether the altered hypocotyl section auxin transport results were reflected in changes in the distribution of endogenous auxin, we measured the levels of free IAA in the shoot apices, apical halves, and basal halves of Arabidopsis hypocotyls from estradiol-treated and untreated whole seedlings from Ws wild-type and R2-4A plants. Our results showed that there was significantly less free IAA found in the basal half of hypocotyls from the estradiol-treated R2-4A line compared with basal halves of hypocotyls from estradiol-treated Ws wild-type plants (Fig. 2A). There was also more free IAA in the shoot apices of estradiol-treated R2-4A seedlings compared with estradiol-treated Ws wild-type apices (P < 0.07). Estradiol treatment had no effect on IAA levels in hypocotyls of Ws wild-type seedlings. The highest level of free IAA was observed in the apices of estradiol-treated R2-4A seedlings, with an average of 6.73 ng g−1 fresh weight IAA, while the lowest level of free IAA was observed in the basal half of the estradiol-treated R2-4A plants, with an average of 1.13 ng g−1 fresh weight IAA (Fig. 2B). These results are consistent with those from the hypocotyl section transport assays, suggesting that reduced basipetal auxin transport leads to a reduction of free IAA in the lower portion of the tissue and increased IAA in the upper portion of the plant when APY1 is suppressed in the R2-4A line.
Figure 2.
Free IAA levels are increased in the shoot apices and decreased in the basal halves of hypocotyls of Arabidopsis seedlings after the induction of apyrase RNAi by estradiol. Free IAA levels were significantly decreased in the basal half of R2-4A (+estradiol) hypocotyls compared with the basal halves of R2-4A (−estradiol) or Ws wild-type (WT; +/−estradiol) hypocotyls, and the increase of free IAA found in the apices of R2-4A (+estradiol) hypocotyls was only marginally insignificant. Free IAA in the apical half of hypocotyls showed no difference among the groups analyzed. A shows a graphical representation of the data shown in B. se values are marked by vertical bars. These results are representative of three or more biological repeats. Statistically evaluated differences between samples are indicated by asterisks (Student’s t test; **P < 0.002, *P < 0.07; n = 3). FW, Fresh weight.
Since polar auxin transport is inhibited in hypocotyls of induced R2-4A plants, we also measured IAA transport in induced R2-4A roots, as eATP has already been implicated in the regulation of root basipetal IAA transport (Tang et al., 2003). In contrast to hypocotyls, IAA moves in two polarities in roots in two distinct transport streams, with basipetal (or shootward) transport occurring in the epidermal cells in the root tip and linked to root gravitropism and elongation (Rashotte et al., 2000). Acropetal or rootward IAA transport occurs in the central cylinder and is linked to the control of lateral root formation (Reed et al., 1998). IAA transport values are reported as percentages of transport in the wild type. Basipetal IAA transport was significantly reduced in induced R2-4A (P < 0.04; Fig. 3). In contrast, acropetal IAA transport was not altered in this RNAi line. This finding is consistent with other regulatory strategies, such as reversible protein phosphorylation, that show differential regulation of these two polarities of root IAA transport (Rashotte et al., 2001; Sukumar et al., 2009). Therefore, we examined the effect of the induction of this RNAi construct on root growth and development and on auxin-induced gene expression in this tissue, focusing on root apical development and growth and gravitropism, as these processes are linked to basipetal IAA transport.
Figure 3.
Polar auxin transport is inhibited in primary roots of Arabidopsis after the induction of apyrase RNAi by estradiol. IAA transport was measured in both the basipetal (shootward) and acropetal (rootward) directions in 8-d-old seedlings 4 d after transfer to estradiol. Basipetal IAA transport was reduced in R2-4A roots when induced by estradiol. The average and se values are reported for greater than 42 seedlings in the basipetal assay and greater than 60 seedlings in the acropetal assay and are a summary of three biological repeats. Basipetal IAA transport levels in the wild type averaged 4.2 fmol, and acropetal IAA transport values in the wild type averaged 8.8 fmol. A statistically significant difference between the wild type and the RNAi line is indicated by the asterisk (Student’s t test; *P < 0.05).
Suppression of APY1 Expression in apy2 DR5:GFP Plants Induces Altered GFP Expression and Morphological Changes in Roots
Both hypocotyl and root growth are inhibited in the estradiol-treated R2-4A seedlings, but primary root length is more dramatically affected in this line. We tested whether the reduced root growth was accompanied by altered auxin-induced gene expression using a DR5:GFP reporter. The transgenic DR5:GFP plants were crossed with the R2-4A RNAi mutant line to introduce DR5:GFP into the RNAi mutant. We performed a time-course treatment of the R2-4A seedlings with estradiol and used confocal microscopy to monitor the GFP signal, thereby evaluating the endogenous auxin response in roots by this indirect method. Treatment with estradiol for 1 or 2 d did not induce changes in the GFP signal or in the morphology of the Ws wild-type or R2-4A root (Supplemental Fig. S2). However, after 3 d of estradiol treatment, when R2-4A roots begin exhibiting reduced APY1 expression (Supplemental Fig. S3) and reduced root growth, changes in the GFP signal begin to appear (Fig. 4, A–D). The GFP signal is reduced in the columella and the lateral root cap and in epidermal cells at the root tip. After 4 d of estradiol treatment, the typical pattern of GFP distribution in Ws wild-type primary roots is even more disrupted in the mutant, with more of the GFP signal observed in root cortex cells associated with the distal root vasculature and epidermal cells in the zone of the root where root hairs begin differentiating (Fig. 4, E–H). These results suggest that the suppression of apyrases mimics the effects of treating wild-type seedlings with high concentrations of ATP, and one mode by which apyrase suppression can inhibit root growth is by disrupting the normal pattern of auxin transport.
