Abstract
Pancreatitis is a complex, progressively destructive inflammatory disorder. Alcohol was long thought to be the primary causative agent, but genetic contributions have been of interest since the discovery that rare PRSS1, CFTR, and SPINK1 variants were associated with pancreatitis risk. We now report two significant genome-wide associations identified and replicated at PRSS1-PRSS2 (1×10-12) and x-linked CLDN2 (p < 1×10-21) through a two-stage genome-wide study (Stage 1, 676 cases and 4507 controls; Stage 2, 910 cases and 4170 controls). The PRSS1 variant affects susceptibility by altering expression of the primary trypsinogen gene. The CLDN2 risk allele is associated with atypical localization of claudin-2 in pancreatic acinar cells. The homozygous (or hemizygous male) CLDN2 genotype confers the greatest risk, and its alleles interact with alcohol consumption to amplify risk. These results could partially explain the high frequency of alcohol-related pancreatitis in men – male hemizygous frequency is 0.26, female homozygote is 0.07.
The exocrine pancreas is a simple digestive gland of only two primary cell types, each with a single function (Supplementary Figure 1). Recurrent acute pancreatic inflammation can, but does not always, progress to irreversible damage of the gland, including fibrosis, atrophy, pain, and exocrine and endocrine insufficiency,1-3 known as chronic pancreatitis Different genetic and environmental factors produce the same clinical phenotype4.
We collected biological samples and phenotypic data from 1000 patients with recurrent acute pancreatitis and chronic pancreatitis plus controls in the North American Pancreatitis Study 2 (NAPS2)5. The primary environmental risk factor identified was heavy alcohol drinking when symptoms of pancreatitis began, based on the assessment of the study physician, called here alcohol-related pancreatitis.
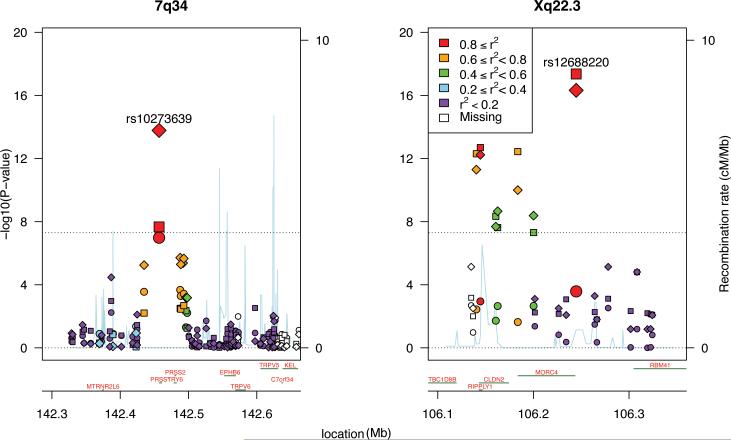
To further define genetic risk, we conducted a two-stage (discovery/replication) genomewide association study (GWAS). The final data set for the Stage 1 cohort included 676 chronic pancreatitis cases and 4507controls of European ancestry (Supplementary Figs. 2-3) genotyped at 625,739 SNPs (Table 1; Supplementary Table 1). Genomewide significant associations (p-value < 5 × 10-8) were identified at two loci. The most highly associated SNP fell in Xq23.3, dubbed the CLDN2 locus, the other in 7q34, the PRSS1-PRSS2 locus (Fig. 1; Table 2; Supplementary Figs. 4-5, Supplementary Table 2). CLDN2 encodes the protein claudin-2, while PRSS1 encodes cationic trypsinogen, and PRSS2 encodes anionic trypsinogen.
Table 1.
Characterization of case subjects used for GWAS*.
| chronic pancreatitis | recurrent acute pancreatitis | chronic pancreatitis + recurrent acute pancreatitis | |||
|---|---|---|---|---|---|
| Stage 1 | 676 | -- | 676 | ||
| Alcohol-related pancreatitis † | Yes | 264 | -- | 264 | |
| No | 411 | -- | 411 | ||
| Unknown | 1 | -- | 1 | ||
| Stage 2 | 331 | 579 | 910 | ||
| Alcohol-related pancreatitis † | Yes | 70 | 113 | 183 | |
| No | 256 | 462 | 718 | ||
| Unknown | 5 | 4 | 9 | ||
| Combined | 930 | 579 | 1506 | ||
| Alcohol-related pancreatitis † | Yes | 334 | 113 | 447 | |
| No | 667 | 462 | 1129 | ||
| Unknown | 6 | 4 | 10 | ||
Does not include information from controls in Stage 1 (n=4514) or from Stage 2 (n=4053). For more complete characterization of samples, please see Supplementary Table 1 in the Appendix.
Alcohol-related pancreatitis was assigned by the study physician at enrollment.
Figure 1.
Manhattan plot showing the negative log (base 10) of the p-value for the association of SNP genotype with affection status for all SNPs passing quality control filters and falling within a selected region of the PRSS1-PRSS2 and CLDN2 loci. Regions selected to highlight the most associated SNPs. Squares indicate Stage 1 results, circles for Stage 2, diamonds for combined Stage 1 and 2 data. After accounting for the most highly associated SNP at each locus, no other SNP approached genomewide-significant association.
Table 2.
