Abstract
Glyoxalase detoxification system composed of glyoxalase (GLO)-I and GLO-II is ubiquitously expressed and implicated in the protection against cellular damage because of cytotoxic metabolites such as advanced glycation end products (AGEs). Recently, ovarian tissue has emerged as a new target of excessive AGE deposition and has been associated with either a high AGE diet in experimental animals or hyperandrogenic disorders such as polycystic ovarian syndrome (PCOS) in humans. This study was designed to investigate the impact of dietary AGEs and androgens in rat ovarian GLO-I activity of normal nonandrogenized (NAN, group A, n = 18) and androgenized prepubertal (AN) rats (group B, n = 29). Both groups were further randomly assigned, either to a high-AGE (HA) or low-AGE (LA) diet for 3 months. The activity of ovarian GLO-I was significantly reduced in normal NAN animals fed an HA diet compared with an LA diet (p = 0.006). Furthermore, GLO-I activity was markedly reduced in AN animals compared with NAN (p ≤ 0.001) when fed with the corresponding diet type. In addition, ovarian GLO-I activity was positively correlated with the body weight gain (rs = 0.533, p < 0.001), estradiol (rs = 0.326, p = 0.033) and progesterone levels (rs = 0.500, p < 0.001). A negative correlation was observed between GLO-I activity and AGE expression in the ovarian granulosa cell layer of all groups with marginal statistical significance (rs = −0.263, p = 0.07). The present data demonstrate that ovarian GLO-I activity may be regulated by dietary composition and androgen levels. Modification of ovarian GLO-I activity, observed for the first time in this androgenized prepubertal rat model, may present a contributing factor to the reproductive dysfunction characterizing PCOS.
INTRODUCTION
Glyoxalase (GLO)-I and GLO-II constitute an ubiquitous scavenging enzymatic system that, under physiological conditions, regulates cell growth and protects cells from damage caused by reactive 2-oxoaldehydes. Examples of reactive 2-oxoaldehydes include glyoxal and methylglyoxal, the major precursors of advanced glycation end products (AGEs) (1–3).
GLO-I, a cytosolic, 42-kDa, dimeric Zn2+ metalloenzyme, is an integral component of this detoxification system catalyzing the conversion of reactive, acyclic α-oxoaldehydes into the corresponding α-hydroxyacids in a glutathione-dependent manner (4). The specificity of GLO-I activity over α-oxoaldehyde decreases the steady-state concentrations of these cytotoxic metabolites and reduces the associated glycation reactions, thus representing a physiological enzymatic defense against glycation (5). Glycation of proteins, nucleotides and basic phospholipids by glyoxal and methylglyoxal is known to potentially damage proteome, genome and lipidome (4). Although GLO-I is ubiquitously expressed, little evidence is available regarding the regulation of this enzyme in human cells. Altered GLO-I activity was implicated in many disorders such as diabetes mellitus (6), Alzheimer’s disease (7) and cancer (8). Data from experimental in vivo model of diabetes have shown that GLO-I overexpression reduces hyperglycemia-induced levels of carbonyl stress, AGE levels and oxidative stress (9,10). Recently, the biochemical activity of GLO-I was found to be significantly decreased in the ovaries of reproductively aged mice in comparison to younger ones (11), implicating advanced glycation scavenging enzymes in the regulation of the female reproductive system regarding tissue specificity of AGE metabolism.
AGEs are known highly reactive molecules, formed from a nonenzymatic reaction of reducing sugars with proteins, lipids and nucleic acids (12). They may induce many structural and vascular changes in several tissues because of insoluble cross-link formation, induction of oxidative stress and subsequent cell activation (13,14). In addition, Westernized diets are considered an alternative important source of exogenously consumed AGEs, since thermally processed common foods have high glycotoxin content (15). Evidence of the last decade generated from data in humans and experimental animals support the contribution of exogenous food–ingested AGEs to elevated serum levels and increased tissue deposition (16–18).
Carboxymethyllysine (CML), being a reactive and widely measured AGE with cross-linking potential, is used as a representative marker of exogenous and endogenous origin of AGEs levels (15–17). In disorders with altered glucose metabolism, AGEs are accumulated in various tissues including the ovarian tissue, implicating their role in the pathogenesis of female reproductive abnormalities (19). A previous study from our group has shown increased AGE deposition in the ovaries of normal female rats fed a high-AGE diet that was further correlated with metabolic and hormonal disturbances (17). In addition, increased AGE localization was detected in the ovarian tissue of women with polycystic ovarian syndrome (PCOS), where elevated endogenous AGE levels were determined as possible pathogenic contributors, being associated both with insulin resistance indices and androgen levels (18,20,21). Moreover, Tatone et al.(11) demonstrated that increased AGE deposition in reproductively aged ovarian tissue was associated with reduced GLO-I activity.
