Abstract
Modulation of the malate content of tomato (Solanum lycopersicum) fruit by altering the expression of mitochondrially localized enzymes of the tricarboxylic acid cycle resulted in enhanced transitory starch accumulation and subsequent effects on postharvest fruit physiology. In this study, we assessed whether such a manipulation would similarly affect starch biosynthesis in an organ that displays a linear, as opposed to a transient, kinetic of starch accumulation. For this purpose, we used RNA interference to down-regulate the expression of fumarase in potato (Solanum tuberosum) under the control of the tuber-specific B33 promoter. Despite displaying similar reductions in both fumarase activity and malate content as observed in tomato fruit expressing the same construct, the resultant transformants were neither characterized by an increased flux to, or accumulation of, starch, nor by alteration in yield parameters. Since the effect in tomato was mechanistically linked to derepression of the reaction catalyzed by ADP-glucose pyrophosphorylase, we evaluated whether the lack of effect on starch biosynthesis was due to differences in enzymatic properties of the enzyme from potato and tomato or rather due to differential subcellular compartmentation of reductant in the different organs. The results are discussed in the context both of current models of metabolic compartmentation and engineering.
Starch is the most important carbohydrate used for food and feed purposes and represents the major resource for our diet (Smith, 2008). The total yield of starch in rice (Oryza sativa), corn (Zea mays), wheat (Triticum aestivum), and potato (Solanum tuberosum) exceeds 109 tons per year (Kossmann and Lloyd, 2000; Slattery et al., 2000). In addition to its use in a nonprocessed form, extracted starch is processed in many different ways, for instance as a high-Fru syrup, as a food additive, or for various technical purposes. As a result of this considerable importance, increasing the starch content of plant tissues has been a major goal for many years, with both classical breeding and biotechnological approaches being taken extensively over the last few decades (Martin and Smith, 1995; Regierer et al., 2002).
The pathway by which carbon is converted from Suc to starch in the potato tuber is well established (Kruger, 1997; Fernie et al., 2002; Geigenberger et al., 2004; Geigenberger, 2011). Imported Suc is cleaved in the cytosol by Suc synthase, resulting in the formation of UDP-Glc and Fru; the UDP-Glc is subsequently converted to Glc-1-P by UDP-Glc pyrophosphorylase. The second product of the Suc synthase reaction, Fru, is efficiently phosphorylated to Fru-6-P by fructokinase (Renz et al., 1993; Davies et al., 2005). Fru-6-P is freely converted to Glc-6-P, in which form it normally enters the amyloplast (Kammerer et al., 1998; Tauberger et al., 2000; Zhang et al., 2008), and once in the plastid, it is converted to starch via the concerted action of plastidial phosphoglucomutase, ADP-Glc pyrophosphorylase (AGPase), and the various isoforms of starch synthase (Martin and Smith, 1995; Geigenberger, 2011). Of these reactions, although some of the control of starch synthesis resides in the plastidial phosphoglucomutase reaction (Fernie et al., 2001b), the AGPase reaction harbors the highest proportion of control within the linear pathway (Sweetlove et al., 1999; Geigenberger et al., 1999, 2004). In addition, considerable control resides in both the Glc-6-P phosphate antiporter (Zhang et al., 2008) and the amyloplastidial adenylate transporter (Tjaden et al., 1998; Zhang et al., 2008) as well as in reactions external to the pathways, such as the amyloplastidial adenylate kinase (Regierer et al., 2002), cytosolic UMP synthase (Geigenberger et al., 2005), and mitochondrial NAD-malic enzyme (Jenner et al., 2001).
As part of our ongoing study of the constituent enzymes of the tricarboxylic acid (TCA) cycle, we made an initially surprising observation that increasing or decreasing the content of malate via a fruit-specific expression of antisense constructs targeted against the mitochondrial malate dehydrogenase or fumarase, respectively, resulted in opposing changes in the levels of starch (Centeno et al., 2011). We were able to demonstrate that these plants were characterized by an altered cellular redox balance and that this led to changes in the activation state of the AGPase reaction. Given that starch only accumulates transiently in tomato (Solanum lycopersicum; Beckles et al., 2001) as a consequence of this activation, the fruits were characterized by altered sugar content at ripening, a fact that dramatically altered their postharvest characteristics (Centeno et al., 2011). Here, we chose to express the antisense fumarase construct in potato in order to ascertain the effect of the manipulation in an organ that linearly accumulates starch across its development. The results obtained are compared and contrasted with those of the tomato fruit and within the context of current models of subcellular redox regulation.
RESULTS
Generation of Transgenic Plants and Screening
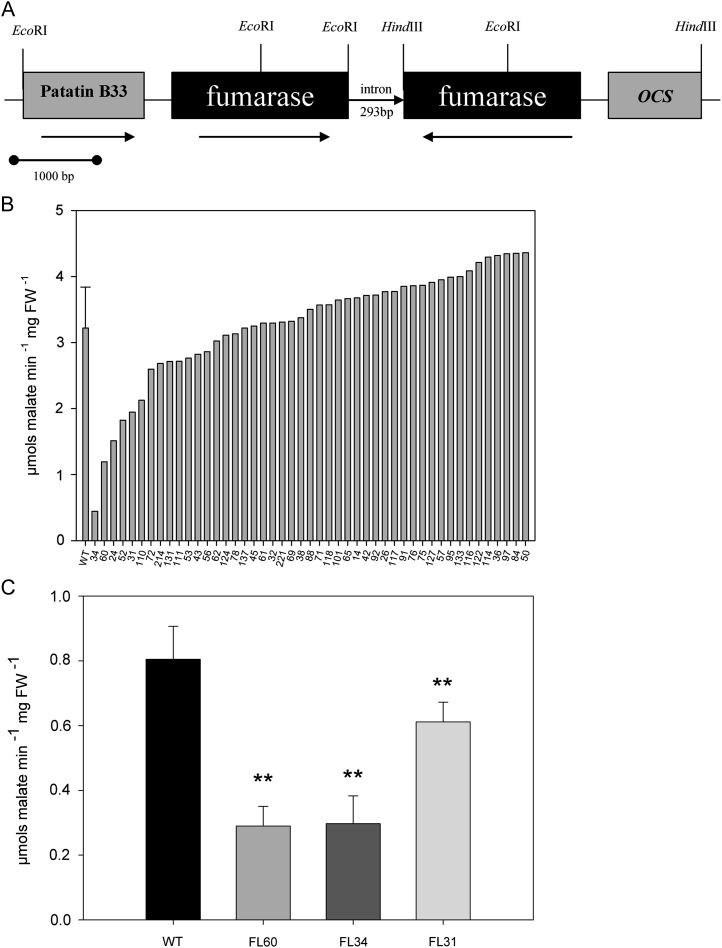
A 1,860-bp fragment of the fumarate hydratase (fumarase) gene from potato (Nast and Müller-Röber, 1996) was cloned using an RNA interference (RNAi) approach into the pBinAR transformation vector under the control of the tuber-specific patatin class I promoter (Twell and Ooms, 1987) and the ocs terminator (Fig. 1A). Plants obtained by Agrobacterium tumefaciens transformation were grown on Murashige and Skoog medium containing kanamycin, and approximately 50 lines resistant to this antibiotic were transferred to the greenhouse and cultivated under normal growth conditions. Tubers were harvested from 10-week-old, nonsenescent plants, and promising lines were selected by using an enzymatic assay for fumarase activity, with three lines, FL60, FL34, and FL31, being confirmed to displays reductions of activity of 65%, 63%, and 24%, respectively (Gibon et al., 2004; Fig. 1, B and C).
