Abstract
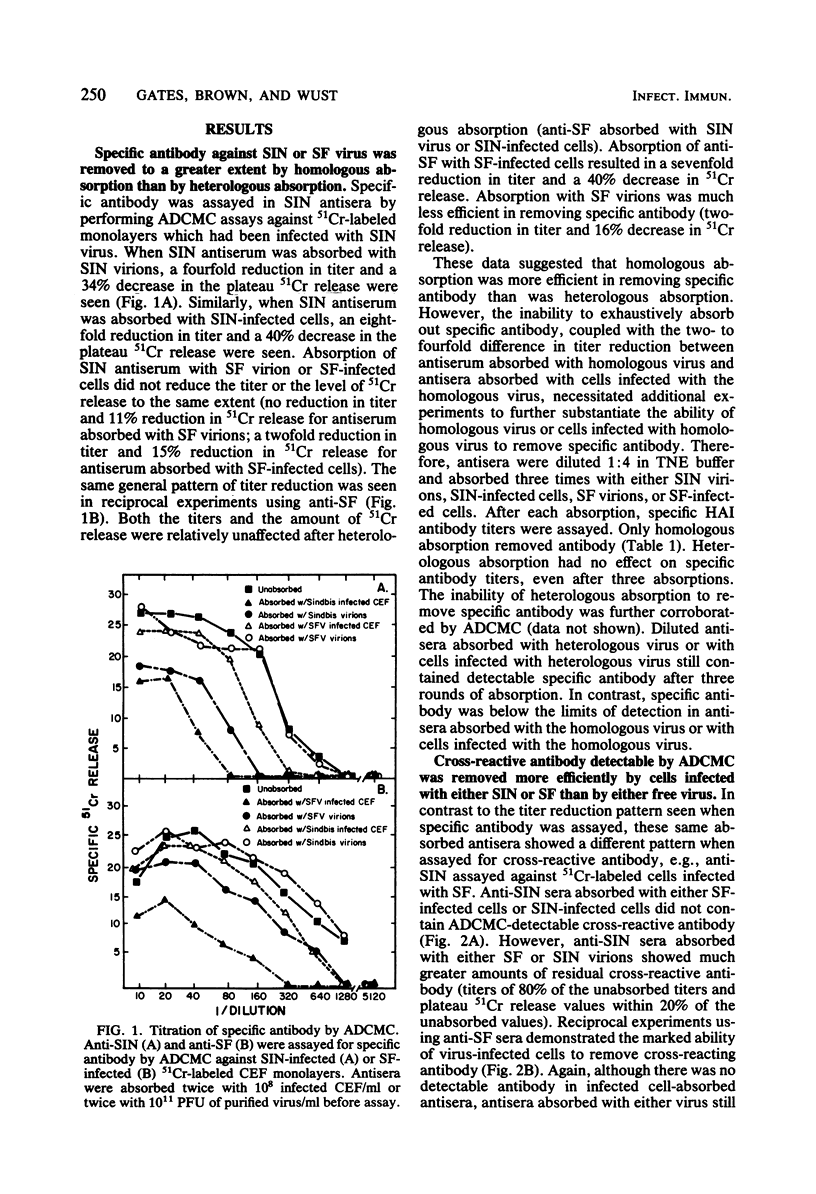
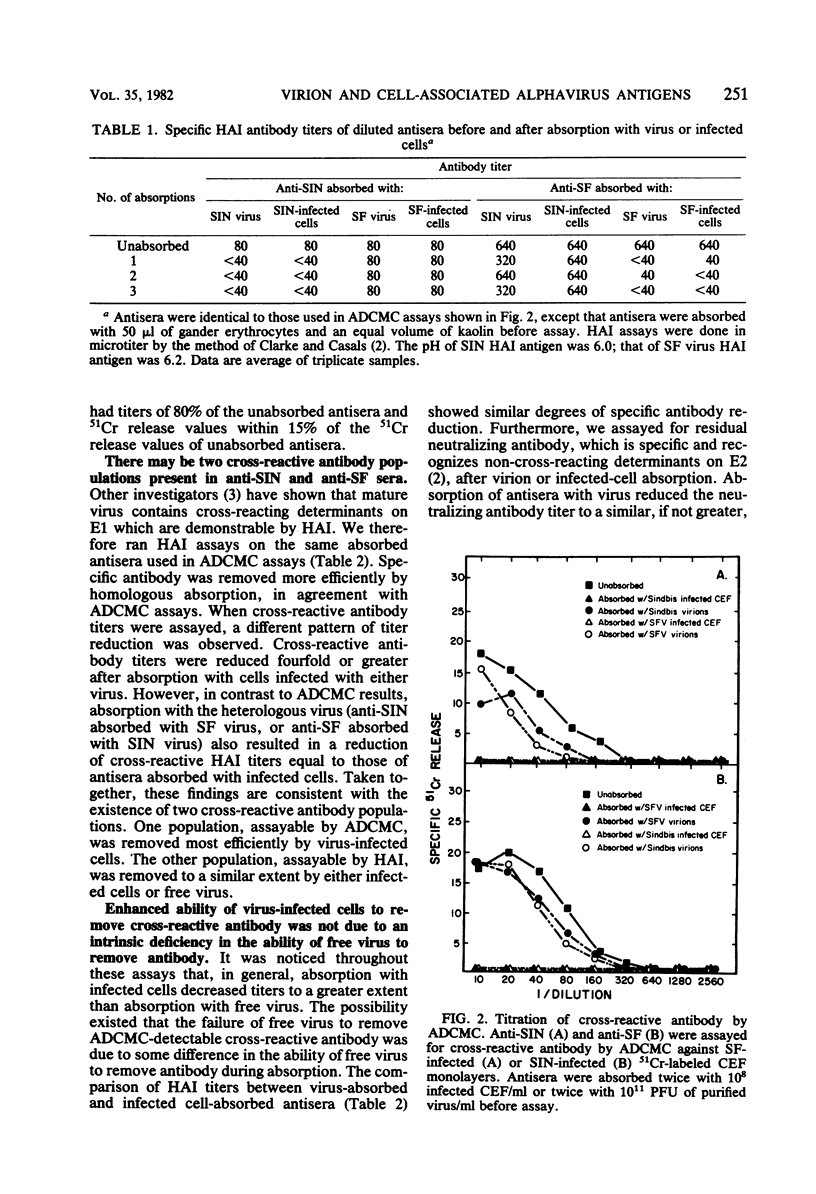
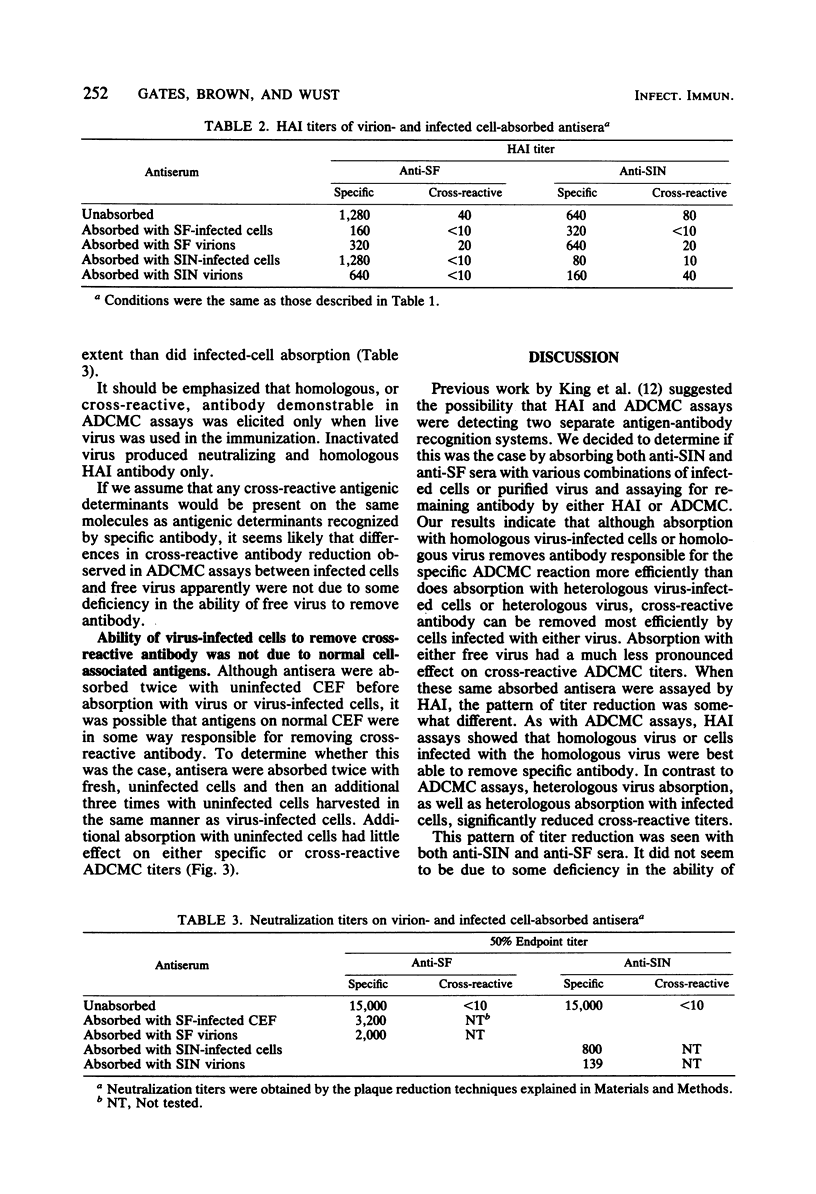
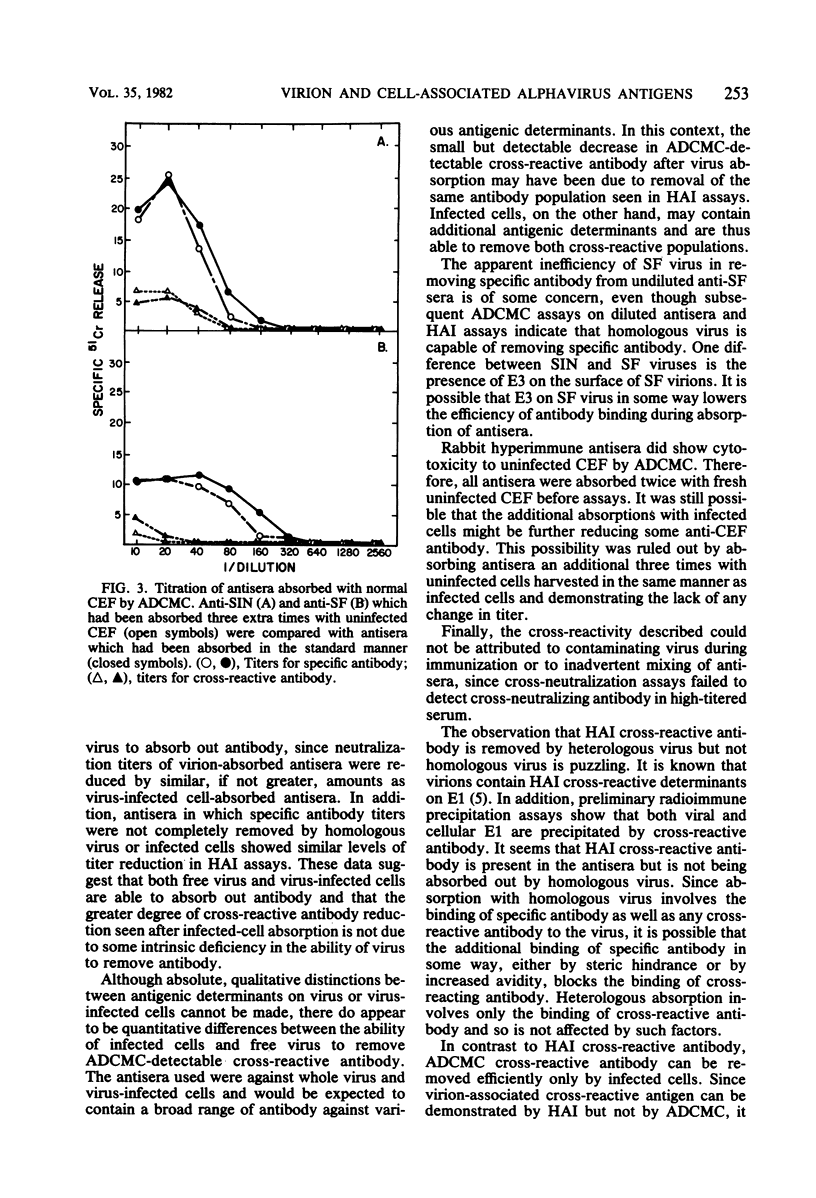
Rabbit hyperimmune antisera against Sindbis (SIN) or Semliki Forest (SF) virus were absorbed with purified SIN virus or SIN virus-infected cells, or with SF virus or SF virus-infected cells. Residual antibody titers were determined by hemagglutination inhibition (HAI) and antibody-dependent, complement-mediated cytolysis (ADCMC) assays. It appeared that absorption with virus-infected cells removed ADCMC-detectable cross-reactive antibody much more efficiently than did absorption with either virus. HAI assays with the same absorbed antisera indicated that both virus and virus-infected cells removed HAI-detectable cross-reactive antibody. On the basis of these and other data, there appeared to be a cross-reactive antigen present on virus-infected cells which was detectable by ADCMC and was distinct from the cross-reactive antigen assayed by HAI.