Results for leading SNPs at the PRSS1-PRSS2 and CLDN2 loci from Stage 1, Stage 2, and joint analysis.
| CP + RAP | CP | CP + RAP | CP + RAP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele Frequency (A1) | Stage 1 | Stage 2 | Combined | ||||||||||||
| CHR | SNP | BP | A1* | A2 | cases | controls | OR | se(OR) | P | OR | se(OR) | P | OR | se(OR) | P |
| 7 | rs10273639 | 142456928 | T | C | 0.350 | 0.424 | 0.712 | 0.044 | 3.0×10-8 | 0.748 | 0.039 | 7.5×10-8 | 0.734 | 0.029 | 2.0×10-14 |
| X | rs7057398 | 106144529 | C | T | 0.374 | 0.281 | 1.493 | 0.075 | 1.4×10-15 | 1.210 | 0.066 | 1.8×10-5 | 1.321 | 0.049 | 4.6×10-17 |
| X | rs12688220 | 106244767 | T | C | 0.367 | 0.261 | 1.612 | 0.081 | 2.4×10-21 | 1.238 | 0.073 | 2.3×10-6 | 1.385 | 0.054 | 2.3×10-22 |
A1 is the allele counted for purposes of computing odds ratio and associated statistics. The model used here includes covariates to control for the two leading eigenvectors for ancestry, as was done in the Plink analyses, but differs in its treatment of the minor allele count for the CLDN2 locus, which resides on the × chromosome (as described in Online Methods). Alleles given are refSNP alleles according to dbSNP. See Supplementary Table 2 for all SNPs passing quality control and showing p-value < 5×10-7 for Stage 1 or Stage 2 or the joint analysis.
The Stage 2 cohort included 910 cases (331 chronic pancreatitis, 579 recurrent acute pancreatitis; Table 1, Supplementary Table 1), again genotyped at 625,739 SNPs, and 4170 controls, most genotyped previously on the Illumina 1M. All subjects were of European ancestry as determined by genetic analyses. Recurrent acute pancreatitis and chronic pancreatitis were modeled as having common susceptibilities, with chronic pancreatitis occurring over time in the presence of additional disease-modifying factors.6 It is possible that this assumption reduces power relative to a study comprising solely chronic pancreatitis or recurrent acute pancreatitis cases. Our primary targets in Stage 2 were the PRSS1-PRSS2 and CLDN2 loci, although we also conducted a joint analysis7 of Stage 1 and Stage 2 data to uncover any new risk loci. After controlling for ancestry, these data demonstrated significant effects for the CLDN2 and PRSS1-PRSS2 loci (Figure 1; Supplementary Table 2-3; Supplementary Figs. 6-7). Quality of SNP genotypes supported the association (Supplementary Fig. 8). The frequencies of the putative risk alleles at these 2 loci were 0.57 for the C allele at rs10273639 (PRSS1-PRSS2 locus), with the minor T allele reducing risk, and 0.26 for the T allele at rs12688220 (CLDN2 locus). No other locus shows association after accounting for SNP genotype quality (Supplementary Figs. 6-8).
PRSS1 gain-of-function mutations, such as p.R122H, increase risk for recurrent acute pancreatitis and chronic pancreatitis8, as do increased copy number9,10. Rare loss-of-function mutations in PRSS2 are protective11. However, rs10273639 is in the 5′ promoter region of PRSS1. Because it is the only highly associated SNP in the locus, we validated its genotypes by independent TaqMan genotyping and also genotyped two SNPs in linkage disequilibrium with it (footnote, Supplementary Table 4)12,13. We screened PRSS1 for rare variants in 1138 subjects: 418 chronic pancreatitis, 350 recurrent acute pancreatitis, and 379 controls. Three known disease-associated variants (A16V, N29I, R122H) were identified in 23 subjects (Supplementary Table 4). These gain-of-function variants occur almost solely in cases (22 out of 23), and two of them, A16V and R122H, likely fall on the C or risk haplotype of this locus (Supplementary Table 4). Nonetheless, with only 19 A16V and R122H events in cases, these rare alleles cannot account for the association observed at this locus.
Sixty-nine control pancreas tissue samples from three sources were genotyped at rs10273639, and cDNA was used to quantify PRSS1 and control gene expression (Supplementary Table 5). For all three sets of quantitative PCR data, the slope relating count of genotype C allele to PRSS1 expression level was positive; together, the samples provide evidence (p = 0.01) that alleles at rs10273639 affect expression of PRSS1: expression levels were highest in patients with two C alleles at rs10273639, intermediate in heterozygotes, and lowest in subjects with two T alleles. Based on this evidence, we posit that reduced trypsinogen production protects the pancreas from injury, as has been observed in genetic mouse models14.
CLDN2 is considered the primary candidate gene within our CLDN2 locus. Claudin-2 is attractive because it serves as a highly regulated tight junction protein forming low-resistance, cation-selective ion and water channels between endothelial cells15,16 and is normally expressed at low levels between cells of the pancreatic ducts and in pancreatic islets17,18. The CLDN2 promoter includes an NFκB binding site19, and gene expression is enhanced in other cells under conditions associated with injury or stress20-22. Claudin-2 can also be expressed by acinar cells when stressed, as reported in porcine models of acute pancreatitis23. Other genes within the CLND2 locus include MORC4, RIPPLY1, and TBC1D8B. MORC4 is expressed at low levels in most tissues, including the pancreas, with higher levels in the placenta and testis24. The MORC4 protein contains a CW four-cysteine zinc-finger motif, nuclear localization signal, and nuclear matrix-binding domain, suggesting that it may be a transcription factor24, but its expression does not appear to correlate with pancreatitis (Supplementary Fig. 9). RIPPLY1 and TBC1D8B are not known to be expressed in the pancreas.