Figure 1.
Generation and screening of transgenic lines expressing the RNAi construct. A, RNAi construct for potato mitochondrial fumarase under the control of a tuber-specific B33 patatin promoter. Black arrows indicate the direction of transcription of the native genes. OCS, Octopine synthase terminator. B, Total fumarase activity in transgenic lines relative to the wild type. C, Total fumarase activity in selected transgenic lines (FL31, FL34, FL60) relative to the wild type (WT). Values are presented as means ± sd of five tubers per line. Bars are as follows: wild type, black bar; FL60, gray bar; FL34, dark gray bar; FL31, light gray bar. Asterisks indicate values that were determined by Student’s t test to be significantly different (P < 0.01) from the wild type. FW, Fresh weight.
Metabolic Profiling Reveals Mild Alterations in the Primary Metabolism of Transgenic Lines
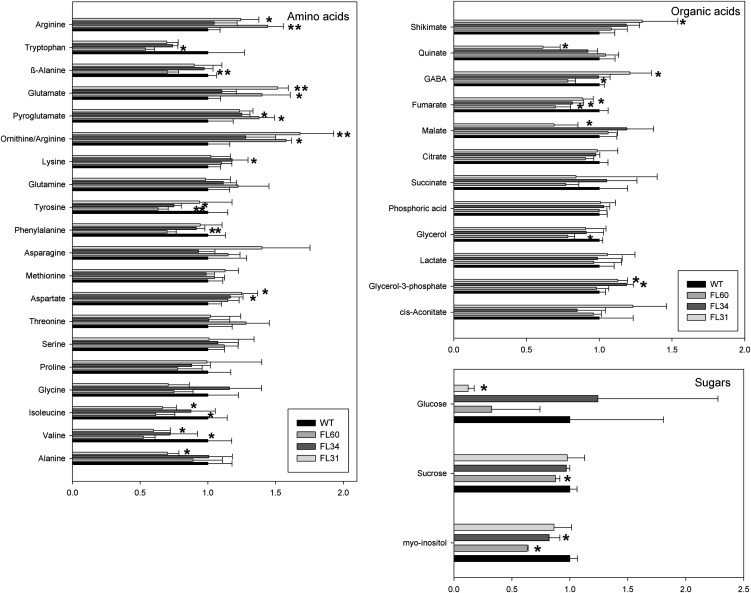
A previously established gas chromatography-mass spectrometry (GC-MS) protocol (Roessner et al., 2001; Lisec et al., 2006) was used in order to gain an overview of primary metabolism in tubers exhibiting reduced fumarase activity. A total of 36 polar metabolites were identified by GC-MS, providing good coverage of the metabolic pathways of starch synthesis and degradation, glycolysis, the TCA cycle, and amino acid metabolism. These studies revealed mild changes in the level of sugars, organic acids, and amino acids (Fig. 2). An additional more sensitive enzymatic assay for malate and fumarate revealed significant decreases in malate (significant for lines FL60 and FL31) and fumarate (all lines; Table I). No major changes, however, were observed for other TCA cycle intermediates, namely citrate and succinate, although in the case of FL60, which presented the strongest decrease in fumarase activity, a reduced level of γ-aminobutyrate was observed. These studies also revealed changes in the levels of amino acids. Notably, Tyr (significant for FL34 and FL60), Phe (FL60), Ile and Val (FL31, FL60) were significantly decreased, while Lys (FL34), Orn (FL31, FL60), and Asp (FL31, FL34) were significantly increased (Fig. 2). Furthermore, metabolic profiling revealed decreased levels of myoinositol (FL34, FL60), Suc (FL60), and Glc (FL31).
Figure 2.
Relative metabolite content in potato tubers from 10-week-old transgenic and wild-type (WT) plants. Data are normalized to the mean response calculated for the wild type of each measured batch. Values are presented as means ± sd of six individual plants per line. Bars are as follows: wild type, black bar; FL60, gray bar; FL34, dark gray bar; FL31, light gray bar. Asterisks indicate values that were determined by Student t test to be significantly different (P < 0.05) from the wild type.
Table I. Relative malate and fumarate content in potato tubers from 10-week-old transgenic and wild-type plants.
Data are normalized to the mean response calculated for the wild type. Values are presented as means ± sd of six individual plants per line. Boldface values were determined by Student’s t test to be significantly different from the wild type (P < 0.05).
| Variable | Wild Type | FL60 | FL34 | FL31 |
|---|---|---|---|---|
| Malate | 1.00 ± 0.13 | 0.66 ± 0.09 | 0.78 ± 0.11 | 0.67 ± 0.08 |
| Fumarate | 1.00 ± 0.09 | 0.71 ± 0.05 | 0.81 ± 0.05 | 0.75 ± 0.06 |
An additional enzymatic assay was performed in order to precisely measure levels of sugars and starch. Levels of spectrophotometrically measured Glc were significantly reduced (P < 0.05) for line FL31 (wild type, 2.8 ± 0.65 μmol Glc g−1 fresh weight; FL31, 1.85 ± 0.13; FL34 2.67 ± 0.47; FL60, 2.2 ± 0.43), with mild reduction for other lines staying in agreement with previous GC-MS measurements. Despite the change in Glc and importantly in malate, no alteration in starch level was observed (Table II). In addition, and in further contrast to the results obtained in tomato (Centeno et al., 2011), there was no significant differences in tuber number, weight, or yield in the transformants (Table II).
Table II. Yield and starch content of potato tubers harvested from 10-week-old transgenic and wild-type plants.
Values are presented as means ± sd of determinations from six individual plants per line.
| Variable | Wild Type | FL31 | FL34 | FL60 |
|---|---|---|---|---|
| Tuber number | 8.2 ± 2.4 | 8.4 ± 1.5 | 6 ± 1 | 12 ± 4.2 |
| Yield (g) | 230 ± 23 | 222 ± 54 | 207 ± 33 | 223 ± 56 |
| Tuber weight (g) | 29.4 ± 6.1 | 27.52 ± 9.46 | 34.91 ± 5.96 | 20.87 ± 9.46 |
| Starch (µmol Glc g−1 fresh weight) | 410.5 ± 53.1 | 416.7 ± 28.1 | 423.8 ± 38.2 | 422.8 ± 25.4 |
Rates of Respiration Remain Unchanged in the Transgenic Lines
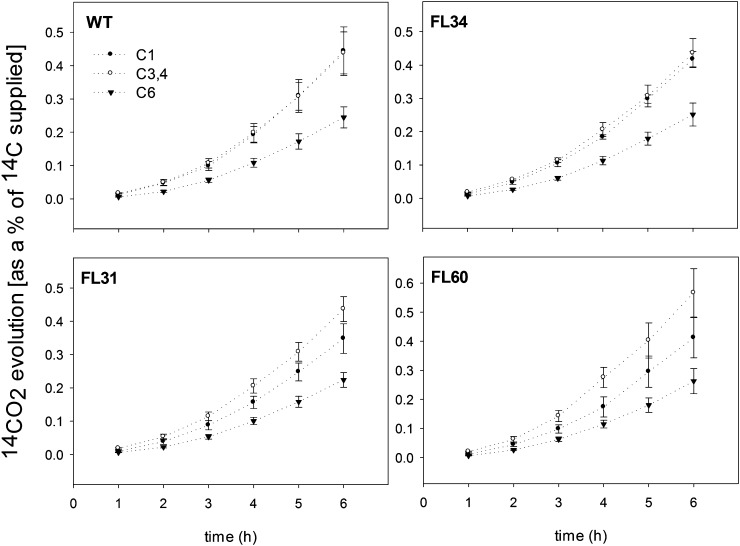
To estimate rates of respiration in the transformants, the evolution of 14CO2 was recorded following incubation of potato tuber discs in positionally labeled [14C]Glc. Freshly cut potato tuber discs were supplied with [1-14C]Glc, [3,4-14C]Glc, and [6-14C]Glc over a period of 6 h. During that time, evolved 14CO2 was collected at hourly intervals, allowing the estimation of fluxes through the oxidative pentose phosphate pathway (OPPP), glycolysis, TCA cycle, and amino acid biosynthesis. While CO2 released from position C1 of Glc is attributed to decarboxylation processes in the OPPP and the TCA cycle, CO2 that is released from positions C3:4 (by the action of pyruvate dehydrogenase or malic enzyme) and C6 (during the third turn of the TCA cycle) is derived from the mitochondria. The ratio of 14CO2 released from the C1 position of Glc to that released from the C6 position gives an estimate of the relative activities of glycolysis and the OPPP (ap Rees and Beevers, 1960). Thus, the C1-C6 ratio of released CO2 reflects a flux through the OPPP, while the ratio of CO2 evolution from the C1 position of Glc to that from the C3:4 position of Glc provides an indication of the relative rate of the TCA cycle with respect to other processes of carbohydrate oxidation (Nunes-Nesi et al., 2005).
