Abstract
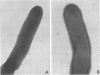
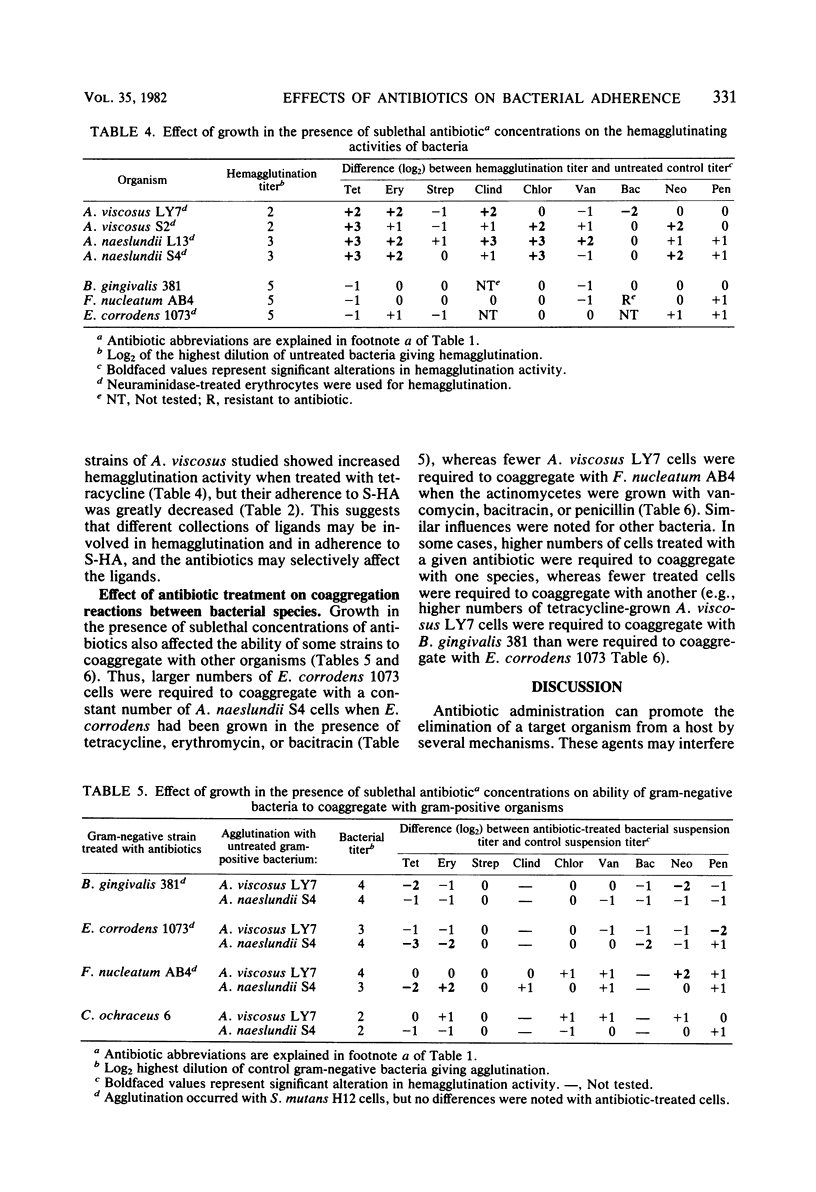
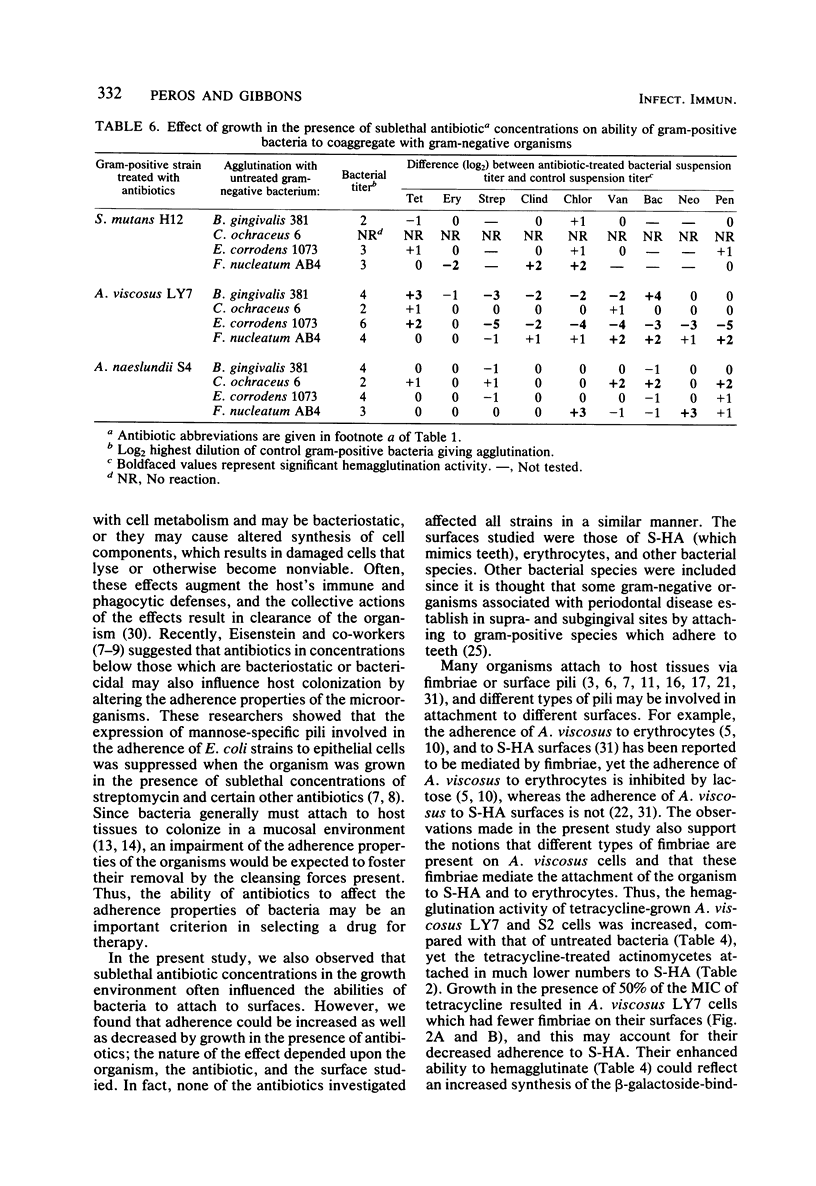
The influence of growth in the presence of sublethal concentrations of nine antibiotics on the ability of certain potentially odontopathic bacteria to attach to saliva-treated hydroxyapatite surfaces which mimic teeth was studied. Cells of Actinomyces viscosus LY7 and S2, Bacteroides gingivalis 381, Capnocytophaga ochraceus 6, and Actinobacillus actinomycetemcomitans N27 attached in lower numbers to saliva-treated hydroxyapatite when grown in the presence of 50% of the minimum inhibitory concentration of tetracycline. Electron microscopic observations of negatively stained preparations indicated that tetracycline-grown A. viscosus LY7 cells had fewer fimbriae than did untreated cells, which may account for the impaired ability of the treated cells to attach. However, cells of Actinomyces naeslundii L13 and S4 attached in higher numbers when grown in the presence of tetracycline, clindamycin, erythromycin, chloramphenicol, or neomycin. Streptococcus mutans strains H12 and JBP also exhibited increased adherence to saliva-treated hydroxyapatite when grown in the presence of 50 or 25% of the minimum inhibitory concentration of penicillin. Thus, growth in the presence of sublethal antibiotic concentrations could increase as well as decrease the adherence of bacteria to saliva-treated hydroxyapatite. Antibiotic-grown cells of the Actinomyces strains showed enhanced hemagglutination activity, but this did not correlate with their ability to attach to saliva-treated hydroxyapatite. Sublethal concentrations of antibiotics in the growth media also affected the coaggregation reactions of several organisms; the effects were specific for one member of the coaggregation pair.
