Abstract
Clonal species normally have low seed production, low recruitment rates and long lifespans, and it is expected that the rates of long-distance dispersal (LDD) of seeds will be low as well. Banksia candolleana is a clonal shrub in Mediterranean-type, fire-prone sclerophyll shrublands of southwestern Australia, whose reproductive biology and population dynamics contrast with those of co-occurring nonclonal congeneric species, all of which are restricted to a mosaic of sand dunes set within a matrix of inhospitable swales. Using microsatellite markers, we genotyped 499 plants in all 15 populations of B. candolleana within a 12-km2 area, assessed population genetic differentiation, and quantified the effective rate of interpopulation seed dispersal through genetic assignment of individuals to populations. We measured life history, reproductive and demographic attributes, and compared these with two co-occurring Banksia species, a non-clonal resprouter and a nonsprouter. B. candolleana has much higher levels of population genetic differentiation, and one-third the rate of interpopulation seed migration, as the other two species (2.2% vs 5.5−6.8% of genotyped plants inferred to be immigrants), though distances reached by LDD are comparable (0.3−2.3 km). The low rate of interpopulation dispersal was supported by an analysis of the age structure of three populations that suggests a mean interdune migration rate of <800 m in 200 years, and 60% of suitable dunes remain uninhabited. Thus, B. candolleana has poor properties for promoting long-distance dispersal. It is unclear if these are idiosyncratic to this species or whether such properties are to be expected of clonal species in general where LDD is less critical for species survival.
Introduction
Landscape genetics addresses how landscape elements, environmental factors and species life-histories influence the process and pattern of genetic connectivity and the spatial distribution of genetic variation within and among populations [1], [2], [3]. Genetic connectivity among plant populations is achieved through the movement of pollen and seeds, but especially through the dynamics of seed dispersal [4], [5]. Most seeds are dispersed within the vicinity of the maternal plant, but long-distance dispersal (LDD) vectors may take seeds into new habitats or different populations. Though rare, these events are disproportionally important for species dynamics at the landscape scale [4], [6]. LDD of seeds, defined by Nathan et al. [7] as dispersal to a distance at least 100 times plant height, plays a crucial role in metapopulation dynamics through colonisation of new habitats and/or recolonisation following local extinction [8]. Indeed, dispersal may be considered a bet-hedging strategy, allowing metapopulation persistence when any single habitat patch is only transiently favorable [9].
Understanding the landscape, environmental and life-history constraints affecting the ability of plants to migrate is increasingly important as species respond to global environmental change [3]. As population fragmentation, environmental heterogeneity, and impacts on LDD vectors increase in many areas, understanding the dispersal dynamics of plants is vital to shaping effective conservation strategies and predicting the magnitude of landscape change for affected populations [3], [10]. LDD modeling is severely constrained by current data limitations [6]. Efforts to quantify LDD events are difficult due to the rare nature of these events and their dependence on non-standard vectors and/or chance occurrences [4], [11]. However, given an appropriate set of conditions [1], genetic assignment tests using molecular markers have had much success in identifying immigrants and their source, quantifying LDD rates, and generating significant detail about the tail of the seed dispersal curve [12], [13], [14], [15]. When combined with demographic data, these genetic approaches provide powerful insights into population connectivity [16].
The South West Australian Floristic Region (SWAFR) comprises 300 000 km2 of sclerophyll forests, woodlands, and shrublands and is internationally recognized as a biodiversity hotspot [17]. Characterized by a Mediterranean-type climate with hot, dry summers and cool, wet winters, the region is periodically subject to wildfire and severe drought. Plant species in the SWAFR are classified by life-history categories that are defined by fire response. Nonsprouters (NS) are killed by fire and produce a new generation from seeds after each fire event. Resprouters (RS) undergo vegetative regrowth after fire from structures insulated from fire heat, as well as producing seedlings immediately post-fire. Because fire response influences many critical plant characteristics, the NS vs. RS dichotomy is often considered a key determinant of Mediterranean plant ecology [18], [19]. The NS vs. RS dichotomy extends to life-history traits including seed set and viability, seedbank size and seedling survival, all of which are lower in resprouting species [19], [20], [21]. These differences are often viewed as the result of a resource mediated tradeoff between resprouting (growth of vegetative structures) and seed production [22], [23], although there is little empirical support [24].
Both RS and NS shrubs and trees in the genus Banksia are dominant plant community members in much of the SWAFR. Three previous studies measured long-distance dispersal of seed in B. hookeriana (NS) [13], [15] and B. attenuata (RS) [14]. In accordance with the general NS/RS dichotomy, B. attenuata produces and stores far fewer viable seeds (10%) than B. hookeriana [20], [22]. In addition, B. attenuata produces seeds twice the weight of B. hookeriana (101 vs 45 mg, [25]) that have a higher terminal velocity (3.49 vs 2.83 ms−1, [25]). B attenuata plants are also shorter (97 vs 147 cm at 12 y after fire, [T. He, unpublished data]). Based on these trait values, it was expected that LDD would be much lower for B. attenuata than for B. hookeriana [9], [14]. However, rates and distances LDD were similar between the two species. For co-occurring metapopulations, 5.5% of B. attenuata individuals genotyped were inferred as immigrants (microsatellite markers) compared with 6.8% (AFLP markers) and 5.5% (microsatellite markers) of B. hookeriana, with a mean/maximum detected dispersal distance of 1.4/2.6 km and 2.0/2.5 km respectively [13], [14], [15]. Thus, seeds of this NS/RS pair appear to be equally mobile and capable of long-distance dispersal, despite the differences in seed production and size.
In order to further assess the impact of life-history traits on LDD and genetic connectivity, we chose a congeneric species with traits representing the extreme of the resprouting class. Here, we examine Banksia candolleana, a (typical) clonal resprouter with seedling recruitment rates and longevity differing markedly from B. attenuata. B. candolleana is an extremely long-lived (up to an estimated 1200 years, see Methods), outcrossing, creeping shrub that co-occurs with B. attenuata and B. hookeriana in the SWAFR. Although its seed bank is comparable with B. attenuata (due to its larger crown), initial postfire seedling establishment is only 5−25% of B. attenuata and seedling survival over the first summer-autumn postfire is negligible [20]. B. candolleana also produces much larger seeds than B. attenuata (213 mg vs 101 mg) that are held close to the ground and are hidden within the interior of the ground-hugging crown. Although winged like those of other banksias, B. candolleana seeds are primarily gravity dispersed. Based on these characteristics, we hypothesized that B. candolleana would experience much lower levels of LDD than the co-occurring B. attenuata and B. hookeriana.
