Abstract
Objective
Previous studies evaluating whether risk factors for gastric cancer are also associated with colorectal cancer (CRC) have shown inconsistent results. We prospectively examined the association of atrophic gastritis, a pre-malignant condition for gastric cancer and long-term sequelae common to many exposure factors, and the risk of incident CRC.
Methods
A total of 20,928 Finnish male smokers, aged 50–69, who were participants in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) had serum pepsinogen I (SPGI) levels measured. Participants with low SPGI levels (<25 µg/l) (n=1,665) were invited for gastroscopy. Of these, 1,059 (63.6%) participants underwent gastroscopy and atrophic gastritis was histologically confirmed in 1,006 (95.0%) participants. We used Cox proportional hazards regression to evaluate the risk of incident CRC.
Results
During a mean follow-up of 11.3 years (236,258 person-years), 425 incident CRC were diagnosed. The incidence rates were 1.82, 1.48, and 1.82 per 1,000 person-years of follow-up for participants with normal SPGI (≥25 µg/l), low SPGI, and histologically-confirmed atrophic gastritis, respectively. Compared to subjects with normal SPGI, there was no increased risk of CRC among subjects with low SPGI (Adjusted Hazard Ratio (HR) = 0.71; 95%CI: 0.47–1.05) and among those with histologically-confirmed atrophic gastritis (Adjusted HR = 0.86; 95%CI: 0.55–1.34).
Conclusions
Atrophic gastritis is not associated with an increased risk of colorectal cancer among male smokers.
Keywords: Serum pepsinogen, atrophic gastritis, colorectal cancer
INTRODUCTION
There has been a considerable interest in evaluating whether risk factors for gastric cancer also predispose to an increased risk of colonic malignancy. Newbold 1 suggested a common histogenesis between intestinal-type gastric cancer and colorectal carcinoma by demonstrating that similar clusters of undifferentiated proliferating columnar cells occur in the mucosal surfaces associated with intestinal metaplasia in the stomach and adenomatous polyps in the colorectum.
Multiple factors that have been suggested to contribute to the development of gastric neoplasia have been examined for an association with colorectal neoplasia with inconsistent results. Previous studies examining the association of chronic Helicobacter pylori infection with colorectal cancer reported inconsistent results with some reporting positive associations 2, 3 while others did not.4, 5 Long-term therapy with proton pump inhibitors (PPI) continues to increase and is likely to increase further with their increasing over-the-counter availability. It is noteworthy that chronic PPI therapy has not been proven to increase the risk of gastric cancer. Nonetheless, studies have suggested that it enhances the development of gastric atrophy with hypergastrinemia, particularly in persons with chronic Helicobacter pylori infection. 6 Gastrin has proliferative effects on colonocytes. 7, 8 However, based on prescription information, recent studies did not find an increased risk of colorectal cancer with PPI use, 9–12 but the duration of PPI exposures in these studies were relatively short given the long time period required for colorectal cancer to develop from a normal mucosa. Moreover, serum gastrin levels were not measured and development of gastric atrophy was not ascertained. Studies that have evaluated the association of hypergastrinemia and colorectal neoplasia have also shown inconsistent results with some, 13–16 but not all, 4, 17–19 reporting positive associations.
We hypothesized that since atrophic gastritis is a pre-malignant condition for gastric cancer which may result as a late sequelae from multiple etiologies including autoimmune pernicious anemia, chronic Helicobacter pylori infections and possibly, long-term treatment with proton pump inhibitors especially in persons with Helicobacter pylori infection, evaluating an association between documented atrophic gastritis and colorectal cancer may shed more light on any association between long-term exposures of these factors and colorectal cancer risk.
Atrophic gastritis may be assessed by measuring serum pepsinogen levels. Low serum pepsinogen I (SPGI) level and/or low serum pepsinogen I/II ratio (SPGI/II ratio) have been validated and used as markers for atrophic gastritis in many previous studies. 20–22 However, the gold standard for diagnosis of atrophic gastritis is histopathological diagnosis following gastroscopy and biopsy of gastric mucosa.
We sought to examine the association between atrophic gastritis and the risk of incident colorectal cancer using both modalities of assessments (SPGI level and histopathology) among participants in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study in Finland.