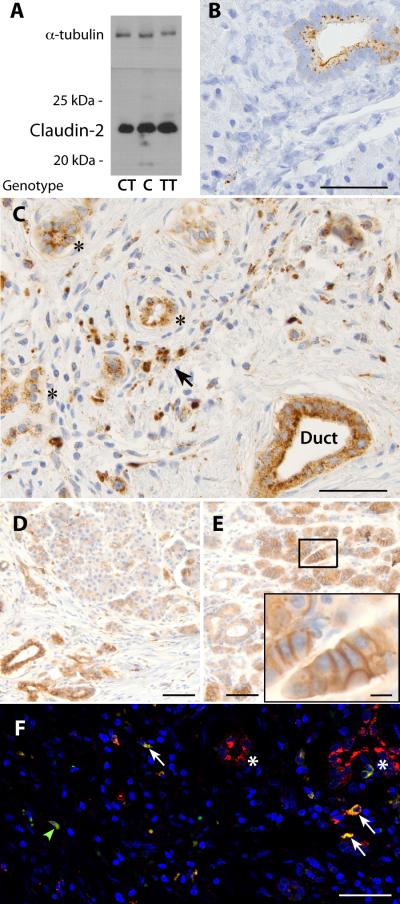
To our knowledge, genetic variations in CLDN2 have never been associated with disease in humans. We assessed DNA sequence variants around CLDN2, RNA, and protein expression for claudin-2 in control tissue classified by histology and genotype (Supplementary Table 6, Supplementary Fig. 10). Evaluating 1000 Genomes data, no exonic variation was identified that could explain the association signal. Using materials and methods described previously for PRSS1 expression, CLDN2 expression levels in control tissues did not correlate with the CLDN2 locus risk genotype (p-value = 0.32). Protein was extracted from the tissue, and only one protein band of the appropriate size was observed with anti-claudin-2 antibodies on Western blot, which correlated with tissue inflammation as determined by systematic grading of histology in adjacent tissue (Fig. 2A, Supplementary Fig. 10). Immunohistochemical staining with anti-claudin-2 antibodies was verified in normal tissue (Fig. 2B), with kidney, duodenum, and bile ducts serving as additional positive controls (not shown). Protein localization was assessed in 12 GWAS cases who underwent pancreatic surgery: 6 with the CLDN2-containing high-risk genotype and 6 without. Claudin-2 cytoplasmic granular staining was markedly increased in both duct and acinar cells in chronic pancreatitis cases (Fig. 2C-E). Only chronic pancreatitis cases with the high-risk CLDN2 genotype demonstrated moderate-to-strong claudin-2 staining along the basolateral membrane of acinar cells (Fig. 2D, 2E, Supplementary Table 6). Claudin-2 was also expressed in macrophages, which could contribute to the pathologic inflammatory process25 (Fig. 2C, F).
Figure 2.
Expression and localization of claudin-2 in the human pancreas using mouse anti-claudin-2 antibodies based on rs12688220 genotype. A. Western blot of anti-claudin-2 antibody from 3 control samples genotyped at rs12688220 (TT is high risk). The antibody reacts with a protein at ~22-23 kDa, consistent with claudin-2. Samples had inflammation and/or fibrosis on histology of adjacent tissue. α-tubulin, loading control. Blots from all controls are presented in Supplementary Figure 8. B. Anti-claudin-2 staining (brown color) of normal-appearing control tissue localizing to ducts but not to acinar cells (scale bar=50μm). C. Severe chronic pancreatitis from a case with the high-risk (T male or TT female) genotype. Claudin-2 staining localizes to the intralobular duct (Duct), atrophic acini (*), and cells with morphologic appearance of macrophages (arrow)(scale bar=50μm). D. Chronic pancreatitis tissue from a patient with the low-risk genotype (CC or CT) with staining localizing to the duct and granular staining in acinar cells (scale bar = 100 μm). E. Chronic pancreatitis, high-risk genotype with intense staining of acinar cell basolateral membrane (scale bar=100 μm, enlarged in inset, scale bar=10μm). F. Immunofluorescence staining of control human pancreatic tissue claudin-2 staining (red) localizing to the ducts (*) and co-localizing with the macrophage marker CD68 (green, colocalized with red is yellow, arrows. Nuclei stained with Hoechst's dye, blue, scale bar = 100μm).
Most studies report excessive alcohol consumption as the major risk factor for adult-onset chronic pancreatitis26-29. However, only 3% of patients who are alcoholics develop chronic pancreatitis30, suggesting a pancreas-targeting risk factor. We compared genotypes based on whether pancreatitis was alcohol-related (yes/no) 5,31. Setting control genotypes counts as the baseline category to be compared with case genotypes, the jointly estimated odds ratios for cases with a positive alcohol-related pancreatitis was greater for both rs10273639 (PRSS1-PRSS2 locus) and rs12688220 (CLDN2 locus) than those estimated for cases with a negative alcohol-related pancreatitis (Table 3). Thus, the effects of both loci appeared to be amplified by alcohol consumption. In a case-only analysis, both loci appear to interact with alcohol-related pancreatitis (Table 3), the CLDN2 locus most prominently (p-value = 4×10-7).