Materials and Methods
Study Area and Sampling
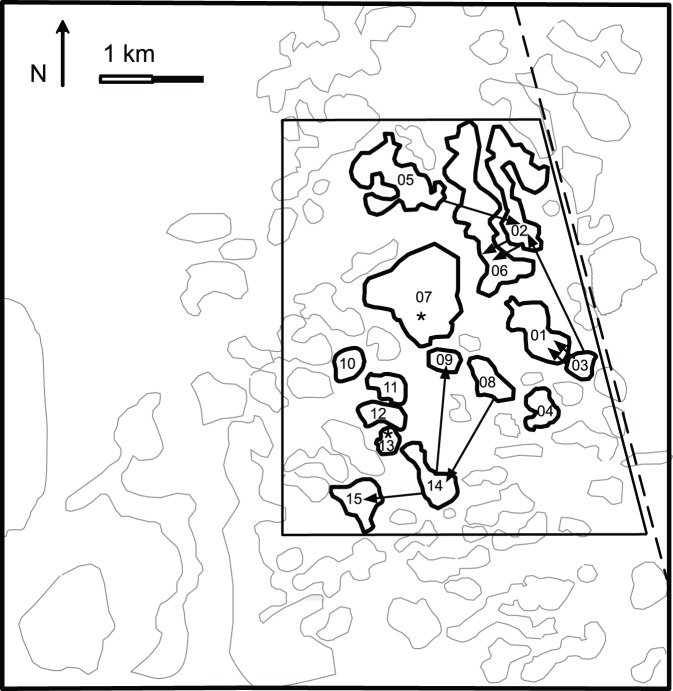
The study site was located on the Eneabba Plain in the SWAFR, in a 3×4 km area centered at E115°13′54″, S29°44′29″. This region has a dry Mediterranean-type climate and a mean fire interval of 17–28 years since 1970 [26]. The sandplain is characterized by a dune and swale system, with plant communities differing between dune crests and swales (as a result of shallower soils and consequent lower water availability in the swales). Banksia attenuata, B. candolleana and B. hookeriana are dominant species of the dune crest shrub/tree communities and are not present in the intervening swales (separating dunes by 65−850 m, Fig. 1). As for B. hookeriana and B. attenuata, we considered B. candolleana to exhibit a metapopulation structure in the area, viewed as an assemblage of local populations that are discrete geographic entities with some interaction via gene flow [27]. We visited all 40 dunes in the study area, identified 15 populations of B. candolleana, and sampled 40 individuals from each population, with the exception of dune BC05, where population size limited sampling to 25 individuals. Sampled individuals were at least 20 m apart to ensure the sampling of unique genets rather than ramets of the same clone. We harvested healthy leaves and stored them in zip-lock bags on ice until they could be frozen at −80°C.
Figure 1. Location of B. candolleana populations (numbered), showing that populations are geographically restricted to dune crests and separated by intervening dune swales.
Arrows indicate seed dispersal events. Asterisks in population BC07 and BC13 indicate immigrant for which the most likely source population was not determined.
Genotyping and Genetic Structure Analysis
Total genomic DNA was extracted following Doyle [28] from 0.5–0.8 g of leaf material with the addition of 5 M potassium acetate and sodium chloride as purification steps. Quantity and quality of extracted DNA were assessed by visualization on 2% agarose gel and Nanodrop (Thermo Fisher, Wilmington, DE). Eleven microsatellite primer pairs were used to genotype 592 samples as described in Merwin et al. [29]. We eliminated 93 samples for which genotyping failed at more than one locus, and continued analysis with the remaining 499 individuals. This reduced sample size per population to 33−39 individuals per population, with one at 24 (Table 1).
Table 1. Sampled Banksia candolleana population characteristics.
| Population | #Samples | Est PopSize | Density(ha−1) | Na | He | Immigrants |
| BC01 | 37 | 400 | 19 | 8.1 | 0.73 | 0 |
| BC02 | 36 | 120 | 9 | 9.0 | 0.77 | 2 |
| BC03 | 37 | 120 | 17 | 8.1 | 0.75 | 1 |
| BC04 | 24 | 30 | 4 | 6.7 | 0.68 | 0 |
| BC05 | 36 | 500 | 10 | 8.2 | 0.73 | 1 |
| BC06 | 37 | 150 | 43 | 7.9 | 0.76 | 2 |
| BC07 | 36 | 4000 | 74 | 8.5 | 0.73 | 1 |
| BC08 | 36 | 210 | 20 | 7.0 | 0.71 | 0 |
| BC09 | 33 | 200 | 20 | 8.0 | 0.76 | 1 |
| BC10 | 38 | 50 | 8 | 6.4 | 0.64 | 0 |
| BC11 | 34 | 40 | 8 | 7.9 | 0.75 | 0 |
| BC12 | 35 | 100 | 34 | 8.0 | 0.71 | 0 |
| BC13 | 39 | 100 | 53 | 8.0 | 0.70 | 1 |
| BC14 | 33 | 50 | 6 | 6.5 | 0.69 | 1 |
| BC15 | 38 | 100 | 6 | 7.8 | 0.72 | 1 |
| Average | 32 | 410 | 22 | 7.7 | 0.72 | 0.7 |
.# = number; Est Pop = Estimated population; Na = mean number of alleles per locus; He = mean expected heterozygosity; Immigrants = number of samples assigned to a population other than that from which it was sampled. All but 2 of these immigrants were confidently assigned to a single source population.
The data were initially analyzed for population genetic parameters of number of alleles (Na) and expected heterozygosity (He), and population differentiation was estimated using both FST and Analysis of Molecular Variance (AMOVA) using GenAlex 6.0 [30]. Deviations from Hardy-Weinberg Equilibrium (HWE) and linkage equilibrium (LE) were tested using GenePop [31], and where consistent deficits of heterozygotes were detected, the possible presence of null alleles was tested using Microchecker [32]. Since B. candolleana is clonal, an analysis of clonality was conducted in GenClone2 [33] so that possible ramets from the same clone could be excluded from further population assignment analysis.
