Abstract
Hepatitis C virus (HCV) infection recurs in liver recipients who are viremic at transplantation. We conducted a randomized controlled trial to test the efficacy and safety of pre-transplant pegylated interferon alpha-2b plus ribavirin (Peg-IFN-a2b/RBV) for prevention of post-transplant HCV recurrence. Enrollees had HCV and were listed for liver transplantation, with either potential living donors or MELD upgrade for hepatocellular carcinoma. Patients with HCV genotypes (G) 1/4/6 (n=44/2/1) were randomized 2:1 to treatment (n=31) or untreated control (n=16); HCV G2/3 (n=32) were assigned to treatment. Overall, 59 were treated and 20 were not. PEGIFN alfa-2b, starting at 0.75 μg/kg/wk, and ribavirin (RBV), starting at 600 mg/d, were escalated as tolerated. Patients assigned to treatment versus control had similar baseline characteristics. Combined virologic response (CVR) included pre-transplant sustained VR (SVR12) and post-transplant VR (pTVR), defined as undetectable HCV RNA 12 weeks after end of treatment or transplant, respectively. In intent-to-treat analyses, 12 (19%) assigned to treatment and 1 (6%) assigned to control achieved CVR (p=0.29); per-protocol values were 13 (22%) and 0 (0%) (p=0.03). Among treated G1/4/6 patients, 23/30 received transplant of whom 22% had pTVR; among treated G2/3 patients 21/29 received transplant, of whom 29% had pTVR. pTVR was 0%, 18%, and 50% in patients treated for <8, 8–16, and >16 weeks, respectively (p=0.01). Serious adverse events (SAEs) occurred with similar frequency in treated versus untreated patients (68% vs. 55%, p=0.30) but the number of SAEs per patient was higher in the treated group (2.7 vs. 1.3, p=0.003).
Conclusion
Pretransplant treatment with PEGIFN/RBV prevents post-transplant recurrence of HCV in selected patients. Efficacy is higher with >16 weeks of treatment, but treatment is associated with increased risk of potentially serious complications.
Keywords: Peginterferon alfa-2b, Ribavirin, Cirrhosis, Waiting List, post-transplant virologic response
Recurrence of hepatitis C virus (HCV) infection is inevitable in viremic patients undergoing liver transplantation (1,2). Aggressive recurrence of hepatitis C is associated with rapid progression to cirrhosis, graft failure, and death or need for liver transplantation (3–5). Prevention of allograft re-infection by pre-transplant antiviral therapy is one strategy for improving graft and patient outcomes in recipients transplanted for chronic hepatitis C.
Virologic response to peginterferon (PEGIFN) and ribavirin (RBV) is reduced in cirrhosis. In the registration trials for PEGIFN/RBV, the rates of sustained virologic response (SVR) were 5% to 15% lower in patients with advanced fibrosis or cirrhosis (6–8). Likelihood of SVR further diminishes with increasing severity of liver disease due to poor tolerability, dose reductions, discontinuation of therapy, and intrinsically compromised response to PEGIFN/RBV (9–11). SVR was only demonstrated in 13% of patients with HCV genotype 1 and decompensated liver disease, two thirds of whom were treatment naive (12).
Despite the reduced rates of SVR among patients with advanced liver disease, on-treatment clearance of HCV RNA from blood can be achieved in 30% to 40% of patients with HCV genotype 1 and 70% to 90% of patients with HCV genotypes 2 or 3. In the setting of liver transplantation, rendering blood free of HCV RNA prior to transplantation could potentially limit the risk for recurrent HCV after liver transplantation. Five published reports have suggested that suppression of HCV RNA in patients with advanced disease is achievable and that 20% to 30% of treated patients may remain free of HCV infection after transplantation (12–16). None of these reports was a randomized trial, limiting conclusions regarding efficacy and more importantly safety.
Herein we report the efficacy and safety of PEGIFN/RBV to prevent recurrence of HCV in a cohort of patients from the Adult-to-adult Living Donor Liver Transplantation Cohort Study (A2ALL).
Patients and Methods
Study Patients
Patients were enrolled from October 2005 to January 2009, and followed through December 2009. Two groups of adult patients with chronic HCV infection listed for liver transplantation were included: those who had a potential living donor, and those with hepatocellular carcinoma (HCC) eligible for Model for End-stage Liver Disease (MELD) waiting list upgrade. These two patient groups were considered the best candidates for pretransplant HCV treatment as they typically had less severe liver decompensation and therefore would be predicted to better tolerate therapy. Another feature of these two groups is a relatively short and predictable duration of time on the waiting list that allowed for timing of treatment. Key inclusion criteria were stable clinical status, HCV RNA positive, MELD ≤ 20 and anticipated time to transplantation of at least 12 weeks. The protocol allowed an investigator to petition for enrollment of a patient with MELD score from 21 to 25. A clinical oversight committee (GE, AL, NT) was created to review the clinical information and determine eligibility for enrollment. Only one case (MELD 22) was approved and enrolled under this provision.
The main exclusion criteria were history of null response to a prior course of full doses of PEGIFN and RBV for at least 12 weeks, symptomatic cardiovascular or psychiatric disease or serious systemic illness, active substance abuse within prior six months, severe cytopenias not responsive to either erythropoietin analogue (EPA) or granulocyte colony stimulating factor (G-CSF) and unstable clinical courses related to ongoing gastrointestinal bleeding, refractory encephalopathy, or HCC beyond Milan criteria. Exclusionary laboratory criteria were creatinine ≥ 2.2 mg/dL, hemoglobin < 10 g/dL, absolute neutrophil count (ANC) < 750/ul, and platelets < 35,000/ul prior to initiation of therapy. EPA and G-CSF were allowed prior to enrollment to achieve these laboratory entry criteria.
The protocol was approved by the Institutional Review Boards at the participating institutions and all subjects provided written informed consent.
Study Design
Eligible patients were enrolled at seven clinical centers; patients infected with genotypes 1/4/6 were randomized 2:1 to treatment or observation using a web-based interface stratified by clinical center, whereas those with HCV genotypes 2/3 were assigned to treatment. The different approach by genotype reflected a known lower virologic response to PEGIFN/RBV for genotypes 1/4/6 than for genotypes 2/3, and the need to include untreated controls to assess treatment risk. The targeted duration of therapy, ≥12 weeks, was anticipated to be necessary to achieve virologic response. The risk of deferring transplantation was also considered. The intent was to treat patients up to the time of transplantation, or for a maximum of 48 weeks. Treatment assignments were not blinded at any stage.