In search of regulatory mechanisms affecting GLO-I activity, in vitro studies in breast and prostate cancer cell lines indicate the role of steroid hormones, particularly of estrogens and testosterone (22,23). Excessive androgen production presents a hallmark of PCOS pathology affecting reproductive system functionality (24).
Taking all these into account, the aim of the present study was to explore whether the activity of GLO-I in ovarian tissue may be altered by prolonged exposure to modified dietary glycotoxin content as well as by exposure to androgen excess.
MATERIALS AND METHODS
Animals
A total of 47 female Wistar rats were allocated to the study, further divided into two main groups. Group A was composed of nonandrogenized animals (NAN), which at 21 d of age, were subdivided randomly in two groups. Subgroup A1 (n = 9) was fed commercial chow high in AGE content (HA), while subgroup A2 (n = 9) was fed commercial chow low in AGE content (LA).
In group B, the animals under study were androgenized (AN) as previously described by Mannerås et al.(25). Briefly, at 21 d of age, the rats were implanted subcutaneously with 90-d continuous- release pellets containing 7.5 mg dihydrotestosterone (DHT) (daily dose 83 μg). The dose of DHT was chosen to mimic the hyperandrogenic state in women with PCOS, whose plasma DHT levels are ~1.7-fold higher than those of healthy controls. Group B was further randomly divided into 2 subgroups. Subgroup B1 (n = 14) were fed commercial chow HA, while subgroup B2 (n = 15) were fed commercial chow LA.
The animals were housed four to five per cage under controlled conditions (21–22°C, 55–65% humidity, 12-h light/12-h dark cycle) and were given pelleted food and water ad libitum at ELPEN (Experimental Research Centre, Athens, Greece). Animal care and experimental procedures conformed to the “Guide for the Care and Use of Laboratory Animals” (Department of Health, Education and Welfare, Athens, Greece) and were approved by the Institutional Animal Care and Use Committee.
The diets used were derived from a single standard rat chow (AIN-93G) purchased from Bioserve (Frenchtown, NJ, USA), consisting of 18% protein, 58% carbohydrate, 7.5% fat, and 3.73 kcal/g. Regular AIN-93G chow is normally prepared by heating at 190°C for 30 min. Analysis of this preparation was performed as previously described (17). Briefly, it contained 76.0 ± 15.3 mg CML/100 g sample (or 436.9 ± 88.1 mg CML/100 g protein), 205.32 ± 22.25 mg fructoselysine/100 g sample (or 1.179.98 ± 127.90 mg fructoselysine/100 g protein) and 52.68 ± 5.71 mg furosine/100 g sample (or 302.78 ± 32.82 mg furosine/100 g protein) and was considered as an HA diet.
The same rodent mix was also prepared without heating. This preparation was of equivalent macro- and micronutrient and energy content but contained 1.3 ± 0.4 mg CML/100 g sample (or 7.7 ± 2.2 mg CML/100 g protein), 104.58 ± 3.08 mg fructoselysine/100 g sample (or 601.01 ± 17.7 mg fructoselysine/100 g protein) and 26.83 ± 0.79 mg furosine/100 g sample (or 154.22 ± 4.54 mg furosine/100 g protein) and was considered an LA diet.
Body weight was monitored weekly. The study was concluded after 3 months, and rats were sacrificed with administration of 20 mg/mL xylazine hydrochloride and 100 mg/mL ketamine hydrochloride, under anesthesia with ether, allowing blood sample collection and tissue retrieval.
Preparation of Ovarian Homogenates
Ovaries were excised from rats and immediately frozen in liquid nitrogen after storage at −80°C. For the determination of GLO-I activity, tissues were thawed and homogenized. The homogenization was performed in 10 mmol/L phosphate buffer (pH 7.0) supplemented with 1 mmol/L dithiothreitol (DTT) with a Potter-Elvehjem homogenizer. The homogenates were centrifuged for 60 min (4°C) at 20,000g, and the supernatants were recovered and used for enzymatic analysis. The protein content was determined according to the Lowry method.
