Abstract
Fertilization begins with binding and fusion of a sperm with the oocyte, a process that triggers a high amplitude calcium transient which propagates through the oocyte and stimulates a series of preprogrammed signal transduction events critical for zygote development. Identification of the pathways downstream of this calcium transient remains an important step in understanding the basis of zygote quality. The present study demonstrates that the calcium-calmodulin sensitive protein tyrosine kinase PYK2 is a target of the fertilization-induced calcium transient in the zebrafish oocyte and that it plays an important role in actin-mediated events critical for sperm incorporation. At fertilization, PYK2 was activated initially at the site of sperm-oocyte interaction and was closely associated with actin filaments forming the fertilization cone. Later PYK2 activation was evident throughout the entire oocyte cortex, however activation was most intense over the animal hemisphere. Fertilization-induced PYK2 activation could be blocked by suppressing calcium transients in the ooplasm via injection of BAPTA as a calcium chelator. PYK2 activation could be artificially induced in unfertilized oocytes by injection of IP3 at concentrations sufficient to induce calcium release. Functionally, suppression of PYK2 activity by chemical inhibition or by injection of a dominant-negative construct encoding the N-terminal ERM domain of PKY2 inhibited formation of an organized fertilization cone and reduced the frequency of successful sperm incorporation. Together, the above findings support a model in which PYK2 responds to the fertilization-induced calcium transient by promoting reorganization of the cortical actin cytoskeleton to form the fertilization cone.
Keywords: fertilization, oocyte, PYK2, calcium, actin
Introduction
Fertilization triggers a series of preprogrammed biochemical steps in the oocyte, which function to establish a block to polyspermy, activate zygote metabolism, and initiate resumption of the meiotic cell cycle and zygote development. An early step in this process is the transient elevation of intracellular free calcium. This fertilization-induced calcium transient is common to oocytes of all species examined including organisms as primitive as the sponge and as complex as mammals (Runft, et al., 2002; Whitaker, 2006). While species differences exist in the subcellular distribution, timing, and biochemical triggers for these calcium transients, the downstream responses are similar among species. Studies in a variety of models have demonstrated that members of the calcium-calmodulin-dependent kinase family play a critical role in meiosis resumption and metabolic activation (Chang, et al., 2009; Miyazaki and Ito, 2006; Ozil, et al., 2005). However other calcium sensitive proteins such as synaptotagmin and calcineurin are activated at fertilization and play important roles as well (Leguia, et al., 2006; Nishiyama, et al., 2007). In addition to pathways regulated directly by calcium, multiple downstream protein kinase-mediated pathways are stimulated indirectly following the calcium transient. Protein tyrosine kinase-mediated events result in phosphorylation of cytoskeleton-associated proteins in the oocyte cortex (McGinnis, et al., 2007; Sharma and Kinsey, 2006; Wright and Schatten, 1995) in species ranging from sea urchins to mouse. Src-family kinases (SFKs) including Fyn kinase were recently shown to become localized to the oocyte cortex in marine invertebrates (Sharma et al., 2006; Townley, et al., 2009), zebrafish (Sharma et al., 2006; Sharma and Kinsey, 2008), frogs (Sakakibara, et al., 2005) and mammals (Levi, et al., 2010). Fyn kinase was also found to function in maintenance of cortical cytoskeleton polarity in mouse oocytes and to contribute to the process of pronuclear congression (Luo, et al., 2010). In the zebrafish oocyte, SFK activation and tyrosine phosphorylation of proteins associated with the cortical actin layer was initiated at the fertilization cone and propagated through the entire oocyte cortex in a manner temporally correlating with the calcium transient (McGinnis, et al., 2011; Sharma et al., 2006). However, our inhibitor studies (unpublished) revealed that suppression of SFK activity did not abolish the fertilization-induced wave of tyrosine kinase signaling suggesting that other PTKs may be part of the response to fertilization.
Our analysis of the PTKs that respond during egg activation revealed the presence of a calcium-calmodulin (Kohno, et al., 2008) dependent protein tyrosine kinase activity in the zebrafish oocyte plasma membrane which we now have identified as proline-rich tyrosine kinase-2 (Pyk2), (also referred to as; cell-associated kinase-beta (CAK-β), calcium-dependent tyrosine kinase (CADTK), related adhesion focal tyrosine kinase (RAFTK)). PYK2 is a member of the focal adhesion kinase (FAK) family of protein kinases. Like FAK, PYK2 is activated initially through a range of stimuli including growth factors, cytokines, integrin ligation and G-coupled receptor agonists (Orr and Murphy-Ullrich, 2004a). PYK2 is unique in that full activation requires participation of calmodulin and elevated intracellular calcium (Wu, et al., 2006) and, in many cases, participation of a Src-family kinase (Cheng, et al., 2002; Sorokin, et al., 2001). PYK2 and FAK are cytoplasmic protein tyrosine kinases that transduce signals from cell surface receptors to cytoplasmic pathways through their unique capability as scaffolding proteins as well as through their kinase activity. The 129 kDA PYK2 protein consists of multiple protein interaction domains (reviewed (Schlaepfer, et al., 1999) such as an N-terminal Ezrin, Radixin, Moesin (ERM) homology domain that includes a calmodulin binding site. The single catalytic site is followed by two proline-rich domains that include SH3 and SH2 binding sites critical to docking functions of the protein. The C-terminal Focal Adhesion Targeting (FAT) domain functions during docking interactions with cytoskeletal components such as paxillin and talin. These diverse protein interaction domains enable PYK2 to integrate a wide variety of extracellular and intracellular signals to regulate Rho activity (Okigaki, et al., 2003) and implement actin cytoskeleton functions relating to cell-cell junction turnover (Sun, et al., 2011), cell process formation (Gil-Henn, et al., 2007) and phagocytosis (Owen, et al., 2007). PYK2 can also function in more complex events such as mechanical and osmotic stress response (Koh, et al., 2001; Klemm, et al., 2010; Takahashi, et al., 2003), proliferation (Cox, et al., 2006), and survival (Hanks, et al., 2003).
The mouse oocyte expresses certain Src-family and FAK-family kinases at levels much higher than most other cell types (McGinnis et al., 2011) suggesting that these two kinase families play important roles in the function of mammalian oocytes and possibly oocytes of other species as well. We have previously detected FAK kinase activity in zebrafish zygotes and early embryos (Tsai, et al., 2005), but that study made use of antibodies targeting the C-terminal domain of FAK which are unable to detect the closely related PYK2 kinase. As a result, the potential role of PYK2 in zebrafish fertilization has remained an unsolved question. The present study has examined the subcellular distribution and activation of PYK2 kinase during fertilization of the zebrafish oocyte with the objective of defining this novel element of the calcium-regulated pathways that are activated by the fertilizing sperm. The results demonstrate that PYK2 is a novel component of the response of the oocyte during fertilization. It was found to be activated initially at the micropyle and subsequently throughout the entire oocyte cortex in response to the fertilization-induced calcium transient. Functional analysis revealed that PYK2 was involved in cytoskeletal rearrangements necessary for sperm incorporation. The discovery of this new component of the oocyte activation pathway provides an important link between the fertilization-induced calcium transient and the changes in the actin cytoskeleton which occur in response to fertilization.