METHODS
ATBC study
The details of the rationale, design, and results of the ATBC have been published. 23,24 In brief, the ATBC was a randomized, double-blind, placebo-controlled, 2 × 2 factorial design, primary prevention trial that tested whether supplementation with alpha-tocopherol and/or beta-carotene could reduce the incidence of lung and other cancers. A total of 29,133 male smokers, aged 50–69 and living in southwestern Finland, were recruited from 1985 to 1988. The intervention was terminated on April 30, 1993, but the participants continue to be followed as a cohort. The ATBC study was approved by the institutional review boards of the National Cancer Institute, Bethesda, Maryland, and the National Public Health Institute, Helsinki, Finland. All subjects gave written informed consent.
At the pre-randomization baseline visit, study participants completed questionnaires on demographic characteristics and provided information regarding their medical, dietary, and smoking history. Their weights and heights were measured by trained study staff. Blood samples were collected from participants at two time points: at baseline (1985–1988) and 3 years after randomization. The sera were stored at −70°C.
Study subjects and serum pepsinogen measurement
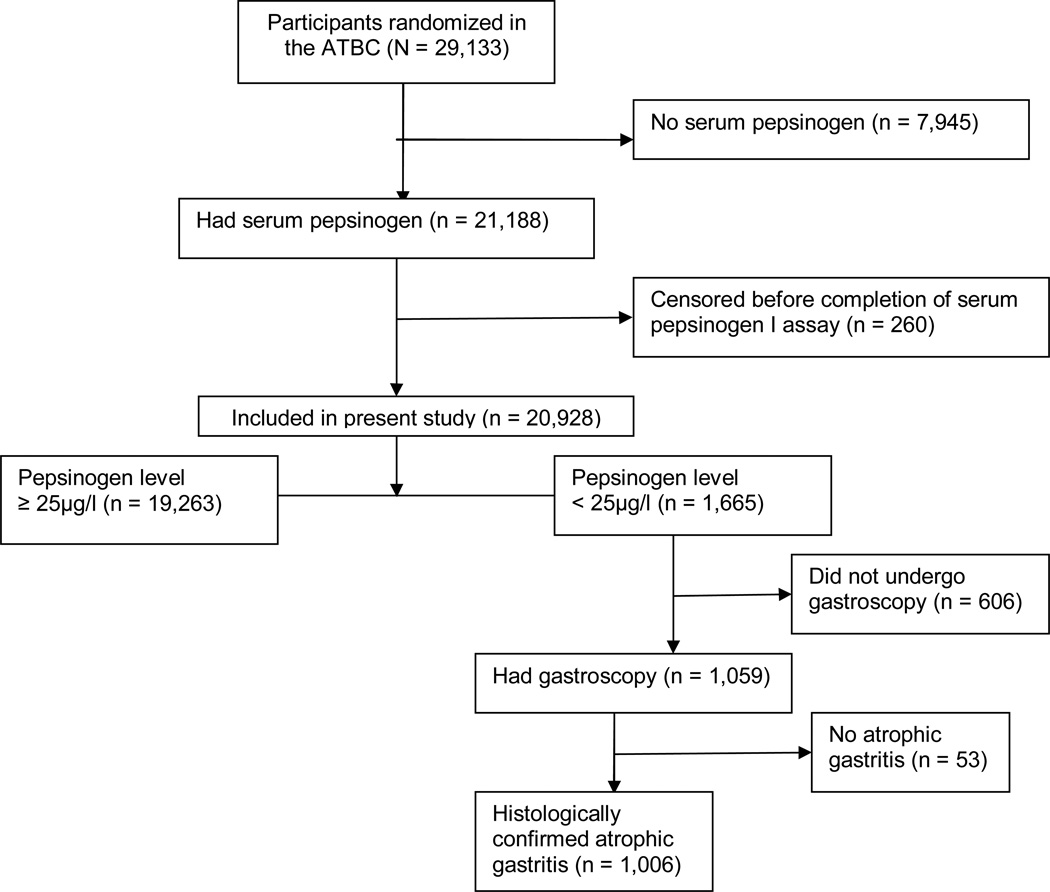
Of the 29,133 randomized subjects in ATBC, 21,188 had sufficient serum available from the second blood draw (3 years after randomization) to have the SPGI assay performed. Of these, 260 participants were censored prior to the date of their SPGI measurement (221 subjects were dead and 39 subjects had personal history of colorectal cancer). Therefore, the remaining 20,928 subjects constitute our final cohort (Figure). SPGI measurements were performed in two laboratories. Serum samples for 6,107 (29.2%) subjects were assayed at the University of California, Los Angeles, California from 1989–1991, but because this laboratory was damaged during an earthquake in 1991, the serum samples for the remaining 14,821 (70.8%) subjects were assayed at the University of Helsinki, Helsinki, Finland from 1992–1993. The analyses were done by radioimmunoassay methods. The results of the SPGI measurements from both laboratories were correlated and standardized. A low SPGI level was defined as <25 µg/l. 25–27
Figure.
