Abstract
In sepsis, toll-like receptor (TLR)-4 modulates the migration of neutrophils to infectious foci, favoring bacteremia and mortality. In experimental sepsis, organ dysfunction and cytokines released by activated macrophages can be reduced by gastrin-releasing peptide (GRP) receptor (GRPR) antagonist RC-3095. Here we report a link between GRPR and TLR-4 in experimental models and in sepsis patients. RAW 264.7 culture cells were exposed to lipopolysaccharide (LPS) or tumor necrosis factor (TNF)-α and RC-3095 (10 ng/mL). Male Wistar rats were subjected to cecal ligation and puncture (CLP), and RC-3095 was administered (3 mg/kg, subcutaneously); after 6 h, we removed the blood, bronchoalveolar lavage, peritoneal lavage and lung. Human patients with a clinical diagnosis of sepsis received a continuous infusion with RC-3095 (3 mg/kg, intravenous) over a period of 12 h, and plasma was collected before and after RC-3095 administration and, in a different set of patients with systemic inflammatory response syndrome (SIRS) or sepsis, GRP plasma levels were determined. RC-3095 inhibited TLR-4, extracellular-signal–related kinase (ERK)-1/2, Jun NH2-terminal kinase (JNK) and Akt and decreased activation of activator protein 1 (AP-1), nuclear factor (NF)-κB and interleukin (IL)-6 in macrophages stimulated by LPS. It also decreased IL-6 release from macrophages stimulated by TNF-α. RC-3095 treatment in CLP rats decreased lung TLR-4, reduced the migration of cells to the lung and reduced systemic cytokines and bacterial dissemination. Patients with sepsis and systemic inflammatory response syndrome have elevated plasma levels of GRP, which associates with clinical outcome in the sepsis patients. These findings highlight the role of GRPR signaling in sepsis outcome and the beneficial action of GRPR antagonists in controlling the inflammatory response in sepsis through a mechanism involving at least inhibition of TLR-4 signaling.
INTRODUCTION
Sepsis remains an important problem with high rates of morbidity and mortality, despite modern advances in critical care management. Sepsis happens when the initial host response fails to limit the infection, leading to systemic inflammation and multiple organ failure (1). Strategies for treating human sepsis, mainly targeting proinflammatory mediators, have only had limited success (2).
Increased levels of circulating cytokines and chemokines, and neutrophil sequestration in the lung, are characteristics of systemic inflammation (3). Reduced neutrophil chemotaxis is associated with illness severity and organ damage (4,5). Expansion of bacterial infection leads to systemic toll-like receptor (TLR) activation, and tumor necrosis factor (TNF) receptors 1 and 2 (TNFR1/R2) appear to be involved in this process (6,7). Endotoxin (lipopolysaccharide [LPS]), a major cell wall component in gram-negative bacteria, can induce systemic inflammation and is a major pathogenic element in infection by gram-negative bacterial (8). Sensing of LPS by toll-like receptor (TLR)-4 in innate immune cells is vital for host defense against gram-negative bacteria. Molecules involved in the TLR-4–activated pathway include the adaptor molecule, myeloid differentiation primary response protein 88 (MyD88), interleukin (IL)-1 receptor–associated kinases and TNF receptor–associated factor 6 (9). This pathway results in activation of several mitogen-activated protein kinases (MAPKs), as well as activation of the transcription factors such as nuclear factor (NF)-κB and activator protein 1 (AP-1), which contribute to the development of septic shock and multiple organ failure with transcriptional regulation of inflammatory genes (10). In this context, TLR-4–defective mice presented neutrophil migration to the peritoneal cavity during sepsis induced by lethal cecal ligation and puncture (CLP) and, as a consequence, are more resistant to sepsis than controls (11). Given its central role in the pathogenesis of sepsis, TLR-4 is a target for the development of novel therapies against sepsis.
Bombesin (BN) is a 14–amino acid peptide isolated from toad skin (12). BN-like immunoreactivity using amphibian BN antibodies was demonstrated in the central nervous system, mammalian gut and lung. Gastrin-releasing peptide (GRP), a BN-like peptide, has been implicated in the pathogenesis of inflammatory conditions (13–19). BN-like receptors such as gastrin-releasing peptide receptor (GRPR), neuromedin B receptor and the orphan BN receptor subtype 3 have been cloned. These receptors are seven transmembrane-spanning G protein– coupled receptors that activate various intracellular signaling pathways associated with neutrophil and macrophages activation by chemokines (20), long known to attract various inflammatory cells (21).
We recently demonstrated that the GRPR antagonist, RC-3095, decreases the release of proinflammatory cytokines and improves survival in sepsis by CLP. In particular, we showed in a CLP model of sepsis and acute lung injury that RC-3095 reduces mortality rates by reducing organ dysfunction and inflammatory infiltration and modulating the release of proinflammatory cytokines by activated macrophages (22). These findings are consistent with the involvement of a GRPR-stimulated inflammatory pathway in the development of sepsis. Given that TLRs are important components of the innate immune response to infection and evidence indicates that these receptors may play a role in the sepsis pathophysiology (23), together with the aforementioned critical role of TLR-4, in particular in neutrophil migration (6,7), we hypothesize that GRPR stimulation may exert its inflammatory effects through a mechanism involving the TLR-4 signaling pathway. Thus, the blockade of GRPR can protect against severe sepsis.
The aim of this study was to investigate the effects of RC-3095 on TLR-4 expression and its signaling pathways in sepsis.
MATERIALS AND METHODS
Animal Experiments
A total of 15 male Wistar rats, 2–3 months old, were used in this study. Rats were subjected to CLP as described (24). The rats were randomly divided into sham-operated, CLP and CLP plus RC-3095 groups, comprising five animals per group. RC-3095 (3 mg/kg subcutaneously; the dose was based on a dose-response curve previously published) (22) was administered immediately after surgery. We previously demonstrated that RC-3095 alone has no effect in this model (22); thus, it was not included in the sham-operated plus RC-3095 group. Six hours after surgery (described below), blood, bronchoalveolar lavage fluid (BALF) and peritoneal lavages were collected and the lung tissue was removed. The experimental procedures with animals were made in accordance with the National Institutes of Health (Bethesda, MD, USA) Guide for Care and Use of Laboratory Animals(25) and the approval of our institutional ethics committee.
Human Subjects
Role of the infusion of RC-3095 of cytokine level in patients
Twelve patients (seven men, five women) admitted to an adult medical intensive care unit (ICU) with a clinical diagnosis of septic shock and failure of three or more organs and who conformed to the consensus conference criteria (26) were enrolled in the study. Patients received a continuous infusion with RC-3095 (3 mg/kg) over a period of 12 h, and plasma samples were collected in heparin-treated vacuum tubes before and after RC-3095 administration for later determination of IL-6 and IL-10. Eleven patients with similar clinical characteristics who did not receive RC-3095 were included as controls. All procedures involving patients and healthy volunteers were made in compliance with the Declaration of Helsinki and National Institutes of Health guidelines and were approved by the institutional ethics committee (protocol number 431/2006). All patients or their relatives gave informed consent before being included in the study.
