Abstract
The rice (Oryza sativa L.) homeobox gene OSH1 causes morphological alterations when ectopically expressed in transgenic rice, Arabidopsis thaliana, and tobacco (Nicotiana tabacum L.) and is therefore believed to function as a morphological regulator gene. To determine the relationship between OSH1 expression and morphological alterations, we analyzed the changes in hormone levels in transgenic tobacco plants exhibiting abnormal morphology. Levels of the plant hormones indole-3-acetic acid, abscisic acid, gibberellin (GA), and cytokinin (zeatin and trans-zeatin [Z]) were measured in leaves of OSH1-transformed and wild-type tobacco. Altered plant morphology was found to correlate with changes in hormone levels. The more severe the alteration in phenotype of transgenic tobacco, the greater were the changes in endogenous hormone levels. Overall, GA1 and GA4 levels decreased and abscisic acid levels increased compared with wild-type plants. Moreover, in the transformants, Z (active form of cytokinin) levels were higher and the ratio of Z to Z riboside (inactive form) also increased. When GA3 was supplied to the shoot apex of transformants, internode extension was restored and normal leaf morphology was also partially restored. However, such GA3-treated plants still exhibited some morphological abnormalities compared with wild-type plants. Based on these data, we propose the hypothesis that OSH1 affects plant hormone metabolism either directly or indirectly and thereby causes changes in plant development.
The molecular mechanisms underlying organ morphogenesis from undifferentiated cells represent one of the most important biological questions. Genes involved in eukaryotic development were first isolated from Drosophila (Garber et al., 1983) and later from several other animal species. The products of these genes share a unique and homologous structure, the homeobox (Gehring, 1987).
In animals cellular differentiation occurs only in the early stages of development, whereas in higher plants undifferentiated cells are maintained as meristems throughout the life of the plant and successively give rise to leaves and floral organs. Recently, the possibility that homeobox genes also play a part in the development and morphogenesis of higher plants has been suggested.
The maize (Zea mays L.) gene KN1 (knotted-1) was the first plant gene shown to encode a homeodomain-containing protein (Hake et al., 1989). Ectopic expression of KN1 in maize or tobacco (Nicotiana tabacum L.) causes altered morphology in the transformed plants (Smith et al., 1992; Sinha et al., 1993). We have also observed that ectopic expression of the rice (Oryza sativa L.) homeobox gene OSH1 causes altered morphology in rice, Arabidopsis (Matsuoka et al., 1993, 1995), and tobacco (Kano-Murakami et al., 1993). For example, OSH1-transformed tobacco plants exhibit abnormally shaped leaves, flowers, and loss of apical dominance. These observations suggest that the OSH1 gene product may regulate the expression of genes related to morphogenesis in plants.
Tobacco plants overexpressing OSH1 exhibit a variety of specific morphological abnormalities. These include wrinkled, slender, or tiny leaves, dwarfing, and pale-colored flowers with dissected margins. The fact that OSH1 overexpression causes pleiotropic morphological alterations in transgenic plants indicates that the activities of plant hormones may also be changed in vivo.
The relationship between plant hormones and development has been the subject of considerable discussion, and it is now widely accepted that plant hormones regulate growth and development of plants by controlling the expression of genes involved in these processes. Phenotypic modifications have been described in transgenic plants overexpressing the Agrobacterium tumefaciens T-DNA genes tms or ipt, which are involved in auxin and cytokinin biosynthesis, respectively (Gaudin et al., 1994). However, little is known about the genes that regulate the biosynthesis or metabolism of plant hormones. In this study, we analyzed the levels of several hormones in OSH1-transformed tobacco plants in an effort to determine whether OSH1-mediated morphological changes may involve alterations in plant hormone levels.
MATERIALS AND METHODS
Plant Material
The preparation of OSH1-transformed tobacco (Nicotiana tabacum cv Samsun NN) plants was as described by Kano-Murakami et al. (1993). T2 seedlings of NOS-OSH1 and 35S-OSH1 transformants were grown under greenhouse conditions at 25°C. Tobacco leaves were frozen in liquid N2 immediately after harvest and stored at −80°C until analysis.