Table 3.
Allele frequencies for rs10273639 (risk allele C) and rs12688220 (risk allele T) when data are stratified by controls or pancreatitis ± alcohol-related diagnosis.
| Status | Alcohol-related | Number of individuals | rs102736391 (C) frequency | rs126882201 (T) frequency |
|---|---|---|---|---|
| Control | -- | 8029 | 0.576 | 0.261 |
| Pancreatitis | No | 1129 | 0.634 | 0.322 |
| Yes | 447 | 0.696 | 0.427 |
Using data from cases only and in a joint analysis of both SNPs, rs12688220 predicts alcohol-related pancreatitis as genotypes (χ2=29.57; DF=2; p-value = 4×10-7) or count of risk alleles (χ2=13.17; DF=1; p-value = 3×10-4). rs10273639 (PRSS1-PRSS2 locus) is a significant predictor (count of risk alleles: χ2=5.68; DF=1; p-value = 0.017; genotypes: χ2=6.05; DF=2; p-value = 0.049), even after accounting for the effects of rs12688220.
We conclude that a common allele in the PRSS1-PRSS2 locus is associated with lower PRSS1 gene expression and that this effect is independent of the previously reported rare gain-of-function PRSS1 variants that increase susceptibility to both recurrent acute pancreatitis and chronic pancreatitis8. For this reason, and because risk variants at the PRSS1-PRSS2 locus exert a similar effect in patients with recurrent acute pancreatitis or chronic pancreatitis, it is reasonable to conjecture that variation at rs10273639 or variation in linkage disequilibrium with it directly affects risk for chronic pancreatitis and recurrent acute pancreatitis through its impact on trypsinogen expression. Variation at the CLDN2 locus, however, is much more strongly associated with chronic pancreatitis than recurrent acute pancreatitis, suggesting that it likely acts as a disease modifier to accelerate transition from recurrent acute pancreatitis to chronic pancreatitis. The significant association of the CLDN2 locus with alcohol suggests that the high-risk allele in the CLDN2 locus may modify risk through a non-trypsin-dependent process. Thus, we have characterized two common genetic risk modifiers for sporadic and alcohol-related chronic pancreatitis.
Online Methods
Subject recruitment
Details of recruitment of cases and controls are reported in Supplementary Table 1. All studies were conducted under institutional review board-approved protocols.
Stage 1 samples
All N = 758 Stage 1 case samples were from the North American Acute Pancreatitis Study (NAPS2 5) were diagnosed with chronic pancreatitis, and were characterized for alcohol-related pancreatitis (Table 1). chronic pancreatitis occurs in less than 0.05% of the population, so a convenience sample provides essentially identical power as a same-sized sample of controls selected for the absence of chronic pancreatitis 32. For controls, we used genotypes from 4076 cases and controls from the Alzheimer Disease Genetics Consortium (ADGC) and 493 NAPS2 subjects, all genotyped on the same platform as the chronic pancreatitis samples.
Stage 2 samples
The Stage 2 samples consisted of N=343 chronic pancreatitis and N=627 recurrent acute pancreatitis cases (Table 1, Supplementary Table 1) as well as 4191 control subjects (3986 from the NeuroGenetics Research Consortium, NGRC, and 205 NAPS2 controls).
Genotypes
All cases and NAPS2 controls were genotyped by the University of Pittsburgh Genomics and Proteomics Core Laboratories using the Illumina HumanOmniExpress Beadchip. Samples were processed and scanned using the manufacturer's recommended protocols with no modifications. ADGC samples33 were also genotyped using Illumina HumanOmniExpress Beadchips, whereas NGRC samples 34 were genotyped on the Illumina Human1M-Duo DNA Analysis BeadChip.
Quality Control (QC) for Stage 1
QC was performed for individuals and then SNPs to determine which samples and SNPs should not be included in the analysis (“dropped’). Assessing sex miscalls based on × chromosome genotypes using Plink35, 7 chronic pancreatitis cases and 20 controls (10 NAPS2; 10 ADGC) were dropped. Based on the requirement for ≥ 95% complete genotypes per individual, 40 cases and 27 controls (20 NAPS2 controls and 7 ADGC controls) were dropped. Searching duplicate or highly related samples based on genotype and using GCTA software 36 (Genetic Relationship Matrix score GRM > 0.4), 35 cases and 78 controls (2 NAPS2, 76 ADGC) were dropped. After these QC filters, 676 cases and 4507 controls remained for association analysis.
SNP QC was first performed using NAPS2 and ADGC samples separately.. Ancestry was estimated using dacGem37 based on 9700 SNPs that had a genotype completion rate of ≥99.9%, a minor allele frequency MAF ≥ 0.05, and were separated by at least 500Kb. Analysis of genotypes from NAPS2 subjects identified 1 significant dimensions of ancestry and clustered subjects into 3 groups (Supplementary Fig. 1). Groups A and B, illustrated in Supplementary Figure 1, delineate 764 and 282 subjects, respectively, of European ancestry (self-identified); SNP QC for MAF and Hardy Weinberg Equilibrium (HWE) were performed on data from these subjects. Of 731,442 SNPs received, 633,790 passed QC filters. SNPs were dropped for the following reasons: 3165 for map location; 11,977 for call rate; 77,300 for MAF < 0.01; and 5219 failed HWE (p-value < 0.005).
