Abstract
Introduction
Sudden cardiac death (SCD) is a large public health problem that warrants on-going evaluation in the general population. While single-year community-based studies have been performed there is a lack of studies that have extended evaluation to multiple years in the same community.
Methods and Results
From the on-going Oregon Sudden Unexpected Death Study, we analyzed prospectively identified SCD cases in Multnomah County, Ore, (population ≈700,000) from February 1, 2002 to January 31, 2005. Detailed information ascertained from multiple sources (first responders, clinical records and medical examiner) was analyzed. A total of 1,175 SCD cases were identified (61% male) with a mean age of 65±18 yrs for men vs. 70±20 for women (P <0.001). The overall incidence rate for the period was 58/100,000 residents/year. One-quarter (24.6%) were ≤55 yrs of age. The most common initial rhythm was ventricular tachycardia or fibrillation (39% of cases, survival 27%) followed by asystole (36%, survival 0.7%) and pulseless electrical activity (23%, survival 6%). Among subjects that underwent resuscitation, the rate of survival to hospital discharge was 12% and overall survival to hospital discharge irrespective of resuscitation was 8%. Of the 68 survivors, 16 (24%) received a secondary prevention ICD.
Conclusion
We report annualized SCD incidence from a multiple-year, multiple-source community-based study, with higher than expected rates of women and subjects age ≤55 yrs. The low implantation rate of secondary prevention ICDs is likely to be multifactorial, but there are potential implications for re-calibration of the projected need for ICD implantation; larger and more detailed studies are warranted.
Keywords: sudden cardiac death, cardiac arrest, ventricular fibrillation, implantable cardioverter defibrillator, cardiopulmonary resuscitation
Introduction
Sudden cardiac death (SCD) is estimated to claim 250,000-300,000 lives annually in the U.S. with significant implications for public health and cost of health care 1, 2 . Since the vast majority of such deaths occur unexpectedly in the field (as opposed to health care facilities) there is a well-recognized need to perform large and detailed population-based evaluations. Due to the challenging nature of sudden cardiac arrest (SCA) with approximately 95% mortality, recent consensus reports have highlighted the need for large studies that employ systematic and comprehensive methodology with consistent definitions for SCD 3, 4.
The Oregon Sudden Unexpected Death Study (Oregon SUDS) is a population-based evaluation of SCD among the residents of the Portland, Oregon metropolitan area ongoing since February 1, 2002. We have previously reported the annual incidence of SCD in this community for a single year (Feb 1, 2002-January 31, 2003) and our findings highlighted the importance of prospective, multiple source evaluation for an accurate assessment of SCD burden 5. Comparisons with death-certificate methodology demonstrated that retrospective methods of surveillance resulted in significant over-estimation (by at least 200%) as well as inaccuracy (positive predictive value 19%) of SCD burden. However, there remains a lack of studies that report evaluation and trends over multiple years in the community 3, 4. Other knowledge gaps in the epidemiology of SCD relate to evaluation of gender and race-ethnicity differences in SCD burden, a more detailed evaluation of the younger (≤55 years old) population and utilization of secondary prevention ICDs in the community. We therefore extended the epidemiology analysis from the Oregon SUDS to 3 years with a particular focus on these knowledge gaps.
Methods
All aspects of this research were approved by the Institutional Review Boards of the Cedars-Sinai Health System, Oregon Health and Science University, as well as all other participating institutions. The Ore-SUDS is an on-going, prospective, community-wide study of out-of-hospital SCD for which methods have been described in detail previously 5, 6. In brief, the study evaluates residents of the Portland, Oregon metropolitan area with a total population of ~1,000,000. However, for the present analysis, the population was limited to Multnomah County, Oregon the largest subset of the metro area residents (population ~700,000).