Diagram of flow of participants through the study
Gastroscopy
Of the 20,928 subjects in the analytic cohort, a total of 1,665 (8.0%) subjects had low SPGI. In all 606 (36.4%) subjects did not have gastroscopy: 109 (18%) were not offered gastroscopy in the primary gastritis study because of its inclusion criteria; 269 (44%) did not respond to the invitation for gastroscopy; 95 (16%) refused; and 133 (22%) were ineligible for gastroscopy because of health reasons (mostly due to coronary artery disease). Therefore, gastroscopy was performed on 1,059 (63.6%) subjects by gastrointestinal endoscopists (Figure). In general, gastroscopy was performed within 2 months after SPGI assay. Routine biopsy specimens were taken under direct visualization as follows: one from the distal and one from the proximal antrum along the lesser curvature, two from the middle corpus, and one from the anterior and one from the posterior wall (total of six biopsies). In addition, multiple biopsy specimens were taken from all endoscopically abnormal lesions (local color changes, ulcers, scars, abnormal folds, polypoid lesions, and tumors).
Histological diagnosis of atrophic gastritis
Gastritis and any related histopathologic appearance in all specimens were classified using the Sydney System. 28 Two independent trial pathologists with expertise in gastrointestinal pathology evaluated the specimens and classified the subjects based on the most severe histopathologic change: no atrophy, atrophic gastritis (mild, moderate, and severe), dysplasia (mild, moderate, and severe), and malignant (adenocarcinoma and carcinoid). For our primary analysis, we categorized subjects only by the absence or presence of atrophic gastritis, irrespective of the severity. Among the 1,059 subjects who underwent gastroscopy, 53 (5.0%) had no evidence of atrophic gastritis in their biopsy samples whereas 1,006 (95.0%) had histologically-confirmed atrophic gastritis (or worse lesions). In a sensitivity analysis, we restricted our analysis to only participants with histologically proven mild, moderate or severe atrophic gastritis on biopsy without dysplasia or malignancy. This was done because we felt that subjects with dysplasia or gastric neoplasia might have had a different course of treatment and pattern of follow-up which may modify their risk of colorectal cancer.
Outcome assessment
Colorectal cancer cases were identified from the Finnish Cancer Registry, which provides almost 100% case ascertainment in Finland. 29 For cases diagnosed through April 1999, medical records were reviewed centrally by two study oncologists for diagnostic confirmation, and cases with histopathologic specimens available were also reviewed and confirmed by one or two pathologists. Information on colorectal cancer cases diagnosed since May 1999 through April 2006 was derived only from the Finnish Cancer Registry. Deaths were identified from the National Death Registry, a branch of Statistics Finland.
Statistical analysis
The reference group was defined as those with normal SPGI (≥ 25 µg/l). Since we defined atrophic gastritis both serologically and histologically, we compared those with low SPGI (< 25 µg/l) and the subset with histologically-confirmed atrophic gastritis with the reference group. We used chi-square test to compare the proportions of dichotomous variables. We used Wilcoxon rank-sum tests to compare continuous variables because several of these variables were not normally distributed.
We determined the incidence rate of colorectal cancer among participants with normal SPGI (reference group), low SPGI (serologic atrophic gastritis), and histologically-confirmed atrophic gastritis. We used Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) comparing the subjects with serologic and histologic atrophic gastritis versus the reference group. The proportional hazard assumption was tested using likelihood-ratio tests. For calculating incidence rates and also for Cox models, we defined the start of follow-up as the date of SPGI assay. We chose this as our start date rather than the date of blood draw (as often done) because we were interested in participants who had histopathologic confirmation of atrophic gastritis following gastroscopy (the gold standard for atrophic gastritis) as our main analysis. The gastroscopy was performed within 2 months of SPGI assay. Therefore, since subjects with normal SPGI was the comparison group for our analyses, using the date of SPGI assay provided a comparable beginning of follow up for this cohort. Subjects were censored at death (from any cause), at diagnosis of colorectal cancer, or at the end of follow-up for this study (April 30, 2006), whichever came first. The number of person-years each participant contributed to the cohort served as the underlying time metric. Of note, when we performed our analysis using the date of blood draw as the start of follow-up and evaluated low- versus normal SPGI level, the results were similar (data not shown).