Treatment, using a low accelerating dose regimen (LADR), was initiated with PEGIFN alfa-2b 0.75 μg/kg/week, and RBV 600 mg/day(d). Dose escalations were performed at weeks 1 (PEGIFN, 1.5 μg/kg/week and RBV 800 mg/d), 2 (RBV 1.0 g/d), and 3 (RBV 1.2 g/d for patients who weighed more than 75 kg) based upon patient tolerance and weekly blood counts. Dose escalation of PEGIFN required ANC>750/μL and platelet count>35,000/μL; dose escalation of RBV required hemoglobin>10g/dL. Once a patient reached the target RBV dose of 1–1.2 g/d (approximately 10.6 to 13.2 mg/kg/d), no further increases in RBV dose were made. Subsequent doses of PEGIFN and RBV were adjusted based upon adverse events, patient tolerability, and blood counts. If the highest tolerated dose of PEGIFN was <0.5 ug/kg, PEGIFN was permanently discontinued. EPA (PROCRIT® 10,000–40,000 U weekly SQ) and G-CSF (NEUPOGEN® 150–300 μg SQ up to three times/week) were allowed before and during antiviral treatment if hemoglobin <12.5 mg/dL or ANC <1000/uL, respectively.
Per protocol, antibiotic prophylaxis was required for patients randomized to treatment who had a history of spontaneous bacterial peritonitis or low protein ascites. After several serious infections the protocol was amended to administer prophylactic antibiotics to all patients with current or past history of ascites.
Patients were seen every two weeks until week 12, then monthly until transplantation or completion of 48 weeks of treatment. Complete blood counts, international normalized ratio of prothrombin time (INR), and chemistry profile were measured at each visit and more often when clinically indicated. For those completing 48 weeks of treatment or undergoing transplantation, follow-up was every 12 weeks for 24 weeks post-treatment or 48 weeks post-transplantation.
Efficacy Assessments
The primary endpoint was post-transplant virologic response (pTVR) defined as undetectable HCV RNA at week 12 after liver transplantation. Pre-transplant sustained VR (SVR12) was defined as undetectable HCV RNA at week 12 after end of treatment. Combined virologic response (CVR) comprised both SVR12 and pTVR.
Patient management required that HCV RNA was quantified locally. Assays differed by clinical center; some assayed HCV RNA by polymerase chain reaction assays (PCR) with limit of detection (LOD) of 50 IU/mL, while others used branched-chain DNA (b-DNA) assays with LOD of 615 IU/mL. Serum samples were also stored for subsequent HCV RNA measurement by a central laboratory. The latter samples were first analyzed by b-DNA assay and all samples with undetectable results (<615 IU/mL) were then retested by transcription-mediated amplification (TMA) with LOD of 5 IU/mL. For data analysis, HCV RNA central laboratory results were supplemented with local results when samples were missing or insufficient for central testing.
Safety Assessments
Safety measures included physical examination, adverse event assessment and laboratory monitoring. Cytopenias with hemoglobin<8 g/dL, ANC<500/ul or platelet count<20,000/uL required treatment interruption or discontinuation. Serious adverse events (SAEs) included standard World Health Organization criteria and specific events related to cirrhosis or liver transplantation. SAEs were evaluated for relationship to antiviral treatment by the site principal investigators. Deaths were reviewed by an oversight committee (GE, AL, NT) and the Data and Safety Monitoring Board (DSMB) for A2ALL to evaluate relationship, if any, to antiviral treatment. The DSMB reviewed safety data quarterly and met twice per year to review study progress.
Statistical Analyses
Efficacy was tested first using intent-to-treat (ITT) analyses and subsequently using per-protocol (PP) analyses; safety analyses were conducted using PP analyses. Descriptive statistics were reported as mean and standard deviation (SD), or percentages as appropriate. Treatment balance over clinical and laboratory characteristics was tested by Student’s t-test for continuous variables and chi-square test for categorical variables. Variables predictive of pTVR were tested by logistic regression; covariates tested included baseline HCV RNA, HCV genotype, graft type, treatment duration, use of growth factors during treatment, and achievement of 80% target dose of PEGIFN and RBV. Proportions of SAEs in treated and untreated patients were compared using two-sided Fisher’s exact tests; SAE rates were compared using Poisson regression. The time to first HCV RNA negativity was estimated by the method of Kaplan-Meier, with censoring at death but not at transplant. The distributions of HCV RNA by treatment week were estimated using the reverse Kaplan-Meier method to account for data below LOD (17).
Sample size was calculated for patients with genotypes 1,4,5, or 6 assuming a 2:1 randomization, α=0.05, and two-sided testing to detect a difference in pTVR of 30% in treated vs. 1% in control patients with 89% power (n=84 transplanted patients). This enrollment target was not achieved (n=47 with genotypes 1/4/6 enrolled, 35 transplanted), and recruitment was terminated to allow complete follow-up of enrolled patients prior to the end of funding. This analysis combines gentoypes 2/3 with genotypes 1/4/6. Although an interim analysis was planned after half of the expected genotypes 1/4/5/6 patients were transplanted (n=40), this analysis was not carried out due to low enrollment. Statistical analyses were performed using SAS 9.2 software (SAS Institute Inc, Cary, NC), with tests performed at a significance level of 0.05.
Results
A total of 145 patients with chronic HCV, including 92 with HCC, were potentially eligible for this study. Eighty-seven (60% of all eligible HCV cases), including 49 with HCC (53% of the HCC cases), were enrolled; eight were screen failures, and 79 were assigned to treatment groups (Figure 1). Reasons for failure to enroll the remaining 58 patients were intolerance to prior interferon, unwillingness to take interferon-based treatment, null response to prior therapy, “too sick”, contraindications to interferon or ribavirin, or inability to comply with visit schedule or study protocol.
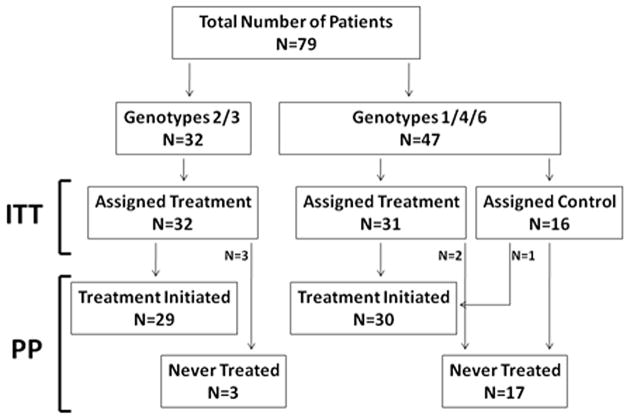
Figure 1.
Enrollment flow chart by HCV genotype, showing both intent-to-treat (ITT) and treatment per-protocol (PP) groups. All 32 patients infected with HCV genotypes 2 or 3 were assigned to treatment but only 29 initiated treatment and 3 never received PEGIFN or RBV. Forty seven patients were infected with HCV genotypes 1, 4, or 6, of whom 31 were assigned to treatment and 16 were assigned to untreated control. Two of the patients assigned to treatment never received PEGIFN or RBV and one control was treated. The 8 screen failures are not reflected in this diagram.