Determination of GLO-I Activity
GLO-I activity was assayed according to Tatone et al.(11). Specifically, the assay solution contained 0.1 mol/L monobasic potassium phosphate (pH 7.2), 2 mmol/L methylglyoxal and 1 mmol/L reduced glutathione (GSH). A total of 30–50 μL of the sample was used for the reaction, which was monitored spectrophotometrically by following the formation of S-d-lactoylglutathione at 240 nm and 25°C. One unit was defined as 1 μmol S-d-lactoylglutathione produced per minute, and the activity was expressed as mU/mg protein.
Biochemical and Hormonal Assays
Rat estradiol (E2) and testosterone were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Calbiotech, CA, USA). Insulin and progesterone (PGR) were also quantified using ELISA immunoassays purchased by Biovendor Laboratory Medicine (Czech Republic) and Neogen Corporation (Lexington, KY, USA), respectively. Serum AGE levels (U/mL) were measured by CML-specific competitive ELISA, as described previously (21).
Immunohistochemical Analysis
Paraffin-embedded sections of formalin-fixed ovarian tissue were deparaffinized by xylene and dehydrated in graded ethanol. Sections were treated in 3% hydrogen peroxide in phosphate-buffered saline (PBS) for 15 min and then rinsed in PBS. To increase the immunoreactivity of AGEs, the sections were placed in 500 mL of 0.01 mol/L citric acid–buffered solution (pH 7.0) and microwaved at 500 W for 5 min. After thorough washing, the sections were incubated with normal rabbit serum for 20 min at room temperature to avoid nonspecific binding of the antibodies. The sections were then incubated overnight at 4°C with the anti-AGE monoclonal antibody 6D12 (0.25 mg/mL stock, dilution 1:50; Research Diagnostics, Concord, MA, USA) in PBS containing 1% bovine serum albumin. Immunoreactivity was detected by the streptavidin–biotin–peroxidase method according to the manufacturer’s protocol. The final reaction product was visualized with 3,3′-diaminobenzidine tetrahy-drochloride (LSAB detection kit; Dako, Carpentaria, CA, USA). Lung tissue sections from diabetic rats were used as positive controls for AGE antibody. Negative controls (for example, ovarian tissue in which the primary antibody was substituted with nonimmune mouse or goat serum) were also stained in each run. The percentage of positive cells was estimated using light microscopy. The evaluation of the immunostained slides was performed blindly and independently by two pathologists. AGE expression was categorized in four levels according to the percentage of positive cells, as follows: minimum (staining 1–10% of cells), low (staining 11–30% of cells), moderate (31–60% of cells) and extensive (>60% of cells) immunostaining. The staining intensity was also assessed in four levels: 1, very weak; 2, weak; 3, moderate; and 4, strong.
Statistical Analyses
The data are expressed as means ± standard error of the mean (SEM), and data analysis was performed using the SPSS 18.0 software for windows. Two-way analysis of variance was used for comparison between parameters, and when p < 0.05, the least significance difference test was used to analyze differences among groups. The Spearman rank correlation test was performed to examine the associations between parameters tested. Statistically significant difference was defined as a p value <0.05.
RESULTS
Somatometric, Metabolic and Hormonal Parameters
To evaluate the impact of dietary AGEs and excessive androgen levels in ovarian GLO-I activity, normal and androgenized prepubertal female rats were evaluated after a 3-month allocation to a specific AGE content diet. Somatometric, metabolic and hormonal parameters (mean ± SEM values) of both groups are described in Table 1, and the respective levels of statistical significance are given in Table 2.
Table 1.
Somatometric, metabolic, hormonal and biochemical parameters in the rat groups.