Extraction and Purification of Plant Hormones
Plant material (10–20 g) was homogenized in 80% aqueous acetone (4:1, v/v) supplemented with 10 mg L−1 butylated hydroxytoluene, and then [4,5,6,7,8,9-13C]IAA (Cambridge Isotope Laboratories, Andover, MA) and [6,6,6-2H]ABA were added as internal standards. The homogenate was filtered and solid residue was further extracted twice with the same solvent. Extracts were combined and mixed and then divided into two equal samples. One sample was used for IAA, ABA, and GA analyses and the remaining sample was used for cytokinin analysis.
For analysis of IAA, ABA, and GAs, the extract was concentrated to an aqueous residue (30 mL) in vacuo, adjusted to pH 2.5 with 6 n H2SO4, and extracted with EtOAc (3 × 10 mL). The aqueous residue was discarded, and the EtOAc extracts were combined and extracted with saturated NaHCO3 (3 × 10 mL). The EtOAc residue was discarded and the NaHCO3 extracts were combined, adjusted to pH 2.5 with 6 n H2SO4, and extracted with EtOAc (3 × 10 mL). The aqueous residue was discarded, and the EtOAc extracts were combined and passed through a column of anhydrous Na2SO4 to remove water. The resulting eluate was evaporated to dryness, dissolved in 80% aqueous MeOH (1 mL), and loaded onto a Sep-Pak C18 cartridge (Millipore). The cartridge was eluted with 80% aqueous MeOH (2 × 5 mL) and the eluate was evaporated to dryness. The residue was dissolved in MeOH (1 mL), transferred to a small vial, and dried under a stream of N2. The residue was subjected to HPLC on a column of PEGASIL ODS (6 mm i.d. x 150 mm, Senshu Kagaku, Tokyo, Japan) and eluted with 0.5% AcOH in 5% aqueous acetonitrile (solvent A) and 0.5% AcOH in 80% aqueous acetonitrile (solvent B) at 40°C as follows: 0 to 5 min, isocratic elution with solvent A; 5 to 50 min, linear gradient of 0 to 33% solvent B; and 50 to 70 min, linear gradient of 33 to 100% solvent B. The flow rate of the solvent was 1.5 mL min−1 and fractions were collected every 1 min. The retention times of IAA, ABA, GA1, and GA4 were 34.4, 42.5, 29 to 30, and 58 to 59 min, respectively. Fractions were evaporated to dryness and methylated using diazomethane, and the fraction that contained IAA was further trimethylsilylated in undiluted N-methyl-N-(trimethylsilyl)-trifluoroacetamide for 20 min at 70°C. Fractions containing IAA or ABA were subjected to GC-MS. Fractions containing GA1 or GA4 (retention time ± 3 min) were assayed by ELISA.
For cytokinin analysis, the extract was concentrated to an aqueous residue (20 mL) in vacuo, 5 mL of AcOH:H2O (1:2, v/v) was added, and the resulting solution was extracted with CH2Cl2 (2 × 10 mL). The aqueous residue was saved and the CH2Cl2 extracts were combined and extracted with AcOH:H2O (1:2, v/v, 3 × 10 mL). The CH2Cl2 extracts were discarded and the AcOH:H2O (1:2, v/v) extracts were combined and added to the former aqueous residue. This aqueous solution was adjusted to pH 9.0 with ammonia solution, and extracted with H2O-saturated BuOH (4 × 25 mL). The aqueous residue was discarded and the BuOH extracts were combined. H2O was added to the BuOH extract and the mixture was evaporated to an aqueous residue, which was loaded onto a Sep-Pak C18 cartridge. The cartridge was washed with 5 mL of H2O and then eluted with 80% MeOH (20 mL). The eluate was evaporated to dryness and purified by HPLC under the conditions described above. The retention times of Z and ZR were 8.4 and 17.7 min, respectively. Each fraction that included a retention time of ± 3 min was assayed by ELISA.
GC-MS
GC-MS was performed with a mass spectrometer (model JMS DX303, Jeol) gas chromatograph. A bonded-phase capillary column (OV-1, 0.53 mm i.d. × 15 m, Gasukuro Kogyo, Tokyo, Japan) was used in a temperature-gradient mode for GC-selected ion monitoring analysis of IAA and ABA.