Case Identification and Ascertainment
Out-of-hospital cardiac arrest cases are identified through multiple sources: Emergency Medical Response Services (EMS), the Medical Examiner’s office, and all local hospitals. EMS for Multnomah County consists of a two-tier advanced cardiac life support system with fire department as well as ambulance company first responders. All available medical records (including EMS and Medical Examiner records) were obtained for each subject and used for detailed analysis of clinical data. Mode of death was evaluated and a diagnosis of SCD assigned by a majority consensus review following an independent in-house adjudication by 3 physicians, who closely evaluated the circumstances of SCD in combination with all available clinical data (including autopsy examination if available). Cases with non-cardiac cause of SCD, terminal illness, or drug overdose were excluded. SCD was defined as an ‘unexpected death without obvious extra-cardiac cause, occurring with a rapid witnessed collapse, or if unwitnessed, occurring within 1 hour after the onset of symptoms’ 3. We also included cases of probable SCD (‘an unexpected death without obvious extra-cardiac cause that occurred within the previous 24 hours’) and SCA (‘SCD cases in which specific resuscitation records are available or the individual has survived the cardiac arrest event’) 3.
All Multnomah County residents were included for calculation of incidence rates and basic demographic data. For detailed analysis, subjects <18 years of age were excluded since these data have been reported previously 7.
Definitions
The presenting arrhythmia was defined as the first documented rhythm at the time of SCA manifestation, obtained from the EMS record. Ventricular tachycardia (VT) and ventricular fibrillation (VF) were defined as pulseless conditions with typical electrocardiographic patterns. Pulseless electrical activity (PEA) was defined as a pulseless condition in the presence of organized electrocardiographic activity. Asystole was defined by absence of both pulse and electrical activity on cardiac monitoring. Response time was calculated as the time from dispatch of EMS to their arrival on the scene in contact with the patient and was treated as a continuous variable. Return of spontaneous circulation (ROSC) was defined as return of a palpable pulse in conjunction with a systolic blood pressure of >60 mm Hg 8, 9. Survival rate was defined as the number of SCA cases discharged alive from hospital divided by the total number of SCA cases. The incidence is reported annually for the each year and calculated as the total number of SCA cases divided by the total number of residents per 100,000. Data on the total number of inhabitants of Multnomah county were obtained from the US Census Bureau 10.
Coronary artery disease (CAD) was defined as 50% lumen stenosis on coronary angiogram prior to SCD event or identified at autopsy, or a history of myocardial infarction or coronary vascularization. A history of clinical conditions such as hypertension, hyperlipidemia, and diabetes mellitus were obtained from the detailed clinical records. In survivors, data on left ventricular (LV) function, assessed by echocardiography or LV angiography following, but unrelated to the cardiac arrest, was obtained from the medical records and presented as LV ejection fraction (LVEF). Body mass index (BMI) was calculated as mass (kg) divided by the height squared (m2).
Statistical Analysis
Continuous variables were expressed as mean values ± standard deviation (SD). Significant differences between groups were determined by using two-sided independent-sample Wilcoxon rank sum test for continuous variables and Fisher’s exact test for discrete variables. For all analyses, a value of P <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Description of SCD cases and Incidence Rates
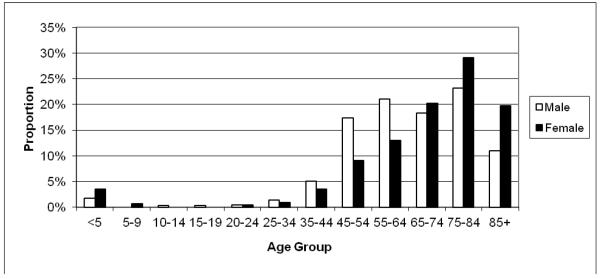
Between February 1, 2002 and January 31, 2005, a total of 1,175 cardiac arrest cases were identified in Multnomah County that met the criteria for SCD. Cases were identified by the EMS in the vast majority (73%) and by the medical examiner’s office in 26%. The remainder (1%) were identified by local hospital emergency departments. SCD cases were comprised of 714 men (61%) and 461 women (39%). The mean age was 65 ± 18 years for men and 70±20 for women (P<0.001). One quarter (24.6%) were ≤55 years and 43% were ≤65 years (Figure 1). The vast majority of the study population were Caucasian (83%), followed by African Americans (9%), and other ethnicities (6%).