Materials and Methods
Oocytes
Oocytes were collected from mature Danio rerio and maintained in filtered (0.2μM) salmon ovarian fluid (Siripattarapravat, et al., 2009) or Hank’s-BSA (Westerfield, 2007) at 28°C, while sperm were maintained on ice in sperm extender solution (Lee, et al., 1999). Fertilization was accomplished by mixing the sperm (5ul) with the eggs, then activating the sperm by addition of 1ml of aquarium water. After the eight cell stage, embryos were transferred into Tubingen E3 medium for culture (Brand, et al., 2002). The effect of PYK2 inhibitors AG17 and AG82 (EMD Millipore, Billerica, MA) and PF04594755 (a generous gift from Pfizer, Inc. Groton, CT) on the capacity of oocytes to be fertilized was determined by fertilization assays. The above inhibitors were added to groups of 20–25 oocytes maintained in 35mM culture plates containing Hanks-BSA and incubated for 45 minutes. Oocytes were then washed twice with Hanks-BSA, the Hanks-BSA was removed, and 2.5 μl of a 10% vol/vol sperm suspension was added followed by 1ml of aquarium water to activate sperm motility. Development was allowed to occur to the pronuclear stage 15 minutes post-insemination (m.p.i.) or to the early cleavage stage (90 m.p.i) and zygotes were fixed in a solution of 4% paraformaldehyde, 0.1% glutaraldehyde, sucrose (4% wt/vol), NaH2PO4 (50mM) pH 7.2. The animal pole of each zygote was then dissected free with a scalpel and stained with DAPI in sucrose (4% wt/vol), NaH2PO4 (50mM) pH 7.2, glycine (10mM), and NP40 (0.5%). Successful fertilization was assayed by imaging the animal caps by fluorescence microscopy at 20X and quantifying the number of oocytes with 2 pronuclei (indicative of sperm penetration) or the number of blastomere nuclei (indicative of successful cleavage).
Membrane preparation and western blot analysis
To overcome the difficulties of analyzing the yolk-rich zebrafish oocyte, western blot detection was performed using the plasma membrane fraction isolated via density gradient centrifugation (Wu and Kinsey, 2004). Samples were prepared from groups of 500–600 oocytes collected at different times after fertilization using solutions that contained the phosphatase inhibitors sodium orthovanadate (100μM) and phenylarsine oxide (40μM) (Sigma Aldrich, St. Louis, MO.). Samples containing 10μg protein were resolved by electrophoresis on a 10% SDS gel and transferred to Nytran+ membranes (EMD Millipore, Billerica, MA) in transfer buffer containing 0.1% SDS. After blocking in 5% dried milk (BioRad Labs, Hercules, CA) containing the above phosphatase inhibitors, blots were probed with a rabbit polyclonal antibody directed against a region of the C-terminal domain of human PYK2 ((P3902) Sigma Aldrich, St Louis, MO), or with phosphorylation site-specific antibodies anti-PYK2-PY402, or anti-PYK2-PY579 (Biosource, Grand Island, NY) and detected with peroxidase-coupled anti-rabbit IgG (Sigma Aldrich, St. Louis, MO) then imaged via chemiluminescence. Band intensity was quantified by densitometric scanning and analyzed with Metamorph 7.1 software (Molecular Devices, Sunnydale, CA).
Immunofluorescence Microscopy
Zebrafish eggs, and zygotes were prepared for immunofluorescence as previously described (Sharma et al., 2006). Where possible, the chorion was dissected away manually prior to permeabilization and blocking, however, in cases where the fertilization cone was to be imaged, the chorion was left in place to avoid destruction of this fragile structure and instead, the fixed chorion was penetrated multiple times with an injection micropipette to facilitate antibody penetration. In cases where pronuclei were to be imaged, glutaraldehyde (0.1%) was added to the fixative. The phosphatase inhibitors sodium orthovanadate (100μM) and phenylarsine oxide (40μM) were included during the fixation and blocking steps. Primary antibodies used included the rabbit anti-PYK2 C-terminal antibody as well as the anti-PYK2-PY402, anti-PYK2-PY579 antibodies described above. Secondary antibodies used include goat-anti-mouse IgG-Alexa-fluor 488 and goat-anti-rabbit IgG-Alexa-fluor 488 (Invitrogen, Carlsbad, CA). Filamentous actin was labeled with alexa 568-phalloidin (Invitrogen, Carlsbad, CA). Nuclei were stained with Hoechst 33258 or with ethidium homodimer (Invitrogen, Carlsbad, CA).
Microinjection and calcium imaging
Oocytes were immobilized either with an oocyte injection chamber (Lee et al., 1999) or with a suction pipette then injected with 1–5nl volumes using beveled glass micropipettes as described (Sharma et al., 2008) driven by a picospritzer II (General Valve Co. Brookshire, TX). Fusion proteins or fluorescent probes were dissolved in injection buffer consisting of KCl, 0.15M; NaCl, 3mM; KH2PO4, 10mM (pH 7.2); glutathione, 1mM; sucrose, 80mM; and EGTA, 10nM. In order to detect changes in [Ca2+]i, oocytes were injected with calcium green-dextran (InVitrogen, Carlsbad, CA) to achieve intracellular concentrations of approximately 0.5μM. In some experiments, a calcium clamping buffer consisting of Tris, 10mM; BAPTA, 20mM; and CaCl2, 12mM was used to block the fertilization-induced calcium transient (Creton et al., 1998; Sharma et al., 2005). The oocytes were then monitored by confocal fluorescence microscopy on a Nikon TE2000U microscope using a 488nm Spectra Physics (Mountainview, CA) laser. Emitted fluorescence images were recorded at 15 sec intervals and the fluorescence intensity of the zygote was quantitated from digital images by centering an elliptical measurement area over the entire zygote as described (Sharma et al., 2008) average pixel density was calculated by Metamorph 7.1 (Molecular Devices, Sunnydale, CA).
Preparation of GST fusion proteins
A GST fusion protein encoding the N-terminal ERM domain of human PYK2 (PERM domain) was prepared by PCR amplification from a kinase inactive human PYK2 plasmid (Origene catalog #SC323519). This was used since the human PYK2 ERM domain is over 70% identical to that of zebrafish (Lo, et al., 2003). The forward primer (5′TGAATTCAATGTCTGGGGTGTCCGAG 3′) and the reverse primer (5′TGCTCGAGGTTTAGCATGGGGAT 3′) contained EcoR1 and Xho1 sites respectively. After amplification with PFU polymerase, the product was ligated into pGEX 4T3 (Agilent Technologies, Santa Clara, CA), expressed as a fusion protein, and purified as previously described (Kinsey and Shen, 2000). Fusion proteins were dialyzed into injection buffer and adjusted to 15–20 mg/ml protein.
Results
Fertilization triggers activation of PYK2 associated with the oocyte plasma membrane
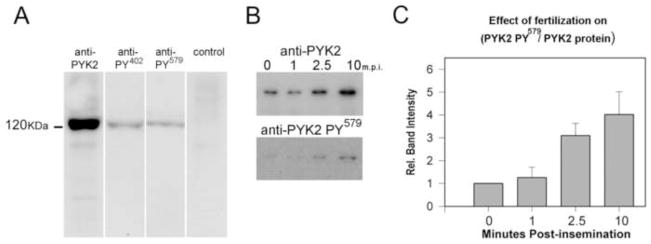
Fertilization triggers localized activation of Src-family and other (as yet not identified) protein tyrosine kinases which are tightly associated with the egg plasma membrane or underlying actin cytoskeletal complex (Sharma et al., 2006). Plasma membrane fractions from unfertilized oocytes exhibit very little SFK activity but do contain an activity that can be stimulated by addition of calcium and calmodulin (Supplemental Fig 1). The apparent Mr of this activity based on electrophoretic mobility (approximately 120 kDA) is similar to the predicted Mr (114 kDA) of zebrafish PYK2 (Lo, et al., 2003) which prompted us to test for the presence of PYK2 protein expression via western blot. PYK2 was not detectable in oocyte homogenates due to its low abundance in the yolk-rich ooplasm, but it was detected in purified egg plasma membranes (Wu and Kinsey, 2000; Wu and Kinsey, 2004) when probed with an antibody directed against amino acids 991 – 1009 of human PYK2, as well as phosphorylation site specific antibodies directed against PYK2 phosphorylated on tyrosine 402 (Y402) and 579 (Y579) (Figure 1A). As mentioned above, PYK2 activation involves autophosphorylation of Y402 which acts as a docking site for Src or Fyn which further increase PYK2 activation by phosphorylating Y579. The phosphorylation state of these tyrosines has often been used to report the relative activation state of PYK2 in numerous systems (Dikic, et al., 1996), and we used a combination of the anti-PYK2 protein antibody and anti- PY579 antibody to determine whether fertilization resulted in a change in amount or activation state of PYK2 associated with the egg plasma membrane fraction. The result presented in Figure 1B provides a typical example of the effect of fertilization on the PYK2 protein and PYK2 PY579 present in plasma membrane fractions purified at different times post-insemination. Fertilization resulted in accumulation of PYK2 protein as well as the phosphorylated PYK2 PY579 in plasma membrane fractions beginning as early as 2.5 m.p.i. When the PYK2 PY579 band intensities were normalized for the amount of PYK2 protein per lane, it became obvious that the ratio of PYK2 PY579 to PYK2 protein also increased as a result of fertilization.
