Abstract
Obesity is a major risk factor for insulin resistance, type 2 diabetes mellitus and cardiovascular disease. The pathophysiology of obesity is associated with chronic low-grade inflammation. Adipose tissue in obesity is significantly infiltrated by macrophages that secrete cytokines. The mechanisms of interaction between macrophages and adipocytes, leading to macrophage activation and increased cytokine release, remain to be elucidated. We reasoned that an adipocyte-derived factor might stimulate activation of macrophages. We have identified pigment epithelium-derived factor (PEDF) as a mediator of inflammation that is secreted by adipocytes and mediates macrophage activation. Recombinant PEDF activates macrophages to release tumor necrosis factor (TNF) and interleukin-1 (IL-1). The PEDF receptor adipose triglyceride lipase (ATGL) is required for PEDF-mediated macrophage activation. Selective inhibition of ATGL on macrophages attenuates PEDF-induced TNF production, and PEDF enhances the phosphorylation of p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. PEDF administration to rats results in increased serum TNF levels, and insulin resistance. Together, these findings suggest that PEDF secreted by adipocytes contributes to the onset and maintenance of chronic inflammation in obesity, and may be a therapeutic target in ameliorating insulin resistance.
INTRODUCTION
Obesity is a global health problem affecting as many as 300 million people worldwide. In the United States, the percentage of the adult population classified as obese has increased from 27.5% to 35.5% from 1999 to 2010 (1). Obesity is further complicated by metabolic disorders, including insulin resistance, type 2 diabetes, fatty liver disease, cardiovascular disease, hypertension, cancers, and cognitive impairments (2–4). The pathophysiology of obesity is associated with chronic low-grade inflammation characterized by increased cytokine production, elevated acute-phase reactants, and activation of a network of inflammatory signaling pathways (5). Overproduction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) leads to significant metabolic changes including hyperlipidemia and insulin resistance. Expanded adipose tissue in obesity is significantly infiltrated with macrophages that produce TNF and IL-1. Approximately 45–60% of cells express the macrophage marker F4/80 in obese adipose tissue compared with only 10–15% F4/80+ cells in adipose tissue from lean mice (6). Interestingly, an increase in macrophage number positively correlates with both adipocyte size and body mass (6,7).
Expression analysis of inflammatory markers in the adipose tissue have implicated macrophages as the primary source of proinflammatory mediators such as TNF, IL-6, macrophage inflammatory protein 1α (MIP1α), macrophage chemoattractant protein 1 (MCP1) and inducible nitric oxide synthase (iNOS) in the adipose tissue (6–8). Proinflammatory cytokines, such as TNF, induce insulin resistance via inhibitor of nuclear factor kappa-B kinase subunit β (IKKβ) and Jun N-terminal kinase (JNK)-mediated inhibitory serine phosphorylation of insulin receptor substrate proteins (9,10). Importantly, selective deletion of IKKβ, a proinflammatory signaling molecule, in myeloid cells or reconstitution of mice with JNK-deficient bone marrow reduces myeloid cell mediated inflammation in adipose tissue, resulting in protection from insulin resistance (11,12). These data collectively demonstrate that activated inflammatory macrophages contribute to the pathogenesis of obesity-induced insulin resistance.
Despite the widespread interest in this field, the mechanisms underlying macrophage activation in obese adipose tissue are not well understood. Transgenic expression of MCP1 in adipose tissue leads to enhanced macrophage recruitment and potentiates obesity-induced insulin resistance (13). However, it is not required for macrophage-mediated inflammation in the obese adipose tissue, as demonstrated by high-fat diet–fed MCP1-knockout mice, which expressed comparable levels of proinflammatory cytokines, TNF, IL-1β and IL-6 in adipose tissue (14). Adipocyte-derived free fatty acids (FFA) promote TNF and IL-6 release by macrophages in a Toll-like receptor 4 (TLR4)-dependent manner and have been implicated in inflammation and insulin resistance (15). TLR4-deficient mice, but not their wild-type lit-termates, are protected from adipose tissue inflammation and systemic insulin resistance in response to systemic lipid infusion. However, recent reports have suggested that FFA-mediated activation of macrophages via TLR4 is not essential for the development of insulin resistance. Mice lacking expression of myeloid differentiation primary response protein 88 (Myd88) are susceptible to increased risk of insulin resistance compared with wild-type controls (16). Similarly, loss of function mutation in TLR4 exacerbates diet-induced obesity and insulin resistance (17). Thus, the identity of the factors that activate an inflammatory phenotype in macrophages in obese adipose tissue is unknown.