Figure 1. Age and gender distribution.
The chart shows proportions of cases per gender and age group (Feb 1, 2002-Jan 31, 2005). A significant proportion of out-of-hospital sudden cardiac deaths occurs in subjects below retirement age.
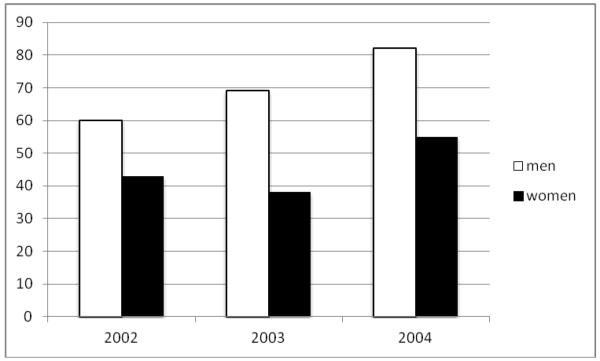
The overall mean incidence rate for the period was 58 per 100,000 inhabitants per year (men 70 per 100,000 and women 45 per 100,000). The incidence rate for each specific year during the follow-up period is presented in Figure 2.
Figure 2. Incidence rates of sudden cardiac death.
Incidence rates per 100,000 inhabitants in Multnomah County, OR for the 3 study years Feb 1, 2002-Jan 31, 2005.
Circumstances of Cardiac Arrest
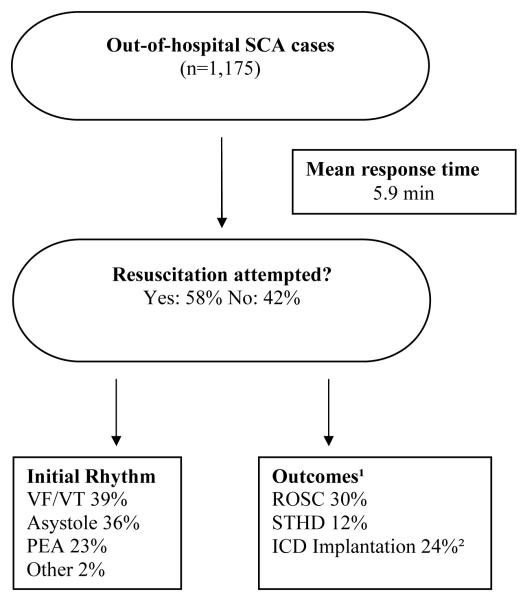
The majority of SCDs (72%) took place in the home (Figure 3). Forty-seven percent of arrests were witnessed and 53% were unwitnessed. There were no significant differences in age or gender between witnessed vs. unwitnessed cases. Resuscitation was attempted more often in the witnessed cases (91% vs. 35%, P<0.0001). The overall mean response time was 5.9 ± 2.9 minutes and there was no difference in response times between SCD at home or outside the home (P=0.18).
Figure 3. Circumstances and outcomes of cardiac arrests.
The flow chart summarizes the circumstances and outcomes of the cardiac arrests in Multnomah county, OR (Feb 1, 2002-Jan 31, 2005).
SCA=sudden cardiac arrest, VF=ventricular fibrillation, VT=ventricular tachycardia, PEA=pulseless electrical activity, ROSC=return of spontaneous circulation, STHD=survival to hospital discharge, ICD=implantable defibrillator-cardioverter.