Population Assignments
We conducted population likelihood assignment tests to infer the seed source population for each sampled individual, following procedures applied previously for B. attenuata and B. hookeriana (14–15). Microsatellite loci that deviated from HWE and LE were excluded from assignment analysis. We assumed that all alleles were shared across candidate source populations. GeneClass2 [34] was first used to calculate the probability that an individual is a resident (i.e. not a first generation migrant) in the population from which it was sampled. The Paetkau et al. [35] Monte Carlo resampling method with 10,000 resampled individuals was used, and an individual with P<0.001 was excluded from classification as a resident. Following Rannala and Mountain’s likelihood Bayesian-based method [36], GeneClass2 [34] was used to estimate allelic frequencies for each population, and the likelihood of originating from each source population was calculated for each individual, resulting in a score (percentage) being given for each population as the source of each sample.
An assignment was accepted as unambiguous when the difference (δ) between the largest and the second largest log-likelihood was above a predetermined threshold stringency level. Adopting too low a level of stringency (i.e. δ near 0) increases the risk of falsely assigning individuals as immigrants due to, for example, inter-population pollen flow [37]. Too high a level of stringency (e.g. δ = 3, i.e 103 times more likely to originate from the population with highest log-likelihood than the second highest log-likelihood) limits the assignment efficiency in terms of the fraction of individuals that can be assigned to any population [38]. In addition, the likelihoods of a particular individual being a resident or an immigrant are not equal, as LDDs are much rarer events than local dispersal events [6], as demonstrated by the low values (5.5% and 6.8%) of immigrants detected for two sympatric congeners [13], [14], [15]. Therefore, we applied a stringency level of δ = 1.0 to accept an individual as an immigrant, i.e. a multilocus genotype had to be at least 10 times more likely to be assigned to a source population other than the (home) population from which it was sampled [38]. This criterion excluded inter-population pollen dispersal events and early crosses between residents and non-residents, as the resulting genotypes would be intermediate between the two parental genotypes and not be confidently assigned to a single population [13].
Our approach provides a stringent and conservative method for identifying seed immigrants but does risk underestimating migration rate [39]. Our choice of the value (P<0.001) at which we exclude an individual as a resident minimizes the probability that local residents are wrongly identified as immigrants, but also potentially underestimates migration rates. As a consequence, we further tested seed migration rate by implementing an assignment-based method that jointly estimates individual population membership and recent migrant proportions to obtain an unbiased migration rate, called BayesAss [40].
Life-history Traits and Demographic Attributes
Life-history traits and demographic and morphological attributes were collected for the three Banksia species. Available information was collated from >30 years of demographic/life-history studies [20], [41], [42], [43], [44] and supplemented with new data as required. These data included fire-caused and inter-fire mortality, canopy seed store (i.e. seed bank of serotinous cones), postfire recruitment rates and recruitment efficiency (number of recruits per plant after fire divided by number of seeds stored per plant at time of fire). Plant height and crown width (in two dimensions) were obtained for 140 mature plants of B. candolleana in an area last burned 12 years ago and spanning a number of low sandy dunes to which the banksias are restricted. Plant density was determined as the number of plants per dune divided by dune size estimated from satellite imagery [45]. Seed production (per ha) was calculated as fecundity per plant × population density (ha−1). Mature resprouts of B. candolleana (and B. attenuata) begin producing seeds again 2–3 years after fire and return to pre-fire height within 4–5 years. B. hookeriana recruits from seed after fire, takes longer to reach maturity (4–5 years) and continues to increase in height with age. By the time stands are 12 years old they are capable of carrying fire again and all three species have reached a large size and accumulated a serotinous seed bank over 7–10 years.
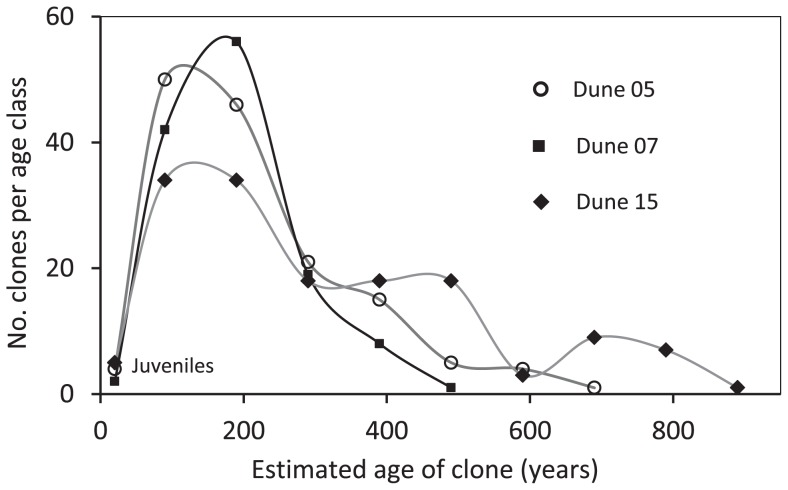
Lifespan was estimated as maximum possible age of an individual plant. Plants of B. candolleana on three large dunes (Dune 05, 07, 15), matched by climate, fire regime and soil-substrate type, were surveyed. The three dunes differ in size but are similar in height (surrogate for soil depth/water availability) and possess the same set of dominant plant species [45]. There was no detectable difference in height of co-occurring B. hookeriana and B. attenuata, two dominant species on the dunes [20], confirming growing conditions were similar. For B. candolleana, below-ground parts of plants were unearthed and the mean length of rhizome produced between fires was measured (7.5±2.5 cm; indicated by horizontal extension of rhizome at the base of vertical branches produced in response to fire). This was divided into the maximum basal radius of clones (n = 140) and multiplied by the estimated mean fire interval (∼15 y, [46]) to give an estimated age for each clone (the maximum width recorded was 1030 cm). A gamma distribution was fitted to the data and extrapolated, giving a maximum estimated longevity of 1200 y (12±4 (SD) m in diameter). Where clumps with otherwise identical leaf and stem morphology were separated by >2 m they were treated as separate plants (genets) but it is possible some were in fact ramets, so that, together with the likelihood of longer fire intervals in the long term as indicated by data for the last 40 y [26], 1200 y must be considered conservative. Data for each of the three populations were put into age (100-cm interval size) classes and used to estimate the time the latest bout of colonisation of the dune occurred (the age of the oldest clone). The right-hand sides of the frequency data were regressed against size classes using negative exponential or logarithmic curves (whichever gave the better fit) in Microsoft Excel version 14.2.3 and the age of the oldest clone was identified by extrapolation to one.