Patients with HCV genotypes 1/4/6 (n=44/2/1) were randomized 2:1 to treatment (n=31) or untreated control (n=16). HCV genotypes 2/3 (n=32) were all assigned to treatment. Overall, 59 were treated with PEGIFN/RBV and 20 were not treated. Of the 47 patients with HCV genotype 1/4/6, two patients assigned to treatment were never treated and one patient assigned to control was treated. Three of the 32 patients with HCV genotype 2/3 did not receive treatment. Reasons for lack of treatment in the five patients assigned to treatment were early transplant, consent withdrawal, death, worsening renal function, and unknown. One control patient requested and received treatment off-protocol.
Baseline characteristics
The demographic, clinical and laboratory characteristics of the patients are shown in Table 1. The assigned treatment and control groups were well matched with respect to age, gender, ethnicity, race, weight, blood counts, baseline HCV RNA levels, and laboratory assessment of liver and renal function. In addition, the mean (± SD) MELD score (12.0±3.3 vs 12.0±3.8), and Child-Turcotte-Pugh (CTP) mean (± SD) score (7.0±1.5 vs 6.3±1.4) were similar. The treatment group had fewer patients with MELD upgrade for HCC (54% vs. 94%, p=0.003). Sixty-two percent of treated patients and 56% of controls were interferon-experienced. The duration of prior therapy and the type of virologic response to prior therapy (relapse, partial response, null response) was not determined.
Table 1.
Baseline characteristics of patients by assigned treatment group
| Characteristic | Treatment (n=63) Mean (SD) or N(%) |
Control (n=16) Mean (SD) or N(%) |
P value |
|---|---|---|---|
| Age, years | 56 (7.0) | 56 (5.4) | 0.71 |
| Gender | |||
| Male | 46 (73%) | 13 (81%) | 0.75 |
| Ethnicity | |||
| Hispanic | 15 (24%) | 2 (12%) | 0.50 |
| Race | |||
| White | 53 (84%) | 11 (69%) | |
| African-American | 3 (5%) | 2 (12%) | 0.28 |
| Other | 7 (11%) | 3 (19%) | |
| Weight (kg) | 84 (15.3) | 88 (13.5) | 0.31 |
| BMI (kg/m2) | 28 (4.4) | 29 (4.9) | 0.45 |
| HCV Characteristics | |||
| Genotype | |||
| 1 | 30 (47%) | 14 (88%) | |
| 2 | 16 (24% | 0 (0%)* | <0.001 |
| 3 | 16 (25%) | 0 (0%)* | |
| 4 or 6 | 1 (4%) | 2 (12%) | |
| Viral Load (log10 IU/mL) | 5.7 (1.0) | 5.7 (0.6) | 0.99 |
| HCC Upgrade | 34 (54%) | 15 (94%) | 0.003 |
| Laboratory Tests | |||
| Hemoglobin (g/dL) | 13.1 (1.7) | 13.5 (1.3) | 0.40 |
| WBC (×103/mm3) | 4.7 (1.7) | 4.2 (1.1) | 0.35 |
| Abs neutrophil count (/mm3) | 794 (1402) | 531 (962) | 0.48 |
| Platelet (×103/mm3) | 92 (53) | 93 (42) | 0.98 |
| Albumin (g/dL) | 3.2 (0.6) | 3.3 (0.6) | 0.83 |
| AST (IU/L) | 111 (69) | 101 (45) | 0.59 |
| ALT (IU/L) | 84 (59) | 79 (40) | 0.74 |
| Total Bilirubin (mg/dL) | 2.1 (1.4) | 2.2 (1.6) | 0.73 |
| Creatinine (mg/dL) | 0.9 (0.3) | 0.9 (0.2) | 0.81 |
| INR | 1.3 (0.2) | 1.3 (0.2) | 1.00 |
| MELD | 12.0 (3.3) | 12.0 (3.8) | 0.96 |
| CTP Score | 7.0 (1.5) | 6.3 (1.4) | 0.11 |
| Previous IFN treatment | 39 (62%) | 9 (56%) | 0.68 |
By study design, all HCV Genotype 2/3 patients were assigned to Treatment.
Virologic Response During Treatment with LADR
The cumulative distributions of HCV RNA results at baseline and during LADR treatment are given in the Supplementary Figure for HCV genotypes 1/4/6 (Panel A) and HCV genotypes 2/3 (Panel B). Patients with HCV genotypes 2/3 had more rapid and greater suppression of HCV RNA during LADR treatment.
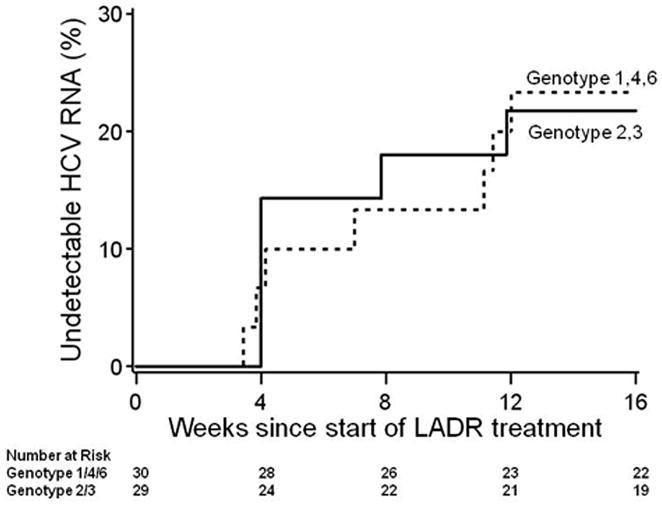
Figure 2 shows the time to the first negative HCV RNA level during LADR treatment for patients achieving CVR. The probability of undetectable HCV RNA at week 16 is similar between patients with HCV genotypes 1/4/6 versus HCV genotype 2/3 (logrank p=0.89). Among the patients who had CVR, all achieved undetectable HCV RNA by week 16.
Figure 2.
The cumulative probability distribution, among treated patients, of time from study enrollment to first HCV RNA negativity for patients who achieved either SVR12 or pTVR, estimated by Kaplan-Meier. Dashed line shows the distribution for patients with HCV genotypes 1/4/6, and solid line for HCV genotypes 2/3.
Combined Virologic Response (CVR)
For the ITT analysis, 12 (19%) of the 63 treatment group patients and 1 (6%) of the 16 control group patients achieved CVR (p=0.29) (Table 2). CVRs included 2 SVRs and 11 pTVRs. The single control CVR was the patient who requested and received treatment, achieved SVR and did not undergo liver transplantation, despite being randomized to no treatment. For the PP analysis, 13 (22%) of the 59 treated (95% CI: 0.12, 0.35) and none of the untreated group achieved CVR (p=0.03).
Table 2.