ADGC data were received in three waves of 1763, 1110, and 1266 subjects. In the first wave, 659,224 SNPs were received, while in waves two and three, 730,525 SNPs were received. After QC as described for the chronic pancreatitis cohort, including harmonization with SNPs passing QC in the chronic pancreatitis cohort, 604,059, 632,761, and 633,023 SNPs remained, respectively. After merging cohorts, 30 related subjects were dropped, leaving 4046 ADGC subjects. Of the 633,615 unique SNPs in this ADGC, QC filters dropped 5 for low MAF and 5316 for HWE, leaving 628,294 SNPs. Combining ADGC and chronic pancreatitis cohorts and performing another round of QC yielded 625,739 SNPs for analysis.
QC for Stage 2
QC for individuals was performed as described for Stage 1. These individual-specific QC filters removed 60 cases, leaving 331 chronic pancreatitis and 579 recurrent acute pancreatitis cases for analysis; 14 controls were also removed, leaving 4177 controls for analysis. We analyzed all SNPs passing QC at Stage 1.
Association analysis
To control confounding due to ancestry, the first 10 major eigenvectors from the spectral decomposition were used as covariates in Stage 1 and Stage 2 analyses38, although only one was significant. We contrasted the genotypes of case subjects and controls via logistic regression and a log-additive (logit) model using Plink35. Genotypes for any SNPs showing association p-value < 5 × 10-7 were manually inspected for valid genotype clustering. SNPs showing poor-quality clustering were excluded. Following Skol et al. 7 and others, we take an overall significance level of 5 × 10-8 and 5 × 10-7 for strongly suggestive association.
To determine whether alcohol interacts with genetic variation to alter risk of pancreatitis, data from cases were fit to a general linear model in which count of alleles or genotypes predicted alcohol etiology (yes/no). The test statistic was obtained as a likelihood ratio chi-square. Note that in these analyses and any analyses other than genomewide association, we model the male genotypes as 0 and 2 39,40. For the genomewide association, Plink encodes the count of minor alleles in males as 0 and 1 and includes a sex effect, but the 0/2 encoding for males is a more powerful approach39,40 .
DNA extraction
DNA was obtained using standard methods41.
Pancreatic tissue processing
Tissue was obtained from two sources [Pitt and Pancreatic Adenocarcinoma Gene-Environment Risk (PAGER) from the University of Pittsburgh and PSU from Pennsylvania State University] and processed in three batches: banked (Pitt) and prospectively collected (PAGER) surgical waste from uninvolved pancreas and normal pancreas specimens from the Gift of Life Program that were not used for transplantation (PSU). PAGER samples were snap-frozen, placed in RNAlater solution (Ambion), and stored at –80°C. PSU pancreas samples were also snap frozen and preserved in formalin or placed in RNAlater solution. RNA was isolated using Trizol reagent (Invitrogen), and its quality examined in 1% agarose gel stained with ethidium bromide. cDNA was transcribed using oligo dt primers and the Superscript II reverse transcriptase kit (Invitrogen).
Gene Expression
Relative expression of PRSS1, PRSS2, CTRC, and 18S was determined by analyzing cDNA using Taqman®-based rtPCR assays (Applied Biosystems). Raw absolute quantitation results were analyzed and converted to relative expression results by software packages SDS V2.3 and DataAssist V1.0 (Applied Biosystems). Assays were repeated in triplicate or quadruplicate. Three sets of samples were assessed, two from Pitt (N=10 and 22) and one from PSU (N=37). PSU results were normalized against 18S, Pitt against CTRC. From each of these three data sets, mean gene expression per sample was regressed against allele count to obtain an estimated slope, standard error, and z-score. We then calculated an overall z-score as a weighted average of the individual z-scores, with weights determined by sample size.
Antibodies
Antibodies against claudin proteins (Invitrogen) were assessed using Western blot for mouse anti-claudin-2 (Catalog No. 32-5600), mouse anti-claudin-4 (Catalog No. 32-9400), and mouse alpha-tubulin antibody (Catalog no. AA12.1 The Developmental Studies Hybridoma Bank at the University of Iowa, http://dshb.biology.uiowa.edu/Antibody-list). Immunohistochemistry was performed using monoclonal antibodies for claudin-2 (Catalog #32-5600,1:1,000 dilution). Immunoflourescence was performed using mouse anti-claudin-2 (Catalog No. 32-5600) and goat anti-human CD68 (Catalog #sc-7082, Santa Cruz Biotechnology Inc.). The secondary antibodies for Immunofluorescence were goat anti-mouse CY3 and anti goat Cy5 from Jackson Immunoresearch.
SDS-PAGE and WESTERN Blotting
Protein homogenates for Western blotting were obtained from snap-frozen tissue that was homogenized and sonicated in lysis buffer supplemented with protease inhibitors. Protein concentration was determined by the Bradford method using a kit from Bio-Rad. Proteins were separated on 12% SDS-PAGE42 followed by transfer to polyvinylidene difluoride (PVDF) membranes43, for Western blotting44. Immunodetection of bound antibodies on PVDF membrane was performed using ECL reagents (Amersham Biosciences). All procedures were carried out according to manufacturer instructions.