Figure 1.
Detection of PYK2 in the oocyte plasma membrane; effect of fertilization. Plasma membrane fractions prepared at 7.5 minutes post- fertilization were analyzed by western blot and probed with an antibody to the mammalian PYK2 protein, anti PY402, anti-PY579, or with control rabbit IgG as described in ‘Materials and Methods’ (panel A). To detect changes in PYK2 phosphorylation after fertilization, plasma membrane samples prepared before (UF) and at 1, 2.5, and 10 minutes post-insemination (m.p.i.), were probed with the anti-PYK2 protein antibody and with the anti- PY579 antibody (panel B) to establish whether the amount or activation state of PYK2 associated with the plasma membrane changed in response to fertilization. Band intensity (‘Materials and Methods’) of the PYK2 PY579 labeled material in panel B was normalized to the PYK2 labeled bands in samples from five membrane preparations and is presented as a bar graph in panel C +/− S.E.M.
Immunofluorescence localization of PYK2 activity
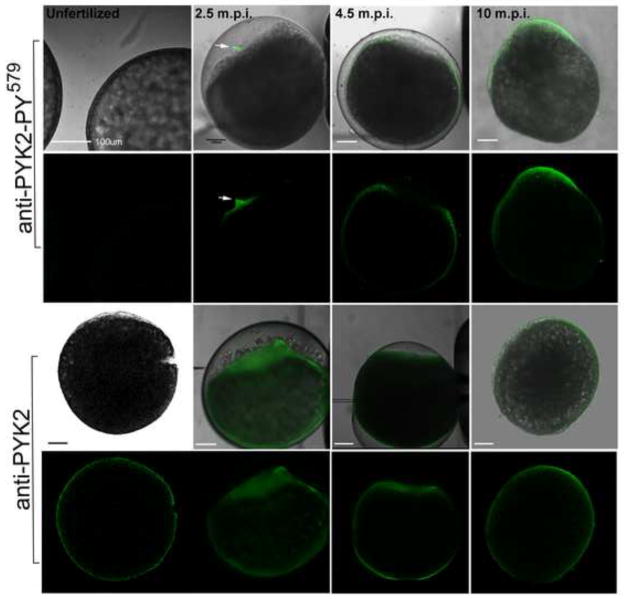
The zebrafish oocyte is a highly polarized cell and in order to determine whether fertilization-induced PYK2 activation occurred uniformly or asymmetrically in the egg plasma membrane, we used the anti-PYK2 protein and the anti-PY579 antibody to detect activated and total PYK2 by confocal immunofluorescence. Oocytes were fixed before and at different times after fertilization and processed under conditions that prevent phosphatase degradation of phosphoproteins. As seen in Figure 2, active PYK2-PY579 was virtually undetectable prior to fertilization despite the fact that PYK2 protein was easily detected concentrated near the oocyte surface and in punctate foci deeper in the cortical cytoplasm (Figure 2, ‘unfertilized’ panels). Within a short time (2.5 m.p.i.) after insemination, phosphorylated PYK2 PY579 was obvious at the site of the developing fertilization cone and by 4.5 minutes post-insemination, PYK2 activation had propagated through the cortical cytoplasm to the vegetal pole. Later (10 m.p.i.), active PYK2 PY579 was broadly distributed throughout the egg cortex but was more concentrated at the animal pole associated with the developing blastodisc. The pattern and timing of PYK2-PY579 immunofluorescence was observed consistently in oocytes recovered from at least 10 different females. Antibodies directed against other tyrosine phosphorylation sites of PYK2 (PYK2-PY402, PYK2-PY580) exhibited a similar pattern of immunofluorescence, however the PYK2-PY579 antibody produced the most intense labeling. In contrast to the localized distribution of activated (phosphorylated) PYK2, the total pool of PYK2 in the oocyte was distributed fairly evenly within the oocyte cortex (Figure 2, lower panels). This result indicates that fertilization initially triggers activation of only a small portion of the total PYK2 pool near the site of sperm entry. While PYK2 and activated PYK2 were fairly uniformly distributed throughout the oocyte cortex at 4.5 m.p.i., both active and total PYK2 pools became progressively concentrated over the blastodisc at 10 m.p.i. The concentration of PYK over the blastodisc is similar to that reported in our earlier study of Fyn and other Src family kinase activation (Sharma and Kinsey, 2006; 2008).
Figure 2.
Localization of activated PYK2 in the fertilized oocyte. Oocytes were fixed before and 2.5, 4.5, and 10 m.p.i., then processed for immunofluorescence, labeled with anti-PYK2-PY579 (upper panels) which would detect the activated form of PYK2 or with anti-PYK2 peptide (lower panels) which would detect the total pool of PYK2 protein. Bound antibody was detected with alexa 488-coupled secondary antibody (green) as described in “Materials and Methods”. Since the chorion formed a barrier to antibody diffusion, it was punctured multiple times with a micropipette after fixation or removed by dissection in samples fixed after it was fully elevated (10m.p.i. and later). Magnification is indicated by the bar which represents 100um.
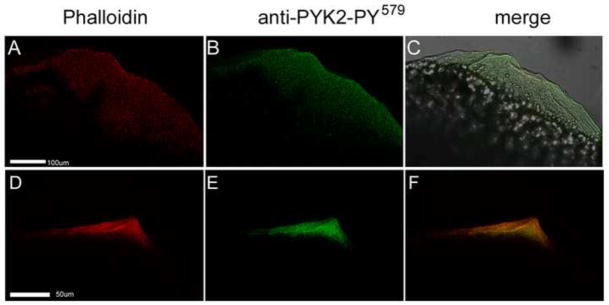
Closer examination of the activation of PYK2 during the earliest stages of fertilization revealed that this kinase was initially activated diffusely in the region of clear cytoplasm that forms near the micropyle and will later form the blastodisc (Figure 3, AC). The earliest stages of fertilization cone formation appear as a conical protrusion characterized by the presence of accumulations of bundled actin filaments that likely provide support for this cytoplasmic protrusion (Figure 3D). Activated PYK2 became more concentrated and closely associated with these actin filament bundles supporting the structural reinforcement of the fertilization cone (Figure 3, E–F).
Figure 3.
PYK2 activation co-localized with actin cytoskeletal reorganization. Oocytes were fixed at 1.0 (panels A–C) and 2.5 m.p.i.(panels D–F), then labeled with alexa 565 - phalloidin (red) and anti-PYK2-PY579 followed by alexa 488-goat anti-rabbit IgG (green). Confocal images are displayed showing actin in the red channel (A, D), phosphorylated PYK2 in the green channel (B,E), or the merged red and green channels (C, F). Magnification is indicated by the bar which represents 100um.
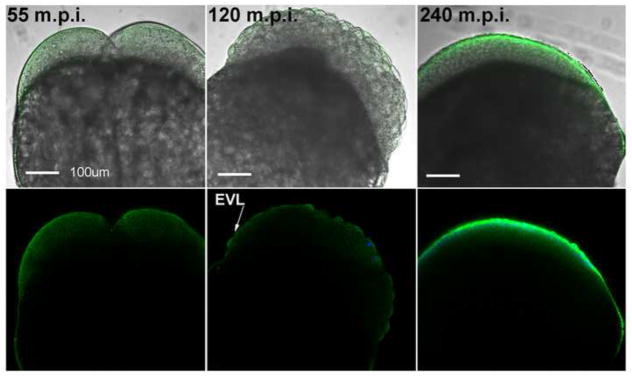
The distribution of activated PYK2 during early embryonic development was also examined by confocal immunofluorescence (Figure 4). At the first mitotic cleavage, activated PYK2 remained associated with the blastomere cortex, however examination of a number of embryos at multiple focal planes indicated that activated PYK2 was largely excluded from the base of the cleavage furrow (Figure 4, left). Early blastula stage embryos exhibited PYK2 activation at the apical region of the enveloping cell layer (EVL), (Figure 4, center) while careful observation detected very little staining within the deeper cell layers. At epiboly, immunofluorescence labeling of activated PYK2 within the EVL was of substantially higher intensity (Figure 4, right).