Surprisingly, there was no significant alteration in 14CO2 evolution in the transgenic lines in comparison with the wild type (Fig. 3). While it was possible to observe a tendency of lower evolution of 14CO2 for the transgenic lines from position C1 and higher release of 14CO2 from the C6 Glc position in the most strongly inhibited line, FL60, these changes were not statistically significant.
Figure 3.
Respiration in potato tubers of 10-week-old transgenic and wild-type (WT) plants. The tuber discs were taken from 10-week-old plants and were incubated in 10 mm MES-KOH solution, pH 6.5, 0.3 mm Glc supplemented with 2.32 kBq mL−1 [1-14C]Glc, [3,4-14C]Glc, or [6-14C]Glc. The 14CO2 liberated was captured (at hourly intervals) in a KOH trap, and the amount of radiolabel released was subsequently quantified by liquid scintillation counting. Values are presented as means ± sd of determinations on six individual plants per line.
Evaluation of Other Major Fluxes in the Transgenic Lines
In order to expand the above study to encompass other major pathways of carbon metabolism, labeling of discs cut from fresh tubers using [U-14C]Glc was performed. Isolated discs were incubated in 2 mL of incubation medium containing [U-14C]Glc. Samples were incubated for 4 h, extracted, and fractionated into organic acids, amino acids, starch, protein, cell wall, phosphoesters, and Suc in order to analyze flux alterations between wild-type and transgenic plants. This time period was chosen based on two considerations. First, it is not so long as to be compromised by wound-induced changes in respiration (Roessner-Tunali et al., 2004); second, it allows labeling of more distant metabolite pools, such as those of the cell wall (which our previous studies in tomato root suggest may be affected following inhibition of TCA cycle activity; van der Merwe et al., 2009, 2010). There were no significant alterations in the level of incorporation of label or in the respired CO2 (Table III). The redistribution of label was unaltered in the transgenic lines. In order to estimate absolute fluxes in the transgenic lines, we calculated the specific activities of the hexose phosphate pool and used these to calculate the fluxes to starch, Suc, cell wall, and respiration using the assumptions documented by Geigenberger et al. (2000). The specific activities of the hexose phosphates were essentially unaltered, as were fluxes to starch, Suc, cell wall, and, importantly, respiration. This final result is in close accordance with the minor impact that diminished fumarase activity had on the respiratory flux and the steady-state metabolite levels determined in the GC-MS analysis.
Table III. Effect of decreased fumarase activity on the metabolism of [U-14C]Glc by potato tubers.
Tuber discs were preincubated in 10 mm MES-KOH (pH 6.5) containing 2 mm Glc in the presence of 10 mm [U-14C]Glc (specific activity of 8.11 MBq mmol−1). Each sample was extracted with boiling ethanol, and the amount of radioactivity in each metabolic fraction was determined as described in “Materials and Methods.” Values are means ± se of six biological replicates. Boldface values were determined by Student’s t test to be significantly different from the wild type (P < 0.05).
| Variable | Wild Type | FL31 | FL34 | FL60 |
|---|---|---|---|---|
| Label incorporated (Bq) | ||||
| Total uptake | 318.22±23.14 | 335.02±26.98 | 391.07±36.51 | 332.45±20.71 |
| Total metabolized | 257.88±25.86 | 292.25±21.84 | 326.05±27.29 | 281.20±20.82 |
| Redistribution of radiolabel (% of total assimilated) | ||||
| CO2 evolution | 8.20±0.65 | 8.42±0.63 | 8.29±0.66 | 8.92±0.67 |
| Organic acids | 20.69±4.16 | 22.39±4.39 | 29.74±1.90 | 25.38±3.41 |
| Amino acids | 9.41±0.76 | 12.55±2.02 | 9.63±0.35 | 11.35±0.84 |
| Suc | 28.62±3.78 | 35.76±3.04 | 21.73±1.96 | 27.51±2.46 |
| Starch | 19.79±4.36 | 9.05±2.77 | 15.95±3.61 | 11.96±3.52 |
| Protein | 3.01±0.23 | 3.59±0.18 | 3.09±0.18 | 3.53±0.30 |
| Cell wall | 7.90±0.92 | 6.57±0.70 | 6.32±0.70 | 7.29±1.05 |
| Fru | 2.38±1.01 | 1.68±1.46 | 5.25±1.52 | 4.06±1.66 |
| Specific activity of hexose phosphates (Bq nmol−1) | 0.40±0.04 | 0.44±0.12 | 0.61±0.05 | 0.45±0.07 |
| Metabolic flux (nmol hexose equivalents g−1 fresh wt h−1) | ||||
| Starch synthesis | 217.94±68.30 | 137.39±48.53 | 146.87±43.79 | 140.98±48.37 |
| Suc synthesis | 245.42±39.73 | 545.17±136.00 | 176.98±12.93 | 359.60±134.36 |
| Respiration | 472.11±28.27 | 627.73±106.49 | 425.62±40.07 | 556.38±118.06 |
| Cell wall synthesis | 77.18±8.55 | 94.13±23.42 | 53.71±9.20 | 98.01±42.02 |
Malate Seems to Play No Role in the Redox Regulation of AGPase in Potato Tuber
Following an extensive metabolic study of tomato fruit development (Carrari and Fernie, 2006), malate was identified as a potential important regulatory metabolite. It was recently demonstrated that alterations in the level of malate resulted in dramatic effects on transitory starch metabolism in tomato fruits: lines displaying low malate content displayed an increased flux to, and accumulation of, starch, whereas those displaying high levels of malate displayed an opposite effect (Centeno et al., 2011). Although malate levels decreased significantly in FL31 and FL60 lines (Table I), no alterations in starch content (Table II) could be observed. Therefore, we next decided to assess the maximal catalytic activities of AGPase enzyme in all transgenic lines (Fig. 4A). No significant changes could be observed, which is most likely the reason for the lack of alteration in starch content (Table II). Since malate control over AGPase redox state was only demonstrated in tomato fruits (Centeno et al., 2011), we next decided to investigate the influence of malate on the potato AGPase activity. Isolated potato discs from 10-week-old wild-type plants were incubated in the presence of 50 mm malate for 2 h. After this time, discs were washed and total AGPase activity was determined as described in “Materials and Methods.” These analyses revealed that, in contrast to the situation in tomato fruits (Centeno et al., 2011), in potato tubers malate does not alter either the total AGPase activity (Fig. 4B) or its activation state (data not shown). We also measured the activation state of potato AGPase in crude extracts isolated in the presence or absence of either the allosteric regulator 3-P-glycerate (3-PGA) or dithiothreitol (DTT). This experiment indicated that the AGPase activation by both 3-PGA and DTT is unaffected in the transgenic lines (Fig. 4C), again in contrast to the situation observed in tomato. Additionally, tuber tissue slices were incubated in the presence of 25 and 50 mm malate together with [U-14C]Glc to estimate the changes in flux toward the starch synthesis (Table IV). These feeding experiments revealed no changes in the starch biosynthetic flux, suggesting that, unlike in the tomato fruit, malate does not affect the flux to starch in potato tubers.