Figure 4.
Suppression of APY1 in the apy2 mutant alters the morphology of roots and the expression of the auxin response reporter DR5:GFP. Shown are primary roots of Arabidopsis seedlings grown for 6 d in the light. A, C, E, and G, Roots of DR5:GFP R2-4A treated for 3 d (A and C) or 4 d (E and G) with the estradiol inducer. B, D, F, and H, DR5:GFP Ws roots treated with estradiol for 3 d (B and D) or 4 d (F and H). These results are representative of 10 or more biological repeats. Bars = 100 µm. WT, Wild type.
The light microscope images of estradiol-treated R2-4A roots indicated that root morphology was greatly altered as apyrase expression was suppressed. In order to better examine the structure and determine the specific regions of the root affected in the R2-4A line, scanning electron microscopy was performed on Ws wild-type roots and mutant roots with suppressed apyrase expression after treatment with estradiol for 6 d. In the mutants, many differentiated root hairs, which are a mark of the maturation zone, were observed extending all the way from the root-shoot junction to near the meristematic zone just basal to the root cap (Fig. 5). The mutant roots also showed a lack of a well-defined meristematic zone, a greatly reduced zone of elongation, as well as a larger diameter near the tip than Ws wild-type seedlings. When DR5:GFP R2-4A seedlings were grown on estradiol for 6 d, the GFP fluorescence accumulated in most cells near the root tip, and the auxin maximum was not apparent in the quiescent zone, in contrast to the strong maximum seen in Ws wild-type roots (Supplemental Fig. S4).
Figure 5.
Scanning electron microscopy images of 6-d-old estradiol-treated Ws wild-type and RNAi R2-4A mutant seedlings showing root swelling and ectopic root hair development. A, Enlarged apical zone of Ws wild-type root. B, Enlarged apical zone of R2-4A root. These results are representative of three or more biological repeats. Bars = 200 µm.
The Root Gravitropic Response Is Altered by Genetic Suppression of Apyrase Expression and Chemical Inhibition of Apyrase Activity
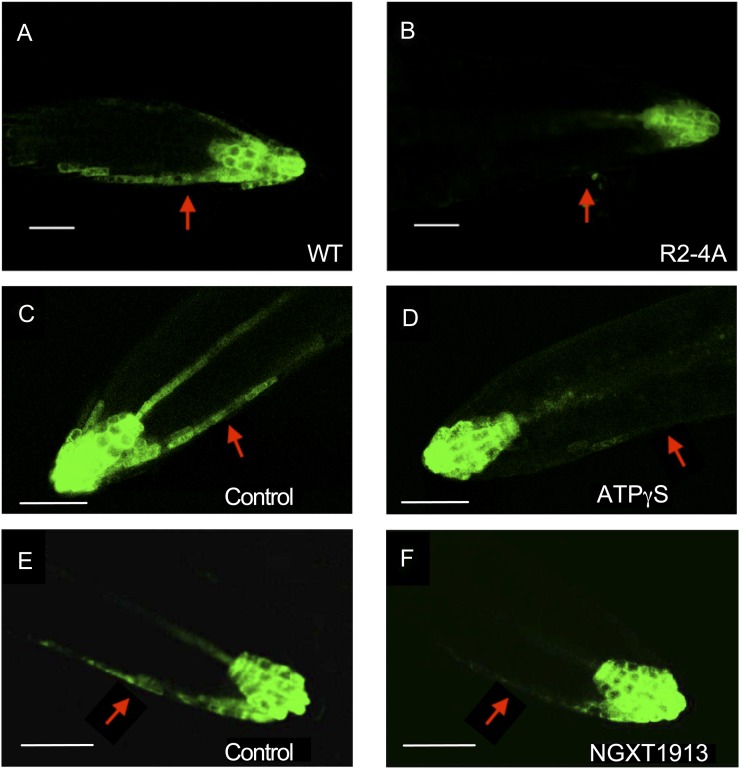
In R2-4A roots that were not treated with estradiol, there was asymmetry of GFP fluorescence, similar to that observed in DR5:GFP Ws wild-type roots (data not shown). In contrast to DR5:GFP seedlings that have normal APY1 and/or APY2 expression, there was no asymmetry of GFP fluorescence in RNAi-suppressed roots of DR5:GFP-expressing seedlings after gravistimulation (Fig. 6, A and B). A similar result was observed in wild-type DR5:GFP seedlings when they were treated with 800 µm ATPγS or with the apyrase inhibitor NGXT1913 (i.e. in these seedlings, there also was no asymmetry of GFP signal after gravistimulation; Fig. 6, C–F). These results suggest that apyrases and extracellular nucleotides may play a role during root gravitropism, so we gravistimulated both RNAi-suppressed plants and Ws wild-type plants treated with a chemical inhibitor or apyrase inhibitor, NGXT1913 (Windsor et al., 2002). The growth of estradiol-induced R2-4A roots is significantly decreased in comparison with Ws wild-type roots (Wu et al., 2007), and this growth inhibition would be expected to also inhibit gravitropic growth, which is what we observed (data not shown). Treatment with the apyrase inhibitor NGXT1913 (Windsor et al., 2002) also decreased both the gravitropic angle and elongation growth of Ws roots reoriented relative to the gravity vector, although its effect on gravitropism was significantly greater than its effect on growth (Supplemental Fig. S5). A similar result (data not shown) was obtained with another apyrase inhibitor, NGXT191 (Windsor et al., 2003). These results are consistent with the inference that apyrase activity also contributes to the lateral transport of auxin that is needed for the gravitropic response.