| NAN-LA | NAN-HA | AN-LA | AN-HA | |
|---|---|---|---|---|
| Body weight | ||||
| Baseline | 77.6667 ± 4.52769 | 83.6667 ± 6.66041 | 99.6667 ± 2.69332 | 95.3571 ± 2.64494 |
| 3 months | 230.7778 ± 5.93431 | 183.5556 ± 12.31430 | 207.0000 ± 8.62996 | 170.7143 ± 9.69107 |
| Body weight gain (%) | 206.55 ± 22.246 | 130.08 ± 22.156 | 110.02 ± 10.516 | 78.71 ± 08.34 |
| Ovarian weight (g) | 0.17189 ± 0.004165 | 1.03333 ± 0.096695 | 0.46000 ± 0.093279 | 1.08646 ± 0.099233 |
| Glucose (mg/dL) | 103.0000 ± 5.21217 | 136.0000 ± 10.64712 | 116.6860 ± 8.76483 | 89.3057 ± 10.04623 |
| Insulin (μIU/mL) | 0.93921 ± 0.131461 | 2.17222 ± 0.254966 | 1.10533 ± 0.073322 | 1.46643 ± 0.102762 |
| Testosterone (ng/mL) | 0.1667 ± 0.01027 | 0.3244 ± 0.02180 | 0.1867 ± 0.01799 | 0.2973 ± 0.02338 |
| E2 (pmol/L) | 19.4113 ± 1.57725 | 10.7150 ± 0.53360 | 15.4329 ± 2.62500 | 9.4543 ± 1.12919 |
| PGR (pmol/L) | 36.4175 ± 2.71724 | 15.1288 ± 2.11061 | 19.3673 ± 1.66433 | 7.8514 ± 0.82163 |
| AGEs (U/mL) | 4.76700 ± 0.319032 | 7.03233 ± 0.288260 | 5.21900 ± 0.208535 | 6.96407 ± 0.350534 |
| GLO-I activity (mU/mg protein) | 369.3300 ± 13.92377 | 284.4322 ± 22.66756 | 171.3453 ± 23.43073 | 161.7169 ± 19.72893 |
| AGE immunostaining granulosa | 1.0000 ± 0.14434 | 2.0000 ± 0.14434 | 1.1333 ± 0.19190 | 2.1429 ± 0.20588 |
Data are means ± SEM.
Table 2.
Levels of statistical significance during the comparison of different parameters and groups.
| NAN-LA versus NAN-HA | NAN-LA versus AN-LA | NAN-HA versus AN-HA | AN-LA versus AN-HA | |
|---|---|---|---|---|
| Body Weight | ||||
| Baseline | 0.467 | <0.001 | 0.075 | 0.265 |
| 3 months | 0.003 | 0.063 | 0.420 | 0.009 |
| Body weight gain (%) | 0.002 | <0.001 | 0.020 | 0.098 |
| Ovarian weight (g) | <0.001 | 0.027 | 0.716 | <0.001 |
| Glucose (mg/dL) | 0.013 | 0.270 | 0.006 | 0.049 |
| Insulin (μIU/mL) | 0.001 | 0.243 | 0.008 | 0.007 |
| Testosterone (ng/mL) | <0.001 | 0.427 | 0.435 | 0.001 |
| E2 (pmol/L) | <0.001 | 0.295 | 0.429 | 0.046 |
| PGR (pmol/L) | <0.001 | <0.001 | 0.001 | <0.001 |
| AGEs (U/mL) | <0.001 | 0.228 | 0.892 | <0.001 |
| GLO-I activity (mU/mg protein) | 0.006 | <0.001 | 0.001 | 0.760 |
| AGE immunostaining granulosa | <0.001 | 0.630 | 0.619 | 0.001 |
Data were considered significant at p ≤ 0.05.
Body weight after the 3 months of feeding was significantly different between NAN-LA– and NAN-HA–fed mice (p = 0.003), and the weight gain was calculated as a percentage (%) increase. Significant difference was observed between LA- and HA-fed rats for ovarian weight (p < 0.001), fasting glucose (p = 0.013), insulin (p = 0.001), serum AGEs (p < 0.001), testosterone (p < 0.001), E2 (p < 0.001) and PGR (p < 0.001). Moreover, statistical significant difference was also observed between AN-LA– and AN-HA–fed animals in relation to ovarian weight (p < 0.001), serum AGEs (p < 0.001), fasting glucose (p = 0.049), testosterone (p = 0.001) and PGR levels (p < 0.001), respectively. Body weight gain and PGR levels were higher in NAN-LA versus AN-LA rats (p = 0.001 and p < 0.001, respectively), whereas insulin, PGR and fasting glucose levels were higher in NAN-HA compared with AN-HA rats (p = 0.008, p = 0.001 and p = 0.006, respectively).
