Abstract
Objective
To study the relation between cherry intake and the risk of recurrent gout attacks among individuals with gout.
Methods
We conducted a case-crossover study to examine associations of a set of putative risk factors with recurrent gout attacks. Individuals with gout were prospectively recruited and followed online for one year. Participants were asked about the following information when experiencing a gout attack: the onset date of the gout attack, symptoms and signs, medications (including anti-gout medications), and potential risk factors (including daily intake of cherries and cherry extract) during the 2-day period prior to the gout attack. We assessed the same exposure information over 2-day control periods. We estimated the risk of recurrent gout attacks related to cherry intake using conditional logistic regression.
Results
Our study included 633 individuals with gout. Cherry intake over a 2-day period was associated with a 35% lower risk of gout attacks compared with no intake (multivariate odds ratio [OR] = 0.65, 95% CI: 0.50-0.85). Cherry extract intake showed a similar inverse association (multivariate OR=0.55, 95% CI: 0.30-0.98). The effect of cherry intake persisted across subgroups by sex, obesity status, purine intake, alcohol use, diuretic use, and use of anti-gout medications. When cherry intake was combined with allopurinol use, the risk of gout attacks was 75% lower than periods without either exposure (OR=0.25, 95% CI: 0.15-0.42).
Conclusions
These findings suggest that cherry intake is associated with a lower risk of gout attacks.
Keywords: Cherry, gout, case-crossover study
Gout is an excruciatingly painful inflammatory arthritis caused by the crystallization of uric acid within joints. The prevalence of gout in the US was estimated to be 3.9% of US adults based on the National Health and Nutrition Examination Survey 2007-2008, which translates into 8.3 million US adults (1, 2). While the pathophysiology of gout is well-characterized and efficacious pharmacological regimens are available, many patients with gout continue to experience recurrent gout attacks (3, 4). Such attacks cause tremendous pain and suffering and are a major cause of morbidity.
Over the past few decades, cherries have garnered considerable public attention and interest from both patients and investigators as potentially effective options in the prevention and management of gout. Small experimental studies in healthy human subjects and animals have demonstrated that cherry consumption lowers serum uric acid levels (5, 6). Others have reported that cherry products contain high levels of anthocyanins (7-9) that possess anti-inflammatory and antioxidant properties (8, 10-12). Furthermore, some cherry producers have claimed that cherry products have the potential to reduce the pain associated with gout (13) and some patients use cherries as a strategy to avoid and/or treat gout attacks (14). However, to our knowledge, no study has assessed whether consumption of cherries lowers the risk of gout attacks, as reflected in warning letters sent to various cherry-based product manufacturers by the Food and Drug Administration about the lack of sufficient data regarding their claims of disease-related benefits of cherry products (13).
To help address this relevant knowledge gap, we analyzed 633 gout patients who were prospectively recruited from across the United States in an online gout study (15). In this study, we used a case-crossover design to quantify the relative risk of gout attack after cherry intake as compared with no cherry intake and its potential modification by allopurinol use and major gout risk factors.
Methods
Study Population and Design
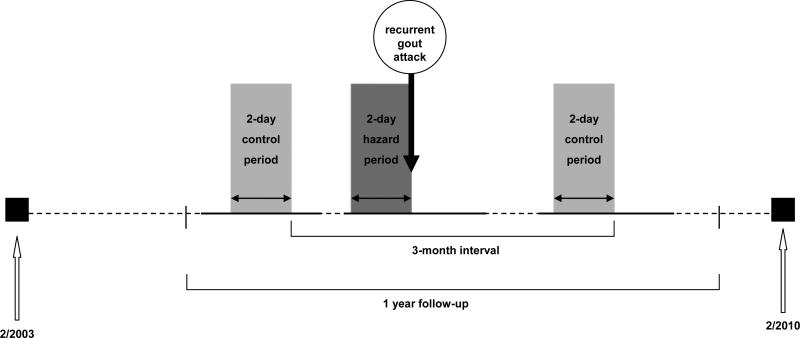
The Boston University online gout study is an ongoing internet-based, case-crossover study that started in February 2003 with primary aims to investigate purported triggers for recurrent gout attacks (15). The study design and timing of exposure measurements in relation to recurrent gout attacks are displayed in Figure 1. As previously described in detail (15), with this study design, each study participant serves as his or her own control, and self-matching eliminates confounding by risk factors that are constant within an individual but that would differ between study subjects during the study period (e.g., genetics, sex, race, education). Such a study design has been successfully utilized in many previous studies where the effect of transient risk factors on the risk of an acute event was evaluated (e.g., triggering factors for myocardial infarction or motor vehicle collision) (16).