Proportions and 95% confidence intervals by genotype of combined virologic response (CVR)† and post-transplant virologic response (pTVR).
| Genotype | Analysis | CVR | pTVR | pTVR |
|---|---|---|---|---|
| Among All Patients | Among Transplanted Patients | Among Transplanted Patients HCV RNA Negative at Transplant | ||
| 1,4,6 | ITT | 6/31=0.19 | 5/24=0.21 | 5/12=0.42 |
| [0.07–0.37] | [0.07–0.42] | [0.15–0.72] | ||
| PP | 7/30=0.23 | 5/23=0.22 | 5/12=0.42 | |
| [0.09–0.42] | [0.07–0.44] | [0.15–0.72] | ||
|
| ||||
| 2,3 | ITT | 6/32=0.19 | 6/22=0.27 | 6/14=0.43 |
| [0.07–0.36] | [0.11–0.50] | [0.18–0.71] | ||
| PP | 6/29=0.21 | 6/21=0.29 | 6/14=0.43 | |
| [0.08–0.40] | [0.11–0.52] | [0.18–0.71] | ||
|
| ||||
| All | ITT | 12/63=0.19 | 11/46=0.24 | 11/26=0.42 |
| [0.10–0.31] | [0.13–0.39] | [0.23–0.63] | ||
| PP | 13/59=0.22 | 11/44=0.25 | 11/26=0.42 | |
| [0.12–0.35] | [0.13–0.40] | [0.23–0.63] | ||
Abbreviations: ITT, Intent-to-Treat; PP, Per-Protocol
Includes patients who achieved sustained virologic response (SVR12 and not transplanted) or pTVR
ITT – analyses of all patients assigned to treatment
PP – analyses of patients who received treatment
A higher proportion of patients with CVR had a >2-log10 IU/mL decrease in HCV RNA by treatment week 4 (89% vs 68%) or week 8 (100% vs 70%), and undetectable HCV RNA by week 12 (100% vs 32%), compared to patients not achieving CVR (Supplementary Table 1).
Liver Transplantation
Fifty-seven patients, 44 treated and 13 controls, underwent liver transplantation: 16 living donor liver transplants (LDLT) and 41 deceased donor liver transplants (DDLT). Due to our selection criteria, more patients who underwent DDLT had HCC upgrade (90% vs. 6%, p<0.0001). As a result, the DDLT group had lower laboratory MELD and CTP scores. Otherwise, LDLT and DDLT groups had similar demographics, HCV genotype distribution, and mean baseline HCV RNA. Among those treated and transplanted, the proportions with pTVR were not significantly different, 5/16 (31%) pTVR in LDLT vs. 6/28 (21%) in DDLT (p=0.49).
Twenty-two patients were not transplanted and seven of these had died, one each from liver failure, renal failure, multiorgan system failure, cardiac arrest, status epilepticus, sepsis, and unknown. Of the 15 who were alive at the end of follow-up, two had achieved SVR and were clinically stable, eight were still listed and awaiting transplantation, three were delisted for progression of HCC, and two were delisted for severe deterioration in liver disease and clinical status.
Virologic Responses in Transplanted Patients
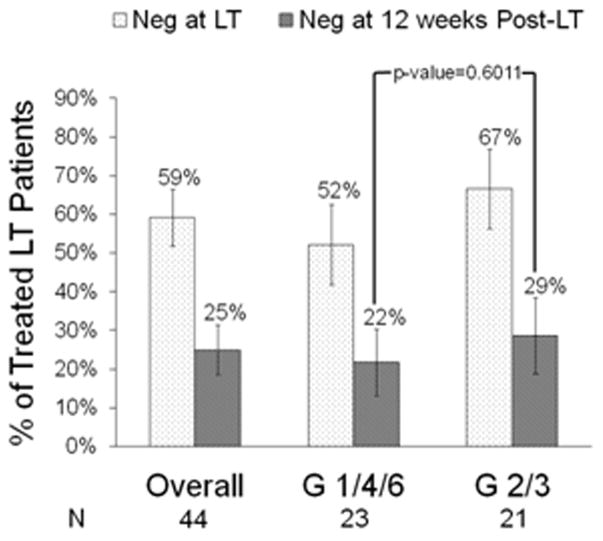
None of the 13 controls but 26/44 (59%) treated patients achieved undetectable HCV RNA by the time of transplantation (p<0.0001). Of the 44 treated patients, 52% with HCV genotypes 1/4/6 and 67% with genotypes 2/3 had undetectable HCV RNA at transplantation (Figure 3).
Figure 3.
Percent of treated patients with undetectable HCV RNA at transplant and at week 12 post-LT by genotype. Overall 59% of 44 treated patients were HCV RNA negative at time of transplant and 25% achieved pTVR (RNA negative at post-transplant week 12). Fifty two percent of 23 genotype (G) 1/4/6 and 67% of 21 G 2/3 were RNA negative at transplant (p=0.33); and 22% of G 1/4/6 and 29% of G 2/3 achieved pTVR (p=0.60). PP analyses; whiskers are +/− 1 standard error.
The proportion with pTVR was comparable in ITT and PP analyses (Table 2). Eleven of the 26 (42%) treated patients who had undetectable HCV RNA at transplantation achieved pTVR, 13 (50%) relapsed, and two (8%) died prior to week 12 post-transplant. None of the controls achieved pTVR (p=0.03). pTVR did not differ between patients with HCV genotypes 1/4/6, (5/23, 22%) versus patients with HCV genotypes 2/3 (6/21, 29%) (p=0.60) (Figure 3). All 11 patients with pTVR were retested at post-transplant week 24 and all remained HCV RNA undetectable.
The LOD of the test used to detect HCV RNA at transplant made a substantial difference in predicting pTVR. For the 26 patients with HCV RNA undetectable at transplant, eight were tested using assays with LOD of 5 IU/mL, compared to 18 tested using assays with LOD >5 IU/mL, and the pTVR was 75% and 28% respectively in these groups (p=0.038).
Predictors of pTVR
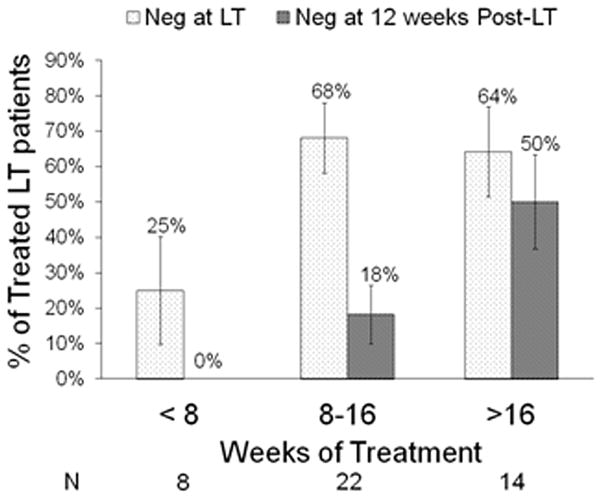
The likelihood of pTVR increased with the duration of treatment. In patients who received <8, 8–16, and >16 weeks of treatment, pTVR was 0%, 18%, and 50%, respectively (p=0.01) (Figure 4). In univariate PP analyses, duration of pre-transplant RBV (categorized, p=0.01) and PEGIFN (categorized, p=0.05) were the only factors significantly associated with pTVR (Table 3). Although other factors were not significant, patients experiencing pTVR were more likely to be infected with HCV genotypes 2/3, had lower baseline HCV RNA, used growth factors during treatment, and achieved target doses of PEGIFN and RBV.