Figure 4.
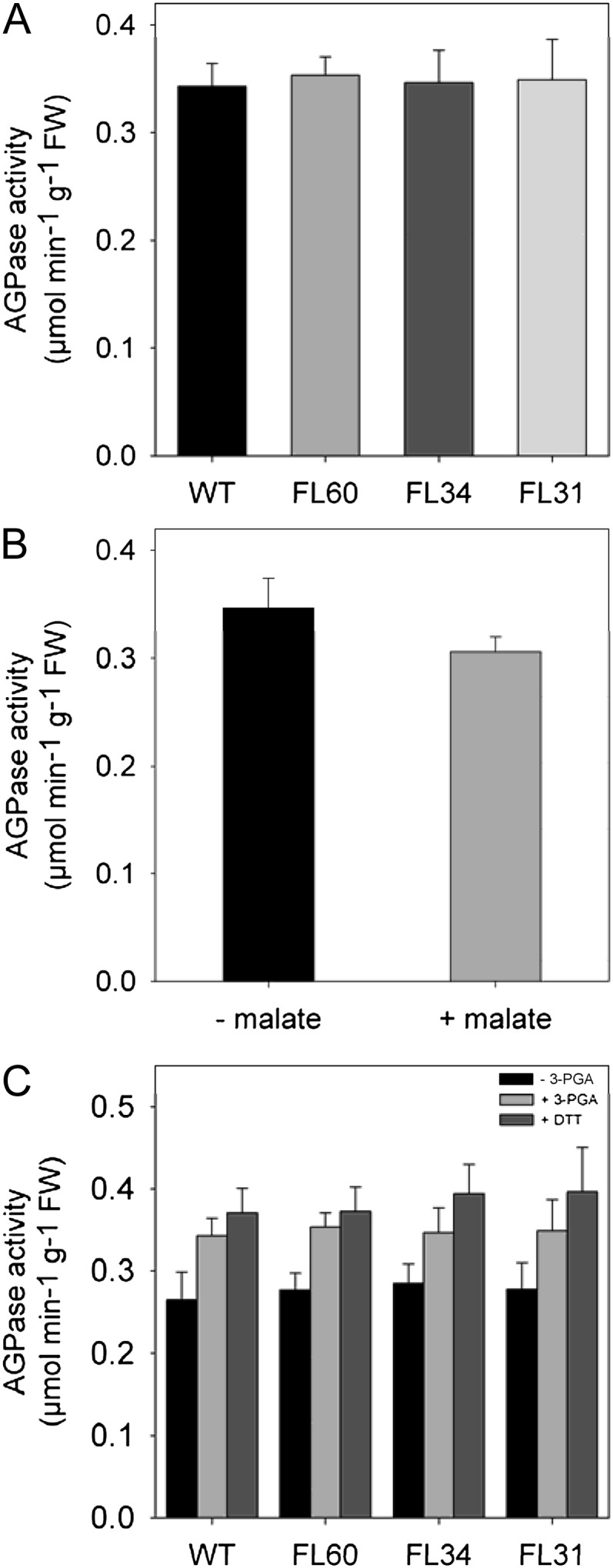
AGPase total activity and activation in potato tubers from 10-week-old transgenic and wild-type (WT) plants. A, AGPase activity measured in tubers of 10-week-old plants from wild-type and transgenic lines. B, Influence of malate on the AGPase activity determined in potato tuber discs from 10-week-old wild-type plants incubated in the presence of 50 mm malate for 2 h. C, AGPase activity in the presence and absence of either the allosteric regulator 3-PGA or DTT as determined in protein extracts from potato tuber harvested from 10-week-old plants. Values are presented as means ± sd of five tubers per line. The statistical differences between values were determined by Student’s t test (P < 0.05). FW, Fresh weight.
Table IV. Effects of malate on the redistribution of radiolabel after incubation in the presence of [U-14C]Glc and absolute fluxes by potato tuber discs.
Tuber discs were preincubated in 10 mm MES-KOH (pH 6.5) containing 2 mm Glc in the absence (control) or presence of varying concentrations of malate for 2 h in addition to 10 mm [U-14C]Glc (specific activity of 8.11 MBq mmol−1). Each sample was extracted with boiling ethanol, and the amount of radioactivity in each metabolic fraction was determined as described in “Materials and Methods.” Values are expressed as means ± se of six biological replicates. No statistical significance from the control (P < 0.05) following the performance of Student’s t tests was observed.
| Malate Concentration |
|||
|---|---|---|---|
| Variable | 0 mm | 25 mm | 50 mm |
| Label incorporated (Bq) | |||
| Total uptake | 565.69±36.95 | 589.44±28.65 | 574.58±22.47 |
| Total metabolized | 450.19±20.44 | 468.05±20.91 | 461.99±21.38 |
| Redistribution of radiolabel (% of total assimilated) | |||
| CO2 evolution | 0.94±0.06 | 1.05±0.15 | 1.21±0.07 |
| Organic acids | 12.79±1.12 | 11.84±1.32 | 12.27±1.16 |
| Amino acids | 10.78±0.67 | 9.82±1.60 | 9.51±1.28 |
| Suc | 14.49±1.24 | 13.69±0.37 | 12.88±0.59 |
| Starch | 21.93±1.63 | 19.94±0.91 | 21.59±1.27 |
| Protein | 3.89±0.39 | 3.61±0.26 | 3.38±0.37 |
| Cell wall | 22.05±1.39 | 20.97±1.09 | 21.64±1.38 |
| Fru | 6.12±0.71 | 6.58±1.02 | 6.52±1.27 |
| Specific activity of hexose phosphates (Bq nmol−1) | 0.65±0.05 | 0.59±0.04 | 0.64±0.06 |
| Metabolic flux (nmol hexose equivalents g−1 fresh wt h−1) | |||
| Starch synthesis | 66.40±7.13 | 69.12±6.95 | 67.33±6.18 |
| Suc synthesis | 76.87±7.91 | 82.15±2.74 | 79.47±4.19 |
| Respiration | 83.65±10.42 | 80.87±10.98 | 81.99±9.17 |
| Cell wall synthesis | 87.15±6.06 | 64.33±2.95 | 68.82±6.42 |
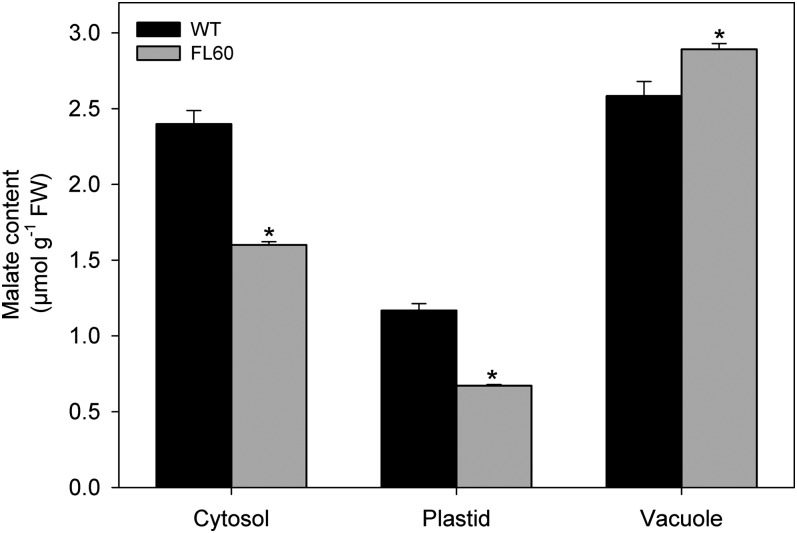
There are two possible explanations for this finding. Either the potato AGPase may be differentially regulated than the tomato, or malate may be differentially compartmented in the two tissue types. In order to discriminate between these two possibilities, we performed a number of studies. First, simple comparison of the gene and protein structures was carried out. In keeping with the close genetic relationship between these two solanaceous species, we found that the gene and protein of the small AGPase subunit were 97% and 98% identical, and the proteins exhibited only 12 different amino acid residues. Perhaps most importantly, the seven Cys residues putatively involved in redox regulation were conserved in the proteins of both origins. Second, we carried out nonaqueous fractionation on tuber material from the wild type and line FL60 (which was chosen on the basis of displaying the greatest reduction in fumarase activity) and determined the subcellular distribution of malate by using the BestFit algorithm (Riens et al., 1991; Klie et al., 2011; Krueger et al., 2011). These analyses revealed that the marker enzyme distribution was similar to that documented previously (Farré et al., 2001; Junker et al., 2006). Despite the fact that the organelle separation was not complete in this analysis, it was sufficient for calculating malate distribution in three main compartments where malate synthesis takes place (cytosol, plastid, and vacuole) using the BestFit algorithm. By comparing the marker enzyme distribution (UDP-Glc pyrophosphorylase and inorganic pyrophosphate-dependent phosphofructokinase for the cytosol; ADP-Glc pyrophosphorylase and shikimate:NADP oxidoreductase for the plastid; α-mannosidase for vacuole) with malate distribution, it was verified that in both wild-type and transgenic lines, malate was localized mainly in the vacuole. Interestingly, the percentages of the total malate pool assigned to the cytosol, plastid, and vacuole were similar between the wild-type and transgenic lines. While in the wild type, 42% of the malate was located in the vacuole, 39% in the cytosol, and 19% in the plastid, in the transgenic line, 56% was located in the vacuole, 31% in the cytosol, and 13% in the plastid (Fig. 5). It is important to note that, despite the fact that the subcellular distribution of malate was slightly modified in the transgenic line, the absolute levels of malate determined for each subcellular compartment were, like the absolute malate content, significantly reduced in both the cytosol and plastid. Intriguingly, however, the storage pool of malate in the vacuole was increased in the transgenics.
Figure 5.
Distribution of malate content in different compartments after nonaqueous fractionation from potato tubers of 10-week-old plants. Malate content was spectrophotometrically measured in tubers of 10-week-old plants from the wild type (WT) and transgenic line FL 60. Values are presented as means ± sd of three individual fractionations per genotype. Asterisks indicate values that were determined by Student’s t test to be significantly different (P < 0.01) from the wild type. FW, Fresh weight.