Immunohistochemistry
Standard automated immunohistochemistry was performed for claudin 2 (antibodies listed above) on formalin-fixed, paraffin-embedded, 5 micron-thick tissue sections. Following deparaffinization in xylene and rehydration in ethanol, antigen retrieval was performed using EDTA pH8 buffer. The Dako Autostainer Plus was used; the slides were incubated for 30 minutes with the primary antibodies, followed by incubation with the secondary reagent (Mach 2 Mouse HRP Polymer from Biocare Medical) for 30 minutes. The chromogen was developed (Dako DAB+) for 10 minutes. The immunohistochemical stains were reviewed by one of the authors (A.M.K.). Cytoplasmic, granular, and membranous staining, predominantly in the lateral cell membranes, were graded on an intensity scale of 0-4 (0, negative; 1, weak; 2, moderate; 3, strong). The staining intensity was very patchy from lobule to lobule in most cases.
Immunofluorescence
Cryostat sections (5 micron) of pancreas were washed 3 times with phosphate-buffered saline (PBS), followed by 3 washes with solution of .5% BSA in PBS. Sections were blocked with 2% BSA solution for 30 minutes. The slides were incubated for 1 hour at room temperature with primary antibody for claudin-2 1:100 and goat anti-human CD68 in 0.5% BSA solution. Slides were washed 3 times with BSA solution and incubated for 1 hour at 20°C with 1:500 dilution anti-goat CY5 and 1:1000 dilution goat anti-mouse CY3 secondary antibodies in BSA solution. Nuclei were stained with Hoeschts dye (bisbenzamide 1mg/100ml water) for 30 seconds. After 3 rinses with PBS, sections were cover slipped with Gelvatol mounting media. Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1.7a). The Cy5 signal (CD68) was pseudocolored as green to show colocalization with the red Claudin signal as yellow.
Supplementary Material
ACKNOWLEDGMENTS
Technical support was provided by Kimberly Stello, Siddhartha Das, Danielle Dwyer, Alexander Rowland, Paul Alexander Blake, Mark Ross, Liz Kish PhD, and Haq Nawaz MD, Shiela Solomon MS CGC and Sharon Boggiano RN from the University of Pittsburgh; Rachel Ostroff, Meredith Goss and Jan Timm from SomaLogic Inc (Boulder CO). Data management by Laurie Silfies and Darina Protivnak, Epidemiology Data Center, Graduate School of Public Health, University of Pittsburgh. Clinical support: Sharon Boggaino RN, Megan Hendricks RN, Beth Elinoff RN MPH CCRC, University of Pittsburgh, Lee McHenry MD, Glen Lehman MD, James Watkins MD, Evan Fogel MD, Laura Lazzell-Pannell, BSN, RN, CCRC, Indiana University, Indianapolis, IN. Additional samples were provided by Frank Burton MD (deceased) Department of Internal Medicine, St. Louis University School of Medicine, St Louis, MO, Simon Lo MD, Department of Medicine, Cedars-Sinai Medical Center, University of California, Los Angeles; Mark T. DeMeo MD, Department of Medicine, Rush University Medical Center, Chicago, IL; William M. Steinberg MD, Washington Hospital Center, Washington DC; Michael L. Kochman MD, Department of Medicine, University of Pennsylvania, Philadelphia, PA; Babak Etemad MD, Department of Gastroenterology and Hepatology, Ochsner Medical Center, New Orleans, LA. Herbert Zeh MD, Arthur James Moser MD, K. Kenneth Lee MD, Department of Surgery, University of Pittsburgh, Pittsburgh PA.
This publication was made possible by Grant Numbers DK061451 (DCW), DK054709 (DCW), DK063922 (DCW), MH057881 (BD/KR), CA117926 (JPS), UL1 RR024153 and UL1TR000005. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. This project used the University of Pittsburgh Genomics and Proteomics Core Laboratories (UL1 RR024153) and the UPCI Clinical Genomics Immunoproteomics and Sequencing Facility (NIH P30CA047904). Jessica LaRusch was supported by Digestive Disease Training Program T32DK063922 (DCW), Narsis Zarnescu MD was supported by the American Gastroenterology Association John I Isenberg MD International Scholar Award. The Liverpool cohort was supported by NIHR Biomedical Research Unit award. Additional support was provided by National Pancreas Foundation (DCW), the Frieda G. and Saul F. Shapira BRCA Cancer Research Program (DCW) and the Wayne Fusaro Pancreatic Cancer Research Fund (DCW) and Gift of Life Foundation
Support for control cohort 1. Alzheimer's Disease Genetics Consortium: The National Institutes of Health, National Institute on Aging (NIH-NIA) supported this work through the following grants: ADGC, U01 AG032984, RC2 AG036528; NACC, U01 AG016976; NCRAD, U24 AG021886; Banner Sun Health Research Institute P30 AG019610; Boston University, P30 AG013846, U01 AG10483, R01 CA129769, R01 MH080295, R01 AG017173, R01 AG025259, R01AG33193; Columbia University, P50 AG008702, R37 AG015473; Duke University, P30 AG028377, AG05128; Emory University, AG025688; Indiana University, P30 AG10133; Johns Hopkins University, P50 AG005146, R01 AG020688; Massachusetts General Hospital, P50 AG005134; Mayo Clinic, P50 AG016574; Mount Sinai School of Medicine, P50 AG005138, P01 AG002219; New York University, P30 AG08051, MO1RR00096, and UL1 RR029893; Northwestern University, P30 AG013854; Oregon Health & Science University, P30 AG008017, R01 AG026916; Rush University, P30 AG010161, R01 AG019085, R01 AG15819, R01 AG17917, R01 AG30146; University of Alabama at Birmingham, P50 AG016582, UL1RR02777; University of Arizona, R01 AG031581; University of California, Davis, P30 AG010129; University of California, Irvine, P50 AG016573, P50, P50 AG016575, P50 AG016576, P50 AG016577; University of California, Los Angeles, P50 AG016570; University of California, San Diego, P50 AG005131; University of California, San Francisco, P50 AG023501, P01 AG019724; University of Kentucky, P30 AG028383, AG05144; University of Michigan, P50 AG008671; University of Pennsylvania, P30 AG010124; University of Pittsburgh, P50 AG005133, AG030653; University of Southern California, P50 AG005142; University of Texas Southwestern, P30 AG012300; University of Miami, R01 AG027944, AG010491, AG027944, AG021547, AG019757; University of Washington, P50 AG005136; Vanderbilt University, R01 AG019085; and Washington University, P50 AG005681, P01 AG03991. The Kathleen Price Bryan Brain Bank at Duke University Medical Center is funded by NINDS grant # NS39764, NIMH MH60451 and by Glaxo Smith Kline. We thank Drs. D. Stephen Snyder and Marilyn Miller from NIA who are ex-officio ADGC members.