Figure 4.
Percent of treated patients with undetectable HCV RNA at transplant and at week 12 post-LT (pTVR) by treatment duration. Virologic response to pre-transplant therapy was linked to treatment duration. Only 25% of the patients treated for less than 8 weeks had undetectable HCV RNA at transplant and none achieved pTVR. In contrast, 64% of patients treated for more than 16 weeks had undetectable HCV RNA at transplant, and 50% achieved pTVR. PP analyses; whiskers are +/− 1 standard error.
Table 3.
Predictors of post-transplant virologic response (pTVR) among those transplanted who were assigned to treatment (ITT, n=46) or who received treatment (PP, n=44)
| Variables | ITT analysis | PP Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Baseline HCV RNA log10 IU/mL | 0.54 | 0.26–1.12 | 0.10 | 0.53 | 0.26–1.10 | 0.09 |
| Baseline HCV RNA >5.5 vs. ≤5.5 log10 IU/mL | 0.34 | 0.08–1.38 | 0.13 | 0.29 | 0.07–1.19 | 0.09 |
| HCV genotype: 1/4/6 vs. 2/3 | 0.70 | 0.18–2.74 | 0.61 | 0.69 | 0.18–2.73 | 0.60 |
| LDLT vs. DDLT | 1.81 | 0.46–7.26 | 0.40 | 1.67 | 0.42–6.69 | 0.47 |
| Duration of pre-liver transplant treatment | ||||||
| PegInterferon | ||||||
| Number of weeks (continuous) | 1.09 | 0.99–1.19 | 0.09 | 1.08 | 0.98–1.19 | 0.14 |
| Categorized | ||||||
| >16 weeks | 1 | 1 | ||||
| 8–16 weeks | 0.36 | 0.08–1.64 | 0.03 | 0.36 | 0.08–1.64 | 0.05 |
| <8 weeks | 0.11 | 0.01–1.11 | 0.13 | 0.01–1.35 | ||
| Ribavirin | ||||||
| Number of weeks (continuous) | 1.09 | 0.99–1.20 | 0.07 | 1.09 | 0.97–1.20 | 0.10 |
| Categorized | ||||||
| >16 weeks | 1 | 1 | ||||
| 8–16 weeks | 0.24 | 0.06–1.08 | 0.004 | 0.24 | 0.06–1.08 | 0.01 |
| <8 weeks | 0.05 | 0.002–1.12 | 0.06 | 0.002–1.45 | ||
| Use of growth factors during treatment | 4.58 | 0.52–40.38 | 0.17 | 3.75 | 0.42–33.61 | 0.24 |
| Ability to achieve 80% of target dose of both PEGIFN and RBV | 3.38 | 0.63–17.96 | 0.15 | 2.93 | 0.54–15.75 | 0.21 |
Abbreviations: ITT, Intent-to-treat; PP-Per Protocol
Serious Adverse Events and Death
SAEs prior to and up to one year after liver transplantation are listed in Table 4. There was no significant difference in the proportion of treated patients and controls that experienced SAEs (68% vs 55%, p=0.30), but the number of SAEs per patient was greater in treated patients (2.7 vs 1.3, p=0.003). There was no association between MELD score and either the total number of SAEs or SAEs due to infection (data not shown).
Table 4.
Serious adverse events (SAEs) in untreated and treated patients pre-liver transplant (LT), in the first 30 days post-LT, and 30 days to 1 year post-LT
| Treated | Untreated | p-value | ||
|---|---|---|---|---|
| Overall | ||||
| Number of patients with SAEs | 40/59 (68%) | 11/20 (55%) | 0.30 | |
| Total number of events | 109 | 14 | ||
| Number of SAEs/patient among those with SAEs | 2.7 | 1.3 | 0.003 | |
| Number of deaths† | 9/59 (15%) | 2/20 (10%) | 0.81‡ | |
|
| ||||
| Pre-LT | ||||
| Number of patients with SAEs | 27/59 (46%) | 4/20 (20%) | 0.04 | |
| Total number of SAEs | 42 | 5 | ||
| Number of events/patient among those with SAEs | 1.6 | 1.3 | 0.52 | |
|
| ||||
| Cytopenia | All | 11 (19%) | 0 (0%) | 0.06 |
| Neutropenia | 6 | 0 | ||
| Thrombocytopenia | 5 | 0 | ||
|
| ||||
| Infection | All | 7 (12%) | 0 (0%) | 0.18 |
| SBP | 3 | 0 | ||
| Other | 4 | 0 | ||
|
| ||||
| Liver-related | All | 8 (14%) | 3 (15%) | 1.00 |
| Liver failure | 6 | 3 | ||
| Other | 2 | 0 | ||
|
| ||||
| Other | 11 (19%) | 1 (5%) | 0.28 | |
|
| ||||
| Post-LT (first 30 days) | ||||
| Number of patients with SAEs | 14/44 (32%) | 2/13 (15%) | 0.31 | |
| Total number of SAEs | 17 | 2 | ||
| Number of events/patient among those with SAEs | 1.2 | 1.0 | 0.46 | |
|
| ||||
| Cytopenia | 0 | 0 | 1.00 | |
|
| ||||
| Infection | 4 (9%) | 0 | 0.56 | |
|
| ||||
| Liver-related | 3 (7%) | 1 (8%) | 1.00 | |
|
| ||||
| Rejection | 1 (2%) | 0 | 1.00 | |
|
| ||||
| Surgical complication | 2 (5%) | 0 | 1.00 | |
|
| ||||
| Other | 7 (16%) | 1 (8%) | 0.67 | |
|
| ||||
| Post-LT (30 days to 1 year) | ||||
| Number of patients with SAEs | 24/44 (55%) | 6/13 (46%) | 0.59 | |
| Total number of SAEs | 50 | 7 | ||
| Number of events/patient among those with SAEs | 2.1 | 1.2 | 0.06 | |
|
| ||||
| Cytopenia | 0 | 2 (15%) | 0.05 | |
|
| ||||
| Infection | 9 (20%) | 3 (23%) | 1.00 | |
|
| ||||
| Liver-related | 2 (5%) | 0 | 1.00 | |
|
| ||||
| Rejection | 0 | 0 | 1.00 | |
|
| ||||
| Surgical complication | 8 (18%) | 0 | 0.18 | |
|
| ||||
| Other | 13 (30%) | 2 (15%) | 0.48 | |
Causes of death in these 11 patients were cardiac arrest (n=2), sepsis (n=2), heart failure (n=2), liver failure (n=1), renal failure (n=1), cerebral edema (n=1), and unknown (n=2)
Based on logrank test. Abbreviation: SBP - Spontaneous Bacterial peritonitis
Prior to liver transplantation, a greater proportion of treated patients had SAEs (46% vs 20%, p=0.04). SAEs of cytopenia (19% vs 0%, p=0.06) and infection (12% vs 0%, p=0.18) were only observed in treated patients. Liver-related SAEs occurred with similar frequency (treated vs control, 14% vs 15%, p=1.0).
