Abstract
Objective
Many different genes or mediators have been implicated in promoting the development of vasculitis, although little is known regarding the mechanisms that normally act to suppress lesion formation. eNOS (NOS3) has been shown to inhibit vascular inflammation in many different model systems, but its roles in the pathogenesis of vasculitis have not been elucidated. The aim of this study was to determine the functions of eNOS in the initiation and progression of vasculitic lesion formation.
Methods
Nos3 mutant MRL/MpJ-Faslpr mice were generated and comprehensively evaluated and compared to controls for the development of autoimmune disease, including vasculitic lesion formation and glomerulonephritis.
Results
Nos3−/− MRL/MpJ-Faslpr mice had accelerated onset and increased incidence of renal vasculitis compared to Nos3+/+ controls. In contrast, no significant differences in severity of glomerulonephritis were observed between groups. Vasculitis was also observed in eNOS deficient mice in other organs, including increased expression in the lung. Ultrastructural analyses of renal lesions revealed the presence of electron dense deposits in affected arteries, while IgG, IgA, and C3 deposition was observed in some vessels in Nos3−/− kidneys. In addition, eNOS deficient mice showed increased levels of circulating IgG-IgA immune complexes at 20 weeks of age compared to Nos3+/+ MRL/MpJ-Faslpr and Nos3−/− C57BL/6 mice.
Conclusion
These findings strongly indicate that eNOS serves as a negative regulator of vasculitis in MRL/MpJ-Faslpr mice, and further suggest that NO produced by this enzyme may be critical for inhibiting lesion formation and vascular damage in human vasculitic diseases.
INTRODUCTION
Vasculitis is a general term used to describe a heterogeneous group of disorders characterized by inflammatory processes leading to destruction of blood vessels (1). It can result in vessel necrosis, occlusion, and subsequently tissue ischemia. Vasculitis is the primary pathologic manifestation of several different diseases, such as Wegener’s granulomatosis, giant cell arteritis, and polyarteritis nodosa, and can also be observed in patients with systemic lupus erythematosus (SLE) and other connective tissue diseases (2–5). It has been proposed that during the initiation of vasculitis, stimuli such as infectious agents, anti-endothelial cell antibodies (AECA), immune complexes, complement proteins, cytokines, and other factors activate endothelial cells, which leads to leukocyte adhesion and infiltration of the vessel wall (5–7). Endothelial and smooth muscle cell damage may then occur through a variety of mechanisms, including neutrophil release of granular contents and reactive oxygen species or from T-cell and macrophage mediated immune mechanisms (8, 9). Priming of neutrophils is also thought to be an important event in the development of vasculitis in some disorders (10). Anti-neutrophil cytoplasmic antibodies (ANCA) and other inflammatory mediators may partially activate neutrophils, which can result in increased interactions of these leukocytes with endothelial cells, promote their respiratory burst, and ultimately lead to endothelial damage (5, 8, 10).
Nitric oxide (NO) is an important regulator of different physiologic and inflammatory responses and has been previously implicated in the development of vasculitis (11–13). NO is produced during the conversion of L-arginine to L-citrulline by three different isoforms known as NO synthases (NOS) (14, 15). Endothelial nitric oxide synthase (eNOS) is a constitutively active enzyme that is expressed in endothelial cells and plays important roles in regulating vasodilatation, inhibiting smooth muscle proliferation and platelet aggregation, modulating leukocyte/endothelium adhesion events, and controlling other key vascular functions (15, 16). Neuronal nitric oxide synthase (nNOS) is the predominate source of NO in neurons and functions in neurotransmission events, but is additionally expressed in muscle and blood vessels (14, 17). Finally, inducible nitric oxide synthase (iNOS) is expressed in many different cells, including macrophages, hepatocytes, and endothelial cells (14, 18). iNOS expression and activity is significantly upregulated in response to inflammatory stimuli (19) and NO produced from this isoform is critical for host defense and other cellular processes (20–22). Published studies of iNOS in vasculitis models suggest that this enzyme significantly contributes to vessel damage (11, 23); however, the role of eNOS or nNOS in relevant in vivo models of vasculitis is yet to be elucidated.
To examine the possible involvement of eNOS in the context of vasculitis, we analyzed Nos3 mutant MRL/MpJ-Faslpr mice for vasculitic lesions in the kidneys and other organs (24). We found that Nos3−/− mice developed severe renal vasculitis and showed significant increases in lesion severity scores compared to non-mutant controls. Loss of eNOS expression also led to the accelerated onset of vasculitis, with renal lesions observed as early as 11 weeks of age. In addition, vasculitis was observed in Nos3−/− MRL/MpJ-Faslpr mice in other organs, including increased lesion formation in the lung. Finally, Nos3−/− MRL/MpJ-Faslpr mice presented with an earlier onset of glomerulonephritis, but did not show a significant increase in the overall severity of glomerular disease at later timepoints. Thus, our findings suggest that eNOS plays a significant role in regulating the development of vasculitis, acting to prevent or restrict the onset and progression of vascular inflammation and damage.
MATERIALS AND METHODS
Mice
MRL/MpJ-Faslpr and Nos3 mutant C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) (25). MRL/MpJ-Faslpr mice deficient in eNOS expression were generated by backcrossing the Nos3 mutation 8 generations onto the MRL/MpJ-Faslpr strain and homozygotes were then generated by intercrossing. Mice were genotyped for the Nos3 mutation by PCR, and in some cases, homozygotes were confirmed by Western blot analysis of liver tissue. Nos3+/+ MRL/MpJ-Faslpr N8 littermates or inbred MRL/MpJ-Faslpr mice were used as controls and approximately equal numbers of males and females were used for all studies. Animal care and experimental manipulations were conducted according to the Guide for the Care and Use of Laboratory Animals and with approval of the UAB IACUC Committee.