GRP plasma levels and sepsis severity
In a different set of subjects, patients consecutively admitted to an adult medical ICU between August 2008 and December 2008 with systemic inflammatory response syndrome (SIRS) or sepsis diagnosis were screened for enrollment. Septic patients (n = 30) were classified as having sepsis, severe sepsis or septic shock, according to the consensus conference (26), by two board-certified internal medicine specialists. Septic patients were paired to SIRS patients (n = 29) in relation to age, sex, severity scores and mortality (demographics summarized in Table 1). Patients were eligible if the time between sepsis diagnosis (this was done by reviewing medical charts and/or contacting the patient’s physician) and ICU admission was no more than 24 h. Blood was collected at ICU admission. In parallel, clinically relevant data were recorded daily for 28 d. Exclusion criteria were as follows: age below 18 years, neoplasia receiving chemotherapy or radiotherapy, chronic hepatic or renal insufficiency and immunodeficiency. Blood was collected from six healthy volunteers to serve as the control.
Table 1.
Patient characteristics.
| SIRS | Sepsis | Severe sepsis | Septic shock | p | |
|---|---|---|---|---|---|
| n | 29 | 9 | 10 | 11 | |
| Age (years) (SD) | 59 (15) | 60(14) | 62(7) | 56(15) | >0.05 |
| Race | |||||
| Black | 0 | 1 | 0 | 1 | >0.05 |
| White | 29 | 8 | 10 | 10 | >0.05 |
| Sex | |||||
| Female | 11 | 4 | 3 | 5 | >0.05 |
| Male | 18 | 5 | 7 | 6 | >0.05 |
| ICU stay (d) | 15 | 17 | 13 | 10 | >0.05 |
| Mortality (n) | 9 | 2a | 4 | 10b | ≤0.01 |
| APACHE II (SD) | 18 (9) | 10 (8)a | 21 (13)b | 24 (10)b | ≤0.01 |
| Sepsis source (n) | |||||
| Abdominal | 0 | 2 | 3 | 5 | >0.05 |
| Respiratory | 0 | 2 | 4 | 3 | >0.05 |
| Surgical cut | 0 | 2 | 1 | 1 | >0.05 |
| Urosepsis | 0 | 1 | 2 | 1 | >0.05 |
| Skin and soft tissue | 0 | 2 | 0 | 1 | >0.05 |
Age, race, sex, ICU length of stay, mortality, APACHE II and sepsis source are quoted as mean (SD) or number (n).
p ≤ 0.01 versus nonsepsis group;
p ≤ 0.01 versus sepsis group.
Drugs
The GRPR antagonist RC-3095, originally synthesized in the laboratory of one of us (A V Schally) by solid-phase methods (27), was obtained from Aeterna Zentaris (Frankfurt am Main, Germany) and is a highly specific inhibitor of the GRPR (20).
RAW 264.7 Experiments
Preparations and treatments
RAW 264.7 macrophages were obtained from UFRJ Cell Bank (Rio de Janeiro, Brazil). To prepare for reverse-transcription polymerase chain reaction (RT-PCR) analysis of TLR-4 mRNA, immunoblotting of phosphorylated extracellular-signal-regulated kinases (pERKs) 1/2, pJNK, phosphorylated Akt (pAkt), electrophoretic mobility shift assay (EMSA) of NF-κB and AP-1, and cytokines, the cells were seeded in 24-well plates (0.5 × 106 cells/well) and incubated for 24 h in RPMI-1640 media supplemented with 10% bovine fetal serum. Cultures were exposed to LPS (Escherichia coli 055:B5; Sigma Aldrich, St. Louis, MO, USA) (100 ng/mL) supplemented medium (RPMI-1640) or RPMI-1640 alone; 4 h later, RC-3095 (10 ng/mL) was added for 2 h. Times after the treatment period (to 60 min, depending on the experiment set [see figure legends]), cells and/or media samples were collected for analysis in RC-3095–free media. In some experiments, cells were exposed to TNF-α (Sigma-Aldrich) supplemented medium (RPMI-1640) or RPMI alone, and 4 h later, RC-3095 (10 ng/mL) was added for 2 h. Cells and/or samples of the medium was collected for later analysis.
In Silico Analysis
Interaction networks of compounds and genes/proteins
To develop a model network for the interaction between LPS and RC-3095, different genes/proteins involved in LPS-activated and RC-3095 pathways were selected; and then, by using STITCH 2.0 (28,29), we screened the possible protein–protein and protein–compound interactions on the basis of experimental knowledge and the database (confident score 0.7, medium). A list with gene symbols and Ensembl protein IDs is provided (Supplementary Table S1). The network connected 45 proteins to LPS and RC-3095, on the basis of their possible actions with each other (“binding” and “reaction”) and different types of associations (“experiments,” “homology,” “databases” and “textmining”) between each component.
Measurements
TLR-4 mRNA analysis
RNA was extracted from RAW 264.7 or lungs by using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration was determined by absorbance at 260 nm, and RNA integrity was confirmed by electrophoresis on 1% agarose gels and staining with 0.1 mg/L ethidium bromide. After DNAse I digestion of the total extracted RNA, 1-μg aliquots of RNA were used to synthesize single-strand cDNA by using oligo dT primer (Promega, Madison, WI, USA) and Impron II reverse transcriptase (Promega). PCR was carried out with 1.0 μL RT product serving as the template. The amplified products were analyzed by ethidium bromide–stained agarose gel electrophoresis. The PCR primer sequences for TLR-4 were 5′ GGCAG CAGGT GGAAT TGTAT 3′ (sense) and 5′ AGGCC CCAGA GTTTT GTTCT 3′ (antisense) and 5′ AAGAG CTGGA ATACC TGGAC 3′ (sense) and 5′ GAAAT GCTAC AGTGG CTACC 3′ (antisense) for cell extracts. The gel was analyzed using Gene Flash and Software Image Gene Tools (Syngene). TLR-4 mRNA levels were expressed as the ratio of signal intensity for the target genes in relation to that for coamplified glyceraldehyde-3-phosphate dehydrogenase.
NF-κB or AP-1 content
The protein content of RAW 264.7 cell nuclear extracts was prepared as described previously (30). EMSA was performed using biotin-labeled oligonucleotides to measure NF-κB or AP-1 content according to the kit manufacturer’s protocol (Pierce).
Phosphorylation of ERK1/2, JNK and Akt
Phosphorylation was measured by Western blotting, wherein whole cell lysates (20 μg for pERK1/2, phospho-JNK and phospho-Akt) were separated by 10% sodium dodecyl sulfate–polyacry-lamide gel electrophoresis (SDS-PAGE) and then electro-transferred to nitrocellu-lose membranes (Amersham International, Buckinghamshire, UK). The membranes were pre-incubated for 1 h at room temperature in Tris-buffered saline (pH 7.6) containing 0.05% Tween 20 and 3% bovine serum albumin. The nitrocellulose membranes were incubated with pERK1/2, phosphorylated JNK and phosphorylated Akt, and the immunore-active bands were detected by incubation with HRP conjugates of anti-rabbit immunoglobulin G and enhanced with chemiluminescence reagents (Amersham).