We reasoned that adipocytes might secrete a previously unrecognized factor(s) that stimulates macrophages to secrete TNF and other cytokines. We isolated a macrophage activating factor from adipocyte conditioned medium (CM), and identified it as pigment epithelium derived factor (PEDF), a glycoprotein member of the serine protease inhibitor (serpin) family. Recombinant PEDF activates macrophage to produce cytokines by signaling via adipose triglyceride lipase (ATGL), and mediates insulin resistance in vivo suggesting a link between adipocyte-derived PEDF and complications of obesity.
MATERIALS AND METHODS
Reagents
Recombinant human PEDF was obtained from Millipore (Billerica, MA, USA). The endotoxin content was below the level of detection with a Limulus assay. Bromoenol lactone (BEL) was purchased from Caymen Chemical (Ann Arbor, MI, USA). Arachidonyl trifluoromethyl ketone (AACOCF3) was obtained from EMD chemical (Gibbstown, NJ, USA). Extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor (U0126), p38 mitogen-activated protein kinase (MAPK) inhibitor (SB203580), anti-ATGL antibody, anti-ERK1/2 antibody, anti-p38 MAPK antibody, anti-phospho ERK1/2 antibody and anti-p38 MAPK antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-laminin receptor (LR) antibody was purchased from Abcam (Cambridge, MA, USA). The following synthetic peptides were used in experiments, a decapeptide from the C-loop of murine epidermal growth factor (EGF33–42) (acetyl-C-(S-Acm)-VIGYSGDR-C(S-Acm)-NH2), and a nonapeptide Lamβ1925–933 (amino acid sequence: CPDGYIGSR), corresponding to the binding region for 67LR on the laminin β1 chain (Princeton Biomolecules, Langhorn, PA, USA).
Animals
Male Sprague Dawley rats, 200–250 g, purchased from Charles River laboratories, were housed at 25°C under light-controlled conditions with a 12-h light/dark cycle. Rats were allowed free access to water and standard rodent chow and were acclimatized for 1 wk prior to experimentation. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research, North Shore-LIJ Health System.
3T3-L1 Adipocyte Culture and PEDF Determination
3T3-L1 cells (American Type Culture Collection [ATCC] Rockville, MD, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco; Life Technologies, Carlsbad, CA, USA) supplemented with 10% bovine calf serum (Mediatech, Manassas, VA, USA), 100μ/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 humidified atmosphere at 37°C. Two-day postconfluent (d 0) cells were stimulated to differentiate with a medium containing 10% fetal calf serum (Gibco; Life Technologies), 1.7 μmol/L insulin (Sigma-Aldrich, St. Louis, MO, USA), 1 μmol/L dexamethasone (Sigma-Aldrich) and 0.5 mmol/L isobutylmethylxanthine (IBMX) for 48 h. Thereafter, fresh medium containing only insulin was added every 2 d for another 6 d. On d 10–12, cells were fully differentiated, and medium was changed to insulin-free medium containing 10% fetal calf serum. For collecting adipocyte conditioned medium (CM), differentiated adipocytes were cultured with serum free Optimem media (Gibco; Life Technologies), and CM was harvested 20 h later.
Ion Exchange Chromatography and Protein Identification
Adipocyte CM was centrifuged at 2000 rpm for 10 min at 4°C. Subsequently, CM was concentrated with Amicon Ultra 15 centrifugal filter devices (Millipore) with a cutoff mass of 5000 Da. Protein concentrations were measured with a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Concentrated CM was dialyzed with buffer (50 mmol/L HEPES buffer) and applied to a 1-mL HiTrap SP-XL column preequilibrated in 50 mmol/L HEPES buffer. The column was washed with 50 mmol/L HEPES buffer until the absorbance at 280 nm of the flow-through was at the baseline. Bound proteins were eluted with 20 mL 0–100% 1 mol/L NaCl in 50 mmol/L HEPES buffer. To measure the TNF-stimulating activity, RAW cells were cultured with individual fractions, and levels of TNF in the cell supernatant were analyzed.