Figure 4.
PYK2 activation during cleavage and epiboly. Developing embryos were collected at 55, 120, and 240 m.p.i., then processed for confocal immunofluorescence with anti-PYK2 PY579 as in Figure 2. Magnification is indicated by the bar which represents 100μm.
Role of calcium signaling in PYK2 activation at fertilization
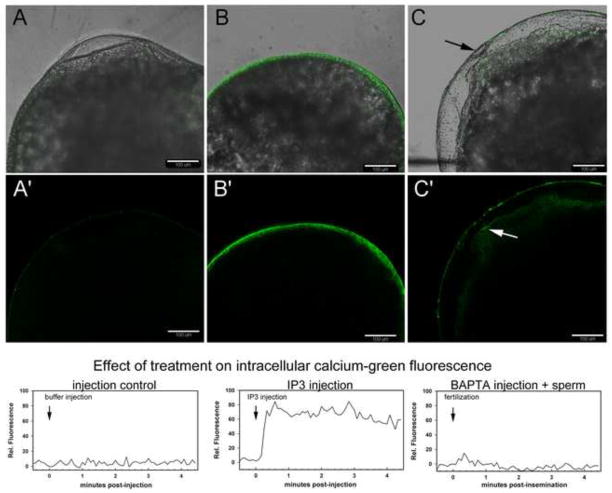
PYK2 is unique among protein tyrosine kinases in that, in the presence of calmodulin, it can be activated by elevated cytoplasmic calcium (Kohno et al., 2008; Wu et al., 2006; Xie, et al, 2008; Yamamoto, Shimizu, et al., 2008) leading to phosphorylation of tyrosines Y579 and Y580. This property raised the possibility that PYK2 could respond to the fertilization-induced calcium wave triggered by fertilization. Zebrafish oocytes parthenogenically activated by hypotonic shock induced by exposure to aquarium water without sperm also exhibit a high amplitude calcium transient, and we observed that PYK2 was also activated in the cortex of these oocytes as well (not shown), suggesting that the calcium transient alone might activate PYK2. In order to test whether an artificially induced calcium transient was sufficient to trigger activation of PYK2 in the absence of sperm, unfertilized oocytes were injected with IP3 to induce a calcium transient (Lee, et al., 2003), then the effect on PYK2 activation was assessed by immunofluorescence. IP3 was added to injection buffer at 25μM then injected into oocytes to produce a final intracellular concentration of 0.25μM. The injected oocytes were maintained in Hank’s-BSA, so they did not experience hypotonic shock. After IP3 injection, oocytes were fixed and processed for confocal immunofluorescence with the anti-PY579 antibody to determine the extent to which PYK2 was activated. The effectiveness of the IP3 and other treatments was assessed in a separate group of oocytes pre-loaded with calcium green-dextran and used to record calcium-induced fluorescence after each treatment (Figure 5, lower panels). As seen in Figure 5A, injection buffer alone did not stimulate PYK2 and had little effect on calcium-induced fluorescence. However, IP3 induced an immediate and intense activation of calcium-induced fluorescence and activated PYK2 throughout the entire oocyte cortex within 2.5 minutes of injection (Figure 5B). In order to test whether the fertilization-induced calcium transient was required to induce PYK2 activation at fertilization, a BAPTA-containing calcium clamping buffer designed to hold intracellular calcium at basal levels (Creton et al., 1995) was used to block the calcium transient during in vitro fertilization. As seen in Figure 5C, the clamping buffer suppressed most but not all of the fertilization-induced activation of PYK2 in the oocyte cortex as detected by anti-PYK PY579 labeling. As indicated by the arrow, many BAPTA-injected oocytes exhibited some localized PYK2 activation even though the global cortical increase in PYK2 PY579 labeling was blocked. This could be explained by the fact that the calcium transient was not completely suppressed by BAPTA as seen by calcium green fluorescence in the bottom panel. In addition, the fact that most BAPTA-injected oocytes exhibited partial elevation of the chorion suggests that some calcium release and cortical granule exocytosis did occur during fertilization in vitro. Nonetheless, the fact that IP3-mediated calcium release in the oocyte can stimulate PYK2 activation, together with the observation that suppression of the fertilization-induced calcium transient blocked PYK2 activation almost completely, suggests that PYK2 is normally activated in the oocyte as a direct response to the calcium transient.
Figure 5.
Effect of changes in intracellular calcium on PYK2 activation state in the zebrafish oocyte.
In order to establish whether oocyte PYK2 could be activated by artificially increasing intracellular calcium levels Oocytes were injected with injection buffer only as a control (A) or with injection buffer containing 25μM IP3 (B) to artificially drive an increase in intracellular free calcium levels. The control and IP3-injected oocytes were maintained in Hanks-BSA for 4.5 minutes then fixed. In order to establish whether prevention of the fertilization-induced calcium transient would block PYK2 activation in response to fertilization, oocytes were injected with calcium clamping buffer (C) then fertilized by a mixture of sperm and aquarium water, followed by fixation at 4.5 m.p.i. Oocytes were then prepared for immunofluorescence detection of activated PYK2 with anti-PYK2-PY579 and images representative of three experiments performed with five oocytes in each group are presented with green fluorescence representing the distribution of PYK2-PY579 labeling. Arrows in panel C point out the position of the micropyle. In separate experiments designed to confirm that the above treatments had the desired effect on intracellular calcium signaling (bottom panels), oocytes were first preloaded with 50μM calcium-green dextran, and allowed to recover for 30 minutes. The injection pipet was replaced, calcium induced fluorescence recording was begun, and a second injection with injection buffer (Panel A, bottom), injection buffer with 25μM IP3 (Panel B, bottom), or with calcium clamping buffer followed by in vitro fertilization (panel C, bottom) was performed after a baseline was established. The relative change in calcium green fluorescence over time is depicted graphically in the bottom panels which show patterns representative of the five oocytes from each group that were recorded.