Cytokine/chemokine level determination
The concentrations of IL-6, IL-10 and monocyte chemotactic protein (MCP)-1 were determined by a standard sandwich enzyme-linked immunosorbent assay (ELISA), by using commercially available kits (R&D Systems, Minneapolis, MN, USA).
TLR-4, NF-κB and MyD88 levels
Protein content of TLR-4, NF-κB and MyD88 was also quantified by immunoblotting. The samples or nuclear extracts (to determine NF-κB) were pooled, minced coarsely and homogenized in extraction buffer (1% Triton-X, 100 mmol/L Tris, pH 7.4, containing 100 mmol/L sodium pyrophosphate, 100 mmol/L sodium fluoride, 10 mmol/L ethylenediamine-tetraacetic acid [EDTA], 10 mmol/L sodium vanadate, 2 mmol/L phenyl-methylsulfonyl fluoride [PMSF] and 0.1 mg aprotinin/mL) at 4°C. The extracts were centrifuged at 8,000g and 4°C for 40 min, and the supernatants were used for protein quantification (31). Extracted proteins were denatured by boiling in Laemmli (32) sample buffer containing 100 mmol/L dithiothreitol; run on SDS-PAGE and transferred to nitro-cellulose membranes and blocked; probed with anti-TLR, anti-p65 and anti-MyD88 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and developed (33). The blots were exposed to preflashed Kodak XAR film with Cronex Lightning Plus intensifying screens at −80°C for 12–48 h. Band intensities were quantitated by optical densitometry (Scion Image Software; Scion Corporation, Frederick, MD, USA) of the developed autoradiographs.
Neutrophil migration in BALF
Migrating neutrophil cell counts in the BALF were performed as previously described (34). The results are expressed as the number of cells/mm3.
Bacterial counts in the peritoneal exudate and blood
Bacterial count was determined as previously described (35). Briefly, after peritoneal lavage with sterile phosphate-buffered saline and blood collection, aliquots of serial dilutions of the samples were plated on Mueller-Hinton agar dishes (Difco; BD, Franklin Lakes, NJ, USA) and incubated at 37°C; colony-forming units (CFUs) were counted after 24 h. The results were expressed as CFU/mL.
Plasma GRP concentrations
Plasma GRP was determined by ELISA commercial assays according to the manufacturer’s instructions (Phoenix Pharmaceuticals, Burlingame, CA, USA).
Statistical Analysis
Results are expressed as means ± standard deviation (SD). Cell culture results represent data from three different experiments with three samples in each experiment. Because of the low number of animals/cell samples in each experiment, analyses were performed using the Kruskal-Wallis test or Wilcoxon test depending on the number of groups. In the human experiments, demographic and clinical characteristics of the study groups were compared by t test, chi-square test or analysis of variance (one-or two-way), followed by a Tukey or Bonferroni post hoc test, as appropriate. A two-sided significance level of ≤0.05 was considered statistically significant. Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores and sepsis severity were entered in the Cox regression analysis to estimate the independent association of each covariate with GRP levels. We could not enter more variables in the regression because of the low number of events. Survival curves were constructed by the Kaplan-Meier method and compared with the log-rank test. The accuracy of GRP concentrations in distinguishing between survivors and non-survivors was examined separately by receiver operator characteristic (ROC) curves. All statistical analyses were performed with SPSS 17.0 for Windows (SPSS, Chicago, IL, USA).
All supplementary materials are available online at www.molmed.org.
RESULTS
GRPR Antagonist RC-3095 Inhibits Expression of TLR-4 and Constituent Molecules of Its Signaling Pathway and Decreases Cytokine/Chemokine Secretion in LPS-Stimulated RAW 264.7 Cells
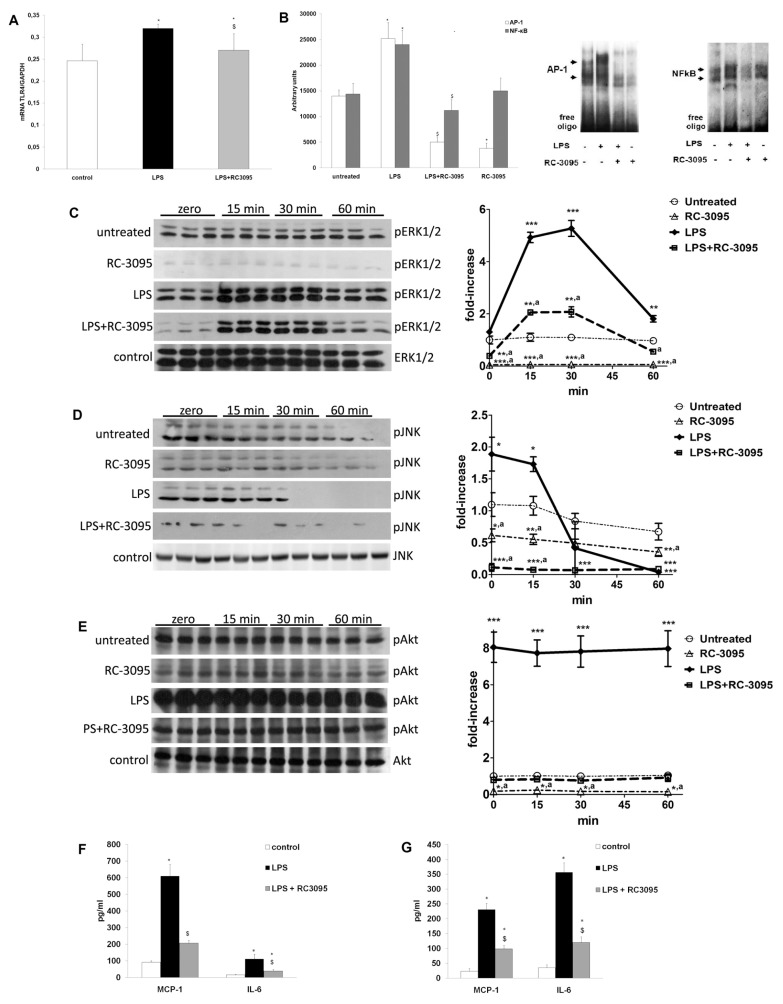
RT-PCR experiments in RAW 264.7 cultures revealed that RC-3095 significantly reduced TLR-4 mRNA levels in macrophages after LPS exposure (Figure 1A, F = 16.4, p = 0.001). Subsequent experiments with EMSA showed that the nuclear extract from LPS-stimulated RAW 264.7 cells had a significant increase in the DNA-binding activity of NF-κB and AP-1 (Figure 1B; F = 235, p < 0.001, to AP-1 and F = 85, p < 0.001, to NF-κB). However, this binding activity was suppressed by exposure to RC-3095, suggesting that suppression of NF-κB and nuclear translocation of AP-1 by RC-3095 was associated with decreased gene expression of TLR-4 and MAP kinase activation (Figure 1C, F = 54 [group versus time], F = 442 [group versus result], F = 135 [time versus result], p < 0.001 to all interactions; Figure 1D, F = 7.6 [group versus time], F = 31 [group versus result], F = 17 [time versus result], p < 0.001 to all interactions; and Figure 1E, F = 0.04, p = 1.0 [group versus time], F = 277 [group versus result], p < 0.001, F = 0.04 [time versus result], p = 1.0). ELISAs revealed elevated MCP-1 and IL-6 levels in RAW 264.7 and peritoneal macrophages exposed to LPS (Figure 1F, F = 1,119, p < 0.001, and Figure 1G, F = 55, p < 0.001) relative to unexposed control cells. Administration of RC-3095 resulted in a significant decrease in MCP-1 and IL-6 titers compared with the corresponding levels in LPS-exposed cells.