We used two Cox models. In model 1 (the parsimonious model), we kept only variables that were significantly associated with pepsinogen levels and the development of colorectal cancer (age, years of smoking and total cigarettes smoked/day). In model 2 (the a priori model), we retained randomization assignment in the ATBC trial and a priori factors that have been suggested in the literature to be risk factors for colorectal cancer (age, body mass index, alcohol intake, cigarettes smoking, family history of colorectal cancer, education, consumption of red meat, and physical activities). In order to assess the effect of any prevalent subclinical colorectal cancer at the time of SPGI measurement, we also repeated our analysis and excluded the first one, three, five and ten years of follow-up. We used Stata ® statistical software version 9 (College Station, Texas) for all analyses. All reported P-values correspond to two-sided tests.
RESULTS
Baseline characteristics of participants
Table 1 shows the baseline characteristics of the 20,928 participants included in the analytic cohort (Figure). The participants were evenly distributed by randomization assignment. The mean age at baseline was 56.9 years and 16.4% of the subjects had junior high school education or higher. The mean duration of smoking was 35.5 years and number of cigarettes smoked per day was 20.2. Approximately 2.7% of participants had a family history of colorectal cancer. Compared to participants with normal SPGI, those with low SPGI or histologically confirmed atrophic gastritis were older; had less formal education; smoked for longer duration but fewer cigarettes per day; had lower body mass index; and were more likely to have a history of peptic ulcer disease, a partial gastrectomy, and family history of stomach cancer (all P-values ≤0.002). However, participants with atrophic gastritis did not differ from those with normal SPGI by family history of colorectal cancer.
Table 1.
Comparison of selected baseline characteristics of subjects based on serological and histological atrophic gastritis
| Baseline characteristics | All participants n = 20,928 |
Normal serum pepsinogen 1 level (≥ 25 µg/l) n = 19,263 |
Low serum pepsinogen 1 level (< 25 µg/l) n = 1,665 |
P value* | Histologically- confirmed atrophic gastritis n = 1,006 |
P value‡ |
|---|---|---|---|---|---|---|
| Randomization assignment | ||||||
| Placebo | 5,229 (25.0) | 4,836 (25.1) | 393 (23.6) | 237 (23.6) | ||
| Beta-carotene | 5,214 (24.9) | 4,801 (24.9) | 413 (24.8) | 0.195 | 251 (25.0) | 0.335 |
| Alpha-tocopherol | 5,248 (25.1) | 4,840 (25.1) | 408 (24.5) | 244 (24.3) | ||
| Alpha-tocopherol and Beta-carotene |
5,237 (25.0) | 4,786 (24.9) | 451 (27.1) | 274 (27.2) | ||
| Age, years (SD) | 56.9 (5.0) | 56.8 (4.9) | 59.8 (5.2) | <0.001 | 58.6 (5.2) | <0.001 |
| Married, n (%) | 17,336 (82.8) | 15,961 (82.9) | 1,375 (82.6) | 0.775 | 848 (84.3) | 0.238 |
| Junior high school education or more, n (%) |
3,440 (16.4) | 3,277 (17.0) | 163 (9.8) | <0.001 | 111 (11.0) | <0.001 |
| Cigarette smoking habits | ||||||
| Duration, years (SD) | 35.5 (8.4) | 35.3 (8.4) | 37.4 (8.7) | <0.001 | 36.8 (9.0) | <0.001 |
| Cigarettes smoked/day (SD) |
20.2 (8.8) | 20.3 (8.9) | 19.4 (8.0) | <0.001 | 19.3 (7.9) | <0.001 |
| Alcohol use, g/day (SD) | 17.3 (20.2) | 17.4 (20.3) | 16.0 (19.8) | <0.001 | 15.7 (18.9) | 0.008 |
| Red meat intake, g/day | 22.2 (10.2) | 22.2 (10.2) | 22.1 (10.2) | 0.546 | 22.3 (9.9) | 0.596 |
| Body mass index, kg/m2 (SD) |
26.3 (3.7) | 26.4 (3.7) | 25.8 (3.8) | <0.001 | 25.8 (3.7) | <0.001 |
| Frequency of 30-minute leisure physical activity per week | ||||||
| <1 | 10,639 (50.9) | 9,798 (50.9) | 841 (50.5) | 506 (50.3) | ||
| 1–2 | 6,268 (30.0) | 5,801 (30.2) | 467 (28.1) | 0.026 | 287 (28.6) | 0.191 |
| ≥ 3 | 3,990 (19.1) | 3,634 (18.9) | 356 (21.4) | 212 (21.1) | ||
| Self-reported medical history | ||||||
| Peptic ulcer disease, n (%) | 3,489 (16.7) | 3,124 (16.2) | 365 (21.9) | <0.001 | 202 (20.1) | 0.001 |
| Partial gastrectomy, n (%) | 653 (3.3) | 449 (2.5) | 204 (13.0) | <0.001 | 119 (12.2) | <0.001 |
| Family cancer history | ||||||