Effect of PYK2 suppression on fertilization and cleavage
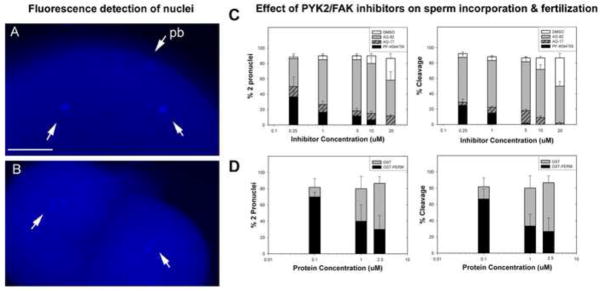
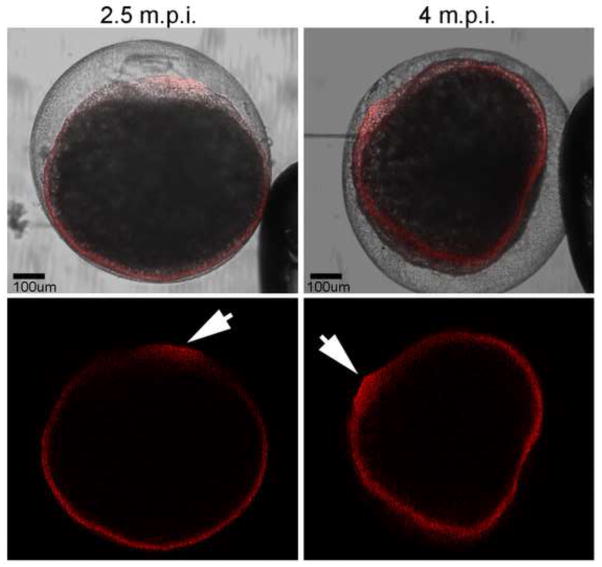
In order to establish whether PYK2 played a significant role in fertilization, chemical inhibitors reasonably selective for PYK2, as well as a dominant-negative GST fusion protein encoding the N-terminal ERM domain of PYK2 (GST-PERM), were used to suppress this kinase in unfertilized oocytes. The impact of PYK2 suppression on fertilization was tested by inseminating the treated oocytes and quantifying the success of sperm incorporation and cleavage. The tyrphostin compound AG17 ((3,5-Di-t-butyl-4-hydroxybenzylidene)-malononitrile; alpha-Cyano-(3,5-di-tert.butyl-4-hydroxy) cinnamonitrile), also referred to as tyrphostin A9, has been shown to inhibit PYK2 activation in cultured cells. However, because AG17 also inhibits other kinases (see discussion), it has been used in combination with the structurally similar AG82 (tyrphostin A25) which preferentially inhibits FAK kinase (Liu et al., 2004) and provides a useful control in addition to the DMSO solvent control. The PYK2-selective inhibitor PF04594755 (Bonnette et al., 2010) was also tested against the AG82 and DMSO controls since structural analogs of this compound are not available. Oocytes were pretreated with the inhibitors, then washed prior to fertilization to avoid possible effects on sperm motility. Successful fertilization was assayed by quantifying sperm penetration (2 or more pronuclei) and by quantifying successful cleavage (2 or more blastomeres with visible nuclei). The results (Figure 6) revealed that AG17 and PF04594755 blocked fertilization as determined by the absence of two pronuclei assessed at 15 m.p.i. and by the reduction in cleavage frequency assessed at 90 m.p.i. In order to provide an alternative method of suppressing PYK2 activity without the possible side effects of chemical inhibitors, dominant-negative PYK2 constructs have been used in a number of systems. For example, GST-fusion proteins encoding protein domains with dominant-negative action in vivo have proven useful in fertilization studies (Calliau and Browaeys-Poly, 2009) where knockdown experiments are less effective because oocytes often express and store signal transduction proteins before oocyte maturation. The GST-PERM fusion protein has been shown to block PYK2 oligomerization needed for full kinase activation (Riggs et al., 2011). When delivered by microinjection of unfertilized oocytes, the GST-PERM protein had an effect on fertilization similar to that of AG17 and PF04594755 although complete inhibition of fertilization and cleavage could not be achieved with this reagent. Morphological examination of oocytes in which development was suppressed following injection of the GST-PERM revealed a failure to develop the normal pyramidal-shaped, actin-rich fertilization cone after fertilization (Figure 7). Cytoplasmic clearing and accumulation of filamentous actin (phalloidin positive material) occurred near the micropyle (arrow), however the well defined pyramidal fertilization cone structure (as shown in Figure 2 and 3) did not occur. None of the treatments disrupted the pre-existing filamentous actin layer extending in the oocyte cortex from animal pole to vegetal pole, so it appears likely that PYK2 suppression blocked reorganization of the actin cytoskeleton needed during fertilization cone formation.
Figure 6.
Effect of PYK2 inhibitors on fertilization. Oocytes were treated with AG17, PF04594755, AG82 (control) or DMSO (solvent control) for 45 minutes, then washed with Hank’s-BSA. Other groups were injected with different concentrations of GST-PERM or GST fusion proteins. The samples were fertilized, then fixed and stained with DAPI at 15 m.p.i. to assess sperm incorporation by the presence of male and female pronuclei, and at 90m.p.i. to assess cleavage by the presence of a cleavage furrow and two somatic nuclei. An example of zygotes derived from control (DMSO) treated oocytes demonstrating the presence of two pronuclei (A, arrows) and a polar body (pb) demonstrates the criteria for sperm penetration. Panel B demonstrates the presence of a cleavage furrow and two somatic nuclei shown (B, arrows) during telophase of the second mitotic division indicating that fertilization was successful. The % of oocytes that successfully incorporated sperm (C, D, left) or cleaved to the 2-cell stage (C,D, right) are presented as the mean of > three experiments +/− S.E.M. Values represent the mean of three or more experiments +/− S.E.M. (*)= significantly different from control (P<0.05).
Figure 7.
Effect of PYK2 inhibitors on actin reorganization post-fertilization. Oocytes injected with GST-PERM (2.5uM final) were fertilized and fixed at 2.5 and 4 m.p.i. The distribution of actin was detected with alexa-565-phalloidin (red). Arrows indicate the position of the defective fertilization cone.
Discussion
Fertilization involves a series of signaling events a key component of which involves IP3-mediated release of intracellular calcium from internal stores (Runft et al., 2002; Whitaker, 2006). The calcium transient delivers a global signal for egg activation acting through the calcium-calmodulin dependent kinase to trigger the cortical reaction as well as cell cycle control events needed to release the oocyte from meiotic arrest and initiate zygote development (Ducibella and Fissore, 2008). The findings presented here identify PYK2 as a novel component of the calcium-induced responses to fertilization. PYK2 is activated early in the response of the oocyte to the fertilizing sperm and becomes associated with the oocyte plasma membrane and with cytoskeletal elements within the fertilization cone. PYK2 activity plays a significant role in events leading up to incorporation of the sperm nucleus and subsequent development.
PYK2 was initially detected in zebrafish as a cDNA (AJ490510) and later confirmed (Lo et al., 2003) to encode a 1004 amino acid polypeptide that is 67% homologous to the human protein. The predicted zebrafish sequence contains all of the major functional domains (ERM, catalytic, proline-rich and FAT) of PYK2 with 70% to 100% homology to the human sequence. PYK2 expression in zebrafish oocytes was initially detected in the present study by analysis of a calcium-sensitive protein tyrosine kinase activity in purified plasma membrane fractions and confirmed by western blot analysis using an antibody directed against the C-terminal domain of human PYK2 which is 79% identical to the zebrafish sequence (Lo et al., 2003). Western blots prepared from whole egg homogenates were inconclusive since high molecular weight yolk proteins obscured any signal from PYK2, however the kinase was easily detected in a plasma membrane fraction purified by sucrose gradient which also contains a large amount of membrane associated actin. Fertilization was accompanied by rapid accumulation of PYK2 protein in the membrane fraction as detected by western blots. Furthermore the ratio of phosphorylated (PYK2 PY579) increased as well indicating that PYK2 was recruited to the plasma membrane and further phosphorylated in response to fertilization. The results derived from western blots were consistent with those obtained by immunofluorescence which appeared to show a fertilization-dependent increase in the amount of PYK2 protein and PYK2 PY579 concentrated in the oocyte cortex or plasma membrane. Phosphorylation of tyrosine 579 as well as tyrosines 402 and 580 is indicative of PYK2 activation as the initial autophosphorylation of tyrosine 402 occurs in response to cell surface stimuli, and the subsequent protein dimerization and transphosphorylation of tyrosines 579 and 580 occurs during full kinase activation (Orr and Murphy-Ullrich, 2004b). The role of calcium/calmodulin and Src family kinases such as Src, Fyn, and Yes in phosphorylation of these sites seems to vary by cell type to cell type(Keogh, et al., 2002) (Chen, et al., 2011), although it is clear that phosphorylation of these sites correlates well with kinase activation.