Figure 1.
RC-3095 decreased TLR4 mRNA expression, signaling pathways and cytokine/chemokine expression in RAW 264.7 cells exposed to LPS. Cell cultures were exposed to LPS (100 ng/mL) and, 4 h later, RC-3095 (10 ng/mL) was added for 2 h. Several times after this period, cells and/or media samples were collected for analysis in RC-3095–free media. (A) RT-PCR, using specific primers to TLR-4, demonstrated that 2-h RC-3095 treatment of LPS-activated RAW 264.7 cells (collected 30 min after RC-3095 treatment) reduced TLR-4 mRNA levels (expressed as the ratio of signal intensity to that of coamplified GAPDH: TLR-4 mRNA/GPDH) (*p < 0.05 versus control and $p < 0.05 versus LPS without RC-3095). (B) RAW 264.7 macrophages were stimulated with LPS; and 30 min after RC-3095 was added, LPS increased NF-κB and AP-1 DNA-binding activity measured by EMSA; and this was attenuated by RC-3095 treatment (*p < 0.05 versus untreated, $p < 0.05 versus LPS). (C–E) Western blot experiments showed that RC-3095 treatment also resulted in a sustained (0–60 min) reduction of pERK1/2, phosphorylated JNK and phosphorylated Akt levels (*p < 0.05, **p < 0.001 and ***p < 0.0001 versus untreated; ap < 0.05 versus LPS). (F) ELISA showed that RC-3095 attenuated MCP-1 and IL-6 increases induced by LPS stimulation in RAW 264.7 cells (G) and in peritoneal macrophages 30 min after the end of treatment (*p < 0.05 versus control and $p < 0.05 versus LPS).
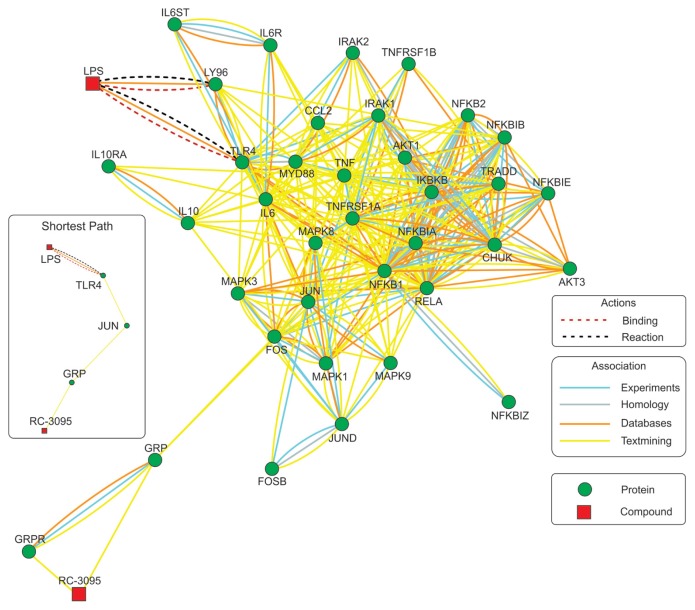
Since the blockade of GRP signaling altered the activation of several different in-tracellular kinases associated with TLR4 activation, we performed an in silico analysis on the interaction of GRP and TLR4 signaling. This analysis gave rise to a network that interconnected 45 genes/proteins with RC-3095 and LPS (Supplementary Table S1). On the basis of experimental data, database and textmining relationships, the RC-3095/LPS network shows the interactions between the components of cell signaling pathways triggered these components (Figure 2). Our analysis shows direct interaction of RC-3095 only with GRPR and GRP, and LPS is connected with the network at first level by interaction with TLR4 and the lymphocyte antigen 96 (LY96). The shortest path linking RC-3095 to LPS connects both GRP and TLR4 to JUN (jun proto-oncogene), which suggests JNK as the first upstream point in the cross-talk between GRP and TLR4 signaling and indicates that effects of RC-3095 on TLR4 activation are mainly secondary to JNK inhibition. Besides, the cross-talk between these two pathways is evidenced by interactions at downstream levels. Components common to both pathways include proinflammatory components (such as IL10, TNF, IL6ST and IL6R), members of the MAPK pathway (such as MAPK3 and MAPK8) and NF-κB and AP-1–related components (such as RELA, IKBKB and FOS), which are connected at several levels to components directly linked to GRP and TLR4.
Figure 2.
Model for RC-3095 and LPS interactions through signaling pathways of common cellular components. In silico analyses on the basis of the experimental data and database are depicted as action-and association-type interactions. This interaction network resulted from the interaction of 45 genes/proteins (Supplementary Table S1) with RC-3095 and LPS (confident score 0.7, medium). The shortest association path between RC-3095 and LPS is shown in the insert.
RC-3095 Inhibits Expression of TLR-4 and Nuclear Content of p65 in the Lung in an Animal Model of Polymicrobial Sepsis
RT-PCR using TLR-4–specific primers demonstrated high levels of TLR-4 mRNA expression in lung tissue 6 h after sepsis and significantly reduced expression of TLR-4 mRNA in RC-3095–treated animals relative to that in the sepsis group (Figure 3A, F = 130, p < 0.001). Immunoblotting experiments showed that the decreased mRNA levels in the lung were followed by decreased TLR-4 protein levels (Figure 3B, F = 100, p < 0.001) and nuclear content of p65 (Figure 3C, F = 129, p < 0.001), but not significant differences in MyD88 (Figure 3D, F = 3, p = 0.07). Thus, pharmacological blockade of the GRP-GRPR system decreased TLR-4 expression and protein content both in vitro and in vivo.
Figure 3.