RAW 264.7 Culture, Assays and TNF Analysis
Mouse macrophagelike RAW 264.7 cells were purchased from the ATCC, and maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 humidified atmosphere at 37°C. To determine the effect of PEDF, cells were cultured in 96-well tissue culture plates at 5 × 105 cells/mL and incubated overnight. The cells were washed once with serum-free Optimem medium, and received 2.5 h of PEDF in Optimem. Supernatants were collected and TNF was analyzed by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA).
mRNA Quantification
RAW 264.7 cells were incubated in duplicates with medium or different concentrations of PEDF. After 60 min total RNA was extracted from cells by use of the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), including on-column DNase treatment (Qiagen), and reverse transcribed to cDNA with an iScript cDNA synthesis kit (Bio-Rad). Semiquantitative measurements of mRNA levels were obtained using the reaction mix LightCycler 480 Probes Master (Roche, Indianapolis, IN, USA) in the LightCycler 480 System (Roche) with the following primers (Invitrogen; Life Technologies) and probes (Roche Universal Probe Library): TNF: forward primer 5′-TCTTC TCATT CCTGC TTGTG G; reverse primer 5′-GGTCT GGGCC ATAGA ACTGA; probe #49, IL1: forward primer 5′-TGTAA TGAAG ACGGC ACACC, reverse primer 5′-TCTTC TTTGG GTATT GCTTG G, probe #78, and IL6: forward primer 5′-GCTAC CAAAC TGGAT ATAAT CAGGA; reverse primer 5′-CCAGG TAGCT ATGGT ACTCC AGAA; probe #6. Values were calculated from a standard curve and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference gene.
Western Blot Analysis
Raw cells were washed once with phosphate buffered saline (Invitrogen; Life Technologies) and lysed with cell lysis buffer (Sigma-Aldrich) containing HaltTM protease inhibitor and phosphatase inhibitor (ThermoFisher, Waltham, MA, USA). Lysates were analyzed by Western blot analysis with antibodies against ATGL, LR, β-actin, total ERK1/2, p38 MAPK, phospho-ERK1/2 and phospho-p38 MAPK.
Insulin Tolerance Test
We administered 0.2 U/kg of regular insulin (Novo Nordisk Inc, Princeton, NJ, USA) intravenously, followed by 200 μL normal saline. Blood samples were taken at time 0, 3, 5, 7, 9, 11, 13, 15 and 20 min and blood glucose levels were analyzed with a glucometer (OneTouch Ultra, LifeScan, Milpitas, CA, USA). KITT, the first order rate constant for the disappearance of glucose, was calculated from the formula KITT = (0.693/t1/2) × 100, where the half-life t1/2 is calculated from the slope of the regression line from 3 to 15 min as described by Bonora et al.(18). Rats were fasted overnight prior to insulin tolerance test (ITT). ITT was performed 90 min after recombinant PEDF (2 mg/kg) infusion.