Histological Analyses and Measurement of Serum Creatinine
Kidneys were collected and fixed in buffered 10% formalin, processed for paraffin sectioning, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E). Duplicate sections were stained with periodic acid-Schiff reagent and hematoxylin (PASH). Vasculitis and glomerulonephritis were evaluated by subjective scoring, without the pathologist’s knowledge of the age or genotype. Vasculitis was assessed by examining the entire area of each section for vascular lesions and recording the type of vessel (arterioles, muscular arteries, elastic arteries, venules, and veins). For each affected vessel, adventitial mononuclear cells, neutrophils, necrosis, and fibrosis; medial mononuclear cells, neutrophils, and necrosis; and intimal mononuclear cells, neutrophils, necrosis, and proliferation were each graded 0, 1, 2, or 3, for absent, mild, moderate, or severe, respectively. Vasculitis scores for individual vessel lesions were calculated from these values according to the formula adventitial mononuclear cells + adventitial neutrophils + adventitial necrosis + adventitial fibrosis + 2*(medial mononuclear cells + medial neutrophils + 2*medial necrosis + intimal mononuclear cells + intimal neutrophils + 2*intimal necrosis + intimal proliferation). Total and average vasculitis scores for each mouse were calculated as the sum of individual vessel lesion scores and the mean score for affected vessels, respectively.
Glomerulonephritis was evaluated as previously described (26). Specific changes included glomerular cellularity, necrosis, crescent and synechia formation, neutrophil accumulation, capillary basement membrane thickening and reduplication, mesangial sclerosis, capsular and periglomerular fibrosis, tubular changes, interstitial inflammatory cell accumulation, and interstitial fibrosis. Each was scored 0, 1, 2, or 3 for normal, mild, or severe, respectively. At least six, and up to 15, glomeruli and adjacent tubules and interstitium were evaluated from both H&E and PASH stained sections from each mouse, and equal numbers of glomeruli from superficial, middle, and deep cortex were examined. Only glomeruli sectioned through the approximate center of the tuft and including the base of the tuft were included. Glomerulonephritis scores for each mouse were calculated as the mean of summed component lesion scores for each glomerulus, with scores for necrosis and crescent formation each weighted by a factor of 2. Lymph nodes, spleen, stomach, intestines, liver, heart, and lungs were also collected from (10) 20 week old Nos3−/− mice, stained with H&E, and examined for vasculitis.
For ultrastructural analyses, kidney tissue was fixed in 2% glutaraldehyde, embedded in plastic, and examined by transmission electron microscopy. Toluidine blue fuschin-stained semi-thin sections were examined by light microscopy prior to performing transmission electron microscopy to ensure that the tissue was representative renal parenchyma. Renal function was assessed by measuring serum creatinine using liquid chromatography tandem mass spectrometry.
Immunohistochemistry, Determination of Serum Immunoglobulin Levels, and Autoantibody Analyses
For detection of T cells, B cells, and macrophages, kidney sections were first deparaffinized in xylene, rehydrated in PBS, and then microwave antigen retrieval was performed. These were then blocked with 10% serum, stained with either rat anti-mouse CD3, B220, or Mac-2 antibodies (Cedarlane Laboratories Ltd, Hornby, Ontario), washed, incubated with a biotin-conjugated anti-rat IgG (Vector Laboratories, Burlingame, CA) and processed with peroxidase ABC (Vector Laboratories). Sections were then developed with diaminobenzidine (Vector Laboratories) and counterstained with hematoxylin. For the analysis of IgG deposition, kidneys wereembedded in OCT and snap-frozen in liquid nitrogen. For IgA and C3 antibody staining, the kidneys were fixed in 4% PFA in PBS, washed in PBS, placed in a 30% sucrose/PBS solution overnight, and then embedded in OCT. Five micrometer sections were fixed in ice-cold acetone (IgG) or 4% PFA (IgA and C3), washed in PBS, treated with 0.5% triton, washed in PBS, blocked with 3% BSA/2% donkey serum, and then incubated with either Cy5-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA), FITC-conjugated goat anti-mouse C3 (MP Biomedicals LLC, Solon, OH), or FITC-conjugated goat anti-mouse IgA (SouthernBiotech, Birmingham, AL) overnight at 4°C. Antibody stained slides were then washed several times with PBS and glass cover slips were mounted to the slides with VECTASHIELD mounting agent (Vector Laboratories). IgG anti-DNA autoantibody titers and total serum IgM, IgA, IgG, IgG1, IgG2a, IgG2b, and IgG3 levels were determined as previously described (26).
Analysis of Glomerular C3, IgG, and IgA Deposition
Glomerular deposition was analyzed by histomorphometry using Image Pro Plus v6.2 (Media Cybernetics, Silver Spring, Maryland). Color images of at least 6 glomeruli were made at 20× objective magnification. The background of each image was thresholded to black (RGB values of 0, 0, and 0), glomeruli were selected as regions of interest, and areas and density sums of areas of staining were collected and exported to Excel spreadsheets that calculated average areas and densities for each mouse.
Determination of IgG-IgA immune complexes in serum samples
Sera were fractionated using a calibrated Superose 6 column. The levels of IgG-IgAimmune complexes in each fraction were determined by capture ELISA. Briefly, plates were coated with 2 ug/ml of the goat anti-mouse IgG. The captured IgG-IgA complexes were then detected with a HRP-labeled goat IgG anti-mouse IgA. The peroxidase chromogenic substrates o-phenylenediamine–H2O2 (Sigma-Aldrich) were then added. The color reaction was stopped with 1 M sulfuric acid, and absorbance at 490 nm was measured using a microplate reader.
Statistical Analyses
Vasculitis and glomerulonephritis scores were analyzed by general linear models analysis of variance supplemented by Tukey’s test for mean comparisons, using Statistix® 9.0 (Tallahassee, FL). Results were considered significant if P ≤0.05.
RESULTS
Generation of eNOS Deficient MRL/MpJ-Faslpr Mice
MRL/MpJ-Faslpr mice spontaneously develop immune-complex-based glomerulonephritis, dermatitis, and vasculitis in many different organs (24). To investigate whether eNOS mediates such vasculitis and disease processes, we backcrossed a Nos3 null mutation onto the MRL/MpJ-Faslpr strain background for eight generations (25). Heterozygotes were then intercrossed to generate Nos3−/− mice. eNOS mutant and control N8 mice displayed the expected clinical features characteristic of the inbred MRL/MpJ-Faslpr strain, including the progressive development of dermatitis and lymphadenopathy. We also analyzed serum immunoglobulin and autoantibody levels in Nos3−/− and Nos3+/+ MRL/MpJ-Faslpr mice. No significant differences were observed at 20 weeks for total serum IgG, IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA, as well as for anti-double stranded and anti-single stranded DNA antibody levels (data not shown).