Acute administration of RC-3095 decreased TLR4 mRNA expression, TLR4 and nuclear content of p65 level in lung in rats subject to lethal sepsis. Male Wistar rats were subjected to CLP and were divided (n = 5 per group) into sham-operated, CLP and CLP plus RC-3095 groups. RC-3095 (3 mg/kg subcutaneously) was administered immediately after surgery. Six hours after surgery, BALF was collected and lung tissue was removed. (A) RT-PCR experiments showed that TLR-4 mRNA levels (reported as TLR-4 mRNA/GPDH) were increased in CLP rats (*p < 0.05 versus sham), and this was reversed in CLP rats treated with RC-3095 ($p < 0.05 versus CLP). (B–D) Immunoblot experiments showed that CLP produced increased TLR-4 and nuclear content of p65 protein levels in lung tissue extracts (*p < 0.05 versus sham) and that RC-3095 treatment decreased this increase ($p < 0.05 versus CLP) but did not show significant differences in MyD88. Scanning densitometry results are expressed as arbitrary units. Bars represent means ± SD of five rats. IB, immunoblot.
RC-3095 Decreases Cytokine/Chemokine Content in an Animal Model of Polymicrobial Sepsis, Cell Migration to the Lung and Bacterial Dissemination
ELISAs revealed elevated MCP-1 and IL-6 levels in the serum and BALF of CLP septic rats (Figure 4A, F = 249, p < 0.001, to MCP-1 and F = 68, p < 0.001, and Figure 4B, F = 155, p < 0.001, to MCP-1 and F = 417, p < 0.001, to IL-6), relative to sham control rats. Administration of RC-3095 resulted in a significant decrease in MCP-1 and IL-6 titers compared with CLP septic rats. In addition, RC-3095 decreased the number of leukocytes in the BALF of CLP animals compared with those in untreated CLP animals (Figure 4C, F = 162, p < 0.001), but maintained the control of infection, since there was a reduced bacterial dissemination in circulation and in peritoneal exudates compared with levels in untreated CLP animals (data not shown).
Figure 4.
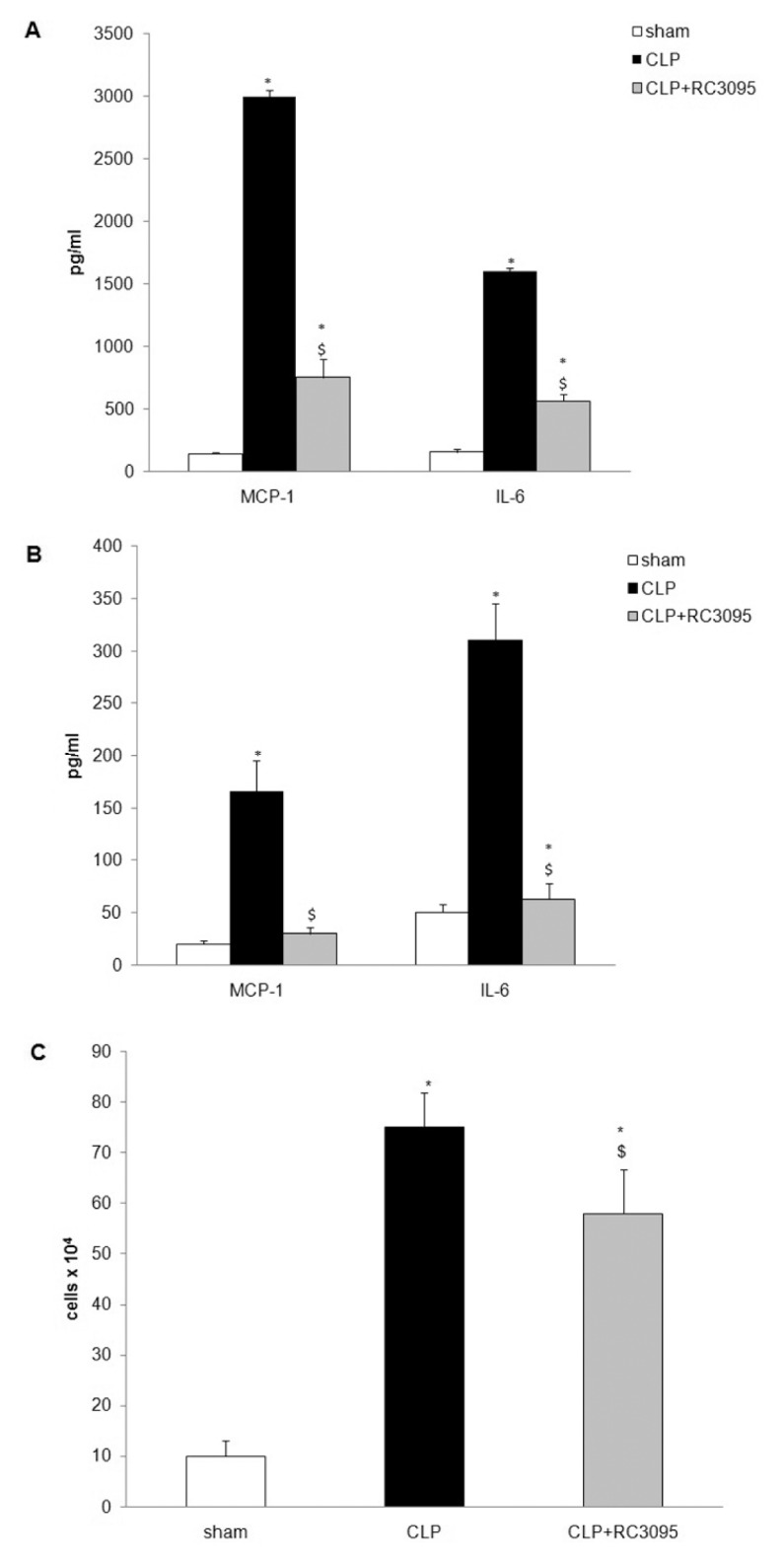
RC-3095 attenuated cytokine/chemokine production and cell inflammatory migration in lung of CLP animals. Male Wistar rats were subjected to CLP and were divided (n = 5 per group) into sham-operated, CLP and CLP plus RC-3095 groups. RC- 3095 (3 mg/kg subcutaneously) was administered immediately after surgery. Six hours after surgery, BALF and serum were collected. (A) ELISA showed that RC-3095 attenuated MCP-1 and IL-6 increases induced by CLP in serum (B) and BALF. Results are expressed as means ± SD (*p < 0.05 versus sham and $p < 0.05 versus CLP). (C) Inflammatory cell migration in the BALF was decreased in RC-3095–treated animals. Results are expressed as means ± SD (*p < 0.05 versus saline and $p < 0.05 versus CLP).