Ovarian GLO-I Activity
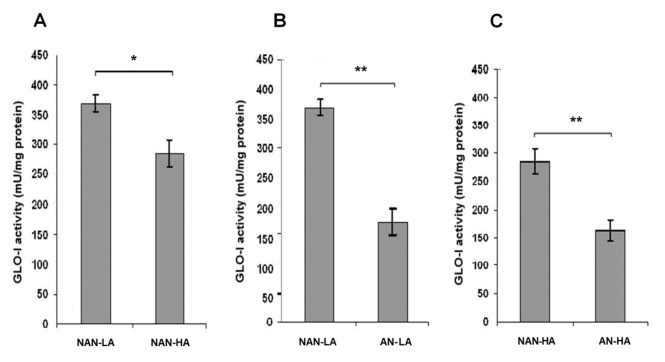
To determine GLO-I activity in the ovaries of both groups, an enzymatic assay was performed in ovarian tissue extracts from all groups. GLO-I activity in NAN-LA–fed rats was higher when compared with NAN-HA rats (p = 0.006) (Figure 1) and higher in NAN-LA rats compared with AN-LA rats (p < 0.001) (Figure 1). The enzymatic activity was also found to be higher in NAN-HA–fed rats compared with AN-HA–fed rats (p = 0.001); however, there was no statistically significant change between AN-LA and AN-HA groups (p = 0.760).
Figure 1.
Effect of the HA diet and androgens on ovarian GLO-I activity. (A) Reduced GLO-I activity is observed in NAN-HA–fed rats compared with NAN-LA–fed rats. (B) GLO-I activity is remarkably reduced in AN-LA compared with NAN-LA. (C) GLO-I activity is decreased in AN-HA–fed compared with NAN-HA–fed rats. *p < 0.05; **p < 0.001.
Correlation Analysis
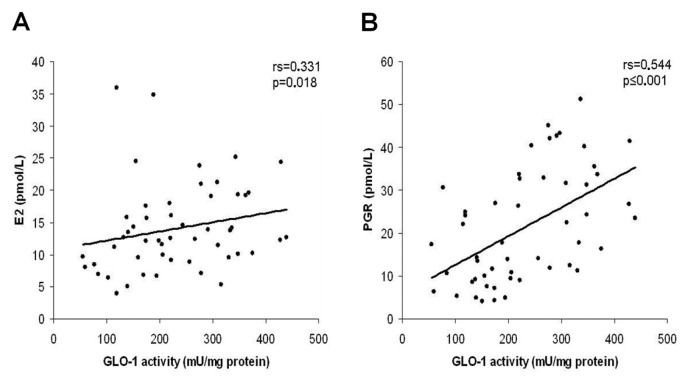
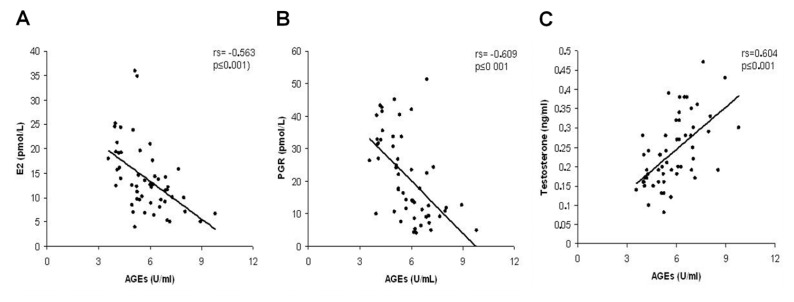
GLO-I activity showed positive correlation with E2 (rs = 0.331, p = 0.018), PGR (rs = 0.544, p = 0.001) and negative correlation with AGE expression in ovarian granulosa cells of marginal significance (rs = −0.263, p = 0.07) (Figure 2). The levels of serum AGEs were positively correlated with testosterone (rs = 0.637, p < 0.001), insulin (rs = 0.470, p = 0.001), ovarian weight (rs = 0.485, p < 0.001) and AGE expression in ovarian granulosa cells (rs = 0.346, p = 0.017) and negatively with E2 (rs = −0.521, p < 0.001) and PGR (rs = −0.508, p < 0.001) (Figure 3).
Figure 2.
Correlation diagrams of GLO-I activity with E2 (A) and PGR (B) levels.
Figure 3.
Correlation diagrams of AGEs with estrogens (E2) (A), testosterone (B) and PGR (C).
Immunohistochemical Localization of AGEs in Ovarian Tissue
To detect variations in AGE deposition among the two main groups and their subgroups, immunostaining was performed in the ovarian tissue of all animals by using a specific anti-CML (6D12) antibody. AGE immunoreactivity was detected in the follicular cell layers (granulosa and theca), in luteinized cells as well as in the ovarian stroma. However, AGE expression was statistically significant only in the granulosa cells (p < 0.001). More specifically, the HA group presented higher AGE expression levels in the granulosa cells when compared with LA (p = 0.001) (Figure 4).
