Pneumocystis is an unusual fungus that is a prototypical opportunistic pathogen, causing an asymptomatic or mild infection in the normal host but fulminate pneumonia (PcP) in the immunocompromised host. Untreated, the mortality rate from PcP approaches 100%. Even with treatment, mortality rates approach 10–20%. It is a ubiquitous organism infecting a wide array of mammalian species. Although the reservoir of infection for Pneumocystis has not been defined, direct airborne transmission from host to host has been proven under experimental conditions using the rat model of PcP [1]. This synopsis will review the evidence suggesting that the reservoir of infection for humans with PcP is other humans, possibly infants and young children.
The study of Pneumocystis has been problematic due to the inability to cultivate the organism or manipulate its cellular or molecular characteristics. As recently as the 1970s, a student studying Pneumocystis would have come away with the following understanding of its basic biology and function as a pathogen: Pneumocystis is an organism of low virulence found in many mammalian species. In humans, Pneumocystis pneumonia (PcP) is a zoonosis resulting from reactivation of a latent infection acquired early in life. This concept of Pneumocystis arose largely through analogy to existing knowledge about other organisms to explain clinical observations, rather than through direct experimentation on the organism. Over the past 25 years, we have learned more about Pneumocystis through controlled studies that have corrected some of the misconceptions contained in the “old” concept of Pneumocystis contagion stated above. What follows is a brief summary of key research observations that give us a better, yet still incomplete, understanding of how Pneumocystis maintains its existence as an opportunistic pathogen.
Pneumocystis Is Not a Zoonosis
In order for Pneumocystis to be classified a zoonosis it would need to be transmissible from one host species to another. This was originally suspected for Pneumocystis because human and rodent Pneumocystis appeared visually similar following histochemical staining procedures. However with the advent of monoclonal antibodies, it was possible to show that Pneumocystis from one host species was phenotypically distinct from Pneumocystis from a different host [2]. Soon thereafter this phenotypic variation was confirmed at the genetic level [3]–[5].
Experiments that directly assessed the ability of Pneumocystis to move between species were published in 1993. This study demonstrated that Pneumocystis could not be transmitted between mice and ferrets [6]. This observation was soon followed by similar results that showed Pneumocystis from rabbits, rats, monkeys, and humans were not infectious for mice [reviewed in 7]. Thus, available data indicates that each mammalian species that contracts PcP has its own strain or species of Pneumocystis.
Pneumocystis Does Not Establish Latency under Laboratory Conditions
To experimentally address the issue of latency, severe combined immunodeficiency disease (scid) mice were allowed to develop active PcP prior to restoration of their immune system by adoptive transfer of normal spleen cells. Immune-reconstituted mice recovered from PcP through an immune response without the use of antibiotics. Pneumocystis was cleared from the lung and could not be detected by three weeks post-reconstitution. To determine if the mice remained latently infected, they were again immunosuppressed and observed for up to 84 days for reactivation of PcP, which did not occur [8]. Similar results, using a somewhat different approach in rats, were obtained by Vargas et al. [9]. Thus, normal immune mechanisms are sufficient to clear Pneumocystis after infection such that latency is unlikely.
Support for the concept that PcP in adults is a result of recent acquisition of Pneumocystis, rather than reactivation of a latent infection, comes from analysis of second episodes of PcP. Keely et al. were able to show that episodes of PcP occurring more than six months apart were caused by genetically distinct strains of Pneumocystis [10]. Furthermore, genetic typing of Pneumocystis isolates from human patients demonstrated that the genotype of the organism causing disease was associated with the patient's place of residence rather than place of birth [11].
Pneumocystis Infects the Normal Host, Producing a Typical Pattern of Contagion Followed by Immune Response and Clearance
To characterize the course of infection in the normal host, we exposed immunocompetent mice to scid mice with active PcP [12]. The normal mice developed a productive infection with growth of organisms through week four, at which time Pneumocystis-specific antibodies appeared in the serum in association with complete clearance of organisms. However, normal mice with this self-limited mild infection were able to transmit Pneumocystis to another normal mouse, thereby serving to maintain Pneumocystis in the environment. Alternatively, if the infected normal mouse encountered an immunosuppressed host, full-blown PcP resulted [13], [14]. Thus the normal host could serve as a reservoir to maintain Pneumocystis in any given mammalian population.