The most striking observation resulting from the immunofluorescence analysis was that within minutes after insemination, PYK2 activation occurred specifically in the newly forming fertilization cone and later spread throughout the entire oocyte cortex. The fact that activated PYK2 was so clearly associated with the actin pyramidal structures forming the body of the fertilization cone suggested that PYK2 might be involved in regulating cytoskeletal remodeling needed to produce this structure which physically tethers and later draws the fertilizing sperm into the egg cytoplasm. As the cortical reaction progressed laterally through the egg cortex, PYK2 activation also was propagated through the cortex toward the vegetal pole although not in association with any large cell processes. The pattern of localization and activation exhibited by PYK2 resembles that of SFKs which were previously shown to become activated in the fertilization cone and later in the oocyte cortex (Sharma et al., 2006; Sharma et al., 2008). Once the blastodisc formed, the total pool of both active and inactive PYK2, as well as the Src-family kinases was concentrated in the cortical region of the blastodisc, likely a result of cytoplasmic streaming.
PYK2 association with cell processes has been observed in several cell types including podosomes of osteoclasts (Gil-Henn et al., 2007), laemellipodia of macrophages (Okigaki et al., 2003), uropod processes as well as target-cell contact regions of NK cells (Sancho, et al., 2002), and focal adhesions and stress fibers of vascular endothelial cells (Tang, et al., 2000). PYK2 associates with a variety of cytoskeletal associated proteins including p130cas, Src, Cbl, integrins, gelsolin, and paxillin (Ivankovic-Dikic, et al., 2000), but the specific functions in modifying actin-based cell processes is not well understood. Suppression of PYK2 pharmacologically (Block, et al., 2011) or by gene knockout has resulted in decreased motility and process formation (Gil-Henn et al., 2007). SFKs such as Src and Fyn have been shown in other model systems to be involved in docking interactions with PYK2 and FAK and participate in the activation of these kinases (Park, et al., 2004), so it is not surprising that they are activated at the same time and in the same place. The simultaneous activation of PYK2 and SFKs in the fertilization cone and oocyte cortex may reflect a multistep co-activation pathway (Orr et al., 2004a) in which PYK2 activation stimulates SFK activation which phosphorylates PYK2 docking sites involved in cytoskeletal interactions that enable PYK2 to promote assembly of actin filament bundles in this highly motile, cell process.
As the fertilized oocyte began to accumulate clear cytoplasm at the blastodisc, activated PYK2 was concentrated in the cortex and plasma membrane over one side of the blastodisc. This asymmetry was retained at the two cell stage and may simply represent persistence of fertilization cone cytoskeletal elements in this region or it may reflect a functional polarity of the zygote cortex. The pre-compaction blastula exhibited only modest PYK2 activation, however as epiboly began, PYK2 was intensely activated in the outermost cell layer (epiblast) in a manner similar to that reported for FAK (Crawford, et al., 2003). The early morphogenetic movements of the zebrafish blastula have been shown to involve intense calcium signaling events (Creton, et al., 1998; Freisinger, et al., 2008; Reinhard et al., 1995; Sharma, et al., 2005; Slusarski and Corces, 2000; Webb and Miller, 2000), and it is likely that PYK2 is responding to calcium signals that are part of that important mechanism. Activation of PYK2 in the cortex of the oocyte and in the EVL of the blastula provides a potential mechanism by which calcium signals may be transduced in the cytoskeleton of these developmentally important structures.
The timing and pattern of PYK2 activation in the zebrafish oocyte suggested that this kinase may respond to the fertilization-induced calcium transient. PYK2 is unique among protein tyrosine kinases in that its activation requires calcium and calmodulin (Kohno et al., 2008). In other systems, the source of calcium for PYK2 activation is usually considered to be plasma membrane cation channels such as the L-type voltage gated calcium channel in gonadotropes (Xie et al., 2008), or the TRPM2 channel in monocytes (Yamamoto et al., 2008). The fact that PYK2 was artificially activated following parthenogenic activation by hypotonic shock in the absence of sperm (known to induce a calcium transient) supported the possibility that the calcium transient might be a trigger for PYK2 activation in the oocyte. Injection of IP3 into quiescent oocytes lacking stimulation by either sperm of hypotonic shock demonstrated that PYK2 can respond to IP3-mediated calcium release. The fertilization-induced activation of PYK2 could be almost completely suppressed by calcium chelation, suggesting that the calcium transient is the primary trigger for PYK2 activation at fertilization. However, the frequent detection of small regions of activated PYK2 closely associated with the micropyle raises the possibility that sperm-egg contact could also induce partial activation of PYK2 in the egg even in the presence of BAPTA. For example, PYK2 has been shown to become activated in response to cell contact in somatic cell models (Hashido, et al., 2006). In the present series of experiments, the rapidity of sperm-oocyte interactions in the zebrafish system made it difficult to define whether PYK2 began to respond to bound sperm prior to sperm-oocyte fusion or after cytoplasmic continuity was established between the two gametes. Identification of conditions that block gamete fusion in this species will be required before this question can be fully resolved.
The functional significance of PYK activation at fertilization was studied by suppressing this kinase via chemical and dominant-negative reagents and observing the impact on fertilization. The close similarity between the catalytic domain of PYK2 and FAK (60% identity) has complicated development of an inhibitor capable of discriminating between PYK2 and FAK. However, compounds that exhibit preferential inhibition of PYK2 over FAK have been used with some success. Tyrphostin AG17 has been used in combination with the structurally similar inhibitor AG82 to selectively block PYK2 function in cultured cells (Fuortes, et al., 1999). The recently developed PF04594755 compound (Bonnette, et al., 2010), a DFG (activation loop)-out inhibitor exerts more specificity by interacting with allosteric sites outside of the ATP binding pocket which are less well conserved between PYK2 and FAK. When administered to 3T3 cells at a concentration of 1uM, PF04594755 inhibits PYK by 96% while suppressing FAK by 42%. However, even this inhibitor has cross specificity since it can inhibit TEK and FLT3 at levels similar to PYK2. In order to control for the unavoidable non-specific effects of the above inhibitors, a dominant-negative approach was also used to block PYK2 function in vivo. The N-terminal ezrin, radixin, moesin interaction domain (ERM) of PYK2 is approximately 58% conserved between PYK2 and FAK and can bind to the catalytic domain causing autoinhibition (Kohno et al., 2008). Despite significant homology, the ERM domains of PYK2 and FAK are functionally distinct, providing specificity of function unique to each protein (Reiske, et al., 2000). GST and GFP fusion proteins encoding the ERM domain of FAK have been shown to exert a dominant-negative effect in intact cells (Cooper, et al., 2003; Lipinski, et al., 2005; Riggs, et al., 2011) and we chose to inject bacterially expressed GST-PERM protein in our study in order to have more direct control of the intracellular concentration of protein than can be obtained by mRNA injection. The results of the inhibitor studies revealed that suppression of PYK2 in oocytes by AG17 and PF04594755 treatment as well as by injection of the GST-PERM protein significantly reduced the frequency of fertilization. The GST-PERM protein was not as effective as the chemical inhibitors in that it could not suppress fertilization by over 70%. This difference may reflect the different mechanisms of inhibition since the PERM domain involves protein-protein interaction outside of the catalytic domain while the chemical inhibitors bind directly to the ATP binding site or to the activation loop. Structural differences between the human PERM sequence fused to GST used here and the native zebrafish protein may also be a factor. In any case, we carefully examined the morphology of oocytes that failed to fertilize in order to determine which stage of fertilization was disrupted. This examination revealed a high frequency of abnormal or absent fertilization cones suggesting that PYK2 was required for assembly or function of the fertilization cone. Sperm incorporation in zebrafish eggs requires a robust mechanism since the process occurs coincident with massive surface disruptions resulting from the cortical reaction and chorion elevation. As a consequence, the zebrafish fertilization cone is relatively large and mobile with a core pyramidal structure reinforced by a large array of bundled actin filaments (Hart, Becker, and Wolenski, 1992). The above results suggest that PYK2 is involved in the assembly or bundling of these actin filaments forming the fertilization cone without which sperm-egg contact might be disrupted before the sperm head is sufficiently stabilized.