Infection was more common in treated patients. As mentioned above, 7 of 59 treated patients (12%) and 0 of 20 controls (0%) experienced an SAE of infection. Nineteen of 59 treated patients (20%) and 2 of 20 controls (10%) experienced an adverse event of infection.
Rates of infection in treated patients may have been reduced by antibiotic prophylaxis. SAE of infection occurred in 10% (5/48) of patients receiving prophylaxis compared to 18% (2/11) not receiving prophylaxis. Adverse event of infection occurred in 29% (14/48) of patients receiving prophylaxis compared to 45% (5/11) not receiving prophylaxis.
SAEs also tended to be more common in treated patients early after transplantation. Within the first 30 days, a greater proportion of treated patients had SAEs compared to controls (32% vs 15%, p=0.31) and infection was only observed in treated patients (9% vs 0%, p=0.56).
Between 30 days and one year post-transplantation, we found a similar proportion of treated patients and controls had experienced an SAE (55% vs 46%, p=0.59), but treated patients had more SAEs per patient (2.1 vs 1.2, p=0.06). Rejection within the first year occurred in one treated patient and in none of the control patients (2% vs 0%, p=1.0).
Despite the greater risk for SAEs, pre-transplant treatment was not associated with increased risk of death - 9 (15%) treated patients and 2 (10%) controls died (logrank p=0.81). Five of the treated patients and two of the untreated patients died pre-LT. Overall, mortality rates were 7.0% (4/57) in transplant recipients compared to 31.8% (7/22) in those without a transplant.
Dose and Duration of Peginterferon and Ribavirin
Patients achieving pTVR had higher exposure to both PEGIFN and RBV. The cumulative doses per kilogram body weight, duration of treatment, and percentages of patients achieving 80% of target doses for both PEGIFN and RBV trended higher in the patients achieving pTVR (Supplementary Table 2).
Growth Factors
Forty-four of the 59 treated patients (75%) received growth factors (9 received G-CSF alone, 14 received EPA alone, and 21 received both G-CSF and EPA). Ten of the 11 patients achieving pTVR (91%) compared to 24 of 33 patients without pTVR (73%) (p=0.41) used growth factors during the course of pre-transplant therapy (Supplementary Table 2).
Discussion
LADR-A2ALL is the first randomized, controlled trial of pre-transplant treatment of chronic hepatitis C using PEGIFN plus RBV to prevent recurrent HCV infection after transplantation. LADR-A2ALL sought to determine the efficacy of pretransplant treatment to prevent recurrent HCV, and included an untreated control group to define the safety of PEGIFN and RBV when used in this setting. Pre-transplant treatment achieved post-transplant clearance of HCV, pTVR, in 25%. However, treatment was associated with an increased frequency of SAEs, including infection.
In LADR-A2ALL, 59% of patients were HCV RNA negative at the time of transplant. This rate of on-treatment viral clearance was higher than noted in previous reports using comparable doses of interferon, or PEGIFN, with RBV. In the reports by Everson (12), Forns (14), and Carrion (16), 32%, 35%, and 35% of patients were HCV RNA negative at the time of transplantation–approximately half the virologic response achieved in LADR-A2ALL. One contributing factor to the higher on-treatment virologic response may have been the relatively higher proportion of patients infected with HCV genotypes 2 and 3. The percentage of patients infected with HCV genotypes 2 or 3 in other studies ranged from 17% to 33% (13–16, 18). In LADR-A2ALL, 53% of treated patients were infected with HCV genotype 2 or 3. Another factor may have been the exclusion from the study of patients with prior null response to PEGIFN and RBV.
The higher on-treatment virologic response in LADR-A2ALL could also have been due to the use of a variety of HCV RNA assays with LODs as high as 615 IU/mL. A significant proportion of patients with undetectable HCV RNA by these insensitive assays were likely still viremic. This conclusion is supported by our TMA results, where pTVR was achieved in 75% of patients with undetectable HCV RNA by TMA compared to only 28% of patients with undetectable HCV RNA by the less sensitive assays. This experience indicates that future treatment trials must use centralized sensitive assays to accurately define viral kinetics and virologic responses.
The percentage pTVR achieved in LADR-A2ALL (25%) was similar to pTVR percentages achieved in the reports by Everson (26%), Forns (23%), and Carrion (23%). The relapse rate in LADR-A2ALL of 50% was higher than that observed by Everson (20%), Forns (33%), and Carrion (33%) (12, 14, 16). The higher rate of relapse in LADR-A2ALL was likely related, at least in part, to the high proportion of patients who received a short duration of treatment. In our univariate analyses, the only factor predictive of pTVR was longer duration of pre-transplant treatment. Shorter duration of treatment was associated with higher likelihood of relapse – 100% of patients receiving less than eight weeks of treatment relapsed.
Maintaining adequate doses and blood levels of RBV may be particularly important when using lower doses or dose reductions in PEGIFN (18). In LADR-A2ALL, patients experiencing pTVR were more likely to have achieved 80% of their targeted dose of RBV (91% vs. 70%) and to have remained on RBV longer (17.3 vs. 12.9 weeks). Patients achieving pTVR were more likely to have used EPA, a factor that could have allowed greater exposure to RBV and improved the likelihood of clearing HCV (19).
Factors predicting virologic response in patients with advanced stages of chronic hepatitis C have been noted in other studies. Everson found three factors predictive of SVR in 124 patients with advanced hepatitis C: infection with HCV genotypes 2 or 3 (compared to genotypes 1/4/6), CTP class A cirrhosis (compared to CTP classes B and C), and dose and duration of interferon (or PEGIFN) and RBV treatment (12). Similar observations were made by Forns, Carrion, and Thomas (14–16). Carrion also demonstrated that “early” virologic response (>2 log10 IU/mL decrease by week 4) during treatment predicted likelihood for pTVR (16). In LADR-A2ALL, pTVR was only possible in the patients who were HCV RNA negative by week 12. The findings of Carrion and LADR-A2ALL suggest that treatment could be discontinued in patients who either fail to achieve a 2 log10 drop in HCV RNA by week 8 when using standard doses of PEGIFN/RBV, or who remain HCV RNA positive at week 12 when treated with LADR.