The 3×4 km study area was surveyed for occurrence of the three Banksia species. For each species, potential habitat occupancy was calculated as the fraction of dunes with the species present. Fresh seeds were removed from their follicles after heating to open them, 20 seeds of each species with undamaged wings weighed individually, and their terminal velocity determined by noting the time to fall a distance of 5.5 m. Results were corrected for initial free fall after Clements ([47], see equation 4). Ten plants of each of the three co-occurring species at a representative site 16 y since fire were assessed for the height of all cones per plant (mean of 10 plants for B. candolleana and B. attenuata, and 20 for B. hookeriana) were held above the ground (release height for seeds) and their vertical distance to the edge of the crown as an index of obstructions to their potential postfire uplift by wind.
One issue was whether limited pollen flow might have contributed to the higher between-population molecular variance of B. candolleana compared with the other two species (see Results). Types of pollinators, levels of pollen production on an inflorescence and plant basis, estimated pollen load per pollinator, and extent of coflowering within plants and populations for the three species, were obtained from the pollinator literature [25], [48], [Enright and Lamont, unpublished], [Lamont, pers. observ]. Since most observations were qualitative (except for B. hookeriana), the data were ranked and averaged to give an estimate of relative interpopulation pollen flow for the three species.
Results
Genetic Variation and Differentiation
We scored eleven microsatellite primer pairs that amplified 177 alleles (7 to 29 alleles per primer pair, average 16.1 per locus, SD = 7.6) across 499 individuals from 15 populations. The mean number of alleles (Na) amplified per locus ranged from 6.4 in population BC05 to 9.0 in population BC02 with an average of 7.7. The expected heterozygosity ranged from 0.638 (BC15) to 0.768 (BC02). A significant and large deficit of observed heterozygotes from HW equilibrium expectations was found for the loci D10 and A6 for all but one population (overall F IS = 0.48 and 0.68, respectively). The possibility of null alleles at these two loci was identified by analysis with Microchecker. Evidence for highly significant linkage disequilibrium was also obtained for 10 of 55 pairwise locus comparisons, 4 of which involved the loci D10 or A6. The AMOVA for all eleven loci partitioned 85% of the total observed genetic variation within populations and 15% among them, both of which were significantly >0 (P<0.001). Overall FST = 0.11, and pairwise population FST ranged from 0.01 to 0.11, with a mean of 0.06. Analysis of clonality confirmed that all sampled individuals were different genets.
Population Assignments
Population assignment tests were conducted with the 9 loci in HW equilibrium (all but D10 and A6). All 499 samples generated at least one probability of population assignment >0.001 for the sampled populations, suggesting that all individuals originated from one of the sampled populations. Eleven individuals from 9 populations were determined to be immigrants (Table 1), i.e. assigned to a source population other than the one from which they were sampled with a δ>1.0 (Table 2). The result gives an immigration rate for B. candolleana in the metapopulation of 2.2%. Among the 11 immigrants, δ between the first and second most likely populations was >1.0 for nine samples, allowing an unambiguous determination of source population. For the remaining two samples, the δ between the first and second most likely populations was <1.0, while the δ between the second most likely and home populations (third most likely) was >1.0. Thus, although these samples were clearly immigrants, they could not be unambiguously assigned to a source population (Table 2). The mean distance travelled by the nine immigrants with an unambiguous source population identified was 1.1 km with a range of 0.3−2.3 km (Fig. 1, Table 3). Seeds dispersed in most directions of the compass, except due south, with no clear bias in other directions. BayesAss provided an unbiased between-population migration rate of 0.022, with a 95% credible range between 0.011 to 0.033.
Table 2. Immigrants, and their source populations, inferred by population assignment test.
| ID | Sink population | Most likely source population (score) | Second most likely source population(score) | δ1 | δ2 |
| 269 | BC02 | BC05 (99.6%) | BC02 (0.3%) | 2.483 | – |
| 302 | BC02 | BC03 (86.6%) | BC07 (6.7%) | 1.112 | 1.128 |
| 053 | BC03 | BC01 (91.5%) | BC03 (8.4%) | 1.038 | – |
| 333 | BC05 | BC07 (80.7%) | BC03 (6.9%) | 1.065 | 1.085 |
| 361 | BC06 | BC02 (81.8%) | BC06 (7.6%) | 1.032 | – |
| 364 | BC06 | BC02 (98.8%) | BC06 (0.6%) | 2.194 | – |
| 212 | BC09 | BC14 (100.0%) | BC13 (0.0%) | 7.719 | 8.119 |
| 220 | BC14 | BC08 (90%) | BC09 (9.0%) | 2.202 | 4.228 |
| 476 | BC15 | BC14 (100.0%) | BC15 (0.0%) | 4.859 | – |
| 328* | BC07 | BC09 (48.9%) | BC03 (33.8%) | 0.004 | 1.264 |
| 498* | BC13 | BC15 (64.2%) | BC12 (0.3%) | 0.279 | 1.501 |
δ1 is the difference in log likelihood between the most likely source population and the second most likely source population. δ2 is the difference in log likelihood between the most likely source population and home population when the home population was not the second most likely source population.
Two potential source populations (δ values between the most likely and the second most likely are smaller than 1.0, while δ values between the second most likely source population and home population are greater than 1.0).
Table 3. F ST, between population variance (AMOVA) and (effective) inter-population seed dispersal rates and the mean among-dune dispersal distance for B. candolleana compared with two other co-occurring banksias.