The present study of mature oocytes and zygotes does not rule out other potential functions for PYK2 in oocyte development. For example, a recent investigation of PYK2 in maturing mouse oocytes revealed that active PYK2 was localized to the cortical cytoskeleton and concentrated in the cleavage furrow during first polar body emission. PYK2 was also detected in association with perinuclear actin at the germinal vesicle stage (Meng, et al., 2006). We did not detect association of activated PYK2 with the pronuclei of zebrafish zygotes or with mitotic nuclei in embryos suggesting that this kinase may have functions unique to oocyte maturation. Also, we were unable to detect activated PYK2 at the base of the mitotic cleavage furrow however the close association between PYK2 and the cortical actin cytoskeleton appear to be conserved between zebrafish and mammals.
The initial activation of PYK2 at the fertilization cone as well as the progressive activation of PYK2 in the fertilized oocyte cortex correlates well with the activation pattern exhibited by SFKs (Sharma et al., 2006; Sharma et al., 2008) suggesting that they may be activated in response to the same stimulus. The calcium-induced activation of PYK2 has the potential to act as a signal leading to activation of SFKs including FYN due to the docking interaction between Y402 and the SH2 domain of Src or Fyn (Dikic et al., 1996). In this model, stimulation of PYK2 by the calcium transient would lead to partial activation and autophosphorylation at tyrosine 402 which would interact with the SH2 domain of SFKs promoting their ‘open’ configuration through an SH2 displacement mechanism (Andreev, et al., 2001; Schlaepfer et al., 1999); (Park et al., 2004; Wang and Reiser, 2003). Such a pathway may accelerate SFK activation in the oocyte cortex, possibly in conjunction with phosphatases such as rPTPα (Wu and Kinsey, 2002) which can activate SFKs but are not calcium sensitive. Once activated, PYK2 would be expected to phosphorylate paxillin, p130CAS (Bonnette et al., 2010) and ArfGTPase activating protein such as ASAP1(Kruljac-Letunic, et al., 2003), which have important functions in cytoskeletal and membrane remodeling events.
PYK2 represents a novel component of the egg activation pathways triggered by fertilization. The fact that PYK2 is activated in the immediate vicinity of sperm-oocyte interaction and its requirement for fertilization cone formation and sperm incorporation place it among the earliest responses of the oocyte to the fertilizing sperm. The fact that PYK2 suppression blocks an early step of successful fertilization will likely complicate analysis of other potential roles in cytoskeletal remodeling later during zygote development, however future studies involving intracytoplasmic sperm injection or artificial activation might be able to more precisely define the downstream actions of this calcium activated kinase and reveal additional roles in zygote development.
Supplementary Material
Highlight.
PYK2 is rapidly activated in response to fertilization of the zebrafish oocyte as a direct response to the calcium transient
PYK2 activation is initiated at the fertilization cone where it is associated with actin-filamentous structures.
PYK2 activation propagates through the cortical cytoplasm from animal pole to vegetal pole.
PYK2 activity is required for proper formation of the fertilization cone and for sperm incorporation.
Acknowledgments
This work was supported by NICHD HD062860 to W.H.K. We would also like to thank Jinping Luo, Lily Zhang and Ling Chen for technical assistance. We would like to thank Jon Wittouck and Joe Gasper at the University of Washington for collecting salmon ovarian fluid for this study. The compound PF04594755 was a generous gift from Pfizer Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 2.Block ER, Tolino MA, Klarlund JK. Extracellular ATP stimulates epithelial cell motility through Pyk2-mediated activation of the EGF receptor. Cell Signal. 2011;23:2051–2055. doi: 10.1016/j.cellsig.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnette PC, Robinson BS, Silva JC, Stokes MP, Brosius AD, Baumann A, Buckbinder L. Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. J Proteomics. 2010;73:1306–1320. doi: 10.1016/j.jprot.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Brand M, Granato M, Nusslein-Vollard C. Keeping and Raising of Zebrafish. In: Hamid O, editor. Zebrafish, A Practical Approach. Oxford University Press; 2002. pp. 7–37. [Google Scholar]
- 5.Calliau K, Browaeys-Poly E. A microinjectable biological system, the xenopus oocyte as an approach to understanding signal transduction protein function. In: Carroll DJ, editor. Methods in Molecular Biology; Microinjection methods and applications. Humana Press; 2009. pp. 43–55. [DOI] [PubMed] [Google Scholar]
- 6.Chang HY, Minahan K, Merriman JA, Jones KT. Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development. 2009;136:4077–4081. doi: 10.1242/dev.042143. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JJ, Chao YJ, Wang DL. Cyclic strain activates redox-sensitive proline-rich tyrosine kinase 2 (PYK2) in endothelial cells. J Biol Chem. 2002;277:48152–48157. doi: 10.1074/jbc.M110937200. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23:8030–8041. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- 11.Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–3081. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creton R, Speksnijder J, Jaffe L. Patterns of free calcium in zebrafish embryos. J Cell Science. 1998;111:1613–1622. doi: 10.1242/jcs.111.12.1613. [DOI] [PubMed] [Google Scholar]
- 13.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 14.Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freisinger CM, Houston DW, Slusarski DC. Image analysis of calcium release dynamics. Methods Mol Biol. 2008;468:145–156. doi: 10.1007/978-1-59745-249-6_11. [DOI] [PubMed] [Google Scholar]
- 16.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 19.Hart NH, Becker KA, Wolenski JS. The sperm entry site during fertilization of the zebrafish egg: localization of actin. Mol Reprod Dev. 1992;32:217–228. doi: 10.1002/mrd.1080320306. [DOI] [PubMed] [Google Scholar]
- 20.Hashido M, Hayashi K, Hirose K, Iino M. Ca2+ lightning conveys cell-cell contact information inside the cells. EMBO Rep. 2006;7:1117–1123. doi: 10.1038/sj.embor.7400821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- 22.Keogh RJ, Houliston RA, Wheeler-Jones CP. Thrombin-stimulated Pyk2 phosphorylation in human endothelium is dependent on intracellular calcium and independent of protein kinase C and Src kinases. Biochem Biophys Res Commun. 2002;294:1001–1008. doi: 10.1016/S0006-291X(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 23.Kinsey WH, Shen SS. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Devel Biol. 2000;225:253–264. doi: 10.1006/dbio.2000.9830. [DOI] [PubMed] [Google Scholar]
- 24.Klemm AH, Kienle S, Rheinlaender J, Schaffer TE, Goldmann WH. The influence of Pyk2 on the mechanical properties in fibroblasts. Biochem Biophys Res Commun. 2010;%19:393, 694–697. doi: 10.1016/j.bbrc.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 25.Koh YH, Che W, Higashiyama S, Takahashi M, Miyamoto Y, Suzuki K, Taniguchi N. Osmotic stress induces HB-EGF gene expression via Ca(2+)/Pyk2/JNK signal cascades in rat aortic smooth muscle cells. J Biochem. 2001;130:351–358. doi: 10.1093/oxfordjournals.jbchem.a002993. [DOI] [PubMed] [Google Scholar]
- 26.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem J. 2008;410:513–523. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- 27.Kruljac-Letunic A, Moelleken J, Kallin A, Wieland F, Blaukat A. The tyrosine kinase Pyk2 regulates Arf1 activity by phosphorylation and inhibition of the Arf-GTPase-activating protein ASAP1. J Biol Chem. 2003;278:29560–29570. doi: 10.1074/jbc.M302278200. [DOI] [PubMed] [Google Scholar]
- 28.Lee KW, Webb SE, Miller AL. A wave of free cytosolic calcium traverses zebrafish eggs on activation. Devel Biol. 1999;214:168–180. doi: 10.1006/dbio.1999.9396. [DOI] [PubMed] [Google Scholar]