Asymptomatic or Subclinical Infection of Human Infants is Common
It has been long known that low titers of antibody to Pneumocystis are common in humans. The role of young children in the contagion cycle was suggested by the observation that Pneumocystis was frequently found in the lungs of infants dying of Sudden Infant Death Syndrome (SIDS) in Santiago, Chile; Oxford, England; Rochester, New York; and Connecticut [15], [16]. While it was initially postulated that Pneumocystis was somehow contributing to SIDS, examination of the lung histology of infants dying of SIDS and who were also infected with Pneumocystis revealed very few organisms and no evidence of an inflammatory reaction. Additional studies do not support any causative role for Pneumocystis in SIDS [17]. They do, however, support the concept that infection of infants is common. These findings are consistent with the findings reported for normal mice infected with Pneumocystis discussed above and are also consistent with studies by Garvy et al., which demonstrate that neonatal mice are highly susceptible to infection with Pneumocystis [18].
To better understand the kinetics of infection, we carried out a prospective study of primary infection in 107 infants in Santiago, Chile. Infants were tested for the presence of serum antibody to Pneumocystis at birth and every two months thereafter for two years. Nasopharyngeal aspirates were also examined for Pneumocystis by PCR during respiratory illnesses. At two months of age, 16% were seropositive and seroconversion occurred at a rate of approximately 5% per month. By 20 months of age, 85% of the cohort of infants had seroconverted. Seventy-four infants were screened for Pneumocystis DNA and 32% were positive [19]. Given that the peak age for SIDS overlaps with the peak time of seroconversion, the presence of Pneumocystis in the lungs of SIDS cases is likely coincidental.
Infants and Children Are a Pneumocystis-Susceptible Population That Could Serve as an Important Reservoir for Maintaining Pneumocystis in the Environment
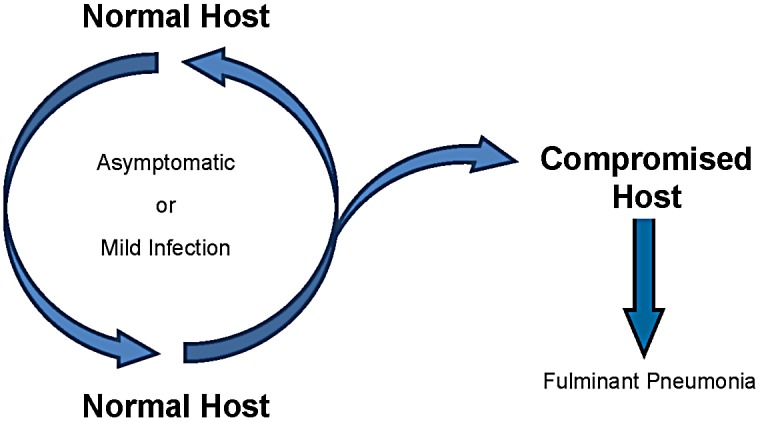
By extending the experimental observations made in animal models of PcP to the epidemiologic observations in infants, one can envision that infants serve to harbor Pneumocystis with little adverse effect on the host. Once infected, an infant could then pass on the infection to another host, either normal or immunocompromised. Resistance to reinfection is of unknown duration but would be compensated for by new births that would serve to maintain a pool of susceptible hosts. As depicted in Figure 1, circulation in the healthy population would result in subclinical or mild infection. It is quite possible that one of the multitude of “viral illnesses” occurring within the first two years of life is actually an infection with Pneumocystis. When an immunocompromised host enters the cycle, the result is active PcP. The compromised host can then spread infection to a compromised patient, as has been suggested by clinic “outbreaks” [20], or back to a normal individual where it can maintain a quasi-symbiotic relationship of limited duration with its human host. Studies have not been done to determine the frequency with which normal older children and adults become infected, so we don't know whether older children and adults also serve as a significant reservoir of infection. However, Pneumocystis DNA has been found in oral washes from adult patients [21].
Figure 1. Proposed transmission cycle for Pneumocystis based on the normal host, likely infants, serving as the reservoir of infection.
So where does Pneumocystis live? Given what we have learned in the past two decades, the most likely answer to this question is: It lives in us. Our concept of Pneumocystis should now read: Pneumocystis is a classic opportunistic pathogen that causes little or no disease after infecting a normal host, while infection of the immunocompromised host results in a uniformly fatal pneumonia if untreated. Each susceptible mammalian species is infected by a unique strain or species of Pneumocystis, but the basis for the host-species specificity remains a mystery. In man, the organism is spread from person to person, and PcP is most likely the result of recent acquisition of Pneumocystis rather than reactivation of a latent infection. The ubiquitous presence of Pneumocystis throughout the population likely explains why this organism is so adept at “finding” immunocompromised patients and suggests that more widespread use of immunomodulatory therapies will increase the risk of developing PcP. Understanding this aspect of the biology of Pneumocystis will aid in developing means to prevent PcP.