Figure 6.
A and B, Fluorescent GFP signals in horizontally positioned primary roots of wild-type (WT; A) and R2-4A (B) plants expressing DR5:GFP. Both wild-type and R2-4A plants were treated with estradiol, which induced the suppression of apyrase expression by RNAi in the R2-4A mutant. C to F, GFP signals in horizontally positioned primary roots of wild-type plants expressing DR5:GFP before and after treatment with 800 µm ATPγS (C and D) or 7.5 µg mL−1 NGXT1913 (E and F). Arrows indicates the lower flank of the primary root, where the GFP signal is evident in the epidermal cells of the wild-type roots but not in the R2-4A roots and not in treated roots. These results are representative of 10 or more biological repeats. Signals were assayed 5 h after roots moved to the horizontal position. Bars = 50 µm.
We also tested the effects of NGXT1913 on the growth of wild-type and R2-4A hypocotyls treated with estradiol. Inhibitor treatment reduced the growth of wild-type hypocotyls by a statistically significant 13% (P < 0.05), but it inhibited the growth of R2-4A mutants significantly less (6.5%; P < 0.05), which would be expected since the mutants already have suppressed growth even without NGXT1913 treatment. These hypocotyl results were averages of four biological repeats, with n ≥ 30 for each repeat.
Both Cell Elongation and Mitosis Are Inhibited When Apyrase Expression Is Suppressed in Primary Roots
After R2-4A plants were treated with estradiol, there was a reduction in the overall length of their primary roots (Table I) due to a combination of factors. The overall dimensions of the quiescent center in both Ws wild-type and R2-4A plants were approximately the same (data not shown), but in R2-4A plants, the length of the mitotic zone and the elongation zone were shorter than in Ws plants (Table I). Measurement of cell lengths in these zones showed that R2-4A plants had much less uniform cell sizes than Ws wild-type plants, a difference that was quantified by showing that a higher percentage of cells in R2-4A plants were smaller than one-half the mean diameter compared with Ws wild-type plants (Supplemental Table S1). Linear counts of cells showed that there were fewer cells in both the mitotic and elongation zones of R2-4A plants than Ws wild-type plants (Table II). When taken as a whole, the data revealed that the reduction in length of R2-4A roots was due to both fewer cells in the mitotic zone and elongation zone and less expansion in the elongation zone.
Table I. Comparison of the lengths of the root tip zone, mitotic zone, and elongation zone in wild-type and R2-4A plants after treatment with estradiol, which suppresses apyrase expression in the R2-4A plants.
All values shown are lengths given in micrometers and are averages measured in seven to 10 different plants each day of treatment.
| Root Tipb |
Mitotic Zonec |
Elongation Zoned |
||||
|---|---|---|---|---|---|---|
| Treateda | Wild Type | R2-4A | Wild Type | R2-4A | Wild Type | R2-4A |
| 3 d | 413.4 | 322.6e | 145.7 | 93.1e | 233.1 | 198.5 |
| 4 d | 405.1 | 254.2e | 143.6 | 93.1e | 227.8 | 130.7e |
| 5 d | 386.7 | 220.4e | 153.8 | 78.4e | 203.7 | 111.9e |
| 6 d | 519.7 | 190.8e | 168.0 | 53.5e | 315.2 | 107.7e |
Days the wild type and R2-4A mutants were treated with estradiol.
Measured from the cap apex to the beginning of the zone of differentiation.
Measured from the first cell adjacent to the quiescent center to the first visibly elongating cell.
Measured from the first visibly elongating cell to the first epidermal cell with a visible root hair bud.
Value is significantly different from the wild-type value (P < 0.05).
Table II. Suppression of apyrase by estradiol-induced RNAi in R2-4A plants reduces the average cell number measured in the mitotic and elongation zones of primary roots.
| Mitotic Zoneb |
Elongation Zoneb |
|||
|---|---|---|---|---|
| Treateda | Wild Type | R2-4A | Wild Type | R2-4A |
| 3 d | 24.0 | 14.8c | 11.5 | 9.1 |
| 4 d | 20.9 | 13.9c | 14.7 | 8.6c |
| 5 d | 21.8 | 10.3c | 14.0 | 6.5c |
| 6 d | 22.5 | 8.8c | 17.9 | 10.8c |
Days the wild type and R2-4A mutants were treated with estradiol.
Zones defined as in Table I. All values are average number of cells in the zone measured in five to 10 different plants.
Value is significantly different from the wild-type value (P < 0.05).
Growth-Inhibiting Levels of Applied Nucleotides Do Not Alter the Localization of PIN1, PIN2, AUX1, or ABCB19 Transporters
Dose-response assays of the effect of applied ATPγS on the growth of etiolated hypocotyls and light-grown roots revealed that concentrations between 500 and 800 µm were needed to have a significant inhibitory effect (Roux et al., 2006; W. Tang and S.J. Roux, unpublished data). However, 800 µm ATPγS treatment did not alter the distribution of either PIN1 or ABCB19, which participate in acropetal IAA transport, or PIN2 and AUX1, which participate in basipetal IAA transport, in light-grown roots (Supplemental Figs. S6 and S7). Additionally, ATPγS treatment significantly inhibited the elongation growth of primary roots in pin2 and aux1 mutants just as it did in wild-type seedlings (data not shown).