DISCUSSION
Improving starch content in potato tubers has been the focus of intensive metabolic engineering strategies over the last two decades (Fernie and Willmitzer, 2001; Jenner, 2003; Smith, 2008). A comprehensive review of these strategies alongside application of the framework of metabolic control analyses revealed that a considerable portion of the control of starch biosynthesis in this organ resided in the reaction catalyzed by AGPase (Geigenberger et al., 2004). In addition, considerable control has been demonstrated to be harbored by the plastidial adenylate and Glc-6-P transporter (Tjaden et al., 1998; Zhang et al., 2008), with a minor yet considerable proportion also vested in the plastidial phosphoglucomutase reaction (Fernie et al., 2002). Studies beyond the direct pathway of starch biosynthesis also indicate important roles for the mitochondrial NAD-malic enzyme (Jenner et al., 2001) and the plastidial adenylate kinase (Regierer et al., 2002). However, although the exact mechanism underlying this link in the former case has yet to be resolved, the latter has been characterized to affect both the substrate levels and activation status of the AGPase reaction (Oliver et al., 2008). In the light of these observations, it is interesting that reciprocal up- and down-regulation of starch biosynthesis was observed in tomato fruits exhibiting depressed expression of mitochondrially localized fumarase and malate dehydrogenase, respectively (Centeno et al., 2011). Rather unexpectedly, in this context, was the fact that down-regulation of fumarase in a tuber-specific manner had no effect on the level of starch in this organ (Table II). This fact was perhaps even more surprising given that the level of malate in the transformants (Table I) was similarly altered to that achieved in the tomato transformants (Centeno et al., 2011). This is striking, as in the tomato study they did not correlate strongly to the activity of fumarase; however, this is likely due to adjustments in other routes of malate production (Fernie and Martinoia, 2009). Thus, two possible explanations for the lack of effects on modulating fumarase activity in potato are (1) that the enzyme is present in massive excess and thus the level of reduction obtained here was not great enough to provoke metabolic consequences, or (2) that subtle compensatory mechanisms are invoked in potato but not in tomato. In support of either of these hypotheses is the fact that other metabolites displayed relatively small changes, implying that the global effect of the reduction of fumarase was relatively minor. However, we think that neither of these explanations is the predominant reason, given that clear changes in malate and fumarate content were observed, and it is important to note that the levels of malate normally observed in tomato fruits (Centeno et al., 2011) are considerably higher than those found in potato tubers (Fig. 5; Farré et al., 2001); thus, despite the fact that small changes in the plastidial malate were observed, these changes may not provoke a large enough change in redox state to affect the activation state of AGPase. Interestingly, although the rate of starch biosynthesis is controlled at least partially at the level of ADP-Glc production by redox regulation of the AGPase enzyme, it has been recently demonstrated that other starch-associated enzymes are also under redox control (Glaring et al., 2012); therefore, much work is still required to clarify the role and significance of redox regulation for both individual enzymes and the pathways in which they operate in vivo.
We had initially thought that the previous observation, that decreased NAD-malic enzyme levels resulted in elevated starch yields in potato, may also have been linked to malate-related redox regulation. However, close inspection of the data reveals that at least the overall levels of malate are unaltered in these high-starch lines (Jenner et al., 2001). Comparison of the mitochondrial fumarase gene and protein revealed high similarity between potato and tomato (Nunes-Nesi et al., 2007), as would be expected given the genetic similarity of these species. Taken together, these data led us to conclude that the influence of malate on plastidial starch biosynthesis is context dependent rather than that the potato tuber enzyme had lost its malate regulability.
Although the data presented here show that the overall cellular malate levels were depleted in the fumarase-deficient potato tubers to a similar extent to that previously reported in tomato fruit (Centeno et al., 2011), several lines of evidence, both direct and indirect, suggest that this did not result in major changes in plastidial metabolism. Other characteristics of metabolism were broadly conserved between tomato fruit and potato tuber, with little effect either on the rate of respiration, as would be predicted from metabolic control analysis studies (Araújo et al., 2012), or the levels of a broad range of metabolites. Thus, we conclude that the fumarase reaction plays little role in the overall control of the respiratory flux. Returning to starch synthesis, the indirect evidence for the lack of change in the plastidial redox state is provided by the lack of effect on the AGPase activation state (Fig. 4), the starch biosynthetic flux (Table III), and the absolute starch accumulation (Table II) in the transgenic lines. While this is essentially circumstantial, considerably stronger evidence is provided by the lack of a major effect on the activation state of the plastidial malate dehydrogenase and by the fact that the changes in plastidial malate levels are relatively minor (Fig. 5). It is also interesting that, in contrast to our expectations, there was no major impact on the flux to the cell wall (Table III).