- 29.Lee KW, Webb SE, Miller AL. Ca2+ released via IP3 receptors is required for furrow deepening during cytokinesis in zebrafish embryos. Dev-Biol. 2003;47:411–421. [PubMed] [Google Scholar]
- 30.Leguia M, Conner S, Berg L, Wessel GM. Synaptotagmin I is involved in the regulation of cortical granule exocytosis in the sea urchin. Mol Reprod Dev. 2006;73:895–905. doi: 10.1002/mrd.20454. [DOI] [PubMed] [Google Scholar]
- 31.Levi M, Maro B, Shalgi R. Fyn kinase is involved in cleavage furrow ingression during meiosis and mitosis. Reproduction. 2010;140:827–834. doi: 10.1530/REP-10-0312. [DOI] [PubMed] [Google Scholar]
- 32.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R, Liote F, Rose DM, Merz D, Terkeltaub R. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal-induced nitric oxide production and matrix metalloproteinase expression in chondrocytes. Arthritis & Rheumatism. 2004;50:247–258. doi: 10.1002/art.11486. [DOI] [PubMed] [Google Scholar]
- 34.Lo J, Lee S, Xu M, Liu F, Ruan H, Eun A, He Y, Ma W, Wang W, Wen Z, Peng J. 15000 unique zebrafish EST clusters and their future use in microarray for profiling gene expression patterns during embryogenesis. Genome Research. 2003;13:455–466. doi: 10.1101/gr.885403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev. 2010;22:966–976. doi: 10.1071/RD09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol Reprod Dev. 2011;78:831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng XQ, Zheng KG, Yang Y, Jiang MX, Zhang YL, Sun QY, Li YL. Proline-rich tyrosine kinase2 is involved in F-actin organization during in vitro maturation of rat oocyte. Reproduction. 2006;132:859–867. doi: 10.1530/rep.1.01212. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100:545–552. doi: 10.1254/jphs.cpj06003x. [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;%20:449, 341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 41.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004a;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 43.Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004b;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 44.Owen KA, Thomas KS, Bouton AH. The differential expression of Yersinia pseudotuberculosis adhesins determines the requirement for FAK and/or Pyk2 during bacterial phagocytosis by macrophages. Cell Microbiol. 2007;9:596–609. doi: 10.1111/j.1462-5822.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 45.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J Biol Chem. 2004;279:33315–33322. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- 47.Reinhard E, Yokoe H, Niebling KR, Allbritton NL, Kuhn MA, Meyer T. Localized calcium signals in early zebrafish development. Dev Biol. 1995;170:50–61. doi: 10.1006/dbio.1995.1194. [DOI] [PubMed] [Google Scholar]
- 48.Reiske HR, Zhao J, Han DC, Cooper LA, Guan JL. Analysis of FAK-associated signaling pathways in the regulation of cell cycle progression. FEBS Lett. 2000;486:275–280. doi: 10.1016/s0014-5793(00)02295-x. [DOI] [PubMed] [Google Scholar]
- 49.Riggs D, Yang Z, Kloss J, Loftus JC. The Pyk2 FERM regulates Pyk2 complex formation and phosphorylation. Cell Signal. 2011;23:288–296. doi: 10.1016/j.cellsig.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: Where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- 51.Sakakibara K, Sato K, Yoshino K, Oshiro N, Hirahara S, Mahbub Hasan AK, Iwasaki T, Ueda Y, Iwao Y, Yonezawa K, Fukami Y. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J Biol Chem. 2005;280:15029–15037. doi: 10.1074/jbc.M410538200. [DOI] [PubMed] [Google Scholar]
- 52.Sancho D, Montoya MC, Monjas A, Gordon-Alonso M, Katagiri T, Gil D, Tejedor R, Alarcon B, Sanchez-Madrid F. TCR engagement induces proline-rich tyrosine kinase-2 (Pyk2) translocation to the T cell-APC interface independently of Pyk2 activity and in an immunoreceptor tyrosine-based activation motif-mediated fashion. J Immunol. 2002;169:292–300. doi: 10.4049/jimmunol.169.1.292. [DOI] [PubMed] [Google Scholar]
- 53.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 54.Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma D, Kinsey WH. Regionalized calcium signaling in zebrafish fertilization. Int J Dev Biol. 2008;52:561–570. doi: 10.1387/ijdb.072523ds. [DOI] [PubMed] [Google Scholar]
- 57.Siripattarapravat K, Busta A, Steibel JP, Cibelli J. Characterization and in vitro control of MPF activity in zebrafish eggs. Zebrafish. 2009;6:97–105. doi: 10.1089/zeb.2008.0527. [DOI] [PubMed] [Google Scholar]
- 58.Slusarski DC, Corces VG. Calcium imaging in cell-cell signaling. Methods Mol Biol. 2000;135:253–261. doi: 10.1385/1-59259-685-1:253. [DOI] [PubMed] [Google Scholar]
- 59.Sorokin A, Kozlowski P, Graves L, Philip A. Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells. J Biol Chem. 2001;276:21521–21528. doi: 10.1074/jbc.M008869200. [DOI] [PubMed] [Google Scholar]
- 60.Sun CK, Ng KT, Lim ZX, Cheng Q, Lo CM, Poon RT, Man K, Wong N, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes cell motility of hepatocellular carcinoma through induction of epithelial to mesenchymal transition. PLoS One. 2011;%20:6, e18878. doi: 10.1371/journal.pone.0018878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi T, Yamashita H, Nagano Y, Nakamura T, Ohmori H, Avraham H, Avraham S, Yasuda M, Matsumoto M. Identification and characterization of a novel Pyk2/related adhesion focal tyrosine kinase-associated protein that inhibits alpha-synuclein phosphorylation. J Biol Chem. 2003;278:42225–42233. doi: 10.1074/jbc.M213217200. [DOI] [PubMed] [Google Scholar]
- 62.Tang H, Zhao ZZJ, Landon EJ, Inagami T. Regulation of calcium-sensitive tyrosine kinase Pyk2 by angiotensin II in endothelial cells - Roles of Yes tyrosine kinase and tyrosine phosphatase SHP-2. J Biol Chem. 2000;275:8389–8396. doi: 10.1074/jbc.275.12.8389. [DOI] [PubMed] [Google Scholar]
- 63.Townley IK, Schuyler E, Parker-Gur M, Foltz KR. Expression of multiple Src family kinases in sea urchin eggs and their function in Ca2+ release at fertilization. Dev Biol. 2009;327:465–477. doi: 10.1016/j.ydbio.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 64.Tsai WB, Zhang X, Sharma D, Wu W, Kinsey WH. Role of Yes kinase during early zebrafish development. Dev-Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Reiser G. The role of the Ca2+-sensitive tyrosine kinase Pyk2 and Src in thrombin signalling in rat astrocytes. J Neurochem. 2003;84:1349–1357. doi: 10.1046/j.1471-4159.2003.01637.x. [DOI] [PubMed] [Google Scholar]
- 66.Webb SE, Miller A. Calcium signalling during zebrafish embryonic development. BioEssays. 2000;22:113–123. doi: 10.1002/(SICI)1521-1878(200002)22:2<113::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 67.Westerfield M. The Zebrafish Book: A guide for th elaboratory use of zebrafish. 4. Univ. of Oregon Press; Eugene, OR: 2007. [Google Scholar]
- 68.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil Cytoskeleton. 1995;30:1122–1135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- 70.Wu SS, Jacamo RO, Vong SK, Rozengurt E. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal. 2006;18:1932–1940. doi: 10.1016/j.cellsig.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Wu W, Kinsey W. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int J Devel Biol. 2000;44:837–841. [PubMed] [Google Scholar]
- 72.Wu W, Kinsey WH. Role of PTPase(s) in regulating Fyn kinase at fertilization of the zebrafish egg. Dev Biol. 2002;247:286–294. doi: 10.1006/dbio.2002.0697. [DOI] [PubMed] [Google Scholar]
- 73.Wu W, Kinsey WH. Detection and Measurement of Membrane-Bound Protein Tyrosine Kinases in the Zebrafish Egg. Meth in Mol Biol. 2004;253:273–283. doi: 10.1385/1-59259-744-0:273. [DOI] [PubMed] [Google Scholar]
- 74.Xie J, Allen KH, Marguet A, Berghorn KA, Bliss SP, Navratil AM, Guan JL, Roberson MS. Analysis of the calcium-dependent regulation of proline-rich tyrosine kinase 2 by gonadotropin-releasing hormone. Mol Endocrinol. 2008;22:2322–2335. doi: 10.1210/me.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.