Life-history Traits and Age of Populations
Of the three species, B. candolleana occupied the fewest dunes with the lowest density per dune; it took longest to reach maturity, with negligible post- and interfire mortality and recruitment (Table 4). This species had the smallest seed bank and recruitment efficiency was an order of magnitude less than the other two species. It had the largest seeds but a similar terminal velocity on the faster side of the other species. Plants were the widest and shortest, with cones located closest to the ground but with the greatest distance for released seeds to reach beyond the crown after fire. Most clones (62.4%) were in the 50−250 y age classes, with juveniles (yet to fruit) comprising 2.6%, tapering to one plant estimated at 490 y (negative exponential fit) in population 7 (one sampled plant actually at 485 y), 690 y (negative exponential) in population 5 (one sampled plant at 640 y) and 940 y (negative logarithmic) in population 14 (three sampled plants at 840 y), all with R2>85% (Fig. 2). Extrapolation of the entire data set collectively took the oldest clone to 1200 y.
Table 4. Life-history traits and demography for B. candolleana and two other co-occurring banksias.
| Traits | B. candolleana | B. attenuata | B. hookeriana |
| Fire response | Resprouter −rhizomatous(clonal) | Resprouter −lignotuberous(non-clonal) | Killed(non-clonal) |
| Habitat occupancy (% of available) | 38 | 100 | 45 |
| Population density (plants ha−1) | 48 | 330 | 827 |
| Crown width ± se (cm) | 208±24 | 126±18 | 187±15 |
| Plant height ± se (cm) | 76±5 | 97±6 | 147±8 |
| Time to 50% with fruits (year) | 40 | 25 | 5 |
| Lifespan (year) | 1200 | 300 | 40 |
| Fire-caused mortality (%) | 0 | 3 | 100 |
| Annual mortality 10−15 y postfire (%) | 0.2 | 1 | 5 |
| Seed bank (seeds plant−1) | 32 | 55 | 370 |
| Recruits per parent | 0.007 | 0.06 | >1.00 |
| Recruitment efficiency (recruits seed−1×10−3) | 0.22 | 1.09 | >2.70 |
| Seed mass ± sd (mg) | 213±50 | 101±12 | 45±6 |
| Seed terminal velocity ± sd (m s−1) | 2.30±0.86 | 3.14±0.91 | 2.64±0.71 |
| Seed release height ± sd (cm) | 22±7 | 105±9 | 105±20 |
| Seed position (distance to edge of crown ± sd) (cm) | 56±9 | 15±4 | 29±11 |
Figure 2. Estimated age distribution of B. candolleana clones in three populations (dunes) as located in Fig. 1.
Juveniles are <40 y old. A colonisation event is identified as age of the oldest plant (extrapolated by curve fit) in the population.
Discussion
Our landscape genetic analysis of the clonal species Banksia candolleana involved the same study site, researchers and methodology as for two other Banksia species [14], [15]. Therefore, our results are directly comparable in at least a relative sense, independent of the absolute accuracy of the population assignments [49]. Concordance with independent analyses of the data, however, suggests that our estimates of migration rate are accurate. Our results thus enable a comprehensive assessment of the factors influencing dispersal potential and connectivity among populations of the three species. These suggest a need to explain the higher genetic differentiation between populations of B. candolleana (FST = 0.11, ΦST = 0.15), compared with the other two study species, especially the resprouter, B. attenuata (FST = 0.02, ΦST = 0.02). Collating data we have on pollinators and relative pollen production and transport (Table 5), interpopulation pollen flow is expected to be only marginally less successful for B. candolleana than for B. attenuata, with both far less efficient than B. hookeriana, a highly successful bird-pollinated species that has strong evidence of interpopulation pollen flow [25], [48]. Therefore, differences in genetic structure can essentially be attributed to an effective LDD rate among seeds in B. candolleana that is one-third that of B. attenuata (Table 3). However, LDD seed dispersal distances were similar. Restricted interpopulation seed dispersal is likely then to be an important driver of the differences in among population genetic differentiation observed between these species, giving more permanence to any genetic bias among the founders. Weaker landscape genetic structure observed with B. attenuata and B. hookeriana is a consequence of much higher rates of seed LDD (and possibly pollen flow in the case of B. hookeriana, Table 5), and more frequent population turnover rates.
Table 5. Pollinators (most important in bold) and relative pollen and co-flowering levels (1 = highest rank) and likelihood of interpopulation pollen flow (Σ/mean for all processes).
| Species | Pollinators | Pollen/inflorescence | Pollen/flowering plant | Pollen load/pollinator | Coflowering/plant | Coflowering/population | Interpopulation pollen flow (Σ/6) |
| B. candolleana | Birds, mammals, insects (2) | 3 | 2 | 2 | 3 | 3 | 2.5 |
| B. attenuata | Birds, mammals, insects (2) | 2 | 3 | 3 | 2 | 2 | 2.3 |
| B. hookeriana | Birds (1) | 1 | 1 | 1 | 1 | 1 | 1 |
See Methods for more details.
To explain the distances reached by B. candolleana interpopulation immigrants, the uplift and transport processes proposed for the other two species (wind vortices, cockatoos; [13], [14]) appear to apply to B. candolleana, as seed structure is identical and terminal velocities show little difference (Table 4). Postfire damaged follicles of B. candolleana [consistent with cockatoos feeding on the (exceptionally large) prerelease seeds] were occasionally observed but removal of entire cones was not. The cones are more difficult to remove as they are held deep within the crown (cauliflory) and are not as rewarding (to birds), usually comprising only one or two follicles per cone. He et al. [15] also proposed that the prevailing winds might be sufficient to lift and sweep seeds of B. attenuata and B. hookeriana to adjacent dunes: in B. candolleana four immigrants travelled less than 500 m to the adjacent dune and could fit into this category (Fig. 1). However, the prominent wing of B. candolleana seeds is easily dislodged (unlike the other two species) when obstacles are encountered, making entrainment (the seeds lie flat on the soil) and carriage less likely. This leaves uplift by wind vortices and horizontal drift back to earth by the prevailing winds as the main transport mechanism. The strong NW-NE trend in dispersal direction evident for B. attenuata [14], consistent with the dominant direction of prevailing winds in autumn, was not clearly demonstrated for B. candolleana, suggesting that the pattern was essentially controlled by the random direction of wind vortices. For all species, however, our detection of population connectivity reveals the existence of vectors with capacity for secondary dispersal of seeds across a heterogeneous landscape matrix.