RESULTS
Adipocyte-Derived PEDF Induces Proinflammatory Cytokine Production and Inflammatory Signaling in Macrophages
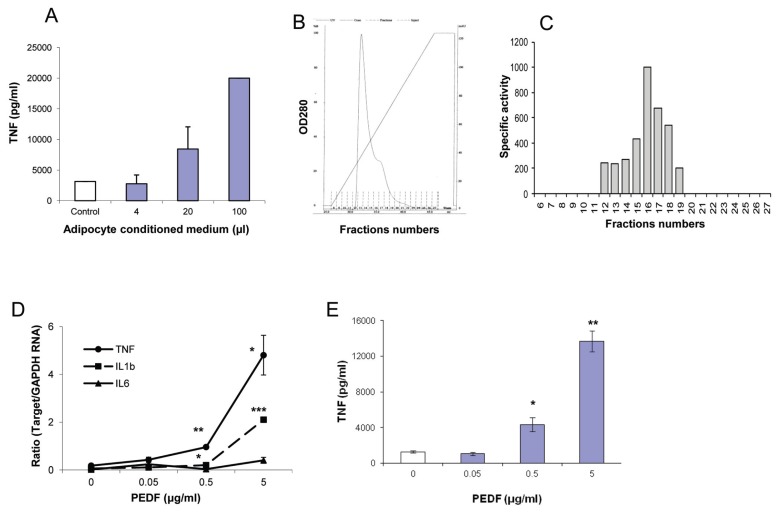
To identify whether adipocytes secrete a factor(s) that stimulates macrophage TNF production, media conditioned by exposure to 3T3-L1 adipocytes was added to macrophage cultures. After 2.5 h, macrophage supernatants were harvested and TNF was measured by ELISA. As shown in Figure 1A, adipocyte CM, but not control medium, increases TNF release by macrophages in a concentration-dependent manner. To isolate the bioactive factor, the adipocyte CM was fractionated by cation exchange chromatography (Figure 1B), and TNF-stimulating bioactivity was determined by adding individual fractions to macrophage cultures. As shown in Figure 1C, most of the TNF-stimulating bioactivity was confined to fraction 16. Mass spectroscopy analysis identified PEDF as the adipocyte-secreted protein in the fraction leading to macrophage activation. To confirm the macrophage-activating functional activity of PEDF, recombinant PEDF was added to macrophage cultures. Recombinant PEDF induces a concentration-dependent increase in the expression of TNF and IL-1 in murine macrophages, and a significant increase in TNF levels in macrophage culture supernatants (Figures 1D, E). A similar increase in TNF production was observed in response to recombinant PEDF in human macrophage cultures (data not shown).
Figure 1.
PEDF secreted by adipocytes increases macrophage TNF production. (A) Different amounts of 3T3-L1 adipocyte CM was added to RAW cells, and levels of TNF in the cell supernatant were quantitated by ELISA. (B) 3T3-L1 adipocyte CM was fractionated with cation exchange chromatography. (C) RAW cells were cultured with individual fraction, and levels of secreted TNF were quantitated with ELISA. Fold change in TNF levels was determined by calculating the increase in TNF levels in response to the individual fraction compared with medium control. Arbitary units of specific activity were determined as fold change per milligram of total protein. (D) RAW cells were cultured with recombinant PEDF, and RNA was isolated after 60 min. Real-time polymerase chain reaction analysis was carried out as described in the Methods. Data are mean ± standard error (SE), *p < 0.05 and **p < 0.005 versus medium alone control. (E) RAW cells were cultured with recombinant PEDF for 2.5 h, and TNF levels were quantitated in the supernatant by ELISA. Data are mean ± SE, *p < 0.05 and **p < 0.0005 versus medium-alone control.
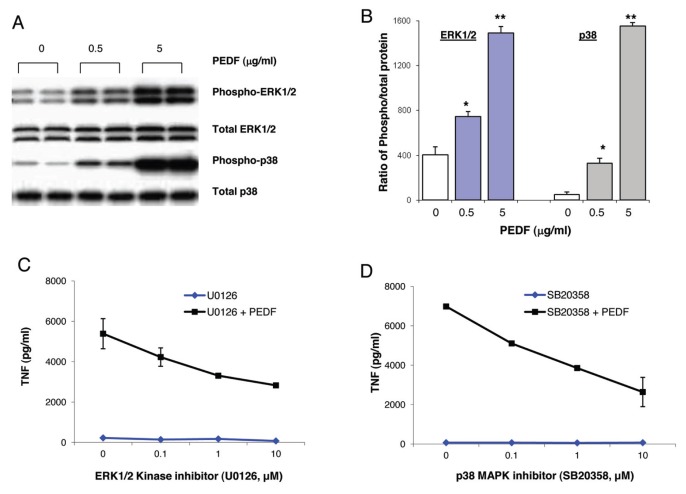
Next we investigated the effect on activation of ERK1/2 and p38 MAPK kinases by exposing macrophages to recombinant PEDF. PEDF induces concentration-dependent increases in the activation of proinflammatory kinases, p38 MAPK and ERK1/2 (Figures 2A, B). To analyze the functional significance of activation of ERK1/2 and p38 for PEDF-mediated inflammatory responses, macrophages were stimulated with PEDF in the presence of an ERK1/2-specific inhibitor, U0126, or a p38-specific inhibitor, SB20358. Inhibition of either ERK1/2 MAPK or p38 MAPK attenuates PEDF-mediated TNF production in a concentration-dependent manner (Figures 2C, D), indicating that ERK1/2 kinase and p38 kinase activation is important for PEDF-mediated inflammatory responses.