Full text
PDF

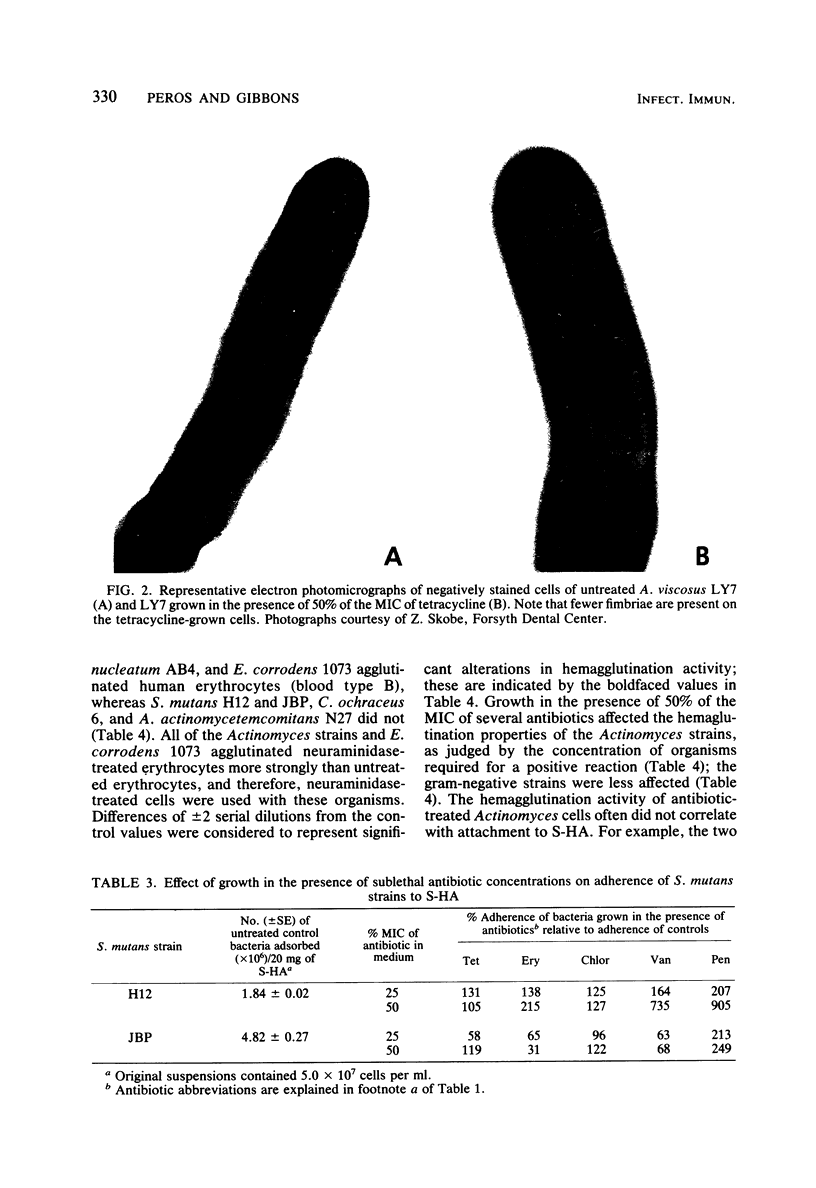


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bammann L. L., Clark W. B., Gibbons R. J. Impaired colonization of gnotobiotic and conventional rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978 Dec;22(3):721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Ofek I. Influence of sublethal concentrations of antibiotics on the expression of the mannose-specific ligand of Escherichia coli. Infect Immun. 1980 Apr;28(1):154–159. doi: 10.1128/iai.28.1.154-159.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Interference with the mannose binding and epithelial cell adherence of Escherichia coli by sublethal concentrations of streptomycin. J Clin Invest. 1979 Jun;63(6):1219–1228. doi: 10.1172/JCI109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Loss of lectin-like activity in aberrant type 1 fimbriae of Escherichia coli. Infect Immun. 1981 Feb;31(2):792–797. doi: 10.1128/iai.31.2.792-797.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hardy L., Jacques N. A., Forester H., Campbell L. K., Knox K. W., Wicken A. J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981 Jan;31(1):78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenberg B., Lindhe J. Juvenile periodontitis. Some microbiological, histopathological and clinical characteristics. J Clin Periodontol. 1980 Feb;7(1):48–61. doi: 10.1111/j.1600-051x.1980.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Qureshi J. V., Gibbons R. J. Differences in the adsorptive behavior of human strains of Actinomyces viscosus and Actinomyces naeslundii to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Jan;31(1):261–266. doi: 10.1128/iai.31.1.261-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Evans R. T., Lobbins P. M., Genco R. J. In vitro antimicrobial susceptibility of Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1980 Jul;18(1):9–12. doi: 10.1128/aac.18.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Mashimo P., Levine M. J., Genco R. J. Periodontal therapy in humans. I. Microbiological and clinical effects of a single course of periodontal scaling and root planing, and of adjunctive tetracycline therapy. J Periodontol. 1979 Oct;50(10):495–509. doi: 10.1902/jop.1979.50.10.495. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]