| Colorectal cancer, n (%) | 472 (2.7) | 440 (2.7) | 32 (2.4) | 0.504 | 19 (2.3) | 0.444 |
| Stomach cancer, n (%) | 2,313 (13.3) | 2,073 (13.0) | 240 (18.1) | <0.001 | 164 (19.6) | <0.001 |
For comparison between participants with normal versus low serum pepsinogen I levels
For comparison between participants with normal serum pepsinogen I level versus histologically-confirmed atrophic gastritis
Compared to the 1,059 participants who underwent gastroscopy and biopsy for atrophic gastritis, the 606 participants with low SPGI who did not were slightly older (mean age 59.5 versus 58.5 years, P < 0.001) and had longer duration of smoking (38.6 versus 36.7 years, P < 0.001). They also had less formal education (junior high school or more: 7.4% versus 11.1%, P = 0.01) and were less likely to be married (79.9% versus 84.1%, P = 0.03). There was no difference in the prevalence of family history of colorectal cancer between the two groups (2.7% versus 2.3%, P = 0.67).
Colorectal cancer risk by low SPGI or histologically confirmed atrophic gastritis versus normal SPGI
A total of 425 colorectal cancers were diagnosed during a mean follow-up of 11.3 years (range 0 – 17.3 years), constituting 236,258 person-years of follow-up. The proportional hazard assumption was satisfied (P value ≥ 0.25 for all our analytic models). Colorectal cancer incidence rate per 1000 person-years of follow-up was 1.82 for subjects with normal SPGI and 1.48 for subjects with low SPGI (Table 2). In our multivariate analysis, we did not observe any association between serologic atrophic gastritis and colorectal cancer risk in the parsimonious model (model 1), (HR = 0.71; 95%CI: 0.47–1.05) and there was no increased risk of colorectal cancer after repeating our analysis with lags of the first year (HR = 0.72; 95%CI: 0.48–1.08), the first 3 years (HR = 0.69; 95%CI: 0.44–1.07), the first 5 years (HR = 0.59; 95%CI: 0.35–1.00) and the first 10 years (HR = 0.89; 95%CI: 0.47–1.70) of follow-up. In the a priori model (model 2), there was also no increased risk of colorectal cancer (HR = 0.81; 95%CI: 0.52–1.25) for low SPGI and there was no change in the risk of colorectal cancer when lags of 1, 3, 5 or 10 years were performed (data not shown).
Table 2.
Incident colorectal cancer in relation to atrophic gastritis
| Normal serum pepsinogen1 level (≥ 25 µg/l) n = 19,263 |
Low serum pepsinogen1 level (< 25 µg/l) n = 1,665 |
Histologically confirmed atrophic gastritis n = 1,006 |
|
|---|---|---|---|
| Number of colorectal cancers diagnosed |
399 | 26 | 21 |
| Mean duration of follow-up, years (range) Person-years of follow- up |
11.35 (0 – 17.26) 218,668 |
10.57 (0.08 – 17.26) 17,590 |
11.44 (0.54– 17.26) 11,510 |
| Incidence rate / 1,000 person-years of follow- up |
1.82 | 1.48 | 1.82 |
| Incidence rate ratio (95% CI) |
Reference | 0.81 (0.52 – 1.20) | 1.00 (0.61 – 1.55) |
| Univariate model, HR (95% CI) |
Reference | 0.82 (0.55 – 1.23) | 1.00 (0.65 – 1.55) |
| Age-adjusted model, HR (95% CI) |
Reference | 0.70 (0.47 – 1.05) | 0.86 (0.55 – 1.33) |
| Multivariate model 1, HR (95% CI)* |
Reference | 0.71 (0.47 – 1.05) | 0.86 (0.55 – 1.34) |
| Multivariate model 2, HR (95% CI)‡ |
Reference | 0.81 (0.52 – 1.25) | 0.98 (0.61 –1.58) |
Model 1 = Parsimonious model with only true confounders (age, years of smoking and total cigarettes smoked/day)
Model 2 = Extended model with randomization assignment in the ATBC trial and a priori factors that have been suggested in the literature to be risk factors for colorectal cancer (age, body mass index, alcohol intake, cigarettes smoking, family history of colorectal cancer, education, consumption of red meat, and physical activities)
Colorectal cancer incidence rates were 1.82 per 1000 person-years for subjects with histologically-confirmed atrophic gastritis. There was also no association with incident colorectal cancer for either the parsimonious model (HR = 0.86; 95%CI: 0.55–1.34) or the a priori model (HR = 0.98; 95%CI: 0.61–1.58). There was also no change in the results for risk of colorectal cancer by histologically-confirmed atrophic gastritis when lags of 1, 3, 5 or 10 years were performed (data not shown).