ELISA Procedure
ELISA was performed according to a modification of the procedure of Atzorn and Weiler (1983). Dynatech 96 immunoplates were coated first with 100 μL of 50 μg mL−1 goat anti-rabbit γ-globulin (in 50 mm NaHCO3 and 0.9% NaCl, pH 7.8) and then with 100 μL of antibodies raised in rabbits against GA1 methyl ester (5 μg mL−1) or with 100 μL of antibodies raised in rabbits against ZR (2 μg mL−1). To each antibody-coated well was added 50 μL of TBS buffer (50 mm Tris-HCl, 1 mm MgCl2, and 0.01% NaN3, pH 9.6) plus 25 μL of a standard in 5% aqueous MeOH or sample solution, and samples were allowed to incubate for 1 h at 4°C. Following this incubation, 25 μL of diluted tracer was added and samples were incubated for a further 3 h. The enzyme activity bound to the immunoplate-adsorbed antibodies was then determined using p-nitrophenyl phosphate as a substrate. The cross-reactivity of antibodies raised against GA1 methyl ester to GA4 methyl ester was 36%, and that of antibodies raised against ZR to Z was 73% under the analytical conditions described above.
Test for GA Sensitivity
The sensitivity of transgenic tobacco to GA was tested by applying 10 μL of a 3 mm aqueous solution of GA3 (Kyowa Hakkokogyo, Tokyo, Japan) to the shoot apex. Treatments were started 40 d after sowing and repeated every 4th d until flowering. Eleven to 13 plants from each phenotype were analyzed.
RESULTS
Transgenic tobacco plants containing OSH1 under the control of the NOS or the 35S promoter were divided into three categories that ranged from mild to severe phenotype (Kano-Murakami et al., 1993). In this study transformants containing the NOS-OSH1 construct were used as mild-phenotype plants and transformants containing the 35S-OSH1 construct were used as severe-phenotype plants. Because the changes in plant hormone levels could be due to differences in developmental stage, mature leaves of the plants that we used in plant hormone analysis were harvested at the stage of vegetative growth.
Levels of Immunoreactive GA in OSH1-Transformed Tobacco Plants
Many GA derivatives are found in plants, and the activities of these GAs in plant growth and development differ. Because GA1 and GA4 are known to be highly active in causing GA-specific responses of plants (Graebe, 1987), we chose these GAs for analysis in this work. Levels of GA1 and GA4 were determined by ELISA, because this method was the most convenient for quantifying large numbers of samples.
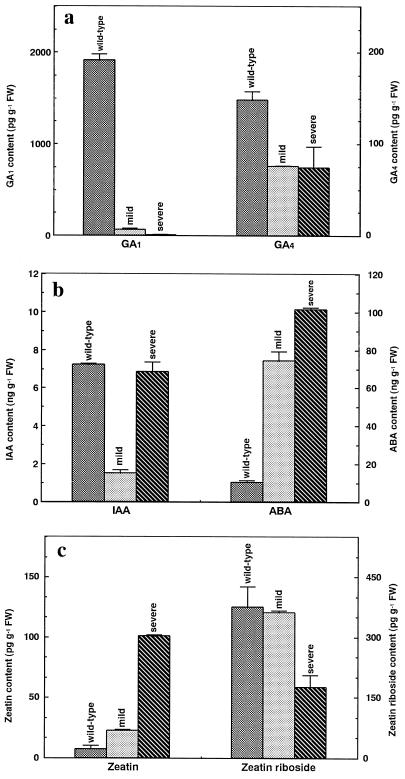
The expression of OSH1 was accompanied by a dramatic decrease of immunoreactive GA in fractions containing GA1 in leaves of transgenic tobacco plants (Fig. 1a; Table I). The immunoreactive GA content in the GA1 fraction of transformants exhibiting a severe or mild phenotype decreased to 0.4 and 3.5% of that seen in wild-type tobacco plants, respectively. In contrast, immunoreactive GA levels in GA4-containing fractions were less severely affected, being reduced by approximately one-half in plants with either phenotype.
Figure 1.
Hormonal contents in transgenic tobacco. a, GA1 and GA4; b, IAA and ABA; and c, Z and ZR. Each column represents the mean ± se of two to four replicates of independently harvested plant material. FW, Fresh weight.
Table I.
Hormonal contents in transgenic tobacco
| Treatment | Phenotype
|
||
|---|---|---|---|
| Wild type | Mild | Severe | |
| GA1 (pg g−1 fresh wt) | 1910 ± 70 | 66.7 ± 14 | 7.79 ± 4.5 |
| GA4 (pg g−1 fresh wt) | 149 ± 9 | 75.9 ± 0.7 | 74.0 ± 23 |
| IAA (ng g−1 fresh wt) | 7.23 ± 0.07 | 1.53 ± 0.15 | 6.83 ± 0.53 |
| ABA (ng g−1 fresh wt) | 10.3 ± 1.1 | 74.3 ± 4.9 | 101 ± 1 |
| Z (pg g−1 fresh wt) | 7.51 ± 2.9 | 22.5 ± 1.2 | 101 ± 1 |
| ZR (pg g−1 fresh wt) | 375 ± 52 | 362 ± 4 | 177 ± 29 |
Each value represents the mean ± se of two to four replications of independently harvested plant material.