As mentioned above, the difference here may be partially due to the fact that absolute malate levels are not of the same magnitude in potato and tomato and partially that the changes in the plastidial levels of tomato would likely be more strongly influenced by the operation of the malate valve. Despite the fact that we were previously unable to provide analogous data for subcellular malate compartmentation in tomato, due to the intractability of this method with tomato fruit material, we were previously able to provide strong cumulative evidence for an altered redox status of the green fruit plastid. This evidence included a strong activation of the plastidial malate dehydrogenase and modulation of pigment metabolism, both of which have been demonstrated to be diagnostic of an altered plastidial redox status (Scheibe, 1991; Foyer et al., 1992; Nashilevitz et al., 2010). We previously postulated that this altered redox status was due to an inhibition of the malate valve caused by the accumulation of malate in the cytosol following the inhibition of fumarase. The malate valve is a well-described mechanism by which excess reductant can be removed from the plastid in order to allow the maintenance of efficient photosynthesis (Scheibe, 2004). The activation of NADP-malate dehydrogenase, which uses excess NADPH to convert oxaloacetate to malate in order to regenerate the electron acceptor NADP, is inhibited by the product NADP, thus switching off its own activity when all NADPH is required and consumed for assimilatory processes and therefore should not be exported as malate (Scheibe 1991). A lowered cytosolic malate level, however, would facilitate the export of malate from the plastid by means of establishing a concentration gradient across the plastid envelope membrane system, likely resulting in an altered plastidial redox state. That plastidial redox balance changes are apparent in the (photosynthetic) green tomato fruit but not in the (heterotrophic) potato tuber most likely reflects the lack of need for an operational plastidial malate valve in the latter situation, given that the plastid is not active as a major source of ATP or reductant in this organ.
It will be interesting in the future to carry out similar studies on the effect of altering the malate levels in photosynthetically active leaf tissues. However, there are already some hints in the literature that the effects may be similar to those observed in the green fruit. First, there are transgenic tomato plants in which the expression levels of mitochondrially localized fumarase (Nunes-Nesi et al., 2007), malate dehydrogenase (Nunes-Nesi et al., 2005), or succinate dehydrogenase (Araújo et al., 2011) were inhibited, resulting in increases (fumarase) and decreases (malate dehydrogenase or succinate dehydrogenase) in leaf malate content, respectively. They were additionally characterized by clearly decreased starch contents in the case of the fumarase antisense lines (Nunes-Nesi et al., 2007) and increased contents in the malate dehydrogenase (Nunes-Nesi et al., 2005) and succinate dehydrogenase (Araújo et al., 2011) antisense lines. Although a similar pattern was not seen in the succinate dehydrogenase knockout mutant of Arabidopsis (Arabidopsis thaliana; Fuentes et al., 2011), it is important to note that the level of malate was not altered in this mutant. Unfortunately, the effect of knocking out both mitochondrial isoforms of malate dehydrogenase in Arabidopsis on starch levels was not documented (Tomaz et al., 2010); however, knocking out the cytosolic fumarase of Arabidopsis resulted in a high-starch leaf phenotype (Pracharoenwattana et al., 2010). The presence of a cytosolic fumarase, however, has not yet been reported in a solanaceous species, despite the fact that the genomes of tomato and potato seem to encode these genes. Indeed, on the basis of cumulative evidence that one is essentially unexpressed, it would seem likely that it is only expressed in a specialized cell type, such as guard cells (Nast and Müller-Röber, 1996). While the levels of starch in Arabidopsis plants deficient in the plastidial NADP-malate dehydrogenase were unaltered (Hebbelmann et al., 2012), it will be interesting in the future to look at the levels of starch in a range of other mutants exhibiting altered cellular or subcellular levels of malate, such as those compromised in the expression of various cellular malate transporters (Emmerlich et al., 2003; Lee et al., 2008). Finally, a clear inverse trend can be seen between malate and starch across a broad range of over 100 Arabidopsis ecotypes (Sulpice et al., 2009, 2010), suggesting that this regulatory mechanism is fairly common.
In summary, we demonstrated here that manipulating tuber malate content is not an effective strategy for improving tuber starch content and, furthermore, that repressing fumarase activity in the tuber only results in minor effects. With the help of subcellular fractionation techniques, however, we were able to comprehend the reason for the failure of this strategy from a biotechnological standpoint and in doing so to better understand differences in malate compartmentation that appear to be dependent on the trophic nature of the tissue under study. These findings, therefore, reinforce the importance of studies aimed at spatially dissecting resolution both for improving our fundamental understanding of metabolic regulation and for facilitating its improvement.
MATERIALS AND METHODS
Materials
Radiolabeled Glcs were purchased from GE Healthcare. All other chemicals were purchased from Sigma-Aldrich or Merck. Potato (Solanum tuberosum ‘Desirée’) was obtained from Saatzucht Lange. Plants were maintained in tissue culture with 8-/16-h day/night cycles on Murashige and Skoog medium (Murashige and Skoog, 1962), which contained 2% Suc. In the greenhouse, plants were grown under the same light regime with a minimum of 250 μmol photons m−2 s−1.
Generation of Transgenic Plants
A 1,860-bp fragment, comprising the entire coding region of the fumarase gene from potato, was cloned using the RNAi approach into the pBinAR transformation vector under the control of the patatin class I promoter (Twell and Ooms, 1987) and the ocs terminator. This construct was introduced into plants by an Agrobacterium tumefaciens-mediated transformation protocol, and plants were selected and maintained as described in the literature (Tauberger et al., 2000). Tubers were harvested from 10-week-old plants and used for the selection of promising lines. This screening allowed the selection of three lines that were used for detailed physiological and biochemical analyses.
TCA Cycle Flux Estimation
Estimations of TCA cycle flux on the basis of 14CO2 evolution were carried out following the incubation of isolated potato tuber tissue discs in 10 mm MES-KOH (pH 6.5) containing 2 μCi of d-[1-14C]Glc, d-[3,4-14C]Glc, or d-[6-14C]Glc. 14CO2 evolved was trapped in KOH (in hourly intervals) and quantified by scintillation counting. The results were interpreted following ap Rees and Beevers (1960) and Nunes-Nesi et al. (2005).
Determination of Enzyme Activities
Enzyme extracts were prepared as described previously (Gibon et al., 2004), except that Triton X-100 was used at a concentration of 1% and glycerol at 20%. Fumarase activity was determined as described (Gibon et al., 2004). Extracts, as well as malate standards (0–20 μm) prepared in the extraction buffer, were incubated in a medium containing 100 mm Tricine/KOH (pH 8.0), 0.2 mm acetyl-CoA, 5 mm phosphate, 5 mm MgCl2, 0.15 mm NAD*, 0.05% (v/v) Triton X-100, 100 units mL−1 malate dehydrogenase, and 1 unit mL−1 citrate synthase. The reaction was started by the addition of fumarate to a final concentration of 0 (blank) or 10 mm (maximal activity). The reaction was stopped with 20 μL of 0.5 m NaOH and heated at 95°C for 5 min. After cooling down, 20 μL of 0.5 m HCl in 0.2 m Tricine/KOH (pH 9.0) was added to neutralize the pH. NADH was measured in the presence of 10 units mL−1 alcohol dehydrogenase in 100 mm Tricine/KOH (pH 9.0), 4 mm EDTA, 0.1 mm phenazine ethosulfate, 0.6 mm methylthiazolyldiphenyl-tetrazolium bromide, and 500 mm ethanol. The absorbances were read at 570 nm and at 30°C until the rates were stabilized. The rates of reactions were calculated as the increase of the absorbance. For AGPase, both the maximal and selective assays were carried out exactly as described previously (Tiessen et al., 2002; Centeno et al., 2011). The activation of AGPase was performed by the addition of 3-PGA in the reaction medium at a final concentration of 2 mm for 20 min.