The Gravity Response of Col-0 Roots Is Inhibited by Applied ATP
The original experiments on the effects of applied ATP on root gravitropic growth were carried out in the Ws ecotype (Tang et al., 2003). To test whether ATP-induced growth effects could be seen in Col-0, the original tests of ATP effects on root growth and gravitropism were repeated in Col-0 seedlings. The results indicated that in this ecotype, 1 mm ATP could significantly inhibit root gravitropism without significantly inhibiting growth (Supplemental Table S2), whereas in Ws, 3 mm was the lowest concentration that had inhibitory effects (Tang et al., 2003). In Col-0, as in Ws, 2 and 5 mm ATP significantly inhibited gravitropism, but in Col-0, unlike in Ws, these higher concentrations also had a significant inhibitory effect on root growth, although the growth effects (less than 2-fold) were much smaller than the curvature effect (more than 4.5-fold). In the Col-0 tests, the pH in ATP-containing medium remained at or above 5.0, a pH that by itself does not inhibit gravitropic growth. Overall, the data indicated that the gravitropic growth of Col-0 ecotype roots was more sensitive to the inhibitory effects of applied ATP than that of Ws roots.
DISCUSSION
In etiolated hypocotyls, pollen tubes, cotton fibers, and root hairs, low concentrations of applied poorly hydrolyzable nucleotides (ATPγS or ADPβS) promote growth, and higher concentrations inhibit growth, so there appears to be an optimal [eATP] for growth (Roux and Steinebrunner, 2007; Wu et al., 2007; Reichler et al., 2009; Clark et al., 2010a, 2010b). These dose-response results are qualitatively similar to the bimodal dose-response curves obtained using auxin (Mulkey et al., 1982) or ethylene (Pierik et al., 2006). Studies in Arabidopsis indicate that APY1 and APY2 may function in part as ectoapyrases to regulate eATP levels outside the cell (Wu et al., 2007). Both are abundant in rapidly growing tissues and appear to be needed to maintain optimal eATP levels for normal plant growth (Wolf et al., 2007; Wu et al., 2007). There is recent evidence that APY1 and APY2 localize to the Golgi, where they could function to regulate protein glycosylation and/or polysaccharide levels (Dunkley et al., 2004; Riewe et al., 2008; Chiu et al., 2012; Parsons et al., 2012; Schiller et al., 2012).
Given the complex interaction of signaling pathways, the steps that lead from eATP to growth control are likely to intersect at some point with transduction steps induced by hormones. Consistent with this idea, applied nucleotides disrupt basipetal auxin transport in maize (Zea mays) and Arabidopsis roots (Tang et al., 2003). This finding led us to hypothesize that suppressing the expression of APY1 and APY2 could affect auxin transport in Arabidopsis seedlings. We tested this idea directly by examining whether plants of the RNAi line R2-4A, which are suppressed in the expression of APY1 in the background of the apy2 null knockout, would show disrupted auxin transport. We demonstrate that both in hypocotyls and roots, reduction in apyrase expression using RNAi leads to reductions in basipetal auxin transport in those tissues in which growth is altered by apyrase suppression. Our data suggest that the growth suppression by APY mRNA interference is at least partially due to auxin transport inhibition.
Correspondingly, suppression of APY1/APY2 expression results in an altered GFP reporter pattern in DR5:GFP-expressing roots, consistent with there being altered auxin distribution (or sensitivity) in the roots of these mutants. Estradiol-treated R2-4A seedlings also have reduced lateral root formation (Wu et al., 2007) and root acropetal IAA transport. This is true also for mutants in genes encoding auxin transport proteins (Marchant et al., 2002; Benková et al., 2003; Lewis et al., 2011) and in wild-type plants treated with inhibitors of polar auxin transport (Reed et al., 1998; Casimiro et al., 2001).
One mechanism by which APY1/APY2 suppression could block auxin transport would be by suppressing the transcript abundance of genes that are involved in auxin transport. Polar auxin transport in plants is well characterized and is mediated by several protein families, AUXIN RESISTANT1/LIKE AUXIN1 (AUX1/LAX) influx carriers, PIN-FORMED (PIN) efflux facilitators, and ATP-binding cassette subfamily B (ABCB) transporters (Peer et al., 2011). To test this hypothesis, we used microarray and quantitative reverse transcription-PCR to examine potential expression changes for AUX1, LAX3, ABCB1, ABCB4, ABCB19, and PIN1 to PIN8. We compared message levels for these genes in both dark- and light-grown R2-4A and wild-type seedlings and found that, although there were major changes in the expression of many genes, there were no significant differences in the message levels of any of these auxin transporters/carriers (data not shown; J. Wu, J.C. Yao, and S.J. Roux, unpublished data). However, as noted below, these results do not rule out the possibility that apyrase suppression could alter the posttranscriptional activity of auxin transporters.