Pre-transplant treatment with PEGIFN plus RBV is only applicable to a select group of patients. We enrolled two types of patients into our study – potential recipients of LDLT who had an identifiable donor undergoing evaluation, and potential recipients of DDLT who met criteria for MELD upgrade for HCC. These patients generally have less severe liver disease compared to patients awaiting DDLT for complications of liver failure, and are therefore able to better tolerate the adverse events associated with PEGIFN/RBV. Additionally, in these two patient groups, the interval between initiation of PEGIFN/RBV treatment and transplantation is somewhat predictable. In the case of LDLT the date of transplantation can be scheduled. In the case of DDLT for HCC after MELD upgrade, transplantation would typically occur within six months after the MELD upgrade. In some centers in the US, liver transplantation may be performed at relatively low MELD scores and the time interval between listing and transplantation can be estimated. Patients with HCV awaiting DDLT in these low-MELD centers might also be candidates for pre-transplant treatment. However, practical logistical issues, such as underlying HCC and need for urgent transplantation or availability of donor liver, compromise the ability to extend treatment duration.
In LADR-A2ALL, treated patients experienced more SAEs and infection. In the case control study of Carrion (16), treated patients had a significantly increased risk for bacterial infection (p<0.001). The increased risk of infection indicates that prophylactic antibiotics during antiviral therapy may be warranted. Experience in both LADR-A2ALL and Barcelona suggested that antibiotic prophylaxis may have lowered the risk for bacterial infection during PEGIFN/RBV treatment.
Nine treated patients (15%) and two control patients (10%) died. In the absence of a control group, the mortality rate in treated patients might be viewed as excessive and related to antiviral therapy. However, the similar mortality rate of controls suggests that our observed mortality rate might represent the underlying risk of mortality in these patients on the waiting list prior to and after hepatic transplantation. Support for this interpretation is provided by the case-control study of Carrion (16). Mortality rates were nearly identical to rates we reported in LADR-A2ALL – 8 deaths in 51 treated patients (16%) versus 7 deaths in 51 case controls (14%).
Limitations of the study include failure to reach enrollment target, inability to complete planned minimum duration of treatment of 12 weeks for many subjects partly due to the timing of DDLT availability, incomplete HCV RNA follow up, inconsistency of limits of detection of HCV RNA assays, and noncompliance with assigned treatment in a few patients. Generalizability of results is primarily for the US Caucasian population. Strengths of the study include randomization among HCV genotypes 1/4/6 allowing comparison of adverse events between treated patients and controls.
In summary, we conducted a randomized, controlled trial of PEGIFN and RBV in 79 patients with advanced hepatitis C who were candidates for liver transplantation. Pre-transplant treatment prevented post-transplant recurrence of HCV infection in 25% of transplanted cases - 22% in HCV genotype 1/4/6 and 29% in HCV genotype 2/3. The strongest predictor of virologic response was duration of treatment. Despite these potentially significant therapeutic benefits, PEGIFN and RBV were poorly tolerated in these “difficult-to-treat” and “difficult-to-cure” patients. SAEs, some potentially life-threatening, occurred during the course of treatment. Future treatments incorporating direct-acting antivirals that accelerate and enhance virologic response should improve rates of pTVR, but will require strategies to limit toxicity.
Supplementary Material
Supplementary Figure 1. The cumulative probability distribution of HCV RNA at baseline (BL), during LADR treatment at weeks 4, 8, 12, and 24, and at transplant (TXP). Panel A shows results for patients with HCV genotypes 1/4/6, and Panel B for HCV genotypes 2/3. The x-axis is log10 HCV RNA, and the y-axis is the probability of a smaller HCV RNA value. The vertical line denotes the limit of detection by the TMA assay (5 IU/mL). For example, in Panel A, the probability that HCV RNA is less than 100 IU/mL (log10 HCV RNA<2) is near 0 at baseline, approximately 30% at weeks 4 and 8, and 50% at week 12. All patients experienced an initial decrease from baseline of ≥1 log, continued decreases with treatment were seen in approximately 60% of G1/4/6 and 90% of G2/3.
Acknowledgments
FINANCIAL SUPPORT
The patients participating in this trial were enrolled in the NIH-sponsored Adult-to-Adult Living Donor Liver Transplantation Cohort Study. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases through cooperative agreements (NIDDK grant numbers: U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531). The trial was also funded by research grants from Schering-Plough, through a cooperative research and development agreement (CRADA) with NIH-NIDDK, and through a Clinical Trial Agreement (CTA) between Ortho-Biotech and NIH-NIDDK. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases through cooperative agreements (listed in parentheses). Additional support was provided by the Health Resources and Services Administration and the American Society of Transplant Surgeons.
The following individuals were instrumental in the planning, conduct, and/or care of patients enrolled in this study at each of the participating institutions:
Columbia University Health Sciences, New York, NY (DK62483): principal investigator, Jean C. Emond, M.D.; coprincipal investigator, Robert S. Brown Jr, M.D., M.P.H.; study coordinators, Scott Heese, B.A., and Taruna Chawla, MD
Northwestern University, Chicago, IL (DK62467): principal investigator, Michael M. I. Abecassis, M.D., M.B.A.; coprincipal investigator, Laura M. Kulik, M.D.; study coordinator, Patrice Al-Saden, R.N., C.C.R.C.
University of Pennsylvania Health System, Philadelphia, PA (DK62494): principal investigator, Abraham Shaked, M.D., Ph.D.; coprincipal investigator, Kim M. Olthoff, M.D.; study coordinators, Brian Conboy, P.A., M.B.A., and Mary Shaw, R.N., B.B.A.
University of Colorado Health Sciences Center, Denver, CO (DK62536): principal investigator, Gregory T. Everson, M.D.; coprincipal investigator, Igal Kam, M.D.; study coordinator, Andrea Herman, R.N.
University of California Los Angeles, Los Angeles, CA (DK62496): principal investigator, Johnny C. Hong, M.D.; coprincipal investigator, Ronald W. Busuttil, M.D., Ph.D.; study coordinator, Janet Mooney, R.N., B.S.N. The PI for LADR was Sammy Saab, MD
University of California San Francisco, San Francisco, CA (DK62444): principal investigator, Chris E. Freise, M.D., F.A.C.S.; coprincipal investigator, Norah A. Terrault, M.D.; study coordinator, Dulce MacLeod, R.N.
University of Michigan Medical Center, Ann Arbor, MI (DK62498): principal investigator, Robert M. Merion, M.D.; data coordinating center staff, Anna S. F. Lok, M.D., Akinlolu O. Ojo, M.D., Ph.D., Brenda W. Gillespie, Ph.D., Margaret Hill-Callahan, B.S., L.S.W., Terese Howell, B.S., C.C.R.C., Lisa Holloway, B. S., C.C.R.C., Monique Lowe, M.S., Abby Smith, B.A., and Abby Brithinee, B.A.
University of North Carolina, Chapel Hill, NC (DK62505): principal investigator, Paul H. Hayashi, M.D., M.P.H.; study coordinator, Tracy Russell, M.A.
University of Virginia (DK62484): principal investigator, Carl L. Berg, M.D.; study coordinator, Jaye Davis, R.N. and Colleen Green, PA. The PI for LADR was Abdullah M. S. Al-Osaimi, MD.