Histological Analyses of Renal Vasculitis and Glomerulonephritis
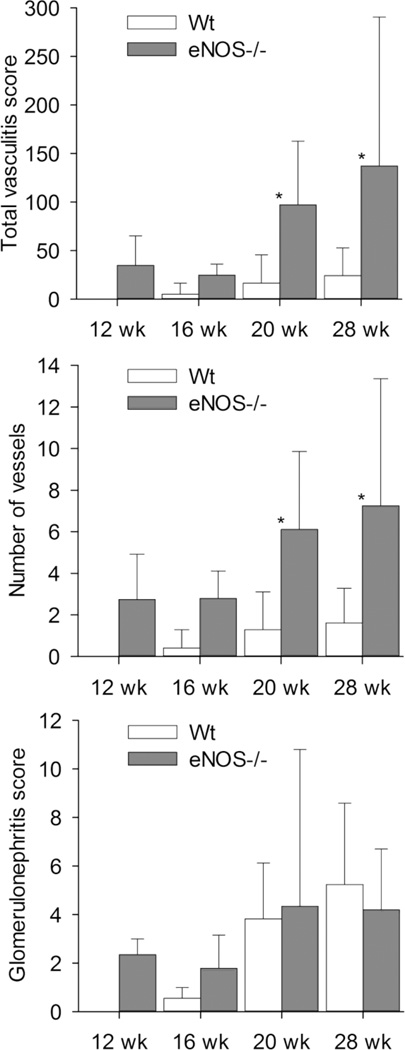
Previous studies of Nos3 mutant mice in other renal inflammation models, including anti-GBM glomerulonephritis, diabetic nephropathy, and remnant kidney systems have shown that eNOS deficiency leads to increased severity of disease, suggesting a regulatory role for this enzyme (27–29). However, with exception of the anti-GBM model, different pathogenic mechanisms are thought to mediate kidney injury, and the development of vasculitis has not been reported in any of these model systems. To determine whether loss of eNOS expression might also lead to similar alterations in the onset or severity of renal inflammation in the MRL/MpJ-Faslpr model, we performed a comprehensive analysis of vasculitis and glomerulonephritis in Nos3−/− and Nos3+/+ mice. Mice were sacrificed at 11 – 13 weeks of age (12 week age group), 16 weeks, 19 – 22 weeks (20 week age group), and 28 weeks or when moribund (28 week age group). These groups were selected because we expected, based on our previous analyses of this model, to see no vasculitis or glomerulonephritis in mice less than 16 weeks old, minimal disease in 16 week old mice, mild to moderate disease in 20 week old mice, and more advanced disease in 28 week old and older mice. (26, 30). Interestingly, we found that loss of eNOS expression resulted in a significant increase in severity of renal vasculitis, but not glomerulonephritis, in mice of the 20 and 28 week age groups (Figure 1). The increase in total vasculitis score was due to increased numbers of affected vessels in Nos3−/− mice, inasmuch as total vasculitis scores closely paralleled numbers of affected vessels.
Figure 1.
Renal vasculitis and glomerulonephritis scores for Nos3+/+ and Nos3−/− MRL/MpJ-Faslpr mice. The total renal vasculitis scores (top), numbers of affected renal vessels (middle), and glomerulonephritis scores (bottom) were analyzed in kidneys from mice sacrificed at the different timepoints listed as described in the Results section. Numbers of Nos3+/+ mice evaluated per timepoint: N=7 (12wk), N=5 (16wk), N=22 (20 wk), and N=6 (28wk or moribund). Numbers of Nos3−/− mice evaluated per timepoint: N=7 (12wk), N=5 (16wk), N=24 (20 wk), and N=11 (28wk or moribund). Means ± standard deviations are shown. Pairs of means marked with asterisks are significantly different (P ≤0.05).
To determine whether partial loss of eNOS expression could also alter renal disease in this model, we evaluated vasculitis and glomerulonephritis in kidneys of Nos3+/− MRL/MpJ-Faslpr mice at 19-22 weeks of age. We found that Nos3+/- mice had an intermediate vasculitis phenotype, although comparisons of means for heterozygotes with those for Nos3−/− and Nos3+/+ mice were not statistically significant (data not shown). In addition, no significant differences were observed in the glomerulonephritis scores among the different genotypes (data not shown). Finally, vasculitis was not observed in the kidneys of Nos3−/− C57BL/6 mice, even at 40 weeks of age (data not shown), suggesting that eNOS deficiency alone is not sufficient for the development of lesions.
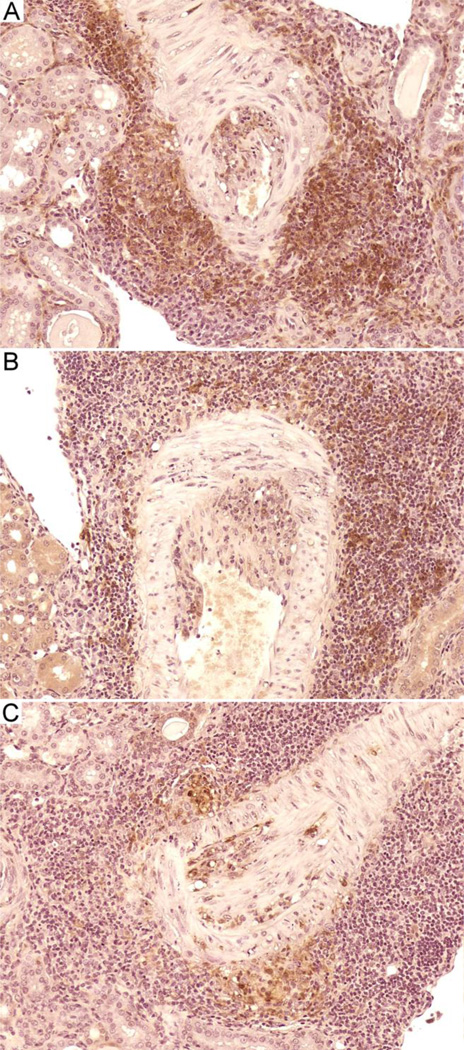
Renal vasculitis involved mainly medium sized arteries with no significant involvement of small blood vessels (Figure 2). Vasculitis in both eNOS mutant and control MRL/MpJ-Faslpr mice was multifocal and affected segmental, arcuate, and interlobular muscular arteries. Lesions were typical of polyarteritis nodosa-like vasculitis previously reported in MRL/MpJ-Faslpr mice (Figure 2 A and B), with intense adventitial accumulation of lymphocytes, macrophages, and neutrophils; medial necrosis and neutrophil accumulation; and intimal inflammatory cell accumulation (31). Occasionally these changes were accompanied by slight to mild intimal proliferation. In contrast, intimal proliferation and inflammation were strikingly prominent in many lesions in Nos3−/− mutant mice (Figure 2 C and D), a feature present in few lesions in Nos3+/+ mice even at 28 weeks of age. Immunohistochemical staining of selected sections showed that cellular accumulations contained CD3+, B220+, and Mac-2+ leukocytes (Figure 3A–C). Although the mean comparisons were not statistically significant, it was noteworthy that vasculitis and glomerulonephritis were present in Nos3−/− mice of the 12 week age group, whereas such lesions were absent in Nos3+/+ mice of that age, suggesting that eNOS deficiency additionally leads to an earlier onset of renal disease (Figure 1).