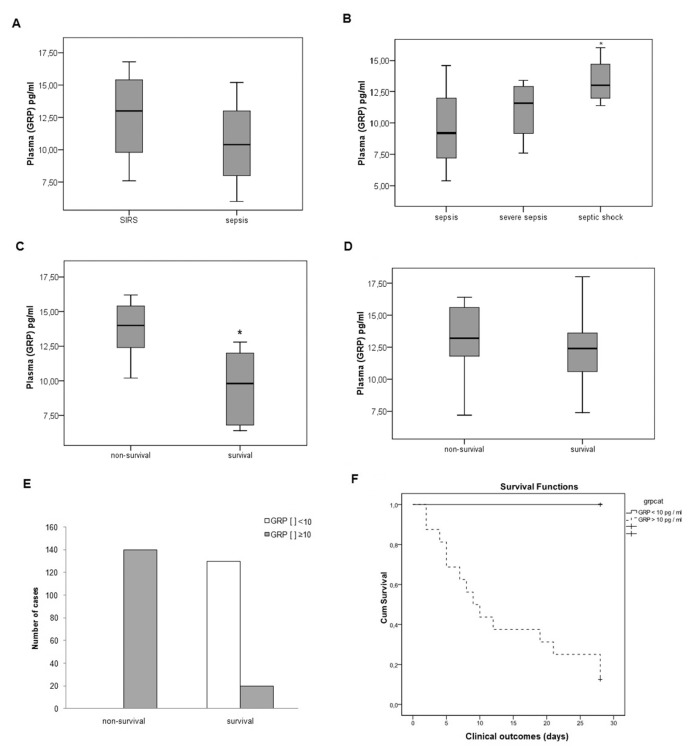
Plasma GRP Levels Can Be Related to Outcome in Septic Patients
The clinical profiles of sepsis patients at all levels of severity (n = 30) were compared with levels of patients with SIRS (n = 29). The data were further analyzed for differences among sepsis patients according to disease severity: sepsis (n = 9), severe sepsis (n = 10) and septic shock (n = 11) patients. The patient groups were similar in terms of race, age, sex, ICU length of stay, sepsis source and SOFA score (data not shown). The median APACHE II score of the mild-to-moderate sepsis group was lower than the scores of the septic shock, severe sepsis and SIRS groups (Table 1). Plasma GRP concentrations, sampled on the patients’ first day in ICU, were similar between the SIRS patients and sepsis patients (sepsis, severe sepsis and septic shock combined; Figure 5A, t = 1.5, p = 0.12), but higher when compared with healthy individuals (4.1 + 1.8 pg/mL, p = 0.02). Comparing patients across levels of sepsis severity, we found that patients with septic shock had greater GRP concentrations than patients with sepsis or severe sepsis (Figure 5B, F = 4.6, p = 0.019). Clinical outcome measures revealed that subjects with the highest GRP concentrations had the highest mortality of the sepsis groups (Figure 5C, t = 5.8, p < 0.001); this association was not apparent in patients with SIRS (Figure 5D, t = 0.24, p = 0.8). Patients with a GRP concentration <10 pg/mL showed no mortality, whereas patients with a GRP concentration ≥10 pg/mL showed a mortality rate of approximately 87% (Figure 5E, χ2 = 22, p < 0.001, and Figure 5, χ2 = 4.7, p < 0.02), with an area under the ROC curve of 0.85. This cutoff value presented a sensitivity of 100% and a specificity of 86%. In the Cox regression analyses, GRP level is not independently associated with outcome only in the septic patients (p = 0.68), but it was independently associated with mortality when including SIRS and septic patients in the regression (p = 0.021).
Figure 5.
GRP plasma levels were elevated in human patients diagnosed with sepsis and correlated with outcome. Patients consecutively admitted to adult medical ICU with SIRS (n = 29) or sepsis diagnosis (n = 30) were screened for enrollment. Septic patients were classified as sepsis, severe sepsis or septic shock, and blood was collected in these patients at ICU admission to determine GRP level by ELISA. (A) When severity of sepsis was not determined, no difference between GRP levels was observed between SIRS and sepsis patients (p < 0.05). (B) Comparison between the severity levels of sepsis (sepsis, severe sepsis and septic shock) revealed greater plasma GRP levels in the septic shock group relative to the other two groups (*p < 0.05). (C) Plasma GRP concentrations differed between sepsis patients who survived and those who did not (*p < 0.01). (D) Plasma GRP concentrations were similar, however, between the survivors and nonsurvivors in the SIRS patient group. (E, F) GRP concentration served as an effective predictor of mortality in septic patients; a GRP concentration below a cutoff value of 10 pg/mL was associated with greater incidence of survival (p < 0.01 versus nonsurvival).
RC-3095 Decreases Plasma IL-6 Levels in Septic Patients
Continuous infusion of RC-3095 (3 mg/kg) for 12 h decreased plasma levels of IL-6 in septic patients (Figure 6A, t = 5.4, p ≤ 0.001), but did not significantly affect plasma levels of IL-10 (Figure 6B, t = 1.9, p = 0.07).
Figure 6.
RC-3095 attenuated plasma IL-6 levels in sepsis patients. Twelve patients with a clinical diagnosis of septic shock and failure of three or more organs received a continuous infusion with RC-3095 (3 mg/kg) over a period of 12 h, and plasma samples were collected before and after RC-3095 administration. The 11 patients who did not receive RC-3095 were included as controls. (A, B) ELISA showed that 12-h infusion of RC-3095 resulted in decreased levels of IL-6 but not IL-10, respectively (*p < 0.05 for T12 versus T0 RC-3095–treated group; $p < 0.05 for T12 sepsis versus T12 sepsis + RC-3095 [with “T0” indicating “before RC-3095 administration” and “T12” “after RC-3095 administration.”].
RC-3095 Effects on TLR-4 Independent Inflammatory Pathways
Because some of the effects mediated by RC-3095 could be mediated by pathways independent of TLR-4 activation, we determined the effects of GRPR antagonism on TNF-α–stimulated RAW 264.7. Treatment with RC-3095 resulted in a significant decrease in IL-6 titers compared with the corresponding levels in TNF-α–stimulated RAW 264.7 (Figure 7, F = 11.16, p < 0.001), suggesting that the effects of RC-3095 was not solely related to the inhibition of TLR-4 signaling.
Figure 7.
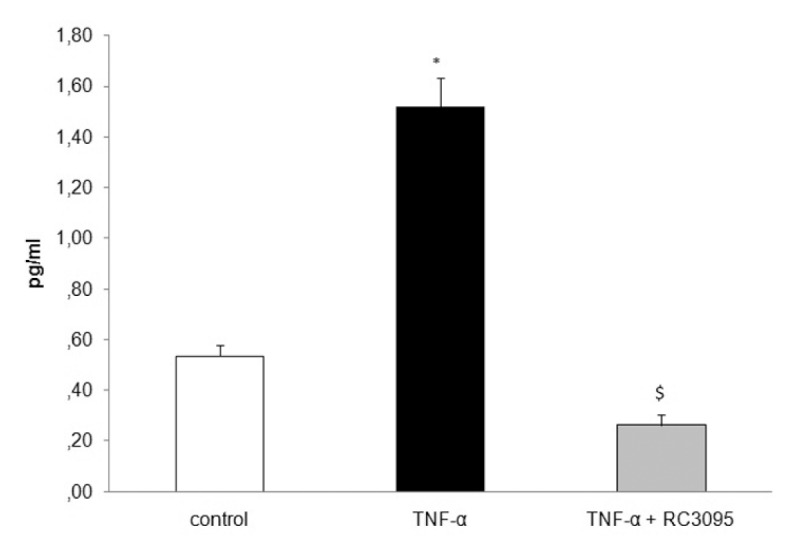
RC-3095 effects were not solely related to the inhibition of TLR-4 signaling. Cells were exposed to TNF-α (Sigma-Aldrich) 4 h later; RC-3095 (10 ng/mL) was added for 2 h. Samples from the medium were collected to determine IL-6 levels. RC-3095 treatment resulted in a significant decrease in IL-6 levels compared with the corresponding levels in TNF-α–stimulated RAW 264.7 (*p < 0.05 versus control and $p < 0.05 versus TNF-α).