Tuber discs (diameter of 10 mm, thickness of 2 mm) were cut directly from growing tubers attached to the fully photosynthesizing mother plant and washed three times with 10 mm MES-KOH (pH 6.5), following an incubation in Erlenmeyer flasks with (1) 3 mL of incubation medium containing 50 mm malate, with pH adjusted to 6.5, or (2) 3 mL of incubation medium containing 50 mm malate (pH 6.5) and 2 mm Glc, containing 1 μCi of [U-14C]Glc (specific activity of 1.4 MBq mmol−1). After 2 h, the discs were harvested, washed three times in MES-KOH buffer (pH 6.5), and frozen in liquid nitrogen to enable further analysis.
Determination of Metabolite Levels
Plant material was ground to a fine powder using a Retsch ball mill. The levels of starch, Suc, Fru, and Glc in the potato tuber tissue were determined exactly as described previously (Fernie et al., 2001b). Malate and fumarate were determined exactly as detailed by Nunes-Nesi et al. (2007). The levels of all other metabolites were quantified by the GC-time of flight-MS method as described by Lisec et al. (2006). Metabolites were manually identified using the reference library mass spectra and retention indices from the Golm Metabolome Database (http://gmd.mpimp-golm.mpg.de; Kopka et al., 2005). Metabolite profiling data are reported following recent recommendations (Supplemental Table S1; Fernie et al., 2011).
Nonaqueous Fractionation
Nonaqueous fractionation of tuber tissue was carried out exactly as determined by Farré et al. (2001). Subcellular metabolite distributions were computed using the new version (version 1.2) of the BestFit command line tool (Klie et al., 2011).
Incubation of Tuber Discs with [U-14C]Glc
Developing tubers were removed from 10-week-old plants, and a 10-mm-diameter longitudinal core was taken. The core was then sliced into 1-mm-thick discs, washed three times in fresh incubation medium (10 mm MES-KOH, pH 6.5), and then incubated (15 discs) in 5 mL of incubation medium containing [U-14C]Glc (1.4 MBq mmol−1) to a final concentration of 2 mm. Samples were then incubated for 2 or 4 h before washing again three times in unlabeled incubation medium and frozen in liquid N2 until further analysis. All incubations were performed in a sealed 100-mL flask at 25°C and shaken at 150 rpm. The evolved 14CO2 was collected in 0.5 mL of 10% (w/v) KOH.
Fractionation of 14C-Labeled Tissue Extracts
Discs were extracted with 80% (v/v) ethanol at 80°C (1 mL per two discs), reextracted in two subsequent steps with 50% (v/v) ethanol (1 mL per two discs for each step), and the combined supernatants were dried under an air stream at 35°C and taken up in 1 mL of water (Fernie et al., 2001c). The soluble fraction was subsequently separated into neutral, anionic, and basic fractions by ion-exchange chromatography; the neutral fraction (2.5 mL) was freeze dried, taken up in 100 µL water, and further analyzed by enzymic digestion followed by a second ion-exchange chromatography step (Carrari et al., 2006). To measure phosphate esters, samples (250 µL) of the soluble fraction were incubated in 50 µL of buffer (10 mm MES-KOH, pH 6.0) with or without 1 unit of potato acid phosphatase (grade II; Boehringer Mannheim) for 3 h at 37°C, boiled for 2 min, and analyzed by ion-exchange chromatography (Fernie et al., 2001c). The insoluble material left after ethanol extraction was homogenized, taken up in 1 mL of water, and counted for starch (Fernie et al., 2001a). Fluxes were calculated as described following the assumptions detailed by Geigenberger et al. (1997, 2000).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Metabolite profiling data.
Acknowledgments
We are grateful to Helga Kulka (Max-Planck-Institut für Molekulare Pflanzenphysiologie) for the excellent care in the production high-quality plants for this work.
Glossary
- AGPase
ADPglucose pyrophosphorylase
- TCA
tricarboxylic acid
- RNAi
RNA interference
- GC
gas chromatography
- MS
mass spectrometry
- OPPP
oxidative pentose phosphate pathway
- 3-PGA
3-P-glycerate
- DTT
dithiothreitol
References
- ap Rees TA, Beevers H. (1960) Pathways of glucose dissimilation in carrot slices. Plant Physiol 35: 830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR. (2012) Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ 35: 1–21 [DOI] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R, Balbo I, Lehmann M, Studart-Witkowski C, Tohge T, et al. (2011) Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture. Plant Cell 23: 600–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckles DM, Craig J, Smith AM. (2001) ADP-glucose pyrophosphorylase is located in the plastid in developing tomato fruit. Plant Physiol 126: 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor M-I, Nunes-Nesi A, Nikiforova V, Centero D, Ratzka A, Pauly M, et al. (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142: 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Fernie AR. (2006) Metabolic regulation underlying tomato fruit development. J Exp Bot 57: 1883–1897 [DOI] [PubMed] [Google Scholar]
- Centeno DC, Osorio S, Nunes-Nesi A, Bertolo ALF, Carneiro RT, Araújo WL, Steinhauser M-C, Michalska J, Rohrmann J, Geigenberger P, et al. (2011) Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell 23: 162–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HV, Shepherd LV, Burrell MM, Carrari F, Urbanczyk-Wochniak E, Leisse A, Hancock RD, Taylor M, Viola R, Ross H, et al. (2005) Modulation of fructokinase activity of potato (Solanum tuberosum) results in substantial shifts in tuber metabolism. Plant Cell Physiol 46: 1103–1115 [DOI] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE. (2003) The plant homolog to the human sodium/dicarboxylic acid cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 16: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L. (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127: 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Aharoni A, Willmitzer L, Stitt M, Tohge T, Kopka J, Carroll AJ, Saito K, Fraser PD, DeLuca V. (2011) Recommendations for reporting metabolite data. Plant Cell 23: 2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Martinoia E. (2009) Malate: jack of all trades or master of a few? Phytochemistry 70: 828–832 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Geigenberger P. (2001a) The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol 125: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Trethewey RN, Willmitzer L. (2001b) The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213: 418–426 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ. (2001c) Fructose 2,6-bisphosphate activates pyrophosphate:fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212: 250–263 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Willmitzer L. (2001) Molecular and biochemical triggers of potato tuber development. Plant Physiol 127: 1459–1465 [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Willmitzer L, Trethewey RN. (2002) Sucrose to starch: a transition in molecular plant physiology. Trends Plant Sci 7: 35–41 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Harbinson J. (1992) Control of the quantum efficiencies of photosystems I and II, electron flow, and enzyme activation following dark-to-light transitions in pea leaves: relationship between NADP/NADPH ratios and NADP-malate dehydrogenase activation state. Plant Physiol 99: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes D, Meneses M, Nunes-Nesi A, Araújo WL, Tapia R, Gómez I, Holuigue L, Gutiérrez RA, Fernie AR, Jordana X. (2011) A deficiency in the flavoprotein of Arabidopsis mitochondrial complex II results in elevated photosynthesis and better growth in nitrogen-limiting conditions. Plant Physiol 157: 1114–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P. (2011) Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol 155: 1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381: 723–740 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Müller-Rober B, Stitt M. (1999) Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta 209: 338–345 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Regierer B, Nunes-Nesi A, Leisse A, Urbanczyk-Wochniak E, Springer F, van Dongen JT, Kossmann J, Fernie AR. (2005) Inhibition of de novo pyrimidine synthesis in growing potato tubers leads to a compensatory stimulation of the pyrimidine salvage pathway and a subsequent increase in biosynthetic performance. Plant Cell 17: 2077–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. (1997) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201: 502–518 [Google Scholar]