Figure 2.
Renal arteritis in Nos3+/+ and Nos3−/− MRL/MpJ-Faslpr mice. A and B, typical granulomatous adventitial inflammation and necrotizing medial and intimal inflammation in Nos3+/+ mice. C and D, similar adventitial and medial inflammation in Nos3−/− mice, but with extensive intimal proliferation and inflammation. H&E. Original magnifications, X20.
Figure 3.
Immunohistochemical staining of renal arteritis lesions in a 20 week old Nos3−/− MRL/MpJ-Faslpr mouse. Representative pictures showing immunohistochemical staining of vasculitic lesions with anti-CD3 (A), anti-B220 (B), and anti-Mac-2 antibodies (C). Original magnifications, X20.
Glomerular lesions were as expected in this SLE model and did not differ in character between Nos3+/+ and Nos3−/− MRL/MpJ-Faslpr mice. Mild glomerulonephritis was characterized primarily by mild to moderate mesangial thickening (sclerosis) and proliferation, with slight to mild neutrophil accumulation (data not shown). Features of more severe lesions in older mice included moderate to severe mesangial thickening and proliferation, moderate to severe neutrophil accumulation, and, in the most severe instances, segmental tuft necrosis, epithelial crescent formation, synechia (adhesion of the tuft to Bowman’s capsule), pericapsular inflammatory cell accumulation, and tubular atrophy (data not shown). Finally, no significant differences in serum creatinine levels were observed between mutant and control mice at 16 or 20 weeks of age, even though Nos3−/− mice showed both an earlier onset and increased severity of vasculitis (data not shown).
Ultrastructural Characterization
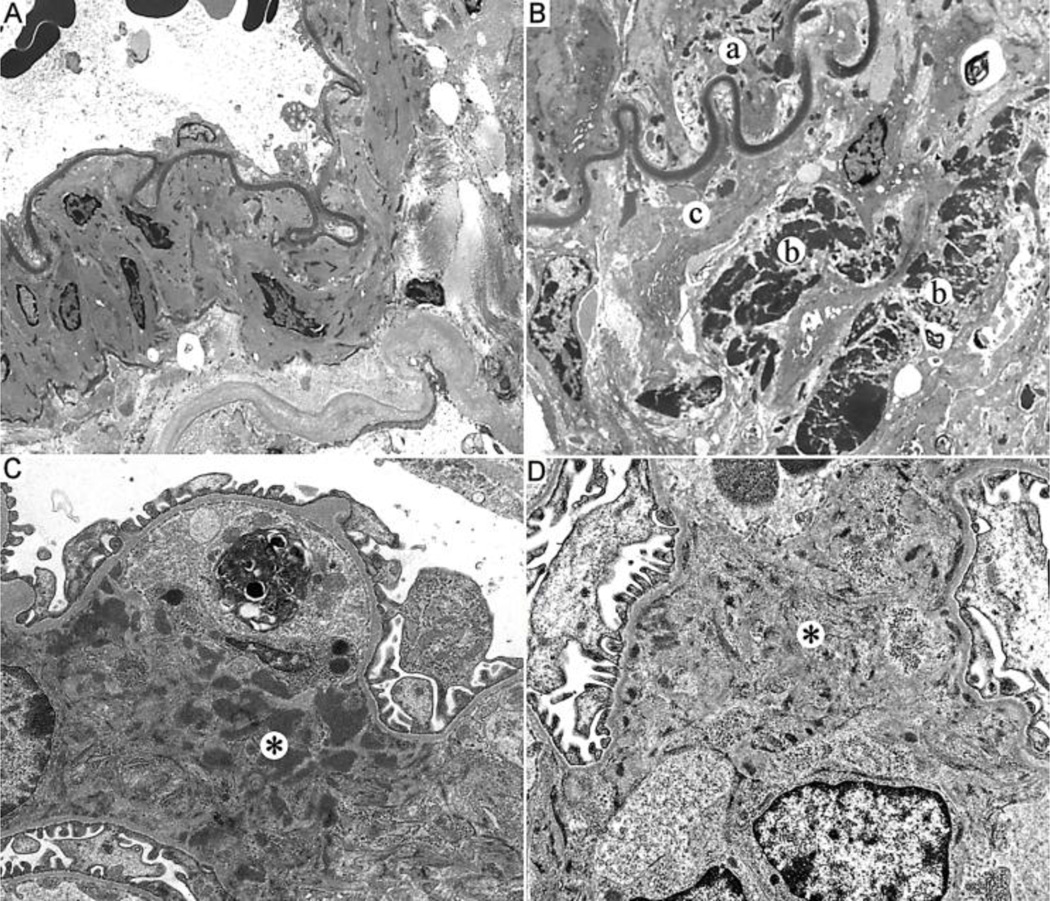
Electron microscopic examination of the larger muscular arteries in the kidney showed marked contrast between Nos3−/− and Nos3+/+ MRL/MpJ-Faslpr mice (Figure 4). Muscular arteries from Nos3+/+ mice had no significant ultrastructural abnormalities. The endothelium, elastic layer, muscular layer and adventitia possessed typical findings of a normal muscular artery without intimal hyperplasia, disruption of the elastic membrane, muscular hypertrophy, adventitial inflammation, or electron dense deposits (Figure 4A). In contrast, the muscular arteries from Nos3−/− mice had intimal thickening with an intact elastic membrane (Figure 4B). There was disruption of the muscular layer and readily identified large electron dense deposits were seen in the area of muscular layer disruption and adventitial tissues. In addition, smaller electron dense deposits were visualized in the subendothelium immediately above the elastic layer. Occasional lymphocytes were present in the adventitia and disrupted muscular layer.