Figure 1. Case-Crossover Study Design and Timing of Exposure Measurements in Relation to Gout Attacks.
Recurrent gout attacks could occur anytime during a 1-year follow-up period in a given patient. Hazard periods refer to the 2-day periods prior to recurrent gout attack. Up to four control periods were selected from the intercritical period every 3 months during the 1-year of follow-up. Exposure to cherry intake and other time-varying factors (potential confounders) were compared between hazard and control periods. The current study spanned February 2003 to February 2010. Horizontal time axis is not drawn to scale, which is reflected by the dotted line.
Subject recruitment
We constructed a study website on an independent secure server in the Boston University School of Medicine domain (https//dcc2.bumc.bu.edu/GOUT). The study was advertised on the Google search engine by linking an advertisement to the search term ‘gout.’ Individuals who clicked on the study advertisement were directed to the study website and were asked to complete the following questions at the entry of the study. These included: socio-demographic information; gout-related data (e.g., diagnosis of initial gout attack, age of onset, medication used for treatment of gout, and number of gout attacks in the last 12 months); and history of other diseases and medication use.
To be eligible for the study, a subject had to report gout diagnosed by a physician, have had a gout attack within the past 12 months, be at least 18 years of age, reside in the United States, and agree to the release medical records pertaining to gout diagnosis and treatment, and provide electronic informed consent. To confirm the diagnosis of gout, we obtained medical records pertaining to the participant's gout history and/or a checklist of the features listed in the American College of Rheumatology (ACR) Preliminary Classification Criteria for Gout (17) completed by the subject's physician. Two rheumatologists (DJH, TN) reviewed all medical records and checklists and determined whether the participants had a diagnosis of gout according to ACR criteria (17). Similar methods of gout diagnosis confirmation have been used in the Health Professional Follow-up Study (18). The study was approved by the Institutional Review Board of Boston University Medical Campus.
Ascertainment of recurrent gout attacks
Data were collected regarding the onset date of the recurrent gout attack, anatomical location of the attack, clinical symptoms and signs (maximal pain within 24 hours or redness), and medications used to treat the attack (i.e. colchicine, NSAIDs, systemic cortico-steroids, and intra-articular cortico-steroid injections). Our method of identifying gout attacks is in keeping with approaches used in acute and chronic gout trials (19-21) and the ACR/EULAR-supported initiative for defining gout attacks that includes only patient-reported elements (22). We further evaluated the robustness of our case definition by restricting recurrent attacks to those treated with at least one anti-gout medication (as listed above), those with podagra, those with maximal pain within 24 hours, those with redness, and those with combinations of these features (i.e., cases with ≥2, ≥3, and all 4 features).
Ascertainment of risk factors
A set of putative risk factors during the 2-day period were assessed for each participant, including purported dietary factors, alcohol use, infections, immunizations, physical activity, geographic location, anti-gout medications, and purported alternative remedies such cherry products. Of note, cherry intake was one of the hypothesized exposures considered to be potentially relevant to the risk of gout attacks since the conception of the Boston University online gout study (Figure 1). Questions on cherry intake (i.e., the fruit) included the number of servings of cherries, with one standard serving size being one half cup or about 10-12 cherries, on each of the prior 2 days during the hazard periods (i.e., 2 days prior to the gout attack). In addition, cherry extract use (yes or no) was assessed on each of the prior 2 days. The same list of exposure data was also collected at the following time points (i.e., control periods, 4 in total): at study entry (for those subjects who entered the study during an intercritical period), and at 3, 6, 9, and 12 months of follow-up (for those subjects who entered the study at the time of a gout attack) (Figure 1). Other potentially time-varying exposures that could be pertinent to gout attack risk were also assessed in the same manner.