- Geigenberger P, Stitt M, Fernie AR. (2004) Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27: 655–673 [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M. (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaring MA, Skryhan K, Kötting O, Zeeman SC, Blennow A. (2012) Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana. Plant Physiol Biochem 58: 89–97 [DOI] [PubMed] [Google Scholar]
- Hebbelmann I, Selinski J, Wehmeyer C, Goss T, Voss I, Mulo P, Kangasjärvi S, Aro E-M, Oelze M-L, Dietz K-J, et al. (2012) Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP-malate dehydrogenase. J Exp Bot 63: 1445–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner HL. (2003) Transgenesis and yield: what are our targets? Trends Biotechnol 21: 190–192 [DOI] [PubMed] [Google Scholar]
- Jenner HL, Winning BM, Millar AH, Tomlinson KL, Leaver CJ, Hill SA. (2001) NAD malic enzyme and the control of carbohydrate metabolism in potato tubers. Plant Physiol 126: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker BH, Wuttke R, Nunes-Nesi A, Steinhauser D, Schauer N, Büssis D, Willmitzer L, Fernie AR. (2006) Enhancing vacuolar sucrose cleavage within the developing potato tuber has only minor effects on metabolism. Plant Cell Physiol 47: 277–289 [DOI] [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge UI. (1998) Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell 10: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klie S, Krueger S, Krall L, Giavalisco P, Flügge UI, Willmitzer L, Steinhauser D. (2011) Analysis of the compartmentalized metabolome: a validation of the non-aqueous fractionation technique. Front Plant Sci 2: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmüller E, Dörmann P, Weckwerth W, Gibon Y, Stitt M, et al. (2005) GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21: 1635–1638 [DOI] [PubMed] [Google Scholar]
- Kossmann J, Lloyd JR. (2000) Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol 35: 141–196 [PubMed] [Google Scholar]
- Krueger S, Giavalisco P, Krall L, Steinhauser MC, Büssis D, Usadel B, Flügge UI, Fernie AR, Willmitzer L, Steinhauser D. (2011) A topological map of the compartmentalized Arabidopsis thaliana leaf metabolome. PLoS ONE 6: e17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger NJ. (1997) Carbohydrate synthesis and degradation. In DT Dennis, DH Turpin, DD Lefebvre, DB Layzelle, eds, Plant Metabolism. Longmann, Harlow, UK, pp 83–104
- Lee M, Choi Y, Burla B, Kim YY, Jeon B, Maeshima M, Yoo JY, Martinoia E, Lee Y. (2008) The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol 10: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Martin C, Smith AM. (1995) Starch biosynthesis. Plant Cell 7: 971–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nashilevitz S, Melamed-Bessudo C, Izkovich Y, Rogachev I, Osorio S, Itkin M, Adato A, Pankratov I, Hirschberg J, Fernie AR, et al. (2010) An orange ripening mutant links plastid NAD(P)H dehydrogenase complex activity to central and specialized metabolism during tomato fruit maturation. Plant Cell 22: 1977–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nast G, Müller-Röber B. (1996) Molecular characterization of potato fumarate hydratase and functional expression in Escherichia coli. Plant Physiol 112: 1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Lytovchenko A, Smith AMO, Loureiro ME, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SN, Tiessen A, Fernie AR, Geigenberger P. (2008) Decreased expression of plastidial adenylate kinase in potato tubers results in an enhanced rate of respiration and a stimulation of starch synthesis that is attributable to post-translational redox-activation of ADP-glucose pyrophosphorylase. J Exp Bot 59: 315–325 [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM. (2010) Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J 62: 785–795 [DOI] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J. (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Renz A, Merlo L, Stitt M. (1993) Partial purification from potato tubers of three fructokinases and three hexokinases which show differing organ and developmental specificity. Planta 190: 156–165 [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW. (1991) Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol 97: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Willmitzer L, Fernie AR. (2001) High-resolution metabolic phenotyping of genetically and environmentally diverse potato tuber systems. Identification of phenocopies. Plant Physiol 127: 749–764 [PMC free article] [PubMed] [Google Scholar]
- Roessner-Tunali U, Liu J, Leisse A, Balbo I, Perez-Melis A, Willmitzer L, Fernie AR. (2004) Kinetics of labelling of organic and amino acids in potato tubers by gas chromatography-mass spectrometry following incubation in (13)C labelled isotopes. Plant J 39: 668–679 [DOI] [PubMed] [Google Scholar]
- Scheibe R. (1991) Redox-modulation of chloroplast enzymes: a common principle for individual control. Plant Physiol 96: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R. (2004) Malate valves to balance cellular energy supply. Physiol Plant 120: 21–26 [DOI] [PubMed] [Google Scholar]
- Slattery CJ, Kavakli IH, Okita TW. (2000) Engineering starch for increased quantity and quality. Trends Plant Sci 5: 291–298 [DOI] [PubMed] [Google Scholar]
- Smith AM. (2008) Prospects for increasing starch and sucrose yields for bioethanol production. Plant J 54: 546–558 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl E-T, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Trenkamp S, Steinfath M, Usadel B, Gibon Y, Witucka-Wall H, Pyl ET, Tschoep H, Steinhauser MC, Guenther M, et al. (2010) Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22: 2872–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Müller-Rober B, Willmitzer L, Hill SA. (1999) The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta 209: 330–337 [DOI] [PubMed] [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN. (2000) Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J 23: 43–53 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JH, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P. (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE. (1998) Altered plastidic ATP/ADP transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J 16: 531–540 [Google Scholar]
- Tomaz T, Bagard M, Pracharoenwattana I, Lindén P, Lee CP, Carroll AJ, Ströher E, Smith SM, Gardeström P, Millar AH. (2010) Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol 154: 1143–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Ooms G. (1987) The 5′ flanking DNA of a patatin gene directs tuber specific expression of a chimeric gene in potato. Plant Mol Biol 9: 365–376 [DOI] [PubMed] [Google Scholar]
- van der Merwe MJ, Osorio S, Araújo WL, Balbo I, Nunes-Nesi A, Maximova E, Carrari F, Bunik VI, Persson S, Fernie AR. (2010) Tricarboxylic acid cycle activity regulates tomato root growth via effects on secondary cell wall production. Plant Physiol 153: 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe MJ, Osorio S, Moritz T, Nunes-Nesi A, Fernie AR. (2009) Decreased mitochondrial activities of malate dehydrogenase and fumarase in tomato lead to altered root growth and architecture via diverse mechanisms. Plant Physiol 149: 653–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Häusler RE, Greiten C, Hajirezaei MR, Haferkamp I, Neuhaus HE, Flügge UI, Ludewig F. (2008) Overriding the co-limiting import of carbon and energy into tuber amyloplasts increases the starch content and yield of transgenic potato plants. Plant Biotechnol J 6: 453–464 [DOI] [PubMed] [Google Scholar]