Figure 4.
Ultrastructural analyses of renal vasculitic lesions and glomerulonephritis. A, Representative electron micrograph of a normal muscular artery of a 20 week old Nos3+/+ MRL/MpJ-Faslpr mouse with no ultrastructural abnormalities in the artery structure or adventitial tissue. B, Representative electron micrograph of a muscular artery from an age-matched Nos3−/− MRL/MpJ-Faslpr mouse demonstrating electron dense deposits beneath the endothelial layer adjacent to elastic layer (a) and within the adventitial tissue (b). In addition, there is disruption of the muscular layer (c). C, Electron micrograph of the mesangium of a representative 20 week old Nos3+/+ MRL/MpJ-Faslpr mouse with expanded matrix and readily identified intermediate to large electron dense deposits (*). D, Electron micrograph of the mesangium of a representative 20 week old Nos3−/− MRL/MpJ-Faslpr mouse with reduced fine small electron dense deposits in mesangium (*). Original magnifications, X5000.
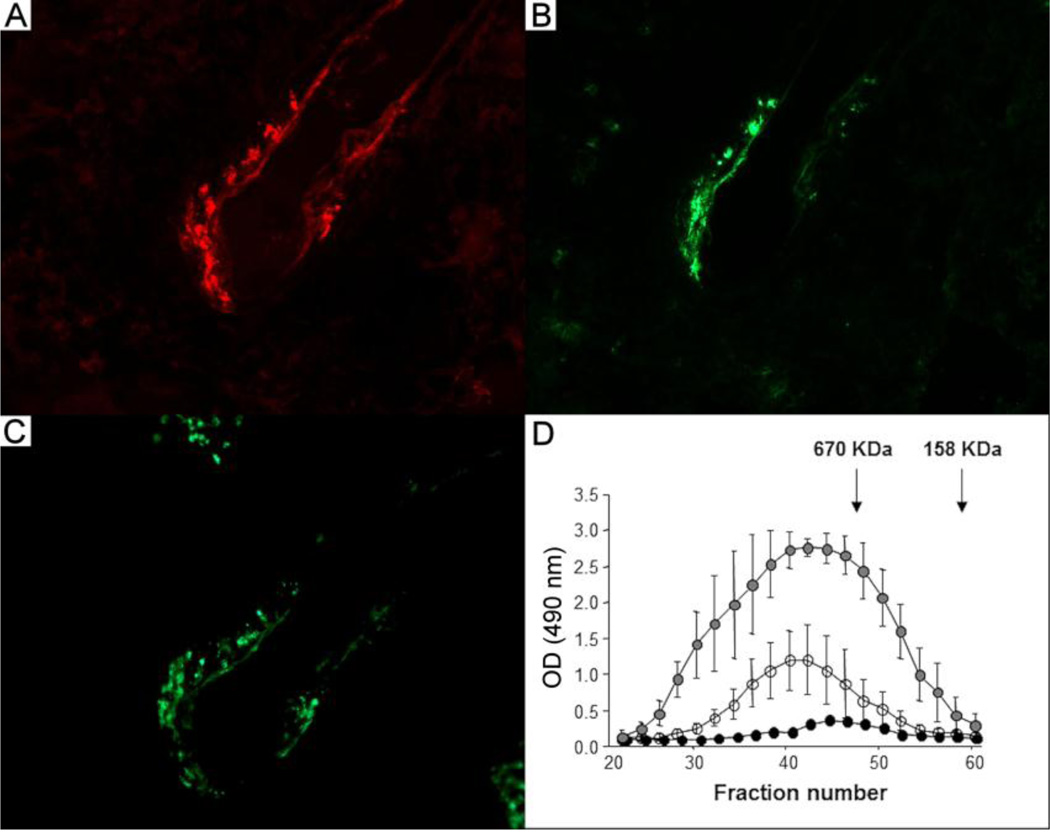
Consistent with these findings, we observed that a small number of the affected vessels in the kidneys of Nos3−/− mice showed deposition of both IgG and IgA (Figure 5A and B), while C3 staining was also evident in these lesions (Figure 5C). However, such deposition was sparse and difficult to find, especially in Nos3+/+ MRL/MpJ-Faslpr mice due to the low frequency of vasculitis in these mice. Based on these observations, we initiated studies of circulating IgG-IgA immune complexes to determine whether eNOS deficiency led to any alterations in their levels or other properties. We found that Nos3−/− MRL/MpJ-Faslpr mice had elevated levels of IgG-IgA immune complexes compared to MRL/MpJ-Faslpr controls and Nos3−/− C57BL/6 mice at 20 weeks of age (Figure 5D). These complexes were large with a molecular mass >670 kDa in both genotypes. In contrast, eNOS deficient C57BL/6 mice had low levels of these complexes and they were of smaller molecular mass. Thus, these findings suggest that circulating immune complexes may play a role in promoting vasculitis in the kidneys of eNOS deficient MRL/MpJ-Faslpr mice, although additional mechanisms for initiating lesion formation must also exist.
Figure 5.
Antibody staining of kidney sections showing arterial wall IgG and IgA deposition and analyses of circulating IgA-IgG immune complexes. Serial sections from a representative kidney of a 20 week old Nos3−/− MRL/MpJ-Faslpr mouse were stained with Cy5-conjugated goat anti-mouse IgG (A), FITC-conjugated goat anti-mouse IgA (B), or FITC-conjugated goat anti-mouse C3 (C). Original magnifications, X40. (D) The levels of circulating IgG-IgAimmune complexes in the serum of Nos3−/− MRL/MpJ-Faslpr mice (gray filled circles, n=5) were elevated compared to Nos3+/+ MRL/MpJ-Faslpr (open circles, n=6) and Nos3−/− C57BL/6 (black filled circles, n=3) mice. Data are expressed as mean ± SD.