Figure 2.
PEDF induces inflammatory signaling pathways in murine macrophages. (A, B) RAW were cultured with different concentrations of recombinant PEDF. Total cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antibodies to phosphorylated and total ERK1/2 and p38 MAPK. Data are mean ± SE. *p < 0.05 and **p < 0.005 versus medium-alone control. (C, D) RAW cells were cultured with recombinant PEDF for 2.5 h in the presence or absence of ERK1/2 inhibitor (U0126) or p38 MAPK inhibitor (SB20358) at the indicated concentrations. TNF levels were measured in the supernatant by ELISA. Data are mean ± SE.
PEDF Receptor ATGL Is Required for PEDF-Mediated Macrophage Activation
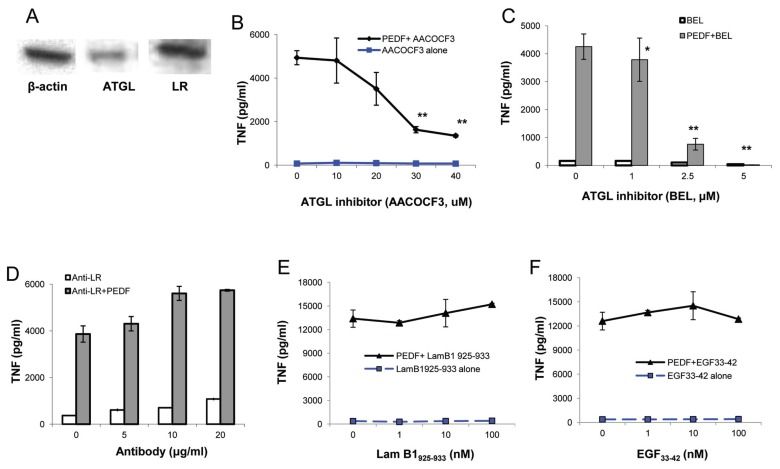
PEDF binds specifically to two known cell membrane receptors, an ATGL/independent phopholipase A2 (iPLA2)/nutrin in neuronal cells, and an LR in endothelial cells (19,20). To investigate receptor expression on macrophages, macrophage cell lysates were probed with anti-ATGL or anti-LR antibodies. Western blot analysis of macrophage cell lysates revealed that macrophages constitutively express PEDF receptors, ATGL and LR (Figure 3A). Next we investigated whether phospholipase enzymatic activity of the ATGL receptor is required for PEDF-induced inflammatory responses. Macrophages were preincubated with the phospholipase inhibitor AACOCF3 (21) prior to stimulation with PEDF. Inhibition of phospholipase activity resulted in concentration-dependent reduction of PEDF-induced TNF production by macrophages (Figure 3B). The requirement for phospholipase activity of ATGL for PEDF-mediated TNF release was further confirmed by culturing macrophages with an iPLA2/ATGL-specific inhibitor, BEL (21), prior to addition of PEDF. BEL attenuates PEDF-induced activation of macrophages in a concentration-dependent manner with more than 80% inhibition at 2.5 μmol/L (Figure 3C), suggesting that Ca2+-independent phospholipase activity is involved in mediating inflammatory effects of PEDF on macrophages.
Figure 3.
ATGL is required for PEDF-mediated macrophage activation. (A) RAW cells express both ATGL and LR. RAW cell lysate was resolved by SDS-PAGE and immunoblotted with antibodies to β-actin, ATGL and LR. RAW cells were cultured with recombinant PEDF for 2.5 h in the presence or absence of (B, C) ATGL inhibitors, AACOCF3 or BEL or (D, E, F) anti-LR antibody or Lam B1925–933 peptide or EGF33–42 peptide at the indicated concentrations. TNF levels were measured in the supernatant by ELISA. Data are mean ± SE, *p < 0.05 and **p < 0.01 versus medium-alone control.