IAA and ABA Levels in OSH1-Transformed Tobacco Plants
Analysis of IAA and ABA was performed by GC-MS using stable isotope-labeled internal standards. This technique is believed to be the most quantitative method currently available for analysis of these hormones.
The IAA content of transgenic tobacco exhibiting a mild phenotype decreased to 21% of that seen in wild-type plants (Fig. 1b; Table I). However, the IAA content of severe-phenotype plants was almost the same as that of wild-type tobacco. This could be due to the presence of numerous abnormal shoots on leaves of severe-phenotype plants that may act as a source of IAA.
Although the role of ABA in plant development is not clearly defined, this hormone is involved in many physiological responses, including stomatal control, dormancy, and adaptation to stress (Zeevaart, 1988). The ABA contents of transgenic tobacco plants with the mild or severe phenotype were approximately 7 and 10 times higher than that of wild-type plants, respectively (Fig. 1b; Table I).
Cytokinin Levels in OSH1-Transformed Tobacco Plants
Cytokinins were first characterized as compounds that promote cell division and are now known to evoke a diversity of responses in plants. Cytokinin derivatives have a wide range of activity. In these derivatives ribosides are an important translocation form, and conversion of cytokinin ribosides to bases is necessary for activity because the latter may be the active form (Letham and Palni, 1983). The cytokinin contents of tobacco plants were separately analyzed as the active form, Z, and its inactive form, ZR, by ELISA after HPLC fractionation. The severity of morphological alteration of OSH1-transformed tobacco plants correlated with an increase in Z levels (Fig. 1c; Table I). In addition, this increase in Z level was accompanied by an increase in the ratio of Z to ZR.
Partial Correction of Abnormal Morphology by Treatment with GA
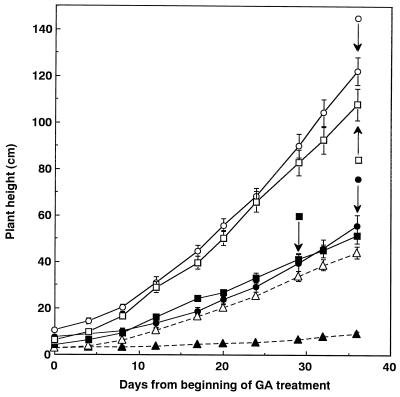
If the morphological alterations in OSH1-transformed tobacco are attributable to the decrease in active GA content, then the application of exogenous GA might be expected to correct the phenotype of transgenic tobacco plants to some extent. In transgenic tobacco exhibiting a mild phenotype, treatment with GA3 reduced the severity of abnormal leaf morphology (Fig. 2). In severe-phenotype transformants, treatment with GA3 also corrected the loss of apical dominance and the severity of leaf abnormalities. Furthermore, treatment with GA3 corrected the abnormal stem elongation in severe-phenotype plants. The stem length of GA3-treated transformants was almost the same as that of wild-type plants (Fig. 3). Finally, in mild-phenotype transformants, flower buds formed 1 week earlier than in wild-type plants. GA3 application restored the time of flower bud formation in these plants to that of wild-type plants (Fig. 3). In severe-phenotype transformants, flower buds formed 16 weeks later than in wild-type plants. GA3 application to these plants accelerated flower bud formation by 8 weeks.
Figure 2.
Phenotypic compensation of transgenic tobacco by GA3 treatment. Wild-type (a) and severe-phenotype (b) transgenic tobacco plants with (left) or without (right) GA3 treatment. The plants were photographed after those on the left had been treated with GA3 for 36 d. For GA3 treatment, 10 μL of 3 mm GA3 was applied to the shoot apex every 4th d, starting 40 d after sowing. c, Phenotypic alteration of leaves of mild-phenotype transformants. d, Phenotypic alteration of leaves of severe-phenotype transformants. a, Bar = 1 m; b, bar = 50 cm; and c and d, bar = 5 cm.
Figure 3.