Ultrastructural analysis of glomeruli from 20 week old Nos3+/+ MRL/MpJ-Faslpr mice showed a moderate increase in mesangial cells and matrix, without segmental lesions or crescents, and there were readily identifiable intermediate to large electron dense deposits within the mesangium and paramesangium, as well as large deposits in the glomerular basement membranes (Figure 4C). The deposits in the basement membranes were subepithelial and often extended into the intramembranous areas and tended to be distinct and not confluent. The basement membranes were markedly thickened in the areas with membranous deposits and the foot processes were attenuated. The visceral epithelial cells were prominent, but lacked cytoplasmic vacuolization. There were no tubular reticular inclusions and tubules and peritubular capillaries lacked electron dense deposits. Finally, there were occasional aggregates of lymphocytes and plasma cells within the interstitium.
In contrast, kidneys from aged-matched Nos3−/− MRL/MpJ-Faslpr mice showed a mild increase in mesangial matrix, and only focal areas with minimally increased mesangial cells (Figure 4D). The mesangium contained fine, small electron dense deposits with mildly increased mesangial matrix. There were fine, small paramesangial deposits also. The basement membranes were of appropriate thickness. The foot processes were well maintained. There were rare small, subendothelial deposits with the basement membranes. The visceral epithelial cells were not prominent and lacked vacuolization and no tubular reticular inclusions were observed. Similar to Nos3+/+ mice, the tubules and peritubular vessels lacked deposits and occasional aggregates of lymphocytes and plasma cells seen.
Analysis of Glomerular C3, IgG, and IgA Deposition
Glomerulonephritis in MRL/MpJ-Faslpr mice has been previously shown to be promoted in part due to immune complex deposition and complement fixation, which activates the inflammatory response and leads to glomerular injury (24, 32). Our ultrastructural analyses suggest that some differences exist between eNOS mutant and control mice in the extent of electron dense deposits in the mesangium, paramesangium, and glomerular basement membranes. Therefore, we performed antibody staining of kidney sections from 20 week old Nos3−/− and Nos3+/+ MRL/MpJ-Faslpr mice along with morphometry to compare the pattern and intensity of staining for glomerular IgG, IgA, and C3. We observed that staining patterns varied considerably among glomeruli within and between individual mice of both genotypes, and that there were no statistically significant differences in staining areas or densities as determined by histomorphometry (data not shown).
Analyses of Vasculitis in other Tissues
In a preliminary assessment of vasculitis in other viscera 20-week-old Nos3−/− MRL/MpJ-Faslpr mice, we found necrotizing inflammation of small to medium arteries in spleen, lymph nodes, and digestive and reproductive tracts, as we and others have previously observed in MRL/MpJ-Faslpr mice (33–35). However, findings in the lung were strikingly different. Seven of the 10 mice had multifocal pulmonary arteritis with endarteritis similar to that in renal vessels (Figure 6). In our experience, true vasculitis, evidenced by degeneration, necrosis, and/or inflammatory cell accumulation within the vessel wall, is rare in pulmonary vessels of MRL/MpJ-Faslpr mice. These findings, along with those in the kidney, suggest that loss of eNOS expression can significantly promote the development of vasculitis in multiple tissues.
Figure 6.
Histopathology of the lung. Representative lung section from a 20 week old Nos3−/− MRL/MpJ-Faslpr mouse. Pulmonary endarteritis with intimal proliferation and accumulation of lymphocytes and neutrophils is observed. H&E. Original magnifications, X20.
DISCUSSION
Mechanistic studies of the immune and inflammatory events leading to the development of vasculitis in different disorders have been hampered by several factors, including the heterogeneity of the diseases themselves, a poor understanding of the genetic and environmental factors involved, and a shortage of appropriate animal models (36). Thus, for many different vasculitic diseases the key genes or molecules that promote or inhibit lesion formation remain largely unidentified, which has further hindered the generation of new agents that may be used for treatment. Previous investigations of eNOS have shown that this enzyme is an important source of NO in vessels for regulating blood pressure, smooth muscle proliferation, inflammatory responses, angiogenesis, and other vascular functions (37, 38). In addition, alterations in eNOS expression or activity have also been correlated with the pathogenesis of diseases such as atherosclerosis, diabetes, and asthma (16, 37, 39). However, studies specifically analyzing the role of this enzyme in the initiation and progression of vasculitis have not been reported.
Here we show using the MRL/MpJ-Faslpr model for SLE and vasculitis that loss of eNOS expression accelerates the onset and increases the number and distribution of affected vessels in the kidney. Renal vasculitic lesions were also detected as early as 11 weeks in Nos3−/− MRL/MpJ-Faslpr mice and primarily targeted medium to large arteries. Although not the primary focus of the current studies, vasculitis was additionally observed in Nos3−/− MRL/MpJ-Faslpr mice in several other organs, including increased expression in the lung compared to Nos3+/+ controls. Nos3+/- mice presented with an intermediate renal phenotype compared to homozygote mutants and controls, suggesting that the overall level of eNOS expression is a critical determinant in regulating lesion formation. Spontaneous development of vasculitis has not been reported in Nos3 mutants in previous studies, which have largely utilized mice on non-autoimmune strain backgrounds (25, 40). In agreement with these investigations, we did not observe lesions in the kidneys of eNOS deficient C57BL/6J mice up to 40 weeks of age. Thus, these findings suggest that decreased eNOS expression does not by itself lead to vasculitis, but requires additional genetic factors to promote lesion formation.
Several mediators are thought to be critical in promoting endothelial injury leading to vasculitis in MRL/MpJ-Faslpr mice, including immune complex deposition, ANCA, and AECA (41–43). However, studies to determine the individual roles of these factors in promoting lesions in MRL/MpJ-Faslpr mice are lacking and other inflammatory stimuli or may also contribute. In our study, we found increased levels of IgG-IgA immune complexes in the circulation and identified both IgG and IgA immunodeposits in some affected blood vessels of the kidneys. Furthermore, we found evidence of complement activation based on C3 staining in these same areas. These findings suggest that eNOS deficiency may render blood vessels more vulnerable to vasculitic lesion formation at sites of immune complex deposition. In addition, they indicate that reduced eNOS expression may also alter the formation or clearance of circulating immune complexes. As this is an unexpected observation, understanding the eNOS-dependent mechanisms that restrict vasculitis development requires further study. Thus, the Nos3 deficient MRL/MpJ-Faslpr mouse model presents a unique opportunity to dissect detailed mechanisms of endothelial cell functions and vasculitis and to identify possible novel functions of eNOS in controlling autoimmunity.