Statistical Analysis
Since each person could have more than one hazard and/or more than one control period and these were matched within a subject, we examined the relation of cherry intake over the prior 2 days to the risk of gout attacks using conditional logistic regression. In a multivariable regression model, we adjusted for purine intake and use of alcohol, diuretics, allopurinol, colchicine, and nonsteroidal anti-inflammatory drugs (NSAIDs). To evaluate a potential dose-response relationship with the risk of gout attacks, we grouped cherry intake over 2 days into five categories: 0, 1, 2, 3 and ≥ 4 servings. We also evaluated the association with cherry extract intake alone, as well as with either cherry or cherry extract intake, using the same multivariable model. Finally, we assessed potential subgroup effects of cherry or cherry extract intake according to sex, body mass index (<30 vs. ≥ 30), purine intake (≤ 1.7 grams [median value] vs. >1.7 grams), use of alcohol (yes vs. no), diuretics (yes vs. no), allopurinol (yes vs. no), colchicine (yes vs. no) and NSAID (yes vs. no) over the prior 2 days. We determined the statistical significance of potential subgroup effects by testing the significance of interaction terms added to our final multivariable models. For all odds ratios, we calculated 95% confidence intervals (CIs). All P values are two-sided. We used SAS 9.2 for all analyses.
Role of the Funding Source
This research was funded by grants from the NIAMS (P60AR047785), Arthritis Foundation, American College Rheumatology Research and Education Fund. The authors have no potential conflicts of interest, including patents, pending patents or other products related to the use of cherry or cherry juice extract for gout.
RESULTS
Six hundred and sixty three gout patients completed both hazard-period and control-period questionnaires over a consecutive 12-month period between February 2003 and February 2010. Of them, 554 (87.5%) participants met the ACR Preliminary Classification Criteria for Gout. The characteristics of the participants are presented in Table 1. The average age of the participants was 54 years. Participants were predominantly men (78%), white (88%), and over half had received a college education. Subjects were recruited from 49 states and the District of Columbia. Approximately 61% of participants consumed alcohol, 29% used diuretics, 45% took allopurinol, 54% used NSAIDs, and 25% took colchicine during either the hazard or control periods.
Table 1.
Characteristics of 633 Participants in the Internet-Based Case-Crossover Study of Gout
| Characteristics | Median or Percent |
|---|---|
| Sex (n, %) | |
| Men | 494 (78.0) |
| Age (median, range) | 54 (21-88) |
| BMI (kg/m2, median, range) | 30.6 (14.7, 69.9) |
| Race (n, %) | |
| Black | 19 (3.0) |
| White | 558 (88.2) |
| Other | 47 (7.4) |
| Refused to answer | 9 (1.4) |
| Education (n, %) | |
| Less than high school graduate | 10 (1.6) |
| High school graduate | 55 (8.7) |
| Some college/technical school | 199 (31.4) |
| College graduate | 157 (24.8) |
| Some professional/graduate school | 70 (11.1) |
| Completed professional or graduate school | 142 (22.4) |
| Household income (n, %) | |
| <25,000 | 51 (8.1) |
| 25,000-49,999 | 127 (20.7) |
| 50,000-74,999 | 121 (19.1) |
| 75,000-99,999 | 89 (14.1) |
| >100,000 | 163 (25.8) |
| Refused to answer | 82 (13.0) |
| BMI (kg/m2, median, range) | 30.6 (14.7-69.9) |
| Years of disease duration (median, range) | 5 (1-49) |
| Alcohol drinkers (n, %) | 383 (60.5) |
| Diuretic users (n, %) | 184 (29.1) |
| Allopurinol users (n, %) | 285 (45.0) |
| Nonsteroidal anti-inflammatory drugs users (n, %) | 342 (54.0) |
| Colchicine users (n, %) | 160 (25.3) |
During the one-year follow-up period, we documented 1,247 gout attacks. Most gout attacks occurred in the lower extremity (92%), particularly in the first metatarsophalangeal joint, and had features of either maximal pain within 24 hours or redness (89%). Approximately 90% of gout attacks were treated with colchicine, NSAIDs, systemic cortico-steroids, intra-articular cortico-steroid injections, or a combination of these medications. The median time between onset of gout attack and completion of the hazard-period questions was 3 days.