The much lower rate of LDD of B. candolleana seeds compared with the other two species may explain its lower dune occupancy rate and population density (Table 4). Both could be expressions of a delay in colonization (Fig. 2), though the latter might be more a function of longer time to maturation and/or lower seed production and recruitment efficiency. Time to maturation and seed production show only minor differences between these clonal and non-clonal resprouters but the 4× greater longevity and 5× lower annual mortality rate of the former more than counter the 9× reduction in its recruitment rate (Table 5). Metapopulation modelling (e.g. as done in [46]) could further determine the overall importance of migration in accounting for population viability of this species.
The dunes have been stable for ∼20,000 years [50] and so would have been subject to many bouts of colonization by, and extinction of, B. candolleana during that time. The three aged populations are 0.4−2.3 km apart (Fig. 1), similar to the distances we recorded as the range of long distance seed dispersal, and were apparently colonized by B. candolleana 500, 700 and 950 y ago based on extrapolation of the best fit curves to one (Fig. 2). Though the population with oldest plants (three at 840 y) is considerably younger than the estimated maximum longevity of clones (1200 y) there is some possibility that it may represent a much older population (>1200 years) because sampling may have missed the oldest plants or they may have died prematurely. This possibility is unlikely for the two youngest populations. Even if the oldest plants are missing, the right-hand slopes of the distribution curves of the extant clones are preserved and extrapolation to one (the colonizer) can still approximate the time of colonization. However, the oldest plants sampled differed in observed age from the extrapolated age by only 5−100 years indicating that little extrapolation was in fact required. A complete shift of the slope to the left, due to massive death of clones older than the median in that population giving a false age of the oldest clone by extrapolation, seems highly unlikely. First, this species has extremely stable population dynamics (Table 4) so that mortality of established plants is negligible, even when subject to frequent fire. Second, we show that the dunes are matched environmentally and biologically (see Methods) so it begs the question why this wholesale shift would occur in one population but not in the others? This means that differences in growth rates also would not apply as an alternative explanation for differences in apparent population age. There is also a possibility that the oldest population has actually persisted over two or more generations, making it impossible to identify the time of colonization. This would mean that the rate of colonization is even slower than our estimates, barring extinction of earlier populations on these dunes. If any well-established prior population had been eliminated, it is likely this would have required a landscape-scale event, such as the most extreme of droughts, that would have exterminated all three populations.
A mean migration rate of <800 m in 200 years is a sobering statistic when contemplating the potential role of plant migration as a response to climate change [51]. Witkowski and Lamont [52] calculated that another clonal banksia, B. goodii, in the absence of LDD mechanisms (considered unlikely because of the forest-environment of this species), would traverse ∼200 m in 200 y and dismissed such a rate as able to contribute to the conservation of this species under projected climate change rates. Interestingly, He et al [15] estimated that a combination of exceptionally wet years (50% more than the mean) and a mean 13-year fire interval would enable B. hookeriana to recruit and ‘creep’ across the occasional bridges (elevated swales) between adjacent dunes every 200 y without needing to invoke LDD. The intermediary plants would die out once drier conditions returned. It is unclear if such a mechanism would be effective for B. candolleana, as it takes 10× as long to mature, though this species appears more tolerant of shallow soils than B. hookeriana [B. Lamont, pers. observ.].
How can the relatively low effective LDD rate of B. candolleana be explained? It cannot be due to its extremely low recruitment rate as dispersal rate is a set fraction of recruitment no matter what level of recruitment occurs. In the absence of any reason to believe that immigrants are less competitive (but see below), whenever recruitment occurs, immigrants among B. candolleana juveniles will make up only a third as many individuals as among B. attenuata and B. hookeriana juveniles. It also cannot be due to the extreme longevity of B. candolleana adults, as a set fraction of adults comprises immigrants independent of their longevity. In the absence of any reason to believe that immigrants are shorter lived at any point in time or at any particular life history stage, immigrants among B. candolleana adults will make up a third as many individuals as among B. attenuata and B. hookeriana adults.
Banksia candolleana has exceptionally large seeds – the largest palatable seeds in the study area apart from the extremely bitter and cryptic seeds of Xylomelum angustifolium (Proteaceae). Large seeds and seedlings are often more visible and attractive to granivores and herbivores [53], [54]. As such, seeds of B. candolleana may be favoured by granivores. In support, many husks of B. candolleana seeds, with their embryos removed by granivorous birds or rodents, were noted after fire in the study area [B. Lamont, pers. observ.]. This might explain why recruitment efficiency is so low in this species (only 1 in 5000 seeds) despite its exceptionally large seeds. It might also affect their relative contribution to the population once established, if seeds are more apparent having alighted anywhere on the soil surface rather than being swept into and buried in the (highly competitive) litter microsites soon after release [55] as proposed by He et al. [14] for B. attenuata to explain the reverse (i.e. why immigrants are successful).
Relative recruitment success is a function of seed size in this environment, as seeds provide an essential source of mineral nutrients for root growth [56], [57], [58]. It is possible that smaller seeds of B. candolleana are more buoyant than larger ones (and therefore more likely to become immigrants but less fit overall), but terminal velocity is not a simple function of seed size (Table 4, [55]), and even the smallest seeds of B. candolleana are much larger than those of B. attenuata (Table 4). While relative buoyancy has little explanatory value in B. candolleana LDD, relative entrainment might. The follicles are the largest in the genus, so that the seed must be lifted 5−6 cm to escape, and they are often pushed up against other stems, so that seed release is not efficient. The average location of follicles is a mere 20 cm above the ground, one-fifth the height of the other two banksias (Table 5). In addition, the winged plate that separates the two seeds and carries them with it as it dislodges from the follicle usually remains attached to the seeds and they fall as a unit to the ground with one side trapping one of the seeds beneath it (this does not occur with the other two species, B. Lamont, pers. observ.). Further, the cones are overtopped by a dense cover of twigs even postfire, 2−4× that of the other two species. In addition, the large wing is soon broken off once reaching the ground, giving less surface area relative to mass for uplift and entrainment. Thus, not only are there considerable obstacles to exposing the seeds to free air, but the critical wind speed for entraining the seeds must be achieved at or near ground level. All these aspects imply that seeds of B. candolleana are more likely than seeds of B. attenuata or B. hookeriana to collect within and around the maternal plant and are less likely to become airborne.