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): principal investigator, Robert A. Fisher, M.D., F.A.C.S.; coprincipal investigator, R. Todd Stravitz, M.D.; study coordinators, April Ashworth, R.N., and Andrea Lassiter, B.S. Charlotte Hoffman, R.N. The PI for LADR was Mitchell Shiffman, MD.
Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: James E. Everhart, M.D.,M.P.H., Averell Sherker, M.D., and Jay H. Hoofnagle, M.D.
The authors wish to acknowledge the contributions of the following study coordinators, co-investigators, and administrative assistants at each of the participating institutions: Andrea Herman, RN, Carlos Garcia, Michelle Jaramillo, and Rita Lerner at the University of Colorado Denver, Aurora, CO; Janet Mooney at the University of California, Los Angeles, CA; Dulce Mac Leod, RN at the University of California, San Francisco, LA; Colleen Green and Royanne Dell, RN at the University of Virginia, Charlottesville, VA; Patrice Al-Saden at Northwestern University, Chicago, IL; Scott Heese at Columbia University, New York, NY; Charlotte Hoffman at the Virginia Commonwealth University, Richmond, VA.
LIST OF ABBREVIATIONS
- A2ALL
Adult to Adult Living Donor Liver Transplant Study
- ANC
absolute neutrophil count
- CTP
Child-Turcotte-Pugh (class or score)
- CVR
combined virologic response
- DDLT
deceased donor liver transplantation
- DSMB
Data and Safety Monitoring Board
- EPA
erythropoietin analogue
- GCRC
General Clinical Research Centers
- G-CSF
granulocyte colony stimulating factor
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ITT
intent-to-treat
- LADR
low accelerating dose regimen
- LDLT
living donor liver transplantation
- LOD
limit of detection
- MELD
model for end-stage liver disease
- PEGIFN
peginterferon alfa-2b
- PCR
polymerase chain reaction
- PP
per-protocol
- pTVR
post-transplant virologic response
- RBV
ribavirin
- SAE
serious adverse event
- SVR 12
pre-transplant sustained virologic response
- SVR
sustained virologic response
- TMA
transcription-mediated amplification
Footnotes
The results of this study were presented, in part, at the American Association for the Study of Liver Diseases (AASLD) meeting in 2009.
The study is registered in ClinicalTrials.gov, number NCT00135798.
CONTRIBUTIONS OF AUTHORS:
Gregory T. Everson, Norah A. Terrault, Robert Brown, Sammy Saab, Mitchell L. Shiffman, AMS Al-Osaimi, and Laura Kulik were principal investigators at the participating clinical centers and were responsible for the overall conduct of the trial, review of the data, presentation of results, and writing of this manuscript. Anna Lok, Brenda Gillespie, and Del Rodrigo provided statistical support, analyses of data, and participated in the writing and review of the manuscript. James E. Everhart was the Project Officer from NIH and participated in all aspects of oversight of the study, analyses of data, and interpretation of results.
FINANCIAL DISCLOSURES
G. T. Everson, N, Terrault, A.S. Lok, R.S. Brown, Sammy Saab, and Abdullah M. S. Al-Osaimi were consultants and received research support from Schering-Plough, Inc. Authors with no financial relationships to disclose include D. Rodrigo, B.W. Gillespie, J.E. Everhart, L. Kulik. M. Shiffman declares the following: Data safety monitoring board, grant support from Abbott. Advisor meeting, and grant support from Achillion, Boehringer-Ingelheim, Globeimmune, Inhibitex, Novartis, Vertex, Zymogenetics. Advisor meeting, data safety monitoring board, grant support from Anadys. Speaker, advisor meetings from Bayer. Consultant for Biolex, Human Genome Sciences, and Romark. Advisor meetings, speaker, grant support for Bristol Myers-Squibb, Gilead, Schering-Plough/Merck. Consultant, advisor meetings, grant support from Conatus. Grant support from Idenix. Advisor meeting for Pfizer. Consultant, advisor meetings, speaker, grant support for Roche/Genentech.
References
- 1.Wiesner RH, Sorrell M, Villamil F the International Liver Transplantation Society Expert Panel. Report of the first international liver transplantation society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9(Suppl):S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, Management and Treatment of Hepatitis C: An Update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 4.Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatology. 2005;42:448–456. doi: 10.1016/j.jhep.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M, Wiesner R. Natural history and management of hepatitis C infection after liver transplantation. Semin Liver Dis. 2004;24(Suppl):S79–88. doi: 10.1055/s-2004-832932. [DOI] [PubMed] [Google Scholar]
- 6.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, et al. Peginterferon alfa-2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, Lok AS, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Everson GT, Hoefs JC, Seeff LB, Bonkovsky HL, Naishadham D, Shiffman ML, Kahn JA, et al. Impact of disease severity on outcome of antiviral therapy for chronic hepatitis C: Lessons from the HALT-C trial. Hepatology. 2006;44:1675–84. doi: 10.1002/hep.21440. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, Moreno-Otero R, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618–1628. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–62. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 13.Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350–355. doi: 10.1053/jlts.2002.31748. [DOI] [PubMed] [Google Scholar]
- 14.Forns X, Garcia-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, Garcia-Valdecasas JC, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389–396. doi: 10.1016/s0168-8278(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 15.Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: A role for interferon therapy before transplantation. Liver Transpl. 2003;9:905–915. doi: 10.1053/jlts.2003.50166. [DOI] [PubMed] [Google Scholar]
- 16.Carrion JA, Martinez-Bauer E, Crespo G, Ramirez S, Perez-del-Pulgar S, Garcia-Valdecasas JC, Navasa M, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: a retrospective study. J Hepatol. 2009;50:719–728. doi: 10.1016/j.jhep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie BW, Chen Q, Reichert H, Franzblau A, Hedgeman E, Lepkowski J, Adriaens P, et al. Estimating population distributions when some data are below a limit of detection by using a reverse Kaplan-Meier estimator. Epidemiology. 2010;21(Suppl 4):S64–S70. doi: 10.1097/EDE.0b013e3181ce9f08. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman ML, Ghany MG, Morgan TR, Wright EC, Everson GT, Lindsay KL, Lok AS, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132(1):103–112. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS, Shiffman ML, Afdahl NH, Reddy KR, McCone J, Lee WM, Herrine SK, et al. Hepatitis C virus treatment-related anemia is associated with higher sustained virologic response rate. Gastroenterology. 2010;139:1602–1611. doi: 10.1053/j.gastro.2010.07.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The cumulative probability distribution of HCV RNA at baseline (BL), during LADR treatment at weeks 4, 8, 12, and 24, and at transplant (TXP). Panel A shows results for patients with HCV genotypes 1/4/6, and Panel B for HCV genotypes 2/3. The x-axis is log10 HCV RNA, and the y-axis is the probability of a smaller HCV RNA value. The vertical line denotes the limit of detection by the TMA assay (5 IU/mL). For example, in Panel A, the probability that HCV RNA is less than 100 IU/mL (log10 HCV RNA<2) is near 0 at baseline, approximately 30