Of 633 participants included in the analysis, 224 (35%) reported only ingesting fresh cherry fruit, 15 (2%) only cherry extract, and 33 (5%) both fresh cherry fruit and cherry extract during hazard, control, or both periods. As shown in Table 2, cherry intake over a two-day period was associated with a 35% lower risk of gout attacks compared with no intake (multivariate OR=0.65, 95% CI: 0.50-0.85. The risk of gout attacks tended to decrease with increasing cherry consumption, up to 3 servings over 2-days; however, further intake did not appear to provide a greater protective effect. Cherry extract intake was associated with a 45% lower risk of gout attacks (multivariate OR=0.55, 95% CI: 0.30-0.98). When intake of either cherries or cherry extract was considered together, the risk of gout attacks was decreased by 37% (multivariate OR=0.63, 95% CI: 0.49-0.82) (Web Appendix Table 1). When we additionally adjusted for caffeinated beverage intake and number of servings of vegetables consumption over the past 2-days, the results did not change materially (OR=0.65, 95% CI: 0.50-0.85).
Table 2.
Cherry or Cherry Extract Intake in Prior 2 Days and Risk of Gout Attacks
| Variable | Number of control periods | Number of hazard periods | Crude OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Cherries Only | ||||
| No | 1318 | 1074 | 1.0 (referent) | 1.0 (referent) |
| Yes (any amount) | 271 | 173 | 0.69 (0.54-0.89) | 0.65 (0.50-0.85) |
| 1 serving | 71 | 53 | 0.92 (0.62-1.36) | 0.98 (0.65-1.48) |
| 2 servings | 98 | 56 | 0.59 (0.40-0.89) | 0.52 (0.34-0.79) |
| 3 servings | 35 | 16 | 0.48 (0.25-0.92) | 0.39 (0.20-0.77) |
| ≥4 servings | 67 | 48 | 0.69 (0.44-1.09) | 0.62 (0.38-1.00) |
| P for trend | <0.003 | <0.001 | ||
| Cherry Extract Only | ||||
| No | 1520 | 1212 | 1.0 (referent) | 1.0 (referent) |
| Yes | 69 | 35 | 0.59 (0.34-1.04) | 0.55 (0.30-0.98) |
| Cherries or Cherry Extract | ||||
| No | 1278 | 1052 | 1.0 (referent) | 1.0 (referent) |
| Yes | 311 | 195 | 0.68 (0.53-0.86) | 0.63 (0.49-0.82) |
Adjusted for purine intake and use of alcohol, diuretics, allopurinol, colchicine and NSAIDs
When we limited the analysis to participants who met to the ACR Preliminary Classification Criteria for Gout (n=554) the multivariable OR of recurrent gout attacks was 0.65 (95% CI: 0.50-0.85) for cherry intake over 2-day period. In addition, when we varied the definition of recurrent gout attacks by requiring specific features individually or in combination, the results did not change materially. For example, when we limited the analysis to those that had at least two of the four features (anti-gout medication use, podagra, maximal pain within 24 hours, and redness) (n=606), the multivariable OR was 0.63 (95% CI, 0.48-0.82).
The inverse association with cherry intake persisted across subgroups by sex and body mass index. Multivariate ORs between cherry intake and the risk of recurrent gout attacks were 0.68 (95% CI: 0.51-0.91) among men and 0.48 (95% CI: 0.27-0.83) among women (P for interaction = 0.28). The corresponding ORs were 0.57 (95% CI: 0.40-0.80) among obese and 0.72 (95% CI: 0.49-1.04) among non-obese individuals (p for interaction = 0.35).
Table 3 summarizes the combined effects of cherry intake and various gout-related risk factors or medications on the risk for gout attacks. Increase of alcohol consumption and purine intake as well as use of diuretic were associated with higher risk of recurrent gout attacks, whereas use of allopurinol and colchicine was associated with lower risk of gout attacks. There was no association between NSAIDs use and risk of recurrent gout attacks (Web Appendix Table 1). The effect of cherry intake on the risk of recurrent gout attacks tended to be stronger when consumed during periods of higher purine intake, alcohol abstention, and when diuretics or NSAIDs were not used. Notably, when cherry intake was combined with allopurinol use, the risk of gout attacks was 75% lower than without either exposure (OR=0.25, 95% CI: 0.15-0.42). None of these factors showed significant interaction with the effect of cherry intake (p values for interaction > 0.17).