Support for control group 2. Data for control group 2 genotypes were obtained from Genome-Wide Association Study of Parkinson Disease: Genes and Environment, dbGaP Study Accession phs000196.v2.p1. This NeuroGenetics Research Consortium (NGRC) is a gene-environment study of Parkinson's disease. Principal Investigator is Haydeh Payami, PhD, New York State Department of Health Wadsworth Center, Albany, NY, USA. Co-investigators are: John Nutt, MD., Oregon Health & Sciences University; Cyrus Zabetian, MS, MD. University of Washington and Puget Sound Veterans Medical Center, Seattle, WA, USA; Stewart Factor, DO. Emory University, Atlanta, GA, USA; Eric Molho, MD. Albany Medical Center, Albany, NY, USA; and Donald Higgins, MD. Albany Medical Center and Albany Veterans Medical Center Albany, NY, USA. Funding Source: 5R01NS36960-10. Genotyping provided by Genotyping Center, Johns Hopkins University Center for Inherited Disease Research (CIDR), Baltimore, MD, USA. Funding Source for Genotyping: HHSN268200782096C. NIH contract “High throughput genotyping for studying the genetic contributions to human disease” from the National Institutes of Health, Bethesda, MD, USA.
Footnotes
AUTHOR CONTRIBUTIONS
Writing Group: A.M.K., B.D., D.C.W., J.L., L.K., M.L.K.
Project design, management, and coordination: B.D., D.C.W., D.Y., J.P.N., M.M.B., M.M.L., N.M.M., S.R.W.
Sample collection and phenotyping: A.B., A.C.M., A.C.N., A.G., A.G.S., A.I.L., A.J.S., A.K., A.M.G., A.P., A.P.L., A.R., A.S., B.G., B.L.M., B.N.V., B.R., B.S.S., B.T.H., C.A.M., C.B.W., C.Cao, C.Cruchaga, C.D., C.E.F., C.E.Y., C.F.L., C.G.L., C.L., C.M.H., C.M.W., C.R., C.T.B., D.A.B., D.Beekly, D.Blacker, D.C., D.C.Marson, D.C.Mash, D.C.W., D.G.C., D.H.C., D.H.G., D.R.G., D.W.D., D.W.T., D.Y., E.A.C., E.D.R., E.H., E.H.B., E.H.K., E.M., E.M.R., E.P., E.R.M., F.M., F.M.L., F.U.W., F.Y.D., G.A.C., G.A.J., G.I.P., G.J., G.L., G.W.B., H.C.C., H.H., H.J.R., H.P., H.V.V., J.A.K., J.A.Schneider, J.A.Sonnen, J.B., J.B.L., J.C.M., J.C.T., J.D., J.D.B., J.D.G., J.E.P., J.F.Q., J.H.G., J.H.K., J.J.L., J.M., J.M.O., J.M.R., J.P.N., J.P.S., J.P.V., J.Q.T., J.R., J.R.B., J.R.G., J.R.M., J.W., J.W.M., K.A.W.B., K.B.F., K.L.H.N., K.L.L., K.M.F., L.B.C., L.E.H., L.G.A., L.J.V.E., L.L.B., L.S.H., L.S.S., L.S.W., L.W.J., L.Y., M.A.A., M.D., M.E.M., M.Ganguli, M.Gearing, M.I.K., M.J., M.L., M.M., M.M.B., M.M.L., M.P.F., M.R., M.R.F., M.S., M.S.A., M.T., N.E.T., N.J.C., N.M.G., N.R.G.R., N.W.K., O.L.L., O.V., P.A.B., P.B.C., P.K., P.S., R.A.H., R.A.S., R.B., R.C.G., R.C.P., R.D., R.E.B., R.E.T., R.K., R.L.A., R.L.H., R.L.W., R.M.C., R.N.R., R.S., S.A., S.E.A., S.F., S.G., S.G.Y., S.L.C., S.S., S.Spina, S.T.A., S.Weintrub, T.B.G., T.D.B., T.G.B., T.J.M., T.M., T.M.F., V.M.V.D., W.A.K., W.G., W.J.M., W.W.P., W.W.S.