To explore whether the LR is also involved in the effects of PEDF on macrophage activation, we stimulated macrophages with recombinant PEDF in the presence or absence of anti-LR antibodies. As shown in Figure 3D, inhibition of PEDF–LR interaction by LR- specific antibodies does not affect PEDF-mediated TNF production by macrophages. We next stimulated LR directly by culturing macrophages with an LR-specific agonist peptide, Lamβ1925–933(22,23). Stimulation of macrophages with agonist Lamβ1925–933 peptide alone did not lead to activation of macrophages. Moreover, costimulation of macrophages with Lamβ1925–933 peptide and PEDF did not result in a synergistic effect on PEDF-mediated macrophage activation (Figure 3E). To confirm that activation of LR is not required for PEDF-mediated macrophage activation, we incubated macrophages with an antagonist peptide, EGF33–42, which inhibits laminin signaling (22,23), and we observed that blocking of LR activity does not attenuate the PEDF-mediated increase in TNF (Figure 3F). Taken together these results indicate that ATGL, and not LR, is required for PEDF- mediated macrophage activation.
PEDF Administration Induces TNF Release and Insulin Resistance in Rodents
To investigate whether PEDF stimulates TNF release in vivo, we administered PEDF intravenously and measured circulating TNF levels in serum. PEDF administration resulted in a significant increase in serum TNF levels (969 ± 197 pg/mL, p < 0.005) compared with nondetectable serum TNF levels following vehicle administration. We next analyzed the effect of PEDF on insulin resistance by administering insulin 90 min after PEDF and measuring blood glucose levels over time. The KITT index, a measure of insulin sensitivity, was calculated as described before (18). The KITT index is inversely proportional to the insulin sensitivity. A significant reduction in the KITT index was observed in the PEDF-receiving group (1.47% ± 0.33 %/min, p < 0.05) compared with the vehicle-receiving group (2.54% ± 0.3 %/min), showing low insulin sensitivity in animals receiving PEDF and high insulin sensitivity in the vehicle-treated groups (Figure 4). These results, together with the observation that animals receiving PEDF also had a significant increase in serum TNF levels, suggest that administration of PEDF induces TNF release and insulin resistance in vivo.
Figure 4.
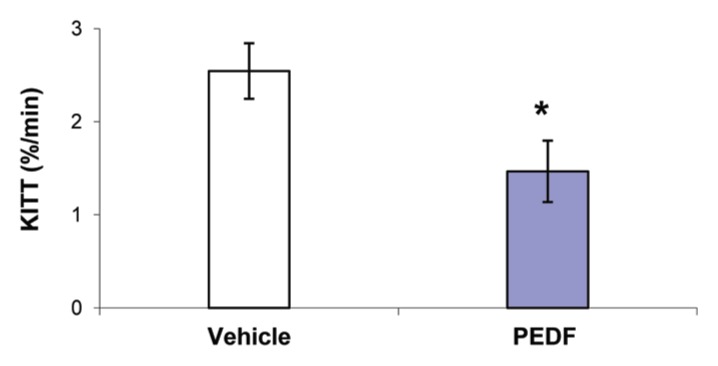
PEDF administration induces TNF release and insulin resistance in rats. Recombinant PEDF (2 mg/kg) was administered to rats, and ITT was carried out 90 min after PEDF administration. KITT, the first-order rate constant for the disappearance of glucose, was calculated as described in Methods. Data are mean ± SE (vehicle group, n = 12; PEDF group n = 9, p < 0.05 versus vehicle control).
DISCUSSION
Here we report that adipocyte-secreted PEDF mediates macrophage activation and induces inflammatory signaling in macrophages. The data reveal that PEDF secreted by adipocytes activates the inflammatory phenotype in macrophages and leads to increasing levels of circulating TNF when administered in vivo that may lead to insulin resistance. Several lines of evidence support this conclusion. First, PEDF is present in the bioactive fraction of adipocyte CM, and recombinant PEDF activates macrophages in a concentration-dependent manner. Second, PEDF induces inflammatory signaling in macrophages. Third, macrophages express PEDF receptors, ATGL and LR. Inhibition of ATGL receptor and not LR attenuates PEDF-mediated macrophage activation. In addition, direct activation of LR by use of agonist peptide, Lamβ1925–933, does not induce macrophage activation. Importantly, in vivo administration of PEDF leads to the inflammatory phenotype and decreases insulin sensitivity in rats.