Table 3.
Cherries or Cherry Extract Intake in Prior 2 Days and Risk of Gout Attacks in Subgroups by Purine Intake, Alcohol Consumption, Use of Diuretics, and Anti-Gout Drugs
| Risk factors over 2 days | Cherry or cherry extract intake over 2 days* | Number of control periods | Number of hazard periods | Adjusted OR (95% CI)** |
|---|---|---|---|---|
| Purine Intake | ||||
| >1.7 grams (high) | No | 536 | 604 | 1.00 (referent) |
| >1.7 grams (high) | Yes | 152 | 119 | 0.60 (0.44-0.83) |
| ≤1.7 grams (low) | No | 742 | 448 | 0.46 (0.37-0.57) |
| ≤1.7 grams (low) | Yes | 159 | 76 | 0.32 (0.22-0.47) |
| Alcohol Drinking | ||||
| Yes | No | 622 | 495 | 1.00 (referent) |
| Yes | Yes | 140 | 98 | 0.92 (0.70-1.20) |
| No | No | 656 | 557 | 0.77 (0.53-1.10) |
| No | Yes | 171 | 97 | 0.50 (0.34-0.74) |
| Diuretic Use | ||||
| Yes | No | 238 | 270 | 1.00 (referent) |
| Yes | Yes | 65 | 58 | 0.74 (0.46-1.20) |
| No | No | 1040 | 782 | 0.37 (0.24-0.58) |
| No | Yes | 246 | 137 | 0.22 (0.13-0.36) |
| Allopurinol Use | ||||
| No | No | 870 | 792 | 1.00 (referent) |
| No | Yes | 205 | 150 | 0.68 (0.50-0.92) |
| Yes | No | 408 | 260 | 0.47 (0.34-0.63) |
| Yes | Yes | 106 | 45 | 0.25 (0.15-0.42) |
| Colchicine Use | ||||
| No | No | 1097 | 930 | 1.00 (referent) |
| No | Yes | 269 | 173 | 0.66 (0.46-0.97) |
| Yes | No | 181 | 122 | 0.61 (0.47-0.80) |
| Yes | Yes | 42 | 22 | 0.52 (0.27-0.98) |
| NSAID Use | ||||
| No | No | 954 | 770 | 1.00 (referent) |
| No | Yes | 223 | 135 | 0.59 (0.44-0.79) |
| Yes | No | 324 | 282 | 1.00 (0.78-1.30) |
| Yes | Yes | 88 | 60 | 0.77 (0.49-1.19) |
Cherry or cherry extract intake over at least one of the prior two days vs. no intake
Adjusted for purine intake and use of alcohol, diuretics, allopurinol, colchicine, and NSAIDs in separate models for corresponding risk factors.
DISCUSSION
In this large study of prospectively recruited patients with preexisting gout, we found that cherry intake was associated with a 35% lower risk of recurrent gout attacks, and cherry extracts showed a similar inverse association. These associations were independent of other risk factors including time-invariant factors such as genetics, sex, race, and education (by study design) and time-varying factors such as purine intake, alcohol consumption, as well as use of anti-gout medications and diuretics. Interestingly, when cherry intake was combined with allopurinol use, the most commonly used urate-lowering drug, the risk of gout attacks was 75% lower than over the period without either exposure.
Several biological mechanisms have been elaborated to link cherry consumption to the risk of gout attacks. A study conducted among 10 healthy women found that consumption of cherries, but not other fruits such as strawberries, grapes, or kiwi fruit, significantly reduced levels of both serum uric acid and plasma creatinine (5). These findings led investigators to speculate that cherries may exert their urate-lowering effect through increasing the glomerular filtration rate or reducing tubular reabsorption. In an animal study, intake of tart cherry juice significantly decreased the levels of serum uric acid in rats with hyperuricemia by inhibiting the hepatic activity of xanthine oxidase and xanthine dehydrogenase, suggesting that cherries may possess the capacity of lowering uric acid production (6). Cherries and cherry extract contain high levels of anthocyanins (7-9) that possess anti-inflammatory properties either through inhibiting cyclooxygenase activity (8, 10-12) or via scavenging of nitric oxide radicals (23). Thus, cherries may also have anti-inflammatory properties against the series of inflammatory responses triggered by monosodium urate crystals. Although cherries contain vitamin C, the amount they include (~ 80mg in 6 servings of cherries) (5) would likely be too low to have an impact on the risk of gout, as the relevant vitamin C doses associated with lower serum uric acid levels and gout risk have been approximately 500mg/daily or higher (24-26).