Rahman et al. (2007) characterized the inhibitory effects of applying IAA or auxin analogs as well as chemical inhibitors of auxin transport on root growth. That study, which used treatment concentrations that decreased root growth rates by 50%, showed that these compounds could be separated into two groups based on their mode of growth inhibition. IAA, 1-naphthalene acetic acid, and triiodobenzoic acid inhibit root growth by decreasing the size of the elongation zone, while 2,4-dichlorophenoxyacetic acid and NPA inhibit root growth, mainly by decreasing the rate of cell production in the meristem. We analyzed morphological changes in root development by performing a time-course evaluation of the effects of estradiol treatment in the R2-4A RNAi line. Our results indicated that apyrase suppression both altered the rate of cell expansion in the elongation zone and disrupted normal patterns of cell division. Thus, the decrease in root length should be attributed to diminished rates of both cell elongation and mitosis, which is an expected result of auxin transport inhibition. The swelling and epidermal cell blebbing also observed in primary roots would suggest an alteration in cell wall integrity, which could be induced by 1-aminocyclopropane-1-carboxylic acid (ACC; Tsang et al., 2011) or ethylene, which both affect and are affected by auxin transport changes in roots (Muday et al., 2012).
Prolonged treatment of wild-type roots with high concentrations of eATP or apyrase inhibitors does not result in the same severe root morphological abnormalities observed in estradiol-treated R2-4A roots (data not shown). Additionally, there are rapid gene expression changes observed in estradiol-treated R2-4A roots, and recent data indicate that APY1 and APY2 localize to and function in the Golgi (Dunkley et al., 2004; Chiu et al., 2012; Parsons et al., 2012; Schiller et al., 2012). Thus, even though suppressing APY1 and APY2 expression increases eATP levels and disrupts basipetal auxin transport, it seems probable that the R2-4A root phenotype may be due to the effects of suppressing apyrase expression that are not directly related to an increase in the eATP concentration.
Applied nucleotides induce a rapid increase in the transcript abundance of genes encoding ACC Synthase6 and Ethylene Response Factor4 in Arabidopsis (Jeter et al., 2004; Song et al., 2006). Correspondingly, higher levels of applied nucleotides can induce ethylene production in the cotton fibers of cultured ovules, and the ACC synthesis inhibitor aminovinyl-Gly can block the effects of applied ATPγS and ADPβS on the growth of fibers (Clark et al., 2010a), hypocotyls (Roux and Steinebrunner, 2007), and root hairs (data not shown). Thus, it would be expected that in addition to auxin-mediated effects on root growth and development, there might also be a role for ACC and/or ethylene in the R2-4A root phenotype. In fact, many of the root-tip anatomical changes described in Tables I and II, Supplemental Table S1, and Figure 4 can be influenced by ethylene (Ma et al., 2003). We also found that application of 1 µm aminovinyl-Gly to estradiol-treated R2-4A seedlings had a slight but statistically significant promotive effect on their root growth (data not shown). The interaction between auxin and ethylene in root growth and development is complex, with these two hormones acting synergistically and antagonistically in different processes in the root (Muday et al., 2012). Furthermore, alterations in auxin transport and auxin levels induce ethylene production in the root elongation zone, while increased ethylene results in increased auxin levels in the root cortex and quiescent center (Bennett and Scheres, 2010).
Chemical inhibition of apyrase activity by the application of apyrase inhibitors results in growth inhibition of pollen (Wu et al., 2007) and cotton fibers (Clark et al., 2010a). Based on the altered auxin transport phenotype observed in the R2-4A line, where expression of APY1 and APY2 is genetically suppressed, one might predict that the application of apyrase inhibitors could also affect polar auxin transport. Our results show that the apyrase inhibitor NGXT1913 does indeed suppress the gravitropic response of roots, but unlike NPA, it does not block polar auxin transport in hypocotyl sections (data not shown). This may reflect its better penetration into roots than into cuticle-covered hypocotyls or its limited disruption of only the lateral transport of auxin needed for gravitropism rather than of the basipetal polar transport measured in the hypocotyl assays.
As discussed earlier, ABCB19 (MDR1) and other ABCB proteins have been shown to facilitate auxin transport (Lewis et al., 2009; Titapiwatanakun et al., 2009). Thomas et al. (2000) described a potential role for ectoapyrases and eATP in regulating the transport activity of ABCB19. The molecular mechanism by which ABCB proteins transport the efflux of compounds is poorly understood, and although hydrolysis of cytoplasmic ATP is required for their activity, it is also possible that a gradient of ATP from inside to outside the cell is needed for their proper function. In agreement with this idea, Lee et al. (2011) have shown that expression of an ABCA1 protein in animal cells results in increased levels of eATP in these cells and that changes in [eATP] regulate ABCA1 transport activity. To the extent that [eATP] could influence ABCB transport activity in plant cells, this could be a plausible mechanism by which the inhibition of ectoapyrase activity, which would raise the [eATP], inhibits auxin transport.
Applied nucleotides induce nitric oxide production in plant cells (Foresi et al., 2007; Torres et al., 2008; Wu and Wu, 2008; Reichler et al., 2009; Clark et al., 2010b). Thus, another possible mechanism by which ectoapyrases and eATP levels could regulate auxin signaling is by inducing increased nitric oxide levels. The two best-documented mechanisms by which nitric oxide can affect plant growth and development are by increasing the level of the cyclic GMP signal and by nitrosylation of the Cys residues of key enzymes, a reversible posttranslational modification that can alter protein function. A recent report found that the TIR1 auxin receptor can be nitrosylated and that its nitrosylation regulates its interaction with AUX/IAA proteins, thereby controlling their degradation (Terrile et al., 2011). Additionally, Fernández-Marcos et al. (2011) showed that high levels of nitric oxide inhibit PIN1-dependent auxin transport in Arabidopsis roots. Speculatively, the increased nitric oxide production induced by apyrase suppression could result in nitrosylation and inhibition of one or more of the proteins that drive auxin transport. This would provide a mechanistic basis for understanding the link between the suppression of APY1/APY2 and the inhibition of polar auxin transport.