Figure 4.
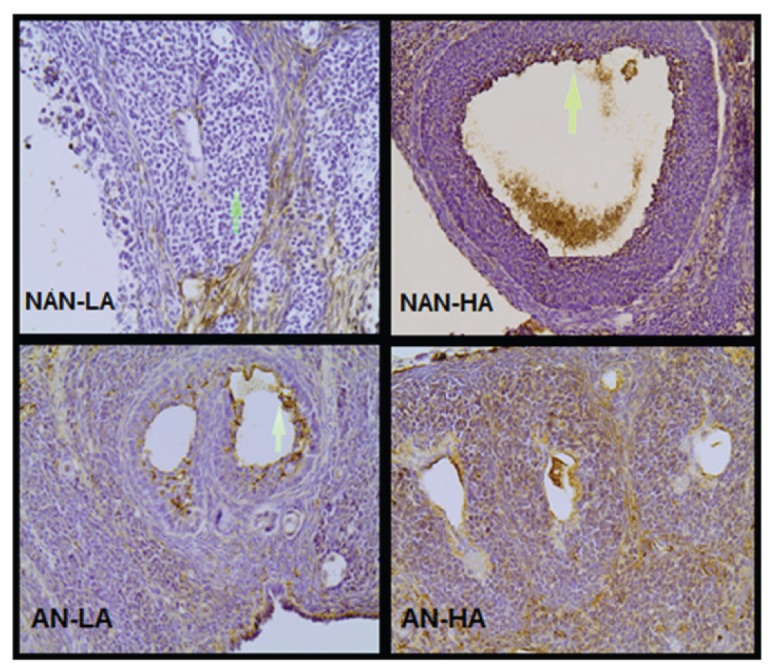
Expression of AGEs in rat ovarian follicles. Granulosa cells (arrow) of NAN-HA display stronger AGE immunoreactivity than NAN-LA. Higher AGE immunoreactivity is observed in AN-HA than in AN-LA. Magnification 200×.
DISCUSSION
The main findings of the present study show the potential of (a) a diet rich in AGEs and (b) androgen excess to impair the activity of ovarian GLO-I, possibly contributing to reduced detoxification and associated ovarian dysfunction observed in modified dietary and hyper -androgenic states.
The rats fed with high AGEs for 3 months showed lower levels of GLO-I activity compared with those fed with low AGEs (Figure 1A). This observation may be in agreement with the findings by Birkenmeier et al.(26), who showed that GLO-I activity is reduced by a redox-dependent manner in a high reactive oxygen species (ROS) environment. In our experiment, the HA diet may promote a high ROS environment, on the basis of elevated serum AGEs, which could negatively influence GLO-I activity.
The decreased GLO-I activity may contribute to increased ovarian AGE deposition as demonstrated previously by immunohistochemical analysis of CML expression in the ovaries of rats fed with an HA diet (17). In the present study, a negative correlation between increased AGE deposition and GLO-I activity proved to be of marginal significance, probably because of the small sample size. Furthermore, it could be suggested that increased ROS environment due to HA intake could contribute to acceleration of the ovarian ageing process by decreasing GLO-I activity. This subsequently would increase AGE deposition in the ovarian tissue, being in accordance with the study of Tatone et al.(11). Therefore, diet modification of AGE content, aiming at factors that may increase GLO-I activity, may play a role in decelerating the ageing process in various tissues, including the ovarian tissue. However, this observation needs further verification and additional studies to be confirmed. In parallel, impaired GLO-I activity could possibly contribute in the aforementioned process, by regulating the intra-ovarian AGE accumulation as well as the pace of their removal.
Furthermore, there is evidence that methylglyoxal itself (a major precursor of AGE and GLO-I substrate) can reduce GLO-I activity through a vicious cycle of ROS overproduction, ATP depletion and induction of apoptosis (26,28,29). More importantly, a recent human study by Jinno et al.(30) demonstrated a significant negative association between serum and follicular AGE levels with poor follicular and embryonic development, resulting in a lower likelihood of ongoing pregnancy. Thus, AGE accumulation in patients with compromised fertility, such as PCOS, could contribute to diminished folliculogenesis and poor oocyte developmental potential.