Our findings suggest that these data on the potential influence of cherries on levels of serum uric acid (5) and potential anti-inflammatory effects (8, 10-12) may be translated into prevention of gout attacks among patients with pre-existing gout. While urate-lowering therapy (e.g., allopurinol in >90% of cases in the US and Europe) can be efficacious in lowering levels of serum urate and the risk of gout when dosed appropriately and used compliantly, the current practice standards limit urate-lowering therapy to specific indications such as frequent gout attacks, tophaceous gout, and advanced gout, primarily due to rare but serious side effects (27). When these indications are not yet met, non-pharmacologic options, such as risk factor modifications, are the only acceptable preventive approach. Should our findings be confirmed by randomized clinical trials, cherries or cherry extracts could provide a novel non-pharmacological option for preventing gout attacks. Furthermore, our findings about combined use with allopurinol also suggest that cherries may add substantially to the effects of allopurinol in preventing gout attacks in gout patients.
Identifying potential triggers for recurrent gout attacks is challenging when using traditional study designs and recruiting methods. To address these issues, we adapted a case-crossover study to examine a set of potential triggers, including cherry intake, in relation to the risk of recurrent gout attacks. This study design is highly adaptable to evaluating the effect of transient exposure as a trigger for acute disease onset, and self-matching of each subject minimizes bias in control selection and removes the confounding effects of the factors that are constant over the study period but differ between participants (e.g., genetic factors, sex, race, education) (28). We also used the internet as a platform to conduct of the study. As ours and other studies have demonstrated that, the internet is an efficient way to access a large number of potential participants and to collect information in real time (29, 30), thereby minimizing recall bias.
Our study has some limitations. While the case-crossover study is an optimal design to assessing the acute effects of cherry intake, it is not ideal for evaluating the long-term effects of habitual cherry consumption. Because cherry consumption was self-reported by questionnaire, some misclassification of exposure is possible despite our use of pictures to depict different serving sizes during our data collection. If gout attacks somehow triggered more recollection of cherry intake, it would have biased its potential protective effects towards the null. Furthermore, our cherry effect estimates were independent of other well-established anti-gout measures such as allopurinol, colchicine, and NSAIDs. Nevertheless, our study was observational; thus, we cannot rule out the possibility that unmeasured factors might have contributed to the observed associations.
We did not collect data on serum uric acid levels; thus, we were unable to assess whether cherry consumption would decrease the risk of gout attacks even among subjects whose serum uric acid levels were below the therapeutic target levels (e.g., 6mg/dL). However, when we limited our analysis to subjects who reported taking allopurinol during the study visits (n = 184), the risk of recurrent gout attacks was still decreased with cherry consumption, even though allopurinol use itself was associated with a lower risk of recurrent gout attacks. In our study, the cherry anti-gout benefits peaked around 3 servings over 2-day period and tended to attenuate in the next higher consumption level, although it remained protective. While certain opposing mechanisms such as fructose included in sweet cherries, as shown with oranges or apples (31, 32), might play a role at certain levels of cherry consumption, we cannot rule out random variations due to relatively small sample sizes of the top consumption categories. Thus, these findings need be confirmed by future studies. Finally, we were unable to estimate the absolute rates of gout attacks using the case-crossover study design, and the distribution of cherry intake among the participants in our study may not be representative of a random sample of US gout patients, but that the biological effects of cherry intake on gout attacks (as reflected in our OR estimates) should be similar.
In conclusion, our study findings suggest that cherry intake is associated with a lower risk of gout attacks. Should our findings be confirmed by randomized clinical trials, cherry products could provide a novel non-pharmacological preventive option against gout attacks.
Supplementary Material
Acknowledgments
Supported by Arthritis Foundation, American College Rheumatology Research and Education Fund, and NIH AR47785.