MATERIALS AND METHODS
Polar Auxin Transport Measurement in Arabidopsis Hypocotyl Sections
Seedlings of Arabidopsis (Arabidopsis thaliana) ecotype Ws, RNAi line R2-4A (apy2 Ws transformed with APY1 mRNA interference), apy1 single knockout, apy2 single knockout, APY1-OE, or APY2-OE were grown in darkness for 6 d and transferred to continuous cool-white fluorescent light (80 μmol m−2 s−1) for 2 d. The assay was performed as described by Liu et al. (2011) with slight modifications. Briefly, agar donor blocks containing 10−7 m [3H]IAA were placed in contact with the apical end of 6-mm hypocotyl sections, an agar receiver block was placed on the basal end of each section, and transport was allowed to occur for 3 h. Agar blocks (1.5%, 2 × 2 mm) were separated from the agar plates by pieces of plastic wrap. Sections were kept upside down in a humid chamber during the transport period. Each section was then split into apical and basal halves, and radioactivity was determined in each half and in the receiver blocks. Data are expressed as dpm in the receiver block plus the basal portion of the tissue as a percentage of total dpm in the tissue plus the receiver blocks (basipetal). Diffusion controls were run with the orientation of the tissue section reversed (acropetal). Polar transport is defined as the percentage of total dpm in the basal portion of the tissue plus the receiver block at the end of the transport period. RNAi was induced by inclusion of 4 µm estradiol in the growth medium.
Measurement of Shootward and Rootward Auxin Transport in Arabidopsis Roots
The assays of shootward (basipetal) and rootward (acropetal) auxin transport in wild-type and apyrase RNAi line R2-4A primary roots were performed as described by Lewis and Muday (2009). For both assays, wild-type and R2-4A seedlings were grown under continuous cool-white fluorescent light for 4 d at 25°C on Murashige and Skoog (MS) medium to ensure that roots were at least 10 mm, then they were transferred to medium containing 4 µm estradiol for 4 d. A droplet of agar (5 µL containing 0.05% MES, 1.25% agar, and 100 nm [3H]IAA [American Radiolabeled Chemicals]) was applied so that it was just touching the root tip for shootward assays or at the root-shoot junction for rootward assays. During the assay, plants were placed under yellow-filtered light to prevent the breakdown of IAA that occurs under white light. For the shootward assays, after 5 h, a 5-mm segment located 2 mm from the root tip was excised. For the rootward assay, a 5-mm segment at the root tip was excised after 18 h. Radioactivity was quantified by scintillation counting. The reported values are averages and se of auxin transport calculated relative to the wild type in each assay and represent several independent trials, in which values from more than 42 individuals of each genotype were pooled for basipetal transport assays and more than 60 individuals were pooled for acropetal transport assays.
Determination of Free IAA Distribution in Arabidopsis Seedlings
Seedlings of Arabidopsis ecotype Ws and RNAi line R2-4A were grown in darkness for 6 d and transferred to continuous cool-white fluorescent light (80 μmol m−2 s−1) for 2 d. The harvesting, dissection, extraction, and assay of the plant material were performed exactly as described by Barkawi et al. (2010), using a SPE 215 liquid handling system (Gilson; Liu et al., 2011). Levels of free IAA were measured in the apices and apical and basal halves of Arabidopsis hypocotyls using isotope dilution gas chromatography-selected ion monitoring-mass spectrometry (Barkawi et al., 2010).
Generation of DR5:GFP RNAi Lines
To generate the DR5:GFP RNAi lines, the R2-4A line was crossed with a transgenic line expressing DR5:GFP. The F1 generation plants were selected on MS medium containing 20 μg mL−1 hygromycin. The plants that could grow on hygromycin plates were heterozygotes of Apy2, RNAi gene, and DR5-GFP gene but homozygotes of Apy1. The seeds harvested from the F1 generation plants were planted on agar in medium containing 4 μm estradiol in 150-mm petri dishes. After 6 d, light-grown seedlings that showed decreased root growth were selected and transferred to soil. The seeds collected from the F2 generation were used to screen for homozygotes of the RNAi mutant expressing DR5:GFP.
Confocal Microscopy
Seedlings treated with estradiol for different numbers of days were originally germinated and grown on MS plates. Both DR5:GFP R2-4A and DR5:GFP wild-type seedlings were transferred to plates containing 4 μm estradiol on day 2 or 3, as noted in the legend to Figure 3. Fluorescence images were captured at day 6 by a Leica SP2 AOBS confocal microscope with the filters set at 488/509 nm for excitation/emission. For root gravitropic response, seedlings were grown in light for 5 d and then rotated 90°. Images were obtained after 5 h of horizontal growth, and the confocal microscope noted above captured fluorescence images of the GFP signals.