Decreased Nos3 expression may also predispose specific areas of the vasculature to inflammation and damage in response to binding of endothelial cell antigens by AECA, or indirectly as a consequence of ANCA activation of neutrophils. Previous studies of eNOS suggest that this enzyme modulates leukocyte/endothelial cell interactions by downregulating endothelial adhesion molecule and chemokine expression through inhibition of NF-κB (44–46). Thus, the increased incidence and severity of vasculitic lesions in Nos3−/− MRL/MpJ-Faslpr mice may result from increased leukocyte activation and/or infiltration of vessels at sites of immune complex deposition, or in areas where the endothelium has undergone stimulation by AECA or other stimuli. An additional contributing factor may also involve the loss of eNOS-mediated suppression of intimal proliferation, which may lead to augmented lumenal occlusion and reduced blood flow following initial lesion formation (47, 48). However, at this time, further studies are necessary to specifically identify the mechanisms by which this enzyme limits or suppresses vasculitis in the MRL/MpJ-Faslpr model, as well as determine whether eNOS plays similar roles in the pathogenesis of human vasculitides.
Earlier studies have linked NO to the progression of the inflammatory disease in MRL/MpJ-Faslpr mice, although these investigations primarily focused on the role of iNOS or used non-specific NO synthase inhibitors. It has been shown that MRL/MpJ-Faslpr overexpress iNOS and overproduce NO in an age-dependent fashion that parallels expression of autoimmune disease (11). In addition, blocking NO production by oral administration of the inhibitor NG-monomethyl-L-arginine reduced the severity of arthritis, glomerulonephritis, and vasculitis in this model (11, 49, 50). Interestingly, Nos2−/− MRL/MpJ-Faslpr mice showed significantly less renal vasculitis compared to wild type mice, suggesting that iNOS may contribute to lesion formation through NO production in infiltrating leukocytes (23). In contrast, iNOS deficiency did not inhibit the development of glomerulonephritis in this model (23). In our studies, we observed that Nos3−/− MRL/MpJ-Faslpr mice presented with an earlier onset of glomerulonephritis compared to controls; however, at later timepoints, eNOS deficient mice did not show any significant increases in the severity of glomerular disease, although some minor ultrastructural differences were noted.
In summary, we herein describe a novel and inhibitory role for eNOS in the context of vascular lesion formation and severity in a well-characterized model of SLE and vasculitis. These findings have the potential for facilitating studies to provide further insights into the pathogenesis of vascular inflammation and also to test novel therapeutic targets in vasculitis.
Acknowledgments
Funding for this research was made possible in part by an American Heart Association Grant-in-Aid Award and a pilot grant from the UAB-UCSD O'Brien Center (NIH P30 DK079337) to DCB. TJ was supported by a National Kidney Foundation Fellowship Award. JN and HS were supported in part by the National Institutes of Health grants DK083663, DK078244, and DK080301.
We would like to acknowledge the UAB-UCSD O'Brien Core Center (NIH P30 DK 079337), Mucosal HIV and Immunobiology Center (NIH 5R24 DK 064400), and the UAB Animal Resources Program Comparative Pathology Laboratory for their technical assistance and support of these studies.
Footnotes
The authors have no competing financial interests.
REFERENCES
- 1.Sharma P, Sharma S, Baltaro R, Hurley J. Systemic vasculitis. Am Fam Physician. 2011;83(5):556–565. [PubMed] [Google Scholar]
- 2.Gibelin A, Maldini C, Mahr A. Epidemiology and etiology of wegener granulomatosis, microscopic polyangiitis, churg-strauss syndrome and goodpasture syndrome: vasculitides with frequent lung involvement. Semin Respir Crit Care Med. 2011;32(3):264–273. doi: 10.1055/s-0031-1279824. [DOI] [PubMed] [Google Scholar]
- 3.Miller DV, Maleszewski JJ. The pathology of large-vessel vasculitides. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S92–S98. [PubMed] [Google Scholar]
- 4.Calamia KT, Balabanova M. Vasculitis in systemic lupus erythematosis. Clin Dermatol. 2004;22(2):148–156. doi: 10.1016/j.clindermatol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Puerta JA, Bosch X. Anti-neutrophil cytoplasmic antibody pathogenesis in small-vessel vasculitis: an update. Amer J Pathol. 2009;175(5):1790–1798. doi: 10.2353/ajpath.2009.090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piggott K, Biousse V, Newman NJ, Goronzy JJ, Weyand CM. Vascular damage in giant cell arteritis. Autoimmunity. 2009;42(7):596–604. doi: 10.1080/08916930903002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilpain P, Mouthon L. Antiendothelial cells autoantibodies in vasculitisassociated systemic diseases. Clin Rev Allergy Immunol. 2008;35(1–2):59–65. doi: 10.1007/s12016-007-8069-3. [DOI] [PubMed] [Google Scholar]
- 8.Pankhurst T, Savage CO, Little MA. Review article: Leukocyte-endothelial dysregulation in systemic small vessel vasculitis. Nephrology. 2009;14(1):3–10. doi: 10.1111/j.1440-1797.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 9.Berden AE, Kallenberg CG, Savage CO, Yard BA, Abdulahad WH, de Heer E, et al. Cellular immunity in Wegener's granulomatosis: characterizing T lymphocytes. Arthritis Rheum. 2009;60(6):1578–1587. doi: 10.1002/art.24576. [DOI] [PubMed] [Google Scholar]
- 10.Hu N, Westra J, Kallenberg CG. Dysregulated neutrophil--endothelial interaction in antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides: implications for pathogenesis and disease intervention. Autoimmun Rev. 2011;10(9):536–543. doi: 10.1016/j.autrev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994;179(2):651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolfson RG, Qasim FJ, Thiru S, Oliveira DB, Neild GH, Mathieson PW. Nitric oxide contributes to tissue injury in mercuric chloride-induced autoimmunity. Biochem Biophys Res Commun. 1995;217(2):515–521. doi: 10.1006/bbrc.1995.2806. [DOI] [PubMed] [Google Scholar]