1 data on outcomes were analysed in a subset of 559 cases with detailed clinical characterization available and resuscitation attempted
2analyzed in the subset of survivors to hospital discharge
The presenting rhythm was available for 759 subjects, comprising 65% of overall cases and 95% of those for whom resuscitation was attempted. VT/VF was observed in 297 subjects (39% of all cases with data available), PEA in 172 (23%), and asystole in 273 (36%). In 13 cases bradycardia was reported and in 4 cases the rhythm was denoted ‘Other’ (e.g., paced rhythm). The survival rate to hospital discharge for victims found with VT/VF was 27%, for PEA 6%, and for asystole 0.7%. There was no difference in response time between VT/VF and PEA/asystole groups (5.8±2.5 vs. 6.0±3.1 min, P=0.95). Subjects with PEA or asystole were older than those with VT/VF (67±20 vs. 65±16 years; P=0.0073) and more likely to be female (42% vs. 31%, P=0.01).
Resuscitation Outcomes and Clinical Characteristics
The overall adult SCA survival rate to hospital discharge irrespective of resuscitation was 8% (95 of 1165 cases with available outcome data). Resuscitation was attempted in 58% of all cases (n=559). In this group, ROSC was observed in 30% and 12% were discharged alive. The survival rate was 14.5% and 0.8% for witnessed and unwitnessed cases, respectively. Survival rates also differed when comparing events occurring at home and outside the home (4.5% vs. 13.4%). Moreover, bystander CPR was more commonly performed outside the home than at home (21.9% vs. 9.4%). The overall survival rate after bystander CPR was 20.6% (26 of 126 individuals survived to hospital discharge). When comparing survivors and non-survivors, the former group was younger (64.5±14.8 vs. 68.7±15.5 years, P=0.041), but there was no significant difference between the two groups regarding gender distribution (P=0.30). Response time was similar for both groups (P=0.59). However, survivors were more likely to have undergone bystander CPR (38% vs. 11%, P<0.0001). The circumstances and outcomes from SCA are summarized in Figure 3.
Detailed Characteristics of Survivors
Of the 68 survivors, 16 (24%) had an ICD implanted. This small subgroup consisted of 37 men and 31 women (P=0.18).
On comparing the 16 recipients of a secondary prevention ICD with the 52 non-recipients, the ICD group was younger (56±18 vs. 67±13 yrs, P=0.010), with a trend toward higher proportion of men (Table). Among the non-recipients, there was a trend towards a greater proportion of individuals discharged to a skilled nursing facility and a non-significant higher rate of significant co-morbidities (Table). Revascularization (PCI or CABG), indicating an ischemic etiology of the cardiac arrest, was performed in 31% of the patients that later received a secondary prevention ICD and in 35% of non-recipients, respectively (P=1.0). There was no evidence (from available ECGs, echocardiograms, autopsy findings, or medical records) of underlying primary arrhythmia syndromes (idiopathic ventricular fibrillation, long- or short-QT syndrome, cathecolaminergic polymorphic ventricular tachycardia, hypertrophic obstructive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, or Brugada syndrome). For 15 of the recipients and 39 of the non-recipients, assessments of LV function following, but unrelated to the cardiac arrest were available for analysis. It was found that non-recipients were more likely to have severe LV dysfunction defined as LVEF≤35% (7/12=77% of non-recipients [mean LVEF 27±5%] vs. 30/39=47% of recipients [mean LVEF 27±6%], P=0.050).
Table.