Sensitivity analysis
We repeated our analysis and restricted the histologically confirmed atrophic gastritis to only 731 subjects with mild, moderate, or severe atrophic gastritis without evidence of dysplasia or malignancy. We undertook this analysis to account for possible differences in treatment and follow-up of participants with dysplasia or neoplasia which may modify their risk of colorectal cancer. Participants with only mild, moderate, or severe atrophic gastritis (n = 731) were followed for a mean duration of 11.6 years (range 0.8–17.3 years) for a total 8,444 person-years of follow-up. Fifteen cases of colorectal cancer were diagnosed for an incidence rate of 1.78 per 1,000 person-years of follow-up. When compared with subjects with normal SPGI, there was no association between this alternate definition of histological atrophic gastritis and colorectal cancer in either analytical model: (HR = 0.86; 95%CI: 0.51–1.43) for the parsimonious model and (HR = 0.99; 95%CI: 0.57–1.72) for the a priori model.
DISCUSSION
In our study, we did not observe an increased risk of colorectal cancer with atrophic gastritis. We assessed atrophic gastritis both biochemically with SPGI as a biomarker, and histopathologically, but we did not observe an increased risk using either modality of assessment. The subjects in our study were 50–69 years-old when they were enrolled in the ATBC trial and were followed up for duration up to 17 years (mean of 11.3 years) after determination of atrophic gastritis. Our follow-up duration was adequate for a large number of cases to be identified and yet, we did not find any suggestion of a positive association.
To the best of our knowledge, there is no previous prospective cohort study that has evaluated the relationship between atrophic gastritis and colorectal cancer for us to compare our findings with. Only one case-control study has evaluated the relationship between atrophic gastritis and colorectal cancer risk. 4 In that study, 113 cases of colorectal cancer and 226 control participants who were enrolled in a health check-up program in Japan had serum pepsinogen, gastrin and Helicobacter pylori antibody measured. The authors reported no association between Helicobacter pylori infection, serum gastrin level or serum pepsinogen level with colorectal cancer.
Other studies have also evaluated factors related to atrophic gastritis and risk of colorectal cancer. 2–5, 9–12 Previous studies examining the association of Helicobacter pylori with colorectal cancer found inconsistent results with some reporting positive associations 2,3 while others did not.4,5 In their study involving 384 subjects with colorectal cancer and 467 matched controls, Zumkeller et al. 3 reported a higher Helicobacter pylori seroprevalence among cases than controls (51% versus 44%), and a positive association between Helicobacter pylori infection and colorectal adenocarcinoma (OR=1.41; 95%CI:1.06–1.87). In contrast, Limburg et al. 5 reported a lack of an association between Helicobacter pylori infection and colorectal cancer risk in a case-control study nested within the ATBC cohort. In that study the serum of 118 cases of colorectal cancer patients and 236 matched controls was assayed for both Helicobacter pylori whole cell and Helicobacter pylori CagA antibodies. There were no differences in the seropositivity results between cases and controls: whole cell (OR=1.05; 95%CI: 0.63–1.74), cagA+ (OR=1.17; 95%CI: 0.74–1.84), and total Helicobacter pylori seropositivity (OR=0.91; 95%CI: 0.53–1.55). Also, no significant association between proton pump inhibitor use and colorectal cancer was found in recent studies. 9–12
Robertson et al. 30 recently evaluated the association between pre-diagnostic serum gastrin level, Helicobacter pylori seropositivity and the risk of recurrent colorectal adenoma using stored sera from two completed chemoprevention trials. The authors reported no association between serum gastrin level and risk of adenoma recurrence (RR=1.10; 95%CI: 0.78–1.54) and advanced adenoma recurrence (RR=0.82; 95%CI: 0.33–2.03), but a reduced risk of adenoma recurrence was observed with Helicobacter pylori seropositivity (RR=0.76; 95%CI: 0.60–0.96) within 3 years of follow-up. This suggests that factors related to atrophic gastritis are not associated with colorectal carcinogenesis even at the early stages of adenoma formation and progression.