Interestingly, the present study demonstrates for the first time that in androgenized animals, GLO-I activity was significantly reduced in low as well as in high AGE–fed animals, suggesting that androgens have an independent suppressing effect on the activity of this detoxifying enzyme. However, the mechanism via which androgens exert their negative effect on GLO-I activity cannot be detracted from the present study. Most prominent suggested mechanisms include the induction of oxidative stress by androgens as well as possible regulation through androgen response elements present in the glyoxalase promoter (22). Nevertheless, it is likely that their effect may be indirect by interference with the ovulatory process. This result is supported by the finding that GLO-I activity has a positive correlation with estrogens and PGR levels, respectively. Two in vitro studies using prostate and breast cancer cell lines have described the effects of androgens and estrogens in the expression and activity of GLO-I; however, their results are controversial, depending on the cell type under study (22,23). Our results appear to be in agreement with the findings of Antognelli et al.(22), where testosterone-dependent cells show positive correlation between estradiol and GLO-I activity as opposed to testosterone-independent cells. The decreased activity of GLO-I in the presence of androgen excess could support the observed increased AGE deposition in the ovaries of women in hyperandrogenic states, such as PCOS. The above is further supported by our findings where AGEs are positively correlated with estrogens and negatively correlated with androgens. In addition, the reduction of GLO-I activity in both groups of androgenized animals was independent of their type of diet, whether low or high AGEs (Figures 1B, C).
Furthermore, in the androgenized group of our study, the low levels of estrogens are likely to contribute to the reduced GLO-I activity, which is in agreement with the observed positive correlation of GLO-I activity with E2 and PGR. Reduced PGR levels were also positively correlated with GLO-I activity, suggesting that androgens may interfere with the ovulatory process via several pathways, including the oxidative ones. However, no further significant decrease of GLO-I activity was observed between the AN-LA and AN-HA groups (Figure 1C), which may indicate that the effect of androgens on the enzymatic pathway prevails over the effect of AGEs, or it may indicate a compensatory mechanism of action. Finally, the marginally significant correlation that was observed between AGE expression in the granulosa and the GLO-I activity could be attributed to the limited number of animals, with a greater number possibly revealing a stronger link between the two parameters.
CONCLUSION
Summarizing the present data, this study shows the impact of androgens and nutritional AGEs on GLO-I activity, possibly contributing to the accumulation of AGEs on the ovarian tissue, increased ROS environment and subsequent ovarian function impairment. The positive correlation of GLO-I with estrogens suggest an additional regulatory mechanism that is particularly relevant to PCOS because of ovulatory dysfunction followed by estrogens deficiency. As shown in our previous studies (17,18,21), it is likely that AGEs play a significant role in the intra-ovarian pathophysiology. In the present study, we showed that GLO-I activity is reduced by dietary AGEs as well as androgens, possibly contributing to further AGE accumulation and, subsequently, an increased ROS environment, leading to worsening of the already disturbed ovarian function in hyperandrogenic states, including PCOS.
Because a low-AGE diet improves GLO-I activity, as shown in our study, it could be useful to explore the low-AGE environment leading to increased GLO-I activity, which may potentially contribute to diminished deposition of AGEs in the ovary, as it has been shown in other tissues (31–33). However, since androgens have an independent effect on the GLO-I activity, the potentially beneficial role of anti-androgens should be further explored alone or in combination with low-AGE diets, targeting both factors in restoration of the enzyme activity.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Principato GB, Locci P, Rosi G, Talesa V, Giovannini E. Activity changes of glyoxalases I–II and glutathione reductase in regenerating rat liver. Biochem Int. 1983;6:249–55. [PubMed] [Google Scholar]
- 3.Kalia S, Pal S, Guha-Mukherjee S. Activation of glyoxalase I during the cell division cycle and its homology with auxin regulated genes. Plant Sci. 1998;132:55–62. [Google Scholar]
- 4.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems: role in ageing and disease. Drug Metabol Drug Interact. 2008;23:125–50. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornalley PJ. Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–8. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 6.Ratliff DM, Vander Jagt DJ, Eaton RP, Vander Jagt DL. Increased levels of methylglyoxal-metabolizing enzymes in mononuclear and poly-morphonuclear cells from insulin-dependent diabetic patients with diabetic complications: aldose reductase, glyoxalase I, and glyoxalase II: a clinical research center study. J Clin Endocrinol Metab. 1996;81:488–92. doi: 10.1210/jcem.81.2.8636255. [DOI] [PubMed] [Google Scholar]