- 13.Oates JC, Gilkeson GS. The biology of nitric oxide and other reactive intermediates in systemic lupus erythematosus. Clin Immunol. 2006;121(3):243–250. doi: 10.1016/j.clim.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16(19):2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49(4–6):134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20(4):223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt N, Pautz A, Art J, Rauschkolb P, Jung M, Erkel G, et al. Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem Pharmacol. 2010;79(5):722–732. doi: 10.1016/j.bcp.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23(2):75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leuk Biol. 2010;88(6):1157–1162. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- 22.Yang GY, Taboada S, Liao J. Induced nitric oxide synthase as a major player in the oncogenic transformation of inflamed tissue. Methods Mol Biol. 2009;512:119–156. doi: 10.1007/978-1-60327-530-9_8. [DOI] [PubMed] [Google Scholar]
- 23.Gilkeson GS, Mudgett JS, Seldin MF, Ruiz P, Alexander AA, Misukonis MA, et al. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997;186(3):365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–391. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 25.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. PNAS USA. 1996;93(23):13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, Raman C, et al. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Amer J Pathol. 2004;165(2):609–616. doi: 10.1016/S0002-9440(10)63325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato W, Kosugi T, Zhang L, Roncal CA, Heinig M, Campbell-Thompson M, et al. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab Invest. 2008;88(9):949–961. doi: 10.1038/labinvest.2008.60. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, et al. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol. 2009;296(2):F317–F327. doi: 10.1152/ajprenal.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, et al. Lack of endothelial nitric oxide synthase aggravates murine accelerated antiglomerular basement membrane glomerulonephritis. Amer J Pathol. 2000;156(3):879–888. doi: 10.1016/S0002-9440(10)64957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Schoeb TR, Panoskaltsis-Mortari A, Zinn KR, Kesterson RA, Zhang J, et al. Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J Immunol. 2006;177(12):8748–8756. doi: 10.4049/jimmunol.177.12.8748. [DOI] [PubMed] [Google Scholar]
- 31.Berden JH, Hang L, McConahey PJ, Dixon FJ. Analysis of vascular lesions in murine SLE. I. Association with serologic abnormalities. J Immunol. 1983;130(4):1699–1705. [PubMed] [Google Scholar]
- 32.Hicks J, Bullard DC. Review of autoimmune (lupus-like) glomerulonephritis in murine models. Ultrastructural pathology. 2006;30(5):345–359. doi: 10.1080/01913120600932677. [DOI] [PubMed] [Google Scholar]
- 33.Bullard DC, King PD, Hicks MJ, Dupont B, Beaudet AL, Elkon KB. Intercellular adhesion molecule-1 deficiency protects MRL/MpJ-Fas(lpr) mice from early lethality. J Immunol. 1997;159(4):2058–2067. [PubMed] [Google Scholar]
- 34.Alexander EL, Moyer C, Travlos GS, Roths JB, Murphy ED. Two histopathologic types of inflammatory vascular disease in MRL/Mp autoimmune mice. Model for human vasculitis in connective tissue disease. Arthritis Rheum. 1985;28(10):1146–1155. doi: 10.1002/art.1780281011. [DOI] [PubMed] [Google Scholar]
- 35.Moyer CF, Strandberg JD, Reinisch CL. Systemic mononuclear-cell vasculitis in MRL/Mp-lpr/lpr mice. A histologic and immunocytochemical analysis. Amer J Pathol. 1987;127(2):229–242. [PMC free article] [PubMed] [Google Scholar]
- 36.Bullard DC, Schoeb TR. Animal Models of Vasculitis. In: Ball GV, Bridges SL, editors. Vasculitis. Second ed. Oxford: Oxford University Press; 2008. pp. 89–96. [Google Scholar]
- 37.Cooke GE, Doshi A, Binkley PF. Endothelial nitric oxide synthase gene: prospects for treatment of heart disease. Pharmacogenomics. 2007;8(12):1723–1734. doi: 10.2217/14622416.8.12.1723. [DOI] [PubMed] [Google Scholar]
- 38.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10(6):1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 39.Singh VK, Mehrotra S, Narayan P, Pandey CM, Agarwal SS. Modulation of autoimmune diseases by nitric oxide. Immunologic Res. 2000;22(1):1–19. doi: 10.1385/IR:22:1:1. [DOI] [PubMed] [Google Scholar]
- 40.Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. 2010;460(6):965–974. doi: 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewicker M, Trautwein G. Sequential study of vasculitis in MRL mice. Lab Anim. 1987;21(4):335–341. doi: 10.1258/002367787781363408. [DOI] [PubMed] [Google Scholar]
- 42.Dimitriu-Bona A, Matic M, Ding W, Yang CP, Fillit H. Cytotoxicity to endothelial cells by sera from aged MRL/lpr/lpr mice is associated with autoimmunity to cell surface heparan sulfate. Clin Immunol Immunopathol. 1995;76(3 Pt 1):234–240. doi: 10.1006/clin.1995.1121. [DOI] [PubMed] [Google Scholar]
- 43.Harper JM, Thiru S, Lockwood CM, Cooke A. Myeloperoxidase autoantibodies distinguish vasculitis mediated by anti-neutrophil cytoplasm antibodies from immune complex disease in MRL/Mp-lpr/lpr mice: a spontaneous model for human microscopic angiitis. Eur J Immunol. 1998;28(7):2217–2226. doi: 10.1002/(SICI)1521-4141(199807)28:07<2217::AID-IMMU2217>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 44.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. PNAS USA. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai A, Miller MJ, Huang X, Warren JS. Nitric oxide modulates MCP-1 expression in endothelial cells: implications for the pathogenesis of pulmonary granulomatous vasculitis. Inflammation. 2003;27(4):213–223. doi: 10.1023/a:1025036530605. [DOI] [PubMed] [Google Scholar]
- 46.Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol. 2005;39(4):595–603. doi: 10.1016/j.yjmcc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Moroi M, Zhang L, Yasuda T, Virmani R, Gold HK, Fishman MC, et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101(6):1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101(4):731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oates JC, Ruiz P, Alexander A, Pippen AM, Gilkeson GS. Effect of late modulation of nitric oxide production on murine lupus. Clin Immunol Immunopathol. 1997;83(1):86–92. doi: 10.1006/clin.1997.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reilly CM, Farrelly LW, Viti D, Redmond ST, Hutchison F, Ruiz P, et al. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int. 2002;61(3):839–846. doi: 10.1046/j.1523-1755.2002.00230.x. [DOI] [PubMed] [Google Scholar]