Studies have shown that countries with high gastric cancer typically have lower colorectal cancer incidence. 31 Finland, at least in the past, was a country with high risk of gastric cancer and low risk of colorectal cancer. 31 The reasons for this inverse ecological association are not well understood, but several speculative theories could be considered. Gastric cancer is usually seen in populations with lower socio-economic status, perhaps because of higher rates of Helicobacter pylori infection, food handling practices and dietary patterns such as heavy consumption of salted and smoke-dried foods. 32–34 In contrast, colorectal cancer is seen more often in higher socioeconomic groups, perhaps due to obesity and less physical activity. It is also quite possible that changes in the gastric micro-environment may be contributing to this inverse ecological association and would merit further research. Nonetheless, it appears that factors that predispose to gastric cancer may not have the same effect in the colon.
Proton pump inhibitors are now very commonly used on a long-term basis in the United States and many other countries of the world. Our study provides some reassuring evidence, by extension, that long-term therapy with proton pump inhibitors, at least in adults, may not increase the risk of colorectal cancer through alteration of gastric homeostasis and perhaps gastrin-related mechanisms.
Potential strengths of our study are that our study population was from a large prospective study; data on risk factors were collected prospectively; dedicated trial pathologists examined the biopsy specimen; and atrophic gastritis was determined using both SPGI and histopathology. We also evaluated the association over a long follow-up period (up to a maximum of 17 years) after SPGI assays using incident colorectal cancer cases ascertained from an accurate cancer registry in a population with relatively low migration.
However, our study has many limitations. Participants in the ATBC were limited to Finnish male smokers which may affect the generalizability of our findings particularly to women and non-smokers, although most previous associations found in this cohort have been corroborated in other cohorts. We did not have any information on PPI therapy as it was not widely prescribed at the beginning of this cohort. We also did not measure serum gastrin levels to confirm hypergastrinemia nor did we perform gastroscopy on all subjects. Hence, it is possible that some subjects in the reference group may actually have had atrophic gastritis despite normal SPGI level, and some subjects may also develop atrophic gastritis during the follow-up period. This may bias our results towards null. However, in a population - based study from Finland, 20 SPGI level was < 25 µg/l in 80% of subjects with severe atrophic corpus gastritis, but in only 2.1% of those without atrophic gastritis. Therefore, we estimate that the effect of such misclassification is minimal. There is also a possibility that participants with atrophic gastritis may modify their behavior which may in turn modify their risk of colorectal cancer. Unfortunately, we could not assess this possibility objectively in our study. However, there was no meaningful difference in the association between atrophic gastritis and colorectal cancer when we excluded participants with gastric dysplasia or malignancy (who are more likely to have lifestyle modification based on the severity of their gastroscopy findings) in sensitivity analysis.
In conclusion, our prospective long-term study did not demonstrate an increased risk of colorectal cancer with atrophic gastritis among Finnish male smokers. Our study suggests that factors which predispose to the development of atrophic gastritis may not increase the risk of colorectal cancer through direct effect on gastric homeostasis.
Acknowledgments
Financial support: This research was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, and the Division of Cancer Prevention, National Institutes of Health, Department of Health and Human Services. The funding agency had a role in the design and reporting of the study and in the decision to submit the manuscript for publication and approved the final version of the manuscript.