DISCUSSION
In the present study, we demonstrated that treatment with RC-3095 can decrease TLR-4 expression and downstream signaling activation in RAW 264.7 cells stimulated by LPS and TNF-α, leading to a decrease in chemokines and cytokines release, probably by inhibition of JNK signaling. These results were supported by our in vivo experiments that showed lower IL-6 and MCP-1 concentrations in RC-3095–treated CLP animals. Furthermore, we showed that treatment with RC-3095 decreased levels of inflammatory cells in BALF, systemic circulation and peritoneal exudate of CLP animals. Our results indicate that administration of RC-3095 limited the spread of infection beyond the abdominal compartment, suggesting that RC-3095 could potentially prevent the development of the multiple organ dysfunction syndrome (36).
There are numerous factors that interact in the long chain of events from pathogen recognition to the diversity of host responses (37,38). Our findings provide support for the notion that TLR-4 is a particularly important element of host defense modulated by GRP during sepsis (23,38). This view is strongly supported by prior research showing that TLR-4–defective mice do not exhibit failure of neutrophil migration to the peritoneal cavity during polymicrobial sepsis induced by lethal CLP and, as consequence, are more resistant to sepsis than controls (11). Furthermore, increased concentration of mRNA for TLR-4 in lung tissue 3 h after CLP surgery has been shown to precede and correlate with death (39). In fact, we observed a huge decrease on TLR4 mRNA and a slight reduction on protein levels, suggesting that posttranslational mechanisms that can eventually modulate TLR-4 levels are not affected by RC-3095. This is of major relevance because, although the complete lack of TLR-4 signaling is beneficial in polymicrobial sepsis, it can have detrimental effects on the basal immune response to gram-negative bacteria (11); thus, the results presented here seem to be of greater clinical significance.
It is well established that immune responses may be influenced by the nervous system (40). Studies support that neuropeptides, which regulate the macrophage response to LPS, affect TLR-4 expression (41) and regulate TLR-4 signaling (42). In this context, and because activated macrophages have been shown to secrete GRP (43) and macrophages seem to be central in the development of sepsis and septic shock (44), we observed a decrease in the expression of TLR-4 mRNA in RAW 264.7 cells stimulated by LPS after treatment with RC-3095. Our findings are consistent with recent reports that increased expression of TLR-2 and TLR-4 during the early phase of sepsis correlates with death in CLP animals (39) and that the downregulation of these receptors increases survival (11). Furthermore, our observation that RC-3095 inhibits upregulation of TLR-4 in polymicrobial sepsis in lung tissue 6 h after CLP, leading to a diminution of lung inflammation, fits with prior research indicating that GRP is present in pulmonary neuroendocrine cells and may be a mediator of acute and chronic lung injury in bronchopulmonary dysplasia (45). The findings also fit with the observation that GRPR antagonism can alleviate alveolar edema and inflammatory infiltration (22).
During endotoxic shock, a massive number of neutrophils and other leukocytes accumulate in the lung—a process entirely dependent on TLR-4. Leukocyte accumulation in the lung is also observed in humans with sepsis (46), where systemic activation of TLR-4 results in immense trapping of leukocytes within lung capillaries (47). One could argue that the effects of TLR-4 antagonists in sepsis will lead only to minor effects, since the TLR-4 activation is extremely rapid; thus, in the clinical scenario, it would already be activated by the time of drug administration. Our data suggest that, in the CLP, TLR4 is upregulated for long times after CLP; thus, although TLR4 activation is extremely rapid, the repeated (or continuous) activation of TLR4 in vivo (due to the delay of source control or antibiotics administration, for example) can be a target to drugs that downregulate TLR4 activation. This idea is supported by septic patient data that demonstrate an upregulation of several genes from the TLR4 pathway that persist in the different stages of sepsis development (48). Furthermore, neuropeptides are known to stimulate cytokine production in macrophages, lymphocytes and mast cells, and substance P is reported to influence LPS-induced production of proinflammatory cytokines, a mechanism that is abolished by neurokinin-1 (NK-1) receptor blocking (49). Arranz et al.(50) showed that proinflammatory cytokines can act synergistically, together with gram-negative bacterial components, to upregulate TLR-4 expression. Thus, it is possible that vasoactive intestinal peptide (VIP)-induced inhibition of TLR-4 upregulation in inflammatory models occurs indirectly via suppression of proinflammatory cytokine production (50). We propose that GRP may serve an autocrine/paracrine role in macrophage activation during sepsis and/or LPS stimulation, leading to a modulation of proinflammatory, but not antiinflammatory, responses (51). In addition, it was recently demonstrated that GRP can directly induce GRPR-mediated neutrophil migration (52); thus, complementary mechanisms of action can be achieved by the inhibition of GRPR, which can be beneficial in treating sepsis.
In addition, we can see that the pathway activated by TNF-α also appears to be associated with decreased proinflammatory response in severe sepsis caused by RC-3095 effects, since our findings show a decrease of IL-6 levels in TNF-α–stimulated cells when treated with RC-3095. The TNFR1/R2 pathways share signaling pathways of TLR-4, resulting in NF-κB activation (23,46). Thus, it was suggested that there is an interaction between GRPR and TLR-4 and TNFR1/R2 pathways, implicating some level of hierarchy or cooperation between these signaling pathways in the generation of inflammation during sepsis. In fact, it was previously demonstrated that there is an interaction between GRPR and CXCR2 (51), suggesting that GRPR can be a central modulator of immune responses during sepsis.
CONCLUSION
Our results indicate that the protective effect of GRPR antagonists can be attributed to an attenuation of TLR-4 or TNFR1/R2 signaling. This attenuation favors neutrophil infiltration, resulting in decreased bacteremia and thus improving sepsis outcome. Taken together, the present results suggest that a GRPR antagonist could be developed as a new alternative therapy for bacterial sepsis.
Supplemental Data
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
F Dal-Pizzol, R Roesler, and G Schwartsmann are inventors in a patent application on the use of GRP antagonists in the treatment of sepsis.