Details of the Survivor Sub-group: Secondary prevention ICD recipients vs. non-recipients
| Survivor Sub-group (n=68) | |||
|---|---|---|---|
| Variable | ICD implanted (n=16) |
No ICD implanted (n=52) |
P-value |
| Age (mean ± SD yrs) | 56 ± 18 | 67 ± 13 | 0.010 |
| Male gender (%) | 75 | 48 | 0.085 |
| Race White/Black/Asian/Other/Unknown |
14/0/1/0/1 | 40/3/3/1/3 | † |
| PCI | 5 (31%) | 15 (29%) | 1.0 |
| CABG | 0 (0%) | 3 (6%) | † |
| LVEF post arrest (%) | 43±18 | 47±14 | 0.46 |
| LVEF ≤35% | 7/12 (47%) | 30/39 (77%) | 0.050 |
| Primary Arrhythmia Syndrome‡ | 0 | 0 | † |
| Discharged to SNF | 1 (6%) | 15 (29%) | 0.09 |
| Refusal | 0 | 1 | † |
| Significant co-morbidity* | 3 (19%) | 13 (25%) | 0.88 |
T-test (two-tailed) and Fisher’s Exact test
End-stage renal disease, Chronic Obstructive Pulmonary Disease, neuropsychiatric conditions
not analyzed due to small numbers
idiopathic ventricular fibrillation, long or short QT syndrome, cathecolaminergic polymorphic ventricular tachycardia, hypertrophic obstructive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, or Brugada syndrome
ICD= implantable cardioverter-defibrillator, PCI= percutaneous coronary intervention, CABG= coronary artery bypass graft surgery, LVEF=left ventricular ejection fraction, SNF=skilled nursing facility
LVEF data was available in 15 of the 16 ICD-recipients and in 39 of the 52 non-recipients
Analysis of clinical history and risk factors
Detailed clinical analysis was performed in 960 adult subjects (82% of adult cases) for whom data was obtainable from medical records, medical examiner and EMS records. Of the 960 cases (585 male [61%] and 385 female [39%]), 411 (43%) had known CAD. Moreover, 567 (59%) had hypertension, 289 (30%) diabetes, and 297 (31%) hyperlipidemia. Data on these comorbidities were missing in 7 subjects. As for other common cardiovascular risk factors, the mean BMI was 28.9±7.7 for men and 29.5±10.9 for women (P=0.44). The proportion of smokers was 30% among males and 20% among females.
Discussion
In this multiple year, multiple source community-based evaluation of SCD incidence and temporal trends, the annualized incidence of 58 per 100,000 inhabitants is consistent with most existing community-based studies11-15. However, there are several new findings that merit discussion and further focused evaluation. We found that women comprised 40% of all cases, 25% of all cases were age ≤55 years and, most importantly, the rate of secondary prevention ICD implantation in the community was significantly lower than anticipated (24%).
The findings that 40% of all SCD cases were women, is significantly different from the 3:1 ratio between men and women that has been reported in several earlier studies 16-18. While the factors for this altered trend need to be evaluated in greater detail it could reflect changing gender trends in prevalence of coronary artery disease 19. However, women still present at older ages than men (mean age 70 vs. 65 years in the present study).
It is well established that the incidence of SCD increases with age and that the majority of cardiac arrest victims are elderly. Becker et al reporting findings from the Chicago CPR project and observed that the annualized incidence of SCA was eight times higher in 75-year-old men compared to 50-year-old men 20. However, in the present study, a surprisingly high proportion (25%) 5 were ≤55 years of age at the time of arrest. Since the societal impact of SCD in terms of productive life-years lost is likely to be the highest in this age-group, this finding needs close examination. Even though the incidence of SCD is low in the general population, the absolute numbers will be significant 1. Taken together with the persisting low survival rates from SCA, this underscores the need for improving risk stratification techniques in the general population. In our study, the overall survival rate was 8%, consistent with previous reports from Europe 21, 22, and the U.S. 23, 24. Also consistent with previous studies, there was a markedly increased survival rate of subjects who received bystander CPR, which highlights the need for further educational activities in the community 25-27.