- 7.Kuhla B, et al. Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiol Aging. 2007;28:29–41. doi: 10.1016/j.neurobiolaging.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ayoub F, Zaman M, Thornalley P, Masters J. Glyoxalase activities in human tumour cell lines in vitro. Anticancer Res. 1993;13:151–5. [PubMed] [Google Scholar]
- 9.Brouwers O, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374–80. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KM, Kim YS, Jung DH, Lee J, Kim JS. Increased glyoxalase I levels inhibit accumulation of oxidative stress and an advanced glycation end product in mouse mesangial cells cultured in high glucose. Exp Cell Res. 2012;318:152–9. doi: 10.1016/j.yexcr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Tatone C, et al. Female reproductive dysfunction during ageing: role of methylglyoxal in the formation of advanced glycation endproducts in ovaries of reproductively-aged mice. J Biol Regul Homeost Agents. 2010;24:63–72. [PubMed] [Google Scholar]
- 12.Niwa T. Mass spectrometry for the study of protein glycation in disease. Mass Spectrom Rev. 2006;25:713–23. doi: 10.1002/mas.20089. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–75. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–52. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg T, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–91. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 16.de Assis AM, et al. High fat and highly thermolyzed fat diets promote insulin resistance and increase DNA damage in rats. Exp Biol Med (Maywood) 2009;234:1296–304. doi: 10.3181/0904-RM-126. [DOI] [PubMed] [Google Scholar]
- 17.Diamanti-Kandarakis E, et al. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J Mol Med. 2007;85:1413–20. doi: 10.1007/s00109-007-0246-6. [DOI] [PubMed] [Google Scholar]
- 18.Diamanti-Kandarakis E, et al. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol. 2007;127:581–9. doi: 10.1007/s00418-006-0265-3. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay S, Mukherjee TK. Bridging advanced glycation end product, receptor for advanced glycation end product and nitric oxide with hormonal replacement/estrogen therapy in healthy versus diabetic postmenopausal women: a perspective. Biochim Biophys Acta. 2005;1745:145–55. doi: 10.1016/j.bbamcr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Kaya C, et al. Advanced oxidation protein products are increased in women with polycystic ovary syndrome: relationship with traditional and nontraditional cardiovascular risk factors in patients with polycystic ovary syndrome. Fertil Steril. 2009;92:1372–7. doi: 10.1016/j.fertnstert.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2005;62:37–43. doi: 10.1111/j.1365-2265.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- 22.Antognelli C, et al. Alteration of glyoxalase genes expression in response to testosterone in LNCaP and PC3 human prostate cancer cells. Cancer Biol Ther. 2007;6:1880–8. doi: 10.4161/cbt.6.12.4961. [DOI] [PubMed] [Google Scholar]
- 23.Rulli A, et al. A possible regulatory role of 17beta-estradiol and tamoxifen on glyoxalase I and glyoxalase II genes expression in MCF7 and BT20 human breast cancer cells. Breast Cancer Res Treat. 2006;96:187–96. doi: 10.1007/s10549-005-9078-7. [DOI] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008;10:e3. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 25.Mannerås L, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–91. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 26.Birkenmeier G, et al. Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS One. 2010;5:e10399. doi: 10.1371/journal.pone.0010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatone C, et al. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum Reprod. 2011;26:1843–59. doi: 10.1093/humrep/der140. [DOI] [PubMed] [Google Scholar]
- 28.de Arriba SG, et al. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells-protection by carbonyl scavengers. Neurobiol Aging. 2007;28:1044–50. doi: 10.1016/j.neurobiolaging.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Howell SK, Sanford RJ, Beisswenger PJ. Methylglyoxal can modify GAPDH activity and structure. Ann N Y Acad Sci. 2005;1043:135–45. doi: 10.1196/annals.1333.017. [DOI] [PubMed] [Google Scholar]
- 30.Jinno M, et al. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum Reprod. 2011;26:604–10. doi: 10.1093/humrep/deq388. [DOI] [PubMed] [Google Scholar]
- 31.Zheng F, et al. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–37. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- 32.Feng JX, et al. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007;71:901–11. doi: 10.1038/sj.ki.5002162. [DOI] [PubMed] [Google Scholar]
- 33.Peppa M, et al. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52:2805–13. doi: 10.2337/diabetes.52.11.2805. [DOI] [PubMed] [Google Scholar]