REFERENCES
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 4.Hedge A, Zhang H, Moochhala SM, Bhatia M. Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis. J Leukoc Biol. 2007;82:678–85. doi: 10.1189/jlb.0407217. [DOI] [PubMed] [Google Scholar]
- 5.Chishti AD, Shenton K, Kirby JA, Baudouin SV. Neutrophil chemotaxis and receptor expression in clinical septic shock. Intensive Care Med. 2004;30:605–11. doi: 10.1007/s00134-004-2175-y. [DOI] [PubMed] [Google Scholar]
- 6.Alves-Filho JC, et al. Regulation of chemokine receptor by toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A. 2009;106:4018–23. doi: 10.1073/pnas.0900196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secher T, et al. Crucial role of TNF receptors 1 and 2 in the control of polymicrobial sepsis. J Immunol. 2009;182:7855–64. doi: 10.4049/jimmunol.0804008. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama K, Muroi M, Tanamoto K. A novel TLR4-binding peptide that inhibits LPS induced activation of NF-KappaB and vivo toxicity. Eur J Pharmacol. 2008;10:152–6. doi: 10.1016/j.ejphar.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Tsung A, et al. A novel inhibitory peptide of toll-like receptor signaling limits lipopolysaccharide-induced production of inflammatory mediators and enhances survival in mice. Shock. 2007;27:364–9. doi: 10.1097/01.shk.0000239773.95280.2c. [DOI] [PubMed] [Google Scholar]
- 10.Hellmich MR, Ives KL, Udupi V. Multiple protein kinase pathways are involved in gastrin-releasing peptide receptor-regulated secretion. J Biol Chem. 1999;274:23901–9. doi: 10.1074/jbc.274.34.23901. [DOI] [PubMed] [Google Scholar]
- 11.Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34:461–70. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- 12.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27:166–7. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam M, et al. Bombesin-like peptides and mast cell responses: relevance to bronchopulmonary dysplasia? Am J Respir Crit Care Med. 2003;168:601–11. doi: 10.1164/rccm.200212-1434OC. [DOI] [PubMed] [Google Scholar]
- 14.Grimsholm O, Rantapaa-Dahlqvist S, Forsgren S. Levels of gastrin releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R416–26. doi: 10.1186/ar1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelio DB, Dal-Pizzol F, Roesler R, Schwartsmann G. Targeting the bombesin/gastrin-releasing peptide receptor to treat sepsis. Recent Pat Antiinfect Drug Discov. 2007;2:178–81. doi: 10.2174/157489107782497344. [DOI] [PubMed] [Google Scholar]
- 16.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roesler R, Henriques JA, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS Neurol Disord Drug Targets. 2006;5:197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- 18.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–22. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 19.Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253–8. doi: 10.1016/s0002-9610(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 20.Schwartsmann G, et al. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest New Drugs. 2006;24:403–12. doi: 10.1007/s10637-006-6886-5. [DOI] [PubMed] [Google Scholar]
- 21.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 22.Dal-Pizzol F, et al. Gastrin-releasing peptide receptor antagonist effects on an animal model of sepsis. Am J Respir Crit Care Med. 2006;173:84–90. doi: 10.1164/rccm.200507-1118OC. [DOI] [PubMed] [Google Scholar]
- 23.Ishii KJ, Akira S. Toll-like receptors and sepsis. Curr Infect Dis Rep. 2004;6:361–6. doi: 10.1007/s11908-004-0034-1. [DOI] [PubMed] [Google Scholar]
- 24.Ritter C, et al. Treatment with Nacetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care. 2004;32:342–9. doi: 10.1097/01.CCM.0000109454.13145.CA. [DOI] [PubMed] [Google Scholar]
- 25.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press; 2011. [cited 2012 Oct 12].Available from: http://oacu.od.nih.gov/regs/ [Google Scholar]
- 26.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–3. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 27.Radulovic S, et al. Biological effects and receptor binding affinities of new pseudononapeptide bombesin/GRP receptor antagonists with Nterminal d-Trp or d-Tpi. Int J Pept Protein Res. 1991;38:593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn M, von Mering C, Campillos M, Jensen LJ, Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36:D684–8. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn M, et al. STITCH 2: an interaction network database for small molecules and proteins. Nucleic Acids Res. 2010;38:D552–6. doi: 10.1093/nar/gkp937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang W, Alvarez-Gonzalez R. The sequence-specific DNA binding of NF-κB is reversibly regulated by the automodification reaction of poly (ADP-ribose) polymerase I. J Biol Chem. 2001;276:47664–70. doi: 10.1074/jbc.M104666200. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.De Souza CT, et al. Peroxisome proliferator-activated receptor gamma coactivator-1- dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia. 2003;46:1522–31. doi: 10.1007/s00125-003-1222-5. [DOI] [PubMed] [Google Scholar]
- 34.Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–23. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- 35.Godshall CJ, et al. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–9. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482–91. doi: 10.1016/j.tips.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Alves-Filho JC, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 38.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 39.Williams DL, et al. Modulation of tissue toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–18. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 40.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–64. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- 41.Tsatsanis C, et al. Corticotropin releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J Immunol. 2006;176:1869–77. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- 42.Taylor AW. The immunomodulating neuropeptide alpha-melanocytestimulating hormone (alpha-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J Neuroimmunol. 2005;162:43–50. doi: 10.1016/j.jneuroim.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253–8. doi: 10.1016/s0002-9610(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 44.Dal-Pizzol F. Alternative activated macrophage: a new key for systemic inflammatory response syndrome and sepsis treatment? Crit Care Med. 2004;32:1971–2. doi: 10.1097/01.ccm.0000139620.32448.12. [DOI] [PubMed] [Google Scholar]
- 45.Degan S, Lopez GY, Kevill K, Sunday ME. Gastrin-releasing peptide, immune responses, and lung disease. Ann N Y Acad Sci. 2008;1144:136–47. doi: 10.1196/annals.1418.022. [DOI] [PubMed] [Google Scholar]
- 46.Kerfoot SM, Kubes P. Local coordination verses systemic deregulation: complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. J Leukoc Biol. 2005;77:862–7. doi: 10.1189/jlb.1004607. [DOI] [PubMed] [Google Scholar]
- 47.Andonegui G, et al. Endothelium-derived toll-like receptor-4 is the key molecule in LPS- induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–20. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salomao R, et al. Toll-like receptor pathway signaling is differently regulated in neutrophils and peripheral mononuclear cells of patients with sepsis, severe sepsis, and septic shock. Crit Care Med. 2009;37:132–9. doi: 10.1097/CCM.0b013e318192fbaf. [DOI] [PubMed] [Google Scholar]
- 49.Dickerson C, Undem B, Bullock B, Winchurch RA. Neuropeptide regulation of proinflammatory cytokine responses. J Leukoc Biol. 1998;63:602–5. doi: 10.1002/jlb.63.5.602. [DOI] [PubMed] [Google Scholar]
- 50.Arranz A, et al. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970–80. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Petronilho F, Roesler R, Schwartsmann G, Dal Pizzol F. Gastrin-releasing peptide receptor as a molecular target for inflammatory diseases. Inflamm Allergy Drug Targets. 2007;6:197–206. doi: 10.2174/187152807783334319. [DOI] [PubMed] [Google Scholar]
- 52.Czepielewski RS, et al. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc Natl Acad Sci U S A. 2012;109:547–52. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.