Scanning Electron Microscopy
Fresh 6-d-old seedlings were fixed in 0.1 m phosphate buffer solution (pH 5.7) containing 1% paraformaldehyde and 2% glutaraldehyde. Samples were fixed under vacuum for 30 min and incubated at 4°C for 12 to 24 h. Fixed seedlings were washed three times in phosphate-buffered saline (pH 7.4) followed by two times in distilled water for 10 min. Samples were dehydrated at room temperature in an ethanol series for 15 min at each step as follows: 15%, 30%, 50%, 70%, 80%, 90%, and 95%, 2× absolute ethanol. Dehydrated specimens were further dried by critical point drying and mounted onto the stubs using double-sided mounting tapes. Dry seedlings were sputter coated with gold immediately after critical point drying. Images were captured by a Philips EM 515 scanning electron microscope operating at an accelerating voltage of 14.7 kV.
Apyrase Inhibitor Assays
Seeds (Ws) were sterilized as described by Tang et al. (2003), kept at 4°C for at least 3 d, and then sown on MS medium with or without the apyrase inhibitor NGXT1913 (Windsor et al., 2002) or NGXT191 (Windsor et al., 2003), which was dissolved in dimethyl sulfoxide. The control plates contained the same final concentration of dimethyl sulfoxide (0.01%) as the treatment plates. Seedlings were grown in darkness for 3 d on vertically oriented plates, then the plates were moved into continuous white light, reoriented 90°, and the seedlings were grown for an additional 18 h. The growth temperature throughout was 23°C. Seedlings were imaged before being reoriented and 18 h later, and ImageJ was used to measure changes in the angle of curvature and overall growth between these two time points.
Anatomical Studies
Ws wild-type and R2-4A plants were grown for 3, 4, 5, and 6 d on plates containing 4 μm estradiol, and the primary root tips were examined by light microscopy. The quiescent center was measured as the distance in µm or number of cells from the first cell interior to the apical epidermal layer to the first cell adjacent to the mitotic zone. The mitotic zone was measured from the first cell adjacent to the quiescent center to the first visibly elongating cell. The elongation zone was measured from the first visibly elongating cell to the first epidermal cell with a visible root hair bud. The zone of differentiation was determined to begin at the first epidermal cell with a visible root hair bud.
Root Gravitropic Assays in the Col-0 Ecotype
Col-0 seedlings (6 d old) were transferred to plates with and without ATP, and roots were assayed for growth and curvature 24 h after gravistimulation as described in table 1 of Tang et al. (2003). The medium pH for all plates was adjusted to approximately 5.0.
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under accession numbers At3g04080 (APY1) and At5g18280 (APY2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ATPγS differentially inhibits etiolated hypocotyl growth of wild-type and mutant seedlings.
Supplemental Figure S2. Treatment with estradiol for 1 or 2 d does not significantly alter the expression of the DR5:GFP in mutant roots.
Supplemental Figure S3. APY1 transcript levels are reduced in R2-4A seedlings after 2.75 d of estradiol treatment.
Supplemental Figure S4. DR5:GFP expression is altered in R2-4A seedlings after 6 d of estradiol treatment.
Supplemental Figure S5. Chemical inhibition of apyrase activity blocks gravitropic growth of roots of Arabidopsis.
Supplemental Figure S6. Distribution of neither PIN1:GFP nor ABCB19:GFP is altered in Arabidopsis roots by treatment with ATPγS.
Supplemental Figure S7. Distribution of neither PIN2:GFP nor AUX1:YFP is altered in Arabidopsis roots by treatment with ATPγS.
Supplemental Table S1. Suppression of apyrase increases the size diversity of cells in the mitotic and elongation zones.
Supplemental Table S2. Gravity response of Arabidopsis Col-0 roots in the absence or presence of ATP 24 h after reorientation.
Acknowledgments
We thank Jerry Cohen for advice and the use of the facilities in his laboratory for auxin analyses, Nathan Miller and Tobias Baskin for helpful discussions, and Trieu Pham and Andrew Cervantes for their assistance in analyzing the gravitropic response of R2-4A plants. We also thank Vibhuti Rana for performing root growth assays and Marianna Grenadier for creating the final figures.