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Teramoto A. Y., Cardiff R. D., Russell P. K. Radioimmune precipitation of group A arboviruses. J Immunol. 1972 Sep;109(3):426–433. [PubMed] [Google Scholar]
- Dalrymple J. M., Vogel S. N., Teramoto A. Y., Russell P. K. Antigenic components of group A arbovirus virions. J Virol. 1973 Nov;12(5):1034–1042. doi: 10.1128/jvi.12.5.1034-1042.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Jones K. J., Scupham R. K., Pfeil J. A., Wan K., Sagik B. P., Bose H. R. Interaction of Sindbis virus glycoproteins during morphogenesis. J Virol. 1977 Feb;21(2):778–787. doi: 10.1128/jvi.21.2.778-787.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G. Early synthesis of Semliki Forest virus-specific proteins in infected chicken cells. J Virol. 1976 Jul;19(1):1–12. doi: 10.1128/jvi.19.1.1-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G., Pauli G. The influence of intramolecular disulfide bonds on the structure and function of Semliki forest virus membrane glycoproteins. Virology. 1980 Apr 30;102(2):300–309. doi: 10.1016/0042-6822(80)90097-5. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Rott R., Schwarz R. T. Carbohydrate-induced conformational changes of Semliki forest virus glycoproteins determine antigenicity. Virology. 1980 Apr 30;102(2):286–299. doi: 10.1016/0042-6822(80)90096-3. [DOI] [PubMed] [Google Scholar]
- King B., Wust C. J., Brown A. Antibody-dependent, complement-mediated homologous and cross-cytolysis of togavirus-infected cells. J Immunol. 1977 Oct;119(4):1289–1292. [PubMed] [Google Scholar]
- Latif Z., Gates D., Wust C. J., Brown A. Cross protection among togaviruses in nude mice and littermates. J Gen Virol. 1979 Oct;45(1):89–98. doi: 10.1099/0022-1317-45-1-89. [DOI] [PubMed] [Google Scholar]
- Peck R. D., Brown A., Wust C. J. Preliminary evidence for cell-mediated immunity in cross-protection among group A arboviruses. J Immunol. 1975 Feb;114(2 Pt 1):581–584. [PubMed] [Google Scholar]
- Peck R., Brown A., Wust C. J. In vitro heterologous cytotoxicity by T effector cells from mice immunized with Sindbis virus. J Immunol. 1979 Oct;123(4):1763–1766. [PubMed] [Google Scholar]
- Peck R., Wust C. J., Brown A. Adoptive transfer of cross-protection among alphaviruses in mice requires allogeneic stimulation. Infect Immun. 1979 Jul;25(1):320–327. doi: 10.1128/iai.25.1.320-327.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M. Two small virus-specific polypeptides are produced during infection with Sindbis virus. J Virol. 1979 Mar;29(3):1186–1195. doi: 10.1128/jvi.29.3.1186-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