The identification of PEDF as an adipocyte-derived factor is consistent with previously reported studies indicating that PEDF is one of the most abundant proteins secreted by human adipocytes derived from primary adipocytes or from human mesenchymal stem cells (24,25) and murine adipocytes derived from differentiation of 3T3-L1 preadipocytes (26,27). PEDF expression and secretion by adipocytes is increased during differentiation (24). Our findings are also in accordance with clinical studies demonstrating a positive association between increased circulating levels of PEDF and visceral adiposity in obese subjects (28) and a corresponding decrease in PEDF levels after weight loss (29).
Proinflammatory effects of PEDF have been described previously in astrocytes, microglia and cerebellar granule cell neurons. PEDF induces expression of proinflammatory mediators such as IL-1β, IL-6, TNF, granulocyte colony-stimulating factor (G-CSF), iNOS and IkB, and chemokines such as MIP1α, MIP-2 and MIP-3α by cultured microglia, astrocytes and neurons (30–32). These studies have demonstrated that in addition to its role as a neurotrophic and antiangiogenic factor, PEDF also acts as a proinflammatory factor. Consistent with this proinflammatory phenotype, we demonstrated that PEDF directly activates inflammatory signaling proteins, p38 MAPK and ERK1/2, in macrophages, and activation of these inflammatory kinases is required for PEDF-mediated macrophage activation. This result is consistent with previous findings demonstrating that PEDF mediates inflammatory signaling in astrocytes and microglial cells via activation of p38 MAPKs, cAMP response element-binding protein (CREB) and nuclear factor-κB (NF-κB). In addition, PEDF mediates activation of proinflammatory signaling proteins, p38 MAPK and NF-κB, in muscle and fat cells in culture (24). Notably, acute in vivo administration of PEDF induces activation of serine/threonine kinases, ERK, JNK and IKKβ, in skeletal muscle and liver (26). Although our studies indicate that PEDF is a major factor in the adipocyte CM that contributes to macrophage activation, it is plausible that other factors, such as IL-1 or adipokine, may also lead to macrophage activation and synergistically enhance TNF release.
It has been proposed that PEDF mediates its diverse biological functions by binding to different cell surface receptors, namely ATGL (also known as calcium independent PLA2/iPLA2) and LR (19,20). Using yeast two-hybrid screening, Notari and colleagues have identified ATGL as a high-affinity PEDF receptor expressed by human retinal pigment epithelium (19). ATGL is expressed as a cell membrane protein, and PEDF binding to ATGL stimulates the enzymatic phospholipase activity of ATGL that liberates FFA (19). Colocalization studies have shown that recombinant PEDF is transported into cells, colocalizes and interacts with ATGL (33). In addition, PEDF regulates lipid metabolism in adipose tissue, skeletal muscle and hepatocytes in an ATGL-dependent manner (33,34). Interaction of PEDF with ATGL has been suggested to mediate its antiangiogenic, antitumorigenic and neurotrophic activities by stimulation of lipid mediators (19). Bernard and colleagues have identified the second known receptor for PEDF (20), a nonintegrin 37/67-kDa LR. Antiangiogenic activity of PEDF is at least partially mediated by binding to LR (20). Macrophages express both ATGL and LR proteins, and interestingly, selective inhibition of ATGL, and not laminin receptor, attenuates PEDF-mediated macrophage activation. ATGL-mediated triacylglycerol hydrolysis is essential for mediating efficient phagocytosis by macrophages (35). Defective lipolysis in macrophages lacking ATGL influences macrophage polarization and actin polymerization, resulting in impaired macrophage migration (36). Bone marrow transplant studies have demonstrated that selective deficiency of ATGL in macrophages results in attenuation of atherosclerotic plaques (37). Triglycerol accumulation in ATGL-deficient macrophages results in mitochondrial dysfunction and programmed cell death (38). In addition, in the absence of functional ATGL, macrophages develop an antiinflammatory M2-like macrophage phenotype (39). Taken together these studies indicate that PEDF induces a proinflammatory phenotype in macrophages via binding to ATGL.