The low implantation rate of secondary prevention ICDs among survivors (24% of survivors to hospital discharge) has significant potential implications for the projected need of ICD implantation and merits close evaluation. While a subgroup of survivors were likely to have reversible conditions that led to SCA, this rate is significantly lower than anticipated. An administrative database of cardiac arrest survivors (1996-2001) showed a ~46% rate of secondary prevention ICD use in the most contemporary portion of the cohort 28, 29. While there could be multiple factors involved, possibilities include higher than anticipated rates of persisting neurological deficits or reversible high-risk cardiac conditions (such as ST-elevation myocardial infarction) among survivors. In the present study, 31% of the ICD recipients underwent revascularization, while this rate was 35% among those not receiving ICD (P=1.0). However, non-recipients were less likely to have a relatively preserved LV function (LV>35%) when compared to recipients (P=0.050). Further, 29% of the patients that did not receive an ICD were discharged to a skilled nursing facility as opposed to 6% of the recipients. Although this difference was not significant (P=0.09), the finding merits further investigation since it might indicate that persisting neurological deficits and/or significant co-morbidities may, in part, explain the low implantation rate of secondary prevention ICDs. Since the number of subjects in the survivor group is relatively small, it would be important to confirm these findings in a larger study in other communities and ethnicities. Several ICD registries have reported underutilization of primary preventive ICDs, particularly in women and minorities 28-32 but these have not been well-evaluated for secondary prevention ICDs. In the present study, women were equally likely to be eligible for the ICD (46% women), but there was a trend toward higher likelihood of implantation in men and those who received an ICD were younger (P=0.010). It would also be of interest to survey potential differences in clinical practice patterns of health care providers, as well as socioeconomic and health insurance status between the two groups, but this information was not available for the present analysis.
Limitations
This study has several possible limitations. Since our analysis was performed in a single US community with a majority Caucasian, European descent population, the results might not be readily generalizable to other communities and ethnicities. Secondly, the inherent nature of community-based SCD evaluations results in a certain proportion of missing information especially for past clinical history, since 40-50% could have SCD as the first manifestation of disease with low rates of visits to health care providers. As a consequence, the clinical information regarding cardiac and non-cardiac morbidities is available in less than 50% of the population. However, the careful adjudication process including multiple source data is likely to exclude a majority of the non-cardiac causes. Furthermore, a proportion of survivors may not have complete neurological recovery, making it difficult to contact and consent them for review of additional information. The alternative of a prospective cohort study of the general population is also not feasible based on the large size of the cohort needed to provide adequate numbers for analysis. Also, we did not have access to data regarding the neurological status at discharge or other measurements of quality-of-life that will need to be evaluated in future studies. Finally, since the survivors constituted a relatively small group, the findings related to survivors should be interpreted with caution.
Conclusion
Using multiple-year, multiple-source surveillance, we report an SCD incidence of 58 per 100,000 with higher than expected proportion of females and subjects with age≤55 years. There was an unexpectedly low ICD implantation rate after survival with potential implications for re-calibration of the projected need for ICD implantation. While our findings suggest that neuropsychiatric or co-morbid conditions may in part account for these findings, further detailed investigation in larger studies is warranted.
Acknowledgements
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments, and the Oregon State Medical Examiner’s office. We thank Dr Eric C. Stecker for his critical review of the manuscript.
Funded in part by National Heart Lung and Blood Institute R01HL088416 and R01HL105170 to SSC. RH is supported by a grant (#2011-1071) from the Swedish Research Council. SSC is the Pauline and Harold Price Professor of Cardiac Electrophysiology at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Footnotes
No disclosures.
References
- [1].Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U. S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- [6].Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- [7].Chugh SS, Reinier K, Balaji S, Uy-Evanado A, Vickers C, Mariani R, Gunson K, Jui J. Population-based analysis of sudden death in children: The Oregon Sudden Unexpected Death Study. Heart Rhythm. 2009;6:1618–1622. doi: 10.1016/j.hrthm.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett P, Becker L, Bossaert L, Delooz H, Dick W, Eisenberg M, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. Task Force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Ann Emerg Med. 1991;20:861–874. [PubMed] [Google Scholar]
- [9].Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa) Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [10]. http://www.census.gov.