Effect of GA3 treatment on plant height. Arrows indicate the emergence of flower buds. Each symbol represents the mean ± se of 11 to 13 replicates. •, Wild type; ○, wild type plus GA; ▴, severe phenotype; ▵, severe phenotype plus GA; ▪, mild phenotype; and □, mild phenotype plus GA.
DISCUSSION
The expression of OSH1 resulted in altered morphology in transgenic tobacco plants and was accompanied by significant changes in hormone levels. Plant hormones are well known to have diverse physiological activities. However, the factors regulating plant hormone metabolism have not yet been elucidated. Schmülling et al. (1993) demonstrated that overexpression of a single rol gene from Agrobacterium rhizogenes resulted in altered morphology and changes of hormonal contents and sensitivity in transgenic tobacco. They suggested that the phenotypic abnormalities of rol-transformed plants were due to complex effects of the altered hormone metabolism and sensitivity. From the fact that it contains a putative DNA-binding domain, the OSH1 gene product is thought to act as a transcription factor (Matsuoka et al., 1993). We propose that OSH1 controls the morphology of tobacco by affecting plant hormone metabolism and/or signal transduction.
We have analyzed the levels of GAs, IAA, ABA, and cytokinins in OSH1-transformed tobacco plants that exhibited morphological abnormalities. We have shown that the GA1 level becomes lower as the morphological aberrations of the transformants become more severe. However, ABA and Z levels become higher in morphologically changed transformants. Transgenic tobacco plants exhibiting a mild phenotype have wrinkled leaves that are thicker, shorter, and more disc-shaped than wild-type leaves. Severe-phenotype plants are dwarfed and their axillary buds develop into vegetative stems, whereas these buds are dormant in wild-type tobacco. The leaves of these plants are not wrinkled but are tiny, and occasionally abnormal shoots arise from them. It is well established that auxin and cytokinin are capable of controlling apical dominance. Under conditions of a high ratio of cytokinin to auxin, plants usually lose their apical dominance and axillary buds develop into vegetative shoots (Tamas, 1987). Based on these results, the loss of apical dominance in severe-phenotype OSH1 transformants could be caused by the higher level of cytokinin, which is shown in Figure 1. In addition, treatment of severe-phenotype OSH1 transformants with GA3 restored apical dominance (Fig. 2b); therefore, the decrease of GA1 level may also be the cause of loss of apical dominance.
In contrast, decreased levels of GA1 seem to be responsible for the observed dwarfism of severe-phenotype plants. GAs are well known to promote stem elongation in a variety of plants, and GA1 is the predominant active GA in tobacco plants, being present at a much higher level than GA4 (Table I). The fact that treatment of severe-phenotype transformants with GA3 restored stem elongation to near that of wild-type plants indicates that dwarfism in these transformants can be at least partly attributed to the decrease in GA1 content.
Auxin is also thought to play a critical role in stem elongation, as demonstrated by the phenotypes of several auxin-resistant mutants of Arabidopsis. The dwf, axr1, and axr2 mutants all exhibit shortened internodes (Mirza and Maher, 1980; Lincoln et al., 1990; Wilson et al., 1990). However, the IAA levels in transgenic tobacco plants did not correlate with the degree of morphological abnormality. In the plant kingdom the primary site of IAA biosynthesis is thought to be in meristems and young, developing organs. We believe that the abnormal shoots present on leaves of severe-phenotype plants could serve as a source of IAA. Therefore, unexpected higher levels of auxin might be detected in leaves of severe-phenotype plants. The production of abnormal shoots on leaves has also been observed in tobacco plants that overexpress a cytokinin biosynthetic gene for isopentenyltransferase (Estruch et al., 1991; Li et al., 1992). In severe-phenotype tobacco plants, the ratio of Z to ZR was strikingly higher than those of mild-phenotype and wild-type plants. The abnormal shoots seen on the leaves of severe-phenotype plants may therefore be due to the increased activity of cytokinins. This imbalance of the auxin to cytokinin ratio may affect the morphology of severe-phenotype plants.
GA was analyzed further in an effort to determine whether the morphological changes in severe-phenotype transformants were due to an inhibition of GA biosynthesis or sensitivity. Exogenous GA3 treatment of transgenic tobacco plants reduced the severity of altered leaf phenotype, dwarfism, and loss of apical dominance and partially restored their flowering time. In addition, RNA-blot analysis revealed that GA3 treatment did not affect the amount of OSH1 transcript present in transgenic tobacco plants (data not shown). These observations suggest that the OSH1 gene product probably does not affect the GA-signaling pathway but instead likely alters the biosynthesis or catabolism of active GA.