Genotyping and expression studies: A.M.K., D.J.H., D.S., G.D.S., J.L., J.L.H., J.P.S., L.A.F., L.O., M.A.P-V., N.O.Z., R.M., V.K.S.
Statistical analysis: B.D., D.Y., K.R., L.K., M.M.B., M.R.O
COMPETING FINANCIAL INTEREST
The authors declare no competing financial interests*.
Note to Editor: Dr Whitcomb notes that he owns stock in Ambry Genetics (a genetic testing company) and also the U.S. patent 6406846 entitled “ Method for determining whether a human patient is susceptible to hereditary pancreatitis, and primers therefore ”. Neither of these interest are directly related to the current manuscript.
References
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: Diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 2.Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2009;10:63–87. doi: 10.1146/annurev-genom-082908-150009. [DOI] [PubMed] [Google Scholar]
- 3.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Whitcomb DC. What is personalized medicine - what does it replace? Nat Rev Gastroenterol Hepatol. 2012 doi: 10.1038/nrgastro.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb DC, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8:520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–45. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nature genetics. 2006;38:209–13. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 8.Whitcomb DC, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature Genetics. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 9.Masson E, et al. Trypsinogen copy number mutations in patients with idiopathic chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:82–8. doi: 10.1016/j.cgh.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Larusch J, Barmada MM, Solomon S, Whitcomb DC. Whole exome sequencing identifies multiple, complex etiologies in an idiopathic hereditary pancreatitis kindred. JOP : Journal of the pancreas. 2012;13:258–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Witt H, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–73. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AD, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–9. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawra R, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217. e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Itallie CM, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. Journal of cell science. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 16.Amasheh S, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of cell science. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, et al. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncology reports. 2011;25:971–8. doi: 10.3892/or.2011.1132. [DOI] [PubMed] [Google Scholar]
- 18.Aung PP, et al. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Archiv : an international journal of pathology. 2006;448:428–34. doi: 10.1007/s00428-005-0120-2. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi T, et al. Cloning of the human claudin-2 5'-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudalrelated homeodomain proteins and hepatocyte nuclear factor-1alpha. The Journal of biological chemistry. 2002;277:21361–70. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- 20.Mankertz J, et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell and tissue research. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. The Journal of biological chemistry. 2011;286:31263–71. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mankertz J, et al. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochemical and biophysical research communications. 2004;314:1001–7. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- 23.Merilainen S, et al. Acute edematous and necrotic pancreatitis in a porcine model. Scandinavian journal of gastroenterology. 2008;43:1259–68. doi: 10.1080/00365520802158580. [DOI] [PubMed] [Google Scholar]
- 24.Liggins AP, et al. MORC4, a novel member of the MORC family, is highly expressed in a subset of diffuse large B-cell lymphomas. British journal of haematology. 2007;138:479–86. doi: 10.1111/j.1365-2141.2007.06680.x. [DOI] [PubMed] [Google Scholar]
- 25.Van den Bossche J, et al. Claudin-1, claudin-2 and claudin-11 genes differentially associate with distinct types of anti-inflammatory macrophages in vitro and with parasite- and tumor-elicited macrophages in vivo. Scandinavian journal of immunology. 2012 doi: 10.1111/j.1365-3083.2012.02689.x. [DOI] [PubMed] [Google Scholar]
- 26.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–8. [PubMed] [Google Scholar]
- 27.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion. 1973;9:447–53. doi: 10.1159/000197473. [DOI] [PubMed] [Google Scholar]
- 28.Robles-Diaz G, Vargas F, Uscanga L, Fernandez-del Castillo C. Chronic pancreatitis in Mexico City. Pancreas. 1990;5:479–83. doi: 10.1097/00006676-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Irving HM, Samokhvalov AV, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP. 2009;10:387–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav D, Eigenbrodt ML, Briggs MJ, Williams DK, Wiseman EJ. Pancreatitis: prevalence and risk factors among male veterans in a detoxification program. Pancreas. 2007;34:390–8. doi: 10.1097/mpa.0b013e318040b332. [DOI] [PubMed] [Google Scholar]
- 31.Yadav D, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–45. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacanu SA, Devlin B, Roeder K. The power of genomic control. American journal of human genetics. 2000;66:1933–44. doi: 10.1086/302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature genetics. 2011;43:436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamza TH, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson's disease modifier gene via interaction with coffee. PLoS genetics. 2011;7:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell S, et al. PLINK: a tool set for whole-genome association and populationbased linkage analyses. American journal of human genetics. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. American journal of human genetics. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klei L, Kent BP, Melhem N, Devlin B, Roeder K. GemTools: a fast and efficient approach to estimating genetic ancestry. 2011 [Google Scholar]
- 38.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 39.Clayton DG. Sex chromosomes and genetic association studies. Genome medicine. 2009;1:110. doi: 10.1186/gm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng G, Joo J, Zhang C, Geller NL. Testing association for markers on the X chromosome. Genetic epidemiology. 2007;31:834–43. doi: 10.1002/gepi.20244. [DOI] [PubMed] [Google Scholar]
- 41.Pfutzer RH, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Analytical biochemistry. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.