We have shown that a species with typical reproductive attributes of the clonal life-form (albeit unusually large seeds) has poor LDD seed properties compared with two congeneric co-occurring non-clonal species, despite essentially the same mode of dispersal and physical landscape context, and that these appear to account for its relatively low rates of LDD. Whether these dispersal attributes are idiosyncrasies of this species or to be expected of long-lived clonal species generally (where the imperative for extensive dispersal via seeds is relaxed) is worthy of further study. For example, in another co-occurring clonal banksia, B. elegans, despite prolific flowering, seed set is usually negligible so that LDD is also negligible [59], [60]. In this regard, there is no substitute for a thorough knowledge of the reproductive biology of the species under study so that taxon-specific attributes can assist interpretation of estimates of population connectivity from genetic data [16]. In addition, the findings suggest that LDD rates of resprouting species might vary as much within the group, depending on whether they are clonal or not, as between resprouters and nonsprouters. As our ability to genotype individuals for population genetic studies becomes increasingly feasible [61], so will the accuracy and power to better reveal the tail of the dispersal curve. When combined with detailed demographic studies on co-occurring species within model metapopulation systems such as ours, landscape genetic approaches will increasingly reveal the effects of life-history variation and landscape patterns on LDD and landscape connectivity.
Acknowledgments
Janet Anthony provided technical assistance; Ben Miller assisted in determining terminal velocities; the Department of Environment and Conservation (Western Australia) permitted us to work on its managed land; the Tinkers (Western Flora Caravan Park, Western Australia) provided invaluable logistic support.
Funding Statement
This research was financed by the Australian Research Council (DP0556767). LM was supported by a Fulbright scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sork VL, Smouse PE (2006) Genetic analysis of landscape connectivity in tree populations. Landscape Ecol 21: 821–836. [Google Scholar]
- 2. Holderegger R, Buehler D, Gugerli F, Manel S (2010) Landscape genetics of plants. Trend Plant Sci 15: 675–683. [DOI] [PubMed] [Google Scholar]
- 3. Luque S, Saura S, Fortin M-J (2012) Landscape connectivity analysis for conservation: insights from combining new methods with ecological and genetic data. Landscape Ecol 27: 153–157. [Google Scholar]
- 4. Cain ML, Milligan MG, Strand AE (2000) Long-distance seed dispersal in plant populations. Am J Bot 87: 1217–1227. [PubMed] [Google Scholar]
- 5. Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trend Ecol Evol 17: 379–386. [Google Scholar]
- 6. Nathan R (2006) Long-distance dispersal of plants. Science 313: 786–788. [DOI] [PubMed] [Google Scholar]
- 7. Nathan R, Perry G, Cronin J, Strand A, Cain M (2003) Methods for estimating long-distance dispersal. Oikos 103: 261–273. [Google Scholar]
- 8. Hanski I (1998) Metapopulation dynamics. Nature 396: 41–49. [Google Scholar]
- 9. Levin SA, Muller-Landau HC, Nathan R, Chave J (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Ann Rev Ecol Evol Syst 34: 575–604. [Google Scholar]
- 10. Segelbacher G, Cushman SA, Epperson BK, Fortin MJ, Francois O, et al. (2010) Applications of landscape genetics in conservation biology: concepts and challenges. Conserv Genet 11: 375–385. [Google Scholar]
- 11. Nathan R, Schurr FM, Speigel O, Steinitz O, Trakhtenbrot A, et al. (2008) Mechanisms of long-distance seed dispersal. Trend Ecol Evol 23: 238–647. [DOI] [PubMed] [Google Scholar]
- 12. Berry O, Tocher MD, Sarre SD (2004) Can assignment tests measure dispersal? Mol Ecol 13: 551–561. [DOI] [PubMed] [Google Scholar]
- 13. He T, Krauss SL, Lamont BB, Miller BP, Enright NJ (2004) Long distance dispersal in a metapopulation of Banksia hookeriana inferred by population allocation from AFLP data. Mol Ecol 13: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 14. He T, Lamont BB, Krauss SL, Enright NJ, Miller BP (2009) Long-distance dispersal of seeds in the fire-tolerant shrub Banksia attenuata . Ecography 32: 571–580. [Google Scholar]
- 15. He T, Lamont BB, Krauss SL Enright NJ (2010) Landscape genetics of Banksia hookeriana in a metapopulation system. Ann Bot 106: 547–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lowe WH, Allendorf FW (2010) What can genetics tell us about population connectivity? Mol Ecol 19: 3038–3051. [DOI] [PubMed] [Google Scholar]
- 17. Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hotspot of biodiversity. Ann Rev Ecol Evol Syst 35: 623–650. [Google Scholar]
- 18. Bell DT (2001) Ecological response syndromes in the flora of southwestern Australia: fire resprouters versus reseeders. Bot Rev 67: 417–440. [Google Scholar]
- 19. Lamont BB, Wiens D (2003) Are seed set and speciation rates always low among species that resprout after fire, and why? Evol Ecol 17: 277–292. [Google Scholar]
- 20. Enright NJ, Lamont BB (1989) Seed banks, fire season, safe sites and seedling recruitment in five co-occurring Banksia species. J Ecol 77: 1111–1122. [Google Scholar]
- 21. Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trend Ecol Evol 16: 45–51. [DOI] [PubMed] [Google Scholar]
- 22. Low AB, Lamont BB (1990) Aerial and below-ground phytomass of Banksia scrub-heath at Eneabba, Western Australia. Aust J Bot 38: 351–359. [Google Scholar]
- 23. Bell TL, Ojeda F (1999) Underground starch storage in Erica species of the Cape Floristic Region - differences between seeders and sprouters. New Phytol 144: 143–152. [Google Scholar]
- 24. Lamont BB, Enright NJ, He T (2011) Fitness and evolution of resprouters in relation to fire. Plant Ecol 212: 1945–1957. [Google Scholar]
- 25. Lamont BB, He T, Enright NJ, Krauss SL, Miller BP (2003) Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. J Evol Biol 16: 551–557. [DOI] [PubMed] [Google Scholar]
- 26.Enright NJ, Clarke M, Keith D, Miller BP (2012). Australian sclerophyllous shrubby ecosystems: heathlands, heathy woodlands and mallee woodlands. In: Bradstock R, Gill A, Williams R, editors. Flammable Australia: Fire Regimes and Biodiversity in a Changing World. CSIRO Publishing, Melbourne. 215–234.