Abbreviations used in this paper
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
- CI
Confidence interval
- CRC
Colorectal cancer
- HR
Hazard ratio
- SPGI
Serum pepsinogen I
Footnotes
Conflict of interest: None
REFERENCES
- 1.Newbold KM. Undifferentiated cells in gastrointestinal mucosa inferring an association between carcinoma of the colon and intestinal type gastric cancer. J Clin Pathol. 1989;42(5):523–524. doi: 10.1136/jcp.42.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol. 2007;12:5, 51. doi: 10.1186/1477-7819-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumkeller N, Brenner H, Chang-Claude J, Hoffmeister M, Nieters A, Rothenbacher D. Helicobacter pylori infection, interleukin-1 gene polymorphisms and the risk of colorectal cancer: evidence from a case-control study in Germany. Eur J Cancer. 2007;43(8):1283–1289. doi: 10.1016/j.ejca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Machida-Montani A, Sasazuki S, Inoue M, et al. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: a case-control study. Helicobacter. 2007;12(4):328–332. doi: 10.1111/j.1523-5378.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 5.Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, et al. Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1095–1099. [PubMed] [Google Scholar]
- 6.Kuipers EJ. Proton pump inhibitors and gastric neoplasia. Gut. 2006;55(9):1217–1221. doi: 10.1136/gut.2005.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a non-transformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 8.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109(4):1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 9.van Soest EM, van Rossum LG, Dieleman JP, et al. Proton pump inhibitors and the risk of colorectal cancer. Am J Gastroenterol. 2008;103(4):966–973. doi: 10.1111/j.1572-0241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sørensen HT. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Gastroenterology. 2007;133(3):755–760. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133(3):748–754. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Siersema PD, Yu S, Sahbaie P, et al. Colorectal neoplasia in veterans is associated with Barrett's esophagus but not with proton-pump inhibitor or aspirin/NSAID use. Gastrointest Endosc. 2006;63(4):581–586. doi: 10.1016/j.gie.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Seitz JF, Giovannini M, Gouvernet J, Gauthier AP. Elevated serum gastrin levels in patients with colorectal neoplasia. J Clin Gastroenterol. 1991;13:541–545. doi: 10.1097/00004836-199110000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Smith JP, Wood JG, Solomon TE. Elevated gastrin levels in patients with colon cancer or adenomatous polyps. Dig Dis Sci. 1989;34:171–174. doi: 10.1007/BF01536047. [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Beardshall K, Waters CM, Calam J, Poston GJ. Postprandial hypergastrinaemia in patients with colorectal cancer. Gut. 1991;32:1352–1354. doi: 10.1136/gut.32.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 17.Kikendall JW, Glass AR, Sobin LH, Bowen PE. Serum gastrin is not higher in subjects with colonic neoplasia. Am J Gastroenterol. 1992;87:1394–1397. [PubMed] [Google Scholar]
- 18.Penman ID, el-Omar E, Ardill JE, et al. Plasma gastrin concentrations are normal in patients with colorectal neoplasia and unaltered following tumor resection. Gastroenterology. 1994;106:1263–1270. doi: 10.1016/0016-5085(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 19.Yapp R, Modlin IM, Kumar RR, Binder HJ, Dubrow R. Gastrin and colorectal cancer. Evidence against an association. Dig Dis Sci. 1992;37:481–484. doi: 10.1007/BF01307566. [DOI] [PubMed] [Google Scholar]
- 20.Varis K, Kekki M, Harkonen M, Sipponen P, Samloff IM. Serum pepsinogen I and serum gastrin in screening of atrophic pangastritis with high risk of gastric cancer. Scand J Gastroenterol Suppl. 1991;186:117–123. doi: 10.3109/00365529109103998. [DOI] [PubMed] [Google Scholar]
- 21.Yanaoka K, Oka M, Mukoubayashi C, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17(4):838–845. doi: 10.1158/1055-9965.EPI-07-2762. [DOI] [PubMed] [Google Scholar]
- 22.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9(4):245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 23.ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 24.Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 25.Samloff IM. Pepsinogens I and II: purification from gastric mucosa and radioimmunoassay in serum. Gastroenterology. 1982;82(1):26–33. [PubMed] [Google Scholar]
- 26.Varis K, Taylor PR, Sipponen P, et al. The Helsinki Gastritis Study Group. Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha-tocopherol and betacarotene. Scand J Gastroenterol. 1998;33:294–300. doi: 10.1080/00365529850170892. [DOI] [PubMed] [Google Scholar]
- 27.Varis K, Sipponen P, Laxén F, et al. The Helsinki Gastritis Study Group. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Scand J Gastroenterol. 2000;35:950–956. doi: 10.1080/003655200750023011. [DOI] [PubMed] [Google Scholar]
- 28.Price AB. The Sydney system: histological division. J Gastroenterol Heptol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort: accuracy and delay. Acta Oncol. 2002;41:381–388. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 30.Robertson DJ, Sandler RS, Ahnen DJ, et al. Gastrin, Helicobacter pylori, and Colorectal Adenomas. Clin Gastroenterol Hepatol. 2009;7(2):163–167. doi: 10.1016/j.cgh.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0. Lyon: IARC Press; 2004. [Accessed January 6, 2009]. (at http://www-dep.iarc.fr/) [Google Scholar]
- 32.Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24(1):33–41. doi: 10.1093/ije/24.1.33. [DOI] [PubMed] [Google Scholar]
- 33.Buiatti E, Palli D, Decarli A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44(4):611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 34.Ramón JM, Serra L, Cerdó C, Oromí J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71(5):1731–1735. doi: 10.1002/1097-0142(19930301)71:5<1731::aid-cncr2820710505>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]