Glossary
- eATP
extracellular ATP
- [eATP]
extracellular ATP concentration
- RNAi
RNA interference
- IAA
indole-3-acetic acid
- Col-0
Columbia
- Ws
Wassilewskija
- NPA
N-1-naphthylphthalamic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- MS
Murashige and Skoog
References
- Barkawi LS, Tam Y-Y, Tillman JA, Normanly J, Cohen JD. (2010) A high-throughput method for the quantitative analysis of auxins. Nat Protoc 5: 1609–1618 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett T, Scheres B. (2010) Root development: two meristems for the price of one? In MCP Timmermans, ed, Plant Development: Current Topics in Developmental Biology, Vol 91. Elsevier Academic Press, San Diego, pp 67–102 [DOI] [PubMed]
- Burnstock G. (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T-Y, Christiansen K, Moreno I, Lao J, Loqué D, Orellana A, Heazlewood JL, Clark G, Roux SJ. (2012) AtAPY1 and AtAPY2 function as Golgi localized nucleoside diphosphatases in Arabidopsis thaliana. Plant Cell Physiol (in press) [DOI] [PubMed] [Google Scholar]
- Clark G, Roux SJ. (2009) Extracellular nucleotides: ancient signaling molecules. Plant Sci 177: 239–244 [Google Scholar]
- Clark G, Roux SJ. (2011) Apyrases, extracellular ATP and the regulation of growth. Curr Opin Plant Biol 14: 700–706 [DOI] [PubMed] [Google Scholar]
- Clark G, Torres J, Finlayson S, Guan X, Handley C, Lee J, Kays JE, Chen ZJ, Roux SJ. (2010a) Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol 152: 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Wu M, Wat N, Onyirimba J, Pham T, Herz N, Ogoti J, Gomez D, Canales AA, Aranda G, et al. (2010b) Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol Biol 74: 423–435 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang ZL, Shin R, Colaço R, Laohavisit A, Shabala S, Davies JM. (2011) Receptor-like activity evoked by extracellular ADP in Arabidopsis root epidermal plasma membrane. Plant Physiol 156: 1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley TPJ, Watson R, Griffin JL, Dupree P, Lilley KS. (2004) Localization of organelle proteins by isotope tagging (LOPIT). Mol Cell Proteomics 3: 1128–1134 [DOI] [PubMed] [Google Scholar]
- Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O. (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA 108: 18506–18511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L. (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CR, Tang WQ, Henaff E, Butterfield T, Roux SJ. (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16: 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Sivaguru M, Stacey G. (2006) Extracellular ATP in plants: visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142: 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Karwatsky J, Ma L, Zha XH. (2011) ABCA1 increases extracellular ATP to mediate cholesterol efflux to ApoA-I. Am J Physiol Cell Physiol 301: C886–C894 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Muday GK. (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK. (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin transport proteins. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Wu G, Ljung K, Spalding EP. (2009) Auxin transport into cotyledons and cotyledon growth depend similarly on the ABCB19 multidrug resistance-like transporter. Plant J 60: 91–101 [DOI] [PubMed] [Google Scholar]
- Liu X, Cohen JD, Gardner G. (2011) Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol 157: 891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM. (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Mulkey TJ, Kuzmanoff KM, Evans ML. (1982) Promotion of growth and shift in the auxin dose/response relationship in maize roots treated with the ethylene biosynthesis inhibitors aminoethoyxvinylglycine and cobalt. Plant Sci Lett 25: 43–48 [Google Scholar]
- Parsons HT, Christiansen K, Knierim B, Carroll A, Ito J, Batth TS, Smith-Moritz AM, Morrison S, McInerney P, Hadi MZ, et al. (2012) Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol 159: 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Blakeslee JJ, Yang HB, Murphy AS. (2011) Seven things we think we know about auxin transport. Mol Plant 4: 487–504 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50: 514–528 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ. (2009) Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot 60: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Fernie AR, Wucke C, Geigenberger P. (2008) The potato-specific apyrase is apoplastically localized and has influence on gene expression, growth, and development. Plant Physiol 147: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux SJ, Song C, Jeter C. (2006) Regulation of plant growth and development by extracellular nucleotides. In F Baluska, S Mancuso, D Volkmann, eds, Communication in Plants. Springer, New York, pp 221–234 [Google Scholar]
- Roux SJ, Steinebrunner I. (2007) Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci 12: 522–527 [DOI] [PubMed] [Google Scholar]
- Schiller M, Massalski C, Kurth T, Steinebrunner I. (2012) The Arabidopsis apyrase AtAPY1 is localized in the Golgi instead of the extracellular space. BMC Plant Biol 12: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Jeter C, Song C, Roux SJ. (2000) Molecular and biochemical comparison of two different apyrases from Arabidopsis thaliana. Plant Physiol Biochem 38: 913–922 [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ. (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar P, Edwards KS, Rahman A, Delong A, Muday GK. (2009) PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis. Plant Physiol 150: 722–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Gilroy S, Jones AM, Stacey G. (2010a) Extracellular ATP signaling in plants. Trends Cell Biol 20: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Swanson SJ, Gilroy S, Stacey G. (2010b) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 154: 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady SR, Sun Y, Muday GK, Roux SJ. (2003) Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol 131: 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrile MC, París R, Calderón-Villalobos LIA, Iglesias MJ, Lamattina L, Estelle M, Casalongué CA. (2011) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE1 auxin receptor. Plant J 70: 492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. (2000) A role for ectophosphatase in xenobiotic resistance. Plant Cell 12: 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, Cheng Y, Adamec J, Nagashima A, Geisler M, et al. (2009) ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Torres J, Rivera A, Clark G, Roux S. (2008) Participation of extracellular nucleotides in the wound response of Dasycladus vermicularis and Acetabularia acetabulum (Dasycladales, Chlorophyta). J Phycol 44: 1504–1511 [DOI] [PubMed] [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS. (2011) Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol 156: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor B, Roux SJ, Lloyd A. (2003) Multiherbicide tolerance conferred by AtPgp1 and apyrase overexpression in Arabidopsis thaliana. Nat Biotechnol 21: 428–433 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Thomas C, Hurley L, Roux SJ, Lloyd AM. (2002) Automated colorimetric screen for apyrase inhibitors. Biotechniques 33: 1024–1030 [DOI] [PubMed] [Google Scholar]
- Wolf C, Hennig M, Romanovicz D, Steinebrunner I. (2007) Developmental defects and seedling lethality in apyrase AtAPY1 and AtAPY2 double knockout mutants. Plant Mol Biol 64: 657–672 [DOI] [PubMed] [Google Scholar]
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ. (2007) Apyrases (nucleoside triphosphate-diphosphohydrolases) play key roles in growth control in Arabidopsis. Plant Physiol 144: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Wu JY. (2008) Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot 59: 4007–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]