- [11].Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- [12].Westfal RE, Reissman S, Doering G. Out-of-hospital cardiac arrests: an 8-year New York City experience. Am J Emerg Med. 1996;14:364–368. doi: 10.1016/S0735-6757(96)90050-9. [DOI] [PubMed] [Google Scholar]
- [13].Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. JAMA. 1994;271:678–683. [PubMed] [Google Scholar]
- [14].Kass LE, Eitel DR, Sabulsky NK, Ogden CS, Hess DR, Peters KL. One-year survival after prehospital cardiac arrest: the Utstein style applied to a rural-suburban system. Am J Emerg Med. 1994;12:17–20. doi: 10.1016/0735-6757(94)90190-2. [DOI] [PubMed] [Google Scholar]
- [15].Fredriksson M, Herlitz J, Nichol G. Variation in outcome in studies of out-of-hospital cardiac arrest: a review of studies conforming to the Utstein guidelines. Am J Emerg Med. 2003;21:276–281. doi: 10.1016/s0735-6757(03)00082-2. [DOI] [PubMed] [Google Scholar]
- [16].Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–2703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- [17].Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- [18].Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. American heart journal. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- [19].Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- [20].Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329:600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- [21].de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- [22].Herlitz J, Rosenfelt M, Bang A, Axelsson A, Ekstrom L, Wennerblom B, Lowhagen O, Palmqvist M, Holmberg S. Prognosis among patients with out-of-hospital cardiac arrest judged as being caused by deterioration of obstructive pulmonary disease. Resuscitation. 1996;32:177–184. doi: 10.1016/0300-9572(96)00970-7. [DOI] [PubMed] [Google Scholar]
- [23].Greene HL. Sudden arrhythmic cardiac death--mechanisms, resuscitation and classification: the Seattle perspective. Am J Cardiol. 1990;65:4B–12B. doi: 10.1016/0002-9149(90)91285-e. [DOI] [PubMed] [Google Scholar]
- [24].Cobb LA, Weaver WD, Fahrenbruch CE, Hallstrom AP, Copass MK. Community-based interventions for sudden cardiac death. Impact, limitations, and changes. Circulation. 1992;85:I98–102. [PubMed] [Google Scholar]
- [25].Herlitz J, Ekstrom L, Wennerblom B, Axelsson A, Bang A, Holmberg S. Effect of bystander initiated cardiopulmonary resuscitation on ventricular fibrillation and survival after witnessed cardiac arrest outside hospital. Br Heart J. 1994;72:408–412. doi: 10.1136/hrt.72.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vaillancourt C, Stiell IG. Cardiac arrest care and emergency medical services in Canada. Can J Cardiol. 2004;20:1081–1090. [PubMed] [Google Scholar]
- [27].Stiell IG, Wells GA, Field B, Spaite DW, Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley D, Luinstra-Toohey L, Campeau T, Dagnone E, Lyver M. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- [28].Voigt A, Ezzeddine R, Barrington W, Obiaha-Ngwu O, Ganz LI, London B, Saba S. Utilization of implantable cardioverter-defibrillators in survivors of cardiac arrest in the United States from 1996 to 2001. J Am Coll Cardiol. 2004;44:855–858. doi: 10.1016/j.jacc.2004.05.053. [DOI] [PubMed] [Google Scholar]
- [29].Saba S, Ravipati LP, Voigt A. Recent trends in utilization of implantable cardioverter-defibrillators in survivors of cardiac arrest in the United States. Pacing and clinical electrophysiology : PACE. 2009;32:1444–1449. doi: 10.1111/j.1540-8159.2009.02509.x. [DOI] [PubMed] [Google Scholar]
- [30].Thomas KL, Al-Khatib SM, Kelsey RC, 2nd, Bush H, Brosius L, Velazquez EJ, Peterson ED, Gilliam FR. Racial disparity in the utilization of implantable-cardioverter defibrillators among patients with prior myocardial infarction and an ejection fraction of <or=35% Am J Cardiol. 2007;100:924–929. doi: 10.1016/j.amjcard.2007.04.024. [DOI] [PubMed] [Google Scholar]
- [31].Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- [32].Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–1524. doi: 10.1001/jama.298.13.1517. [DOI] [PubMed] [Google Scholar]