CONCLUSION
Circulating levels of PEDF are positively associated with obesity and related complications such as metabolic syndrome, type 2 diabetes mellitus, and insulin resistance in humans (28,40,41). In a murine model of high-fat diet–induced obesity, adipocyte PEDF expression and serum levels are elevated in obese mice and are reduced upon weight loss (26). Prolonged administration of PEDF leads to development of insulin resistance in lean mice (26). The mechanism by which PEDF induces insulin resistance is not yet clear. Here we show that acute administration of PEDF in rodents induces insulin resistance in vivo, and this onset of insulin resistance is accompanied by increase in serum TNF levels. TNF plays an important role in mediating insulin resistance in obesity (5,42,43). TNF infusions in humans lead to impairment of whole body insulin-mediated glucose uptake (44). Chronic inflammation in fat, specifically mediated by macrophage activation, plays a crucial role in the development of obesity-related insulin resistance (45). Given the proinflammatory role of PEDF and increased levels of circulating PEDF in obesity, it is plausible that adipocyte-derived PEDF leads to activation of macrophages in obesity. Collectively these findings indicate a previously unrecognized role of PEDF in mediating obesity-associated inflammation and suggest the possibility of utilizing therapeutic strategies to attenuate PEDF-mediated inflammation in the treatment of insulin resistance and associated complications.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Berrington dG, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 11.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 12.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Kamei N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 14.Inouye KE, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–50. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijay-Kumar M, et al. Loss of function mutation in toll-like receptor-4 does not offer protection against obesity and insulin resistance induced by a diet high in trans fat in mice. J Inflamm. 2011;8:2. doi: 10.1186/1476-9255-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonora E, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–8. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- 19.Notari L, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–37. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 20.Bernard A, et al. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284:10480–90. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des. 2005;11:1301–12. doi: 10.2174/1381612053507521. [DOI] [PubMed] [Google Scholar]
- 22.Gebarowska D, et al. Synthetic peptides interacting with the 67-kd laminin receptor can reduce retinal ischemia and inhibit hypoxia-induced retinal neovascularization. Am J Pathol. 2002;160:307–13. doi: 10.1016/S0002-9440(10)64374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson J, et al. Murine epidermal growth factor peptide 33–42 binds to a YIGSR-specific laminin receptor on both tumor and endothelial cells. J Biol Chem. 1996;271:26179–86. doi: 10.1074/jbc.271.42.26179. [DOI] [PubMed] [Google Scholar]
- 24.Famulla S, et al. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes. 2011;35:762–72. doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 25.Chiellini C, et al. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol. 2008;9:26. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowe S, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10:40–7. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Kratchmarova I, et al. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002;1:213–22. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, et al. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with visceral adiposity in Japanese patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:52–6. doi: 10.1002/dmrr.820. [DOI] [PubMed] [Google Scholar]
- 29.Sabater M, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–8. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 30.Takanohashi A, Yabe T, Schwartz JP. Pigment epithelium-derived factor induces the production of chemokines by rat microglia. Glia. 2005;51:266–78. doi: 10.1002/glia.20203. [DOI] [PubMed] [Google Scholar]
- 31.Sanagi T, Yabe T, Yamada H. The regulation of pro-inflammatory gene expression induced by pigment epithelium-derived factor in rat cultured microglial cells. Neurosci Lett. 2005;380:105–10. doi: 10.1016/j.neulet.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Yabe T, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-inflammatory genes in neonatal astrocytes through activation of NF-kappa B and CREB. Glia. 2005;50:223–34. doi: 10.1002/glia.20171. [DOI] [PubMed] [Google Scholar]
- 33.Chung C, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–8. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Borg ML, et al. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–66. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandak PG, et al. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J Biol Chem. 2010;285:20192–201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aflaki E, et al. Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol Life Sci. 2011;68:3933–47. doi: 10.1007/s00018-011-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammers B, et al. Macrophage adipose triglyceride lipase deficiency attenuates atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:67–73. doi: 10.1161/ATVBAHA.110.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aflaki E, et al. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J Biol Chem. 2011;286:7418–28. doi: 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aflaki E, et al. Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol Life Sci. 2011;68:3933–47. doi: 10.1007/s00018-011-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagishi S, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–50. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins A, et al. Increased serum pigment epithelium derived factor levels in Type 2 diabetes patients. Diabetes Res. Clin. Pract. 2008;82:e5–7. doi: 10.1016/j.diabres.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108–14. doi: 10.1152/ajpendo.00471.2005. [DOI] [PubMed] [Google Scholar]
- 45.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]