Funding Statement
The authors received no specific funding for this study.
References
- 1. Hughes WT (1982) Natural mode of acquisition for de novo infection with Pneumocystis carinii . J Infect Dis 145: 842–848. [DOI] [PubMed] [Google Scholar]
- 2. Gigliotti F, Stokes DC, Cheatham AB, Davis DS, Hughes WT (1986) Development of murine monoclonal antibodies to Pneumocystis carinii . J Infect Dis 154: 315–322. [DOI] [PubMed] [Google Scholar]
- 3. Sinclair K, Wakefield AE, Banerji S, Hopkin JM (1991) Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol Biochem Parasitol 45: 183–184. [DOI] [PubMed] [Google Scholar]
- 4. Smulian AG (2001) Pneumocystis carinii: genetic diversity and cell biology. Fungal Genet Biol 34: 145–154. [DOI] [PubMed] [Google Scholar]
- 5. Wright TW, Simpson-Haidaris PJ, Gigliotti F, Harmsen AG, Haidaris CG (1994) Conserved sequence homology of cysteine-rich regions in genes encoding glycoprotein A in Pneumocystis carinii derived from different host species. Infect Immun 62: 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ (1993) Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun 61: 2886–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durand-Joly I, Aliouat el M, Recourt C, Guyot K, Francois N, et al. (2002) Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol 40: 1862–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen W, Gigliotti F, Harmsen AG (1993) Latency is not an inevitable outcome of infection with Pneumocystis carinii . Infect Immun 61: 5406–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vargas SL, Hughes WT, Wakefield AE, Oz HS (1995) Limited persistence in and subsequent elimination of Pneumocystis carinii from the lungs after P. carinii pneumonia. J Infect Dis 172: 506–510. [DOI] [PubMed] [Google Scholar]
- 10. Keely SP, Stringer JR, Baughman RP, Linke MJ, Walzer PD, et al. (1995) Genetic variation among Pneumocystis carinii f. sp. hominis isolates in recurrent pneumocystosis. J Infect Dis 172: 595–598. [DOI] [PubMed] [Google Scholar]
- 11. Beard CB, Carter JL, Keely SP, Huang L, Pieniazek NJ, et al. (2000) Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis 6: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. An CL, Gigliotti F, Harmsen AG (2003) Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. carinii f. sp. muris and host immune response. Infect Immun 71: 2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gigliotti F, Harmsen AG, Wright TW (2003) Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun 71: 3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chabe M, Dei-Case E, Creusy C, Fleurisse L, Respaldiza N, et al. (2004) Immunocompetent hosts as a reservoir of Pneumocystis organisms: histological and rt-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis 23: 89–97. [DOI] [PubMed] [Google Scholar]
- 15. Vargas SL, Ponce CA, Hughes WT, Wakefield AE, Weitz JC, et al. (1999) Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin Infect Dis 29: 1489–1493. [DOI] [PubMed] [Google Scholar]
- 16. Morgan DJ, Vargas SL, Reyes-Mugica M, Walterspiel JN, Carver W, et al. (2001) Identification of Pneumocystis carinii in the lungs of infants dying of sudden infant death syndrome. Pediatr Infect Dis J 20: 306–309. [DOI] [PubMed] [Google Scholar]
- 17. Vargas SL, Ponce CA, Galvez P, Ibarra C, Haas EA, et al. (2007) Pneumocystis is not a direct cause of sudden infant death syndrome. Pediatr Infect Dis J 26: 81–83. [DOI] [PubMed] [Google Scholar]
- 18. Garvy BA, Harmsen AG (1996) Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect Immun 64: 3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, et al. (2001) Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis 32: 855–861. [DOI] [PubMed] [Google Scholar]
- 20. Gianella S, Haeberli L, Joos B, Ledergerber B, Wuthrich RP, et al. (2010) Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis 12: 1–10. [DOI] [PubMed] [Google Scholar]
- 21. Vargas SL, Pizarro P, Lopez-Vieyra M, Neira-Aviles P, Bustamante R, et al. (2010) Pneumocystis colonization in older adults and diagnostic yield of single versus paired noninvasive respiratory sampling. Clin Infect Dis 50: e19–21. [DOI] [PubMed] [Google Scholar]