REFERENCES
- 1.Zhu YY, Pandya BJ, Choi H. Prevalence of Gout and Hyperuricemia in the US General Population:The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011 doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 2.Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29:2403–6. [PubMed] [Google Scholar]
- 3.Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J Rheumatol. 2006;33:104–9. [PubMed] [Google Scholar]
- 4.Gutman AB. The past four decades of progress in the knowledge of gout, with an assessment of the present status. Arthritis Rheum. 1973;16:431–45. doi: 10.1002/art.1780160402. [DOI] [PubMed] [Google Scholar]
- 5.Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133:1826–9. doi: 10.1093/jn/133.6.1826. [DOI] [PubMed] [Google Scholar]
- 6.Haidari F, Mohammad SM, Keshavarz S, Rashidi M. Inhibitory Effects of Tart Cherry (Prunus cerasus) Juice on Xanthine Oxidoreductase Activity and its Hypouricemic and Antioxidant Effects on Rats. Mal J Nutr. 2009;15:53–64. [PubMed] [Google Scholar]
- 7.Wang H, Nair MG, Strasburg GM, Booren AM, Gray JI. Novel antioxidant compounds from tart cherries (Prunus cerasus). J Nat Prod. 1999;62:86–8. doi: 10.1021/np980268s. [DOI] [PubMed] [Google Scholar]
- 8.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–9. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 9.Kirakosyan A, Seymour EM, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009;115:20–5. [Google Scholar]
- 10.Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136:981–6. doi: 10.1093/jn/136.4.981. [DOI] [PubMed] [Google Scholar]
- 11.Schlesinger N, Rabinowitz R, Schlesinger MH. Effect of cherry juice concentration on the secretion of interleukins by human monocytes exposed to monosodium urate crystals in vitro. Ann Rheum Dis. 2010;69(Suppl3):610. [Google Scholar]
- 12.He YH, Zhou J, Wang YS, Xiao C, Tong Y, Tang JC, et al. Anti-inflammatory and anti-oxidative effects of cherries on Freund's adjuvant-induced arthritis in rats. Scand J Rheumatol. 2006;35:356–8. doi: 10.1080/03009740600704155. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration [November 20, 2011];List of Firms Receiving Warning Letters Regarding Cherry and other Fruit-Based Products with Disease Claims in Labeling. ( http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/ComplianceEnforcement/ucm081724.htm)
- 14.Harrold LR, Mazor KM, Velten S, Ockene IS, Yood RA. Patients and providers view gout differently: a qualitative study. Chronic Illn. 2010;6:263–71. doi: 10.1177/1742395310378761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Chaisson CE, McAlindon T, Woods R, Hunter DJ, Niu J, et al. The online case-crossover study is a novel approach to study triggers for recurrent disease flares. J Clin Epidemiol. 2007;60:50–5. doi: 10.1016/j.jclinepi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677–83. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 17.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 19.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–8. doi: 10.1002/art.27327. [DOI] [PubMed] [Google Scholar]
- 20.Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 306:711–20. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 21.So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–76. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 22.Gaffo AL, Schumacher HR, Saag KG, Taylor WJ, Allison J, Chen L, et al. Developing American College of Rheumatology and European League against Rehumatism Criteria for Definition of a Flare in Patients with Gout. Arthritis & Rheumatism. 2009;60(supplement):s563. [Google Scholar]
- 23.van Acker SA, Tromp MN, Haenen GR, van der Vijgh WJ, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem Biophys Res Commun. 1995;214:755–9. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- 24.Huang HY, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EP, et al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52:1843–7. doi: 10.1002/art.21105. [DOI] [PubMed] [Google Scholar]
- 25.Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169:502–7. doi: 10.1001/archinternmed.2008.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C Intake and Serum Uric Acid Concentration in Men. J Rheumatol. 2008;35:1853–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 29.McAlindon T, Formica M, Kabbara K, LaValley M, Lehmer M. Conducting clinical trials over the internet: feasibility study. BMJ. 2003;327:484–7. doi: 10.1136/bmj.327.7413.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorig KR, Laurent DD, Deyo RA, Marnell ME, Minor MA, Ritter PL. Can a Back Pain E-mail Discussion Group improve health status and lower health care costs?: A randomized study. Arch Intern Med. 2002;162:792–6. doi: 10.1001/archinte.162.7.792. [DOI] [PubMed] [Google Scholar]
- 31.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.