OSH1 causes pleiotropic morphological changes and, therefore, may have diverse effects on the metabolism of plant hormones, which are known to have physiological activity in plant growth and development. The signal transduction pathway from the OSH1 gene product has not yet been elucidated, and that from plant hormones also remains obscure. The data presented here suggest the possibility that OSH1 affects plant hormone metabolism either directly or indirectly and thereby causes changes in plant development. The mechanism(s) by which OSH1 affects hormonal activity remains to be elucidated.
ACKNOWLEDGMENTS
We would like to thank Y. Ohashi (National Institute of Agrobiological Resources, Tsukuba, Ibaraki, Japan) for kindly supplying us with wild-type tobacco plants, M. Nakajima and M. Hasegawa (University of Tokyo, Japan) for skillful technical assistance, and T. Maotani (National Institute of Fruit Tree Science, Tsukuba, Ibaraki, Japan) for helpful comments.
Abbreviations:
- AcOH
acetic acid
- BuOH
1-butanol
- EtOAc
ethyl acetate
- MeOH
methanol
- NOS
nopaline synthase
- Z
trans-zeatin
- ZR
trans-zeatin riboside
LITERATURE CITED
- Atzorn R, Weiler EW. The immunoassay of gibberellins. II. Quantification of GA3, GA4 and GA7 by ultra-sensitive solid-phase enzyme immunoassay. Planta. 1983;159:7–11. doi: 10.1007/BF00998807. [DOI] [PubMed] [Google Scholar]
- Estruch JJ, Prinsen E, Van Onckelen H, Schell J, Spena A. Viviparous leaves produced by somatic activation of an inactive cytokinin-synthesizing gene. Science. 1991;254:1364–1367. doi: 10.1126/science.254.5036.1364. [DOI] [PubMed] [Google Scholar]
- Garber RL, Kuroiwa A, Gehring WJ. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO J. 1983;2:2027–2036. doi: 10.1002/j.1460-2075.1983.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Vrain T, Jouanin L. Bacterial genes modifying hormonal balances in plants. Plant Physiol Biochem. 1994;32:11–29. [Google Scholar]
- Gehring WJ. Homeo boxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Graebe JE. Gibberellin biosynthesis and control. Annu Rev Plant Physiol. 1987;38:419–465. [Google Scholar]
- Hake S, Vollbrecht E, Freeling M. Cloning Knotted, the dominant morphological mutant in maize using Ds2 as a transposon tag. EMBO J. 1989;8:15–22. doi: 10.1002/j.1460-2075.1989.tb03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano-Murakami Y, Yanai T, Tagiri A, Matsuoka M. A rice homeotic gene, OSH1, causes unusual phenotype in transgenic tobacco. FEBS Lett. 1993;334:365–368. doi: 10.1016/0014-5793(93)80713-5. [DOI] [PubMed] [Google Scholar]
- Letham DS, Palni LMS. The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol. 1983;34:163–197. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco that overproduce cytokinins in specific tissues and organs. Dev Biol. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Ichikawa H, Saito A, Tada Y, Fujimura T, Kano-Murakami Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 1993;5:1039–1048. doi: 10.1105/tpc.5.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Tamaoki M, Tada Y, Fujimura T, Tagiri A, Yamamoto N, Kano-Murakami Y. Expression of rice OSH1 gene is localized in developing vascular strands and its ectopic expression in transgenic rice causes altered morphology of leaf. Plant Cell Rep. 1995;14:555–559. doi: 10.1007/BF00231937. [DOI] [PubMed] [Google Scholar]
- Mirza JI, Maher EP. More 2,4-D-resistant mutants. Arabidopsis Inf Serv. 1980;17:103–107. [Google Scholar]
- Schmülling T, Fladung M, Grossmann K, Schell J. Hormonal content and sensitivity of transgenic tobacco and potato plants expressing single rol genes of Agrobacterium rhizogenes T-DNA. Plant J. 1993;3:371–382. [Google Scholar]
- Sinha NR, Williams RE, Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Smith L, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, KNOTTED-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- Tamas IA. Hormonal regulation of apical dominance. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Boston, MA: Martinus Nijhoff; 1987. pp. 393–410. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]