- 27.Hanski I, Gaggiotti OE (2004) Ecology, Genetics and Evolution of Metapopulations. Elsevier Academic Press, Amsterdam.
- 28.Doyle JJ (1991) DNA protocols for plants. In: Hewitt GM, editor. Molecular techniques in taxonomy. Springer. 283–293.
- 29. Merwin L, He T, Krauss SL (2010) Isolation and characterization of polymorphic microsatellite DNA markers for Banksia candolleana (Proteaceae). Conserv Genet Resources 2: 345–347. [Google Scholar]
- 30. Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Mol Ecol Notes 6: 288–295. [Google Scholar]
- 31. Rousset F (2008) Genepop '007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resources 8: 103–106. [DOI] [PubMed] [Google Scholar]
- 32. Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P (2004) Microchecker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4: 535–538. [Google Scholar]
- 33. Arnaud-Haond S, Belkhir K (2007) GENCLONE: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Mol Ecol Notes 7: 15–17. [Google Scholar]
- 34. Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, et al. (2004) GeneClass2: a software for genetic assignment and first-generation migrant detection. J Hered 95: 536–539. [DOI] [PubMed] [Google Scholar]
- 35. Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13: 55–65. [DOI] [PubMed] [Google Scholar]
- 36. Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94: 9197–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roques S, Duchesne P, Bernatchez L (1999) Potential of microsatellites for individual assignment: the North Atlantic redfish (genus Sebastes) species complex as a case study. Mol Ecol 8: 1703–1717. [DOI] [PubMed] [Google Scholar]
- 38. Campbell D, Duchesne P, Bernatchez L (2003) AFLP utility for population assignment studies: analytical investigation and empirical comparison with microsatellites. Mol Ecol 12: 979–991. [DOI] [PubMed] [Google Scholar]
- 39. Robledo-Arnucio JJ (2012) Joint estimation of contemporary seed and pollen dispersal rates among plant populations. Mol Ecol Resources 12: 299–311. [DOI] [PubMed] [Google Scholar]
- 40. Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163: 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Enright NJ, Lamont BB (1992) Recruitment variability in the resprouting shrub Banksia attenuata and non-sprouting congeners in the northern sandplain heaths of south-western Australia. Acta Oecologica 13: 727–741. [Google Scholar]
- 42. Enright NJ, Marsula R, Lamont BB, Wissel C (1998a) The ecological significance of canopy seed storage in fire-prone environments: a model for nonsprouting shrubs. J Ecol 86: 946–959. [Google Scholar]
- 43. Enright NJ, Marsula R, Lamont BB, Wissel C (1998b) The ecological significance of canopy seed storage in fire-prone environments: a model for resprouting shrubs. J Ecol 86: 960–973. [Google Scholar]
- 44. Lamont BB, Enright NJ, Witkowski ETF, Groeneveld J (2007) Conservation biology of banksias: insights from natural history to simulation modeling. Aust J Bot 55: 280–292. [Google Scholar]
- 45. He T, Lamont BB, Krauss SL, Enright NJ, Miller BP (2008) Covariation between intraspecific genetic diversity and species diversity within a plant functional group. J Ecol 96: 956–961. [Google Scholar]
- 46. Groeneveld J, Enright NJ, Lamont BB (2008) Simulating the effects of different spatio-temporal fire regimes on plant metapopulation persistence in a Mediterranean-type region. J Appl Ecol 45: 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clements HB (1977) Lift-off of forest firebrands USDA Forest Service Research Paper SE-159.
- 48. Krauss SL, He T, Barrett L, Lamont BB, Miller BP, et al. (2009) Contrasting impacts of pollen and seed dispersal on spatial genetic structure in the bird-pollinated Banksia hookeriana . Heredity 102: 274–285. [DOI] [PubMed] [Google Scholar]
- 49. Manel S, Schwartz M, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18: 189–197. [Google Scholar]
- 50. Krauss SL, He T, Lamont BB, Miller BP, Enright NJ (2006) Late Quaternary climate change and spatial genetic structure in the shrub Banksia hookeriana . Mol Ecol 15: 1125–1137. [DOI] [PubMed] [Google Scholar]
- 51.Midgley GF, Thuiller W, Higgins SI (2007) Plants species migration as a key uncertainty in predicting future impacts of climate change on ecosystems: progress and challenges. In. Canadell J, editor. Terrestrial Ecosystems in a Changing World. Springer-Verlag, Berlin. 149–160.
- 52. Witkowski ETF, Lamont BB (2006) Resilience of two Banksia species to global change: Comparing results of bioclimatic modelling, demographic and translocation studies. Int J Biodivers Sci Managem 2: 1–14. [Google Scholar]
- 53. Garb J, Kotler BP, Brown JS (2000) Foraging and community consequences of seed size for coexisting Negev Desert granivores. Oikos 88: 291–300. [Google Scholar]
- 54. Rafferty CM, Lamont BB, Hanley ME (2010) Herbivore feeding preferences in captive and wild populations. Austral Ecol 35: 257–263. [Google Scholar]
- 55. Lamont BB, Witkowski ETF, Enright NJ (1993) Post-fire litter microsites: safe for seeds, unsafe for seedlings. Ecology 74: 501–512. [Google Scholar]
- 56. Leishman MR, Westoby M (1994) The role of seed size in seedling establishment in dry soil conditions − experimental evidence from semi-arid species. J Ecol 82: 249–258. [Google Scholar]
- 57. Lamont BB, Witkowski ETF (1995) A test for lottery recruitment among four Banksia species based on their demography and biological attributes. Oecologia 101: 299–308. [DOI] [PubMed] [Google Scholar]
- 58. Lamont BB, Groom PK (2002) Green cotyledons of two Hakea species control seedling mass and morphology by supplying mineral nutrients rather than organic compounds. New Phytol 153: 101–110. [Google Scholar]
- 59. Lamont BB, Barrett GJ (1988) Constraints on seed production and storage in a root-suckering Banksia . J Ecol 76: 1069–1082. [Google Scholar]
- 60. Lamont BB (1989) Sexual versus vegetative reproduction in Banksia elegans . Bot Gaz 149: 370–375. [Google Scholar]
- 61. Tautz D, Ellegren H, Weigel D (2010) Next generation molecular ecology. Mol Ecol 19: 1–3. [DOI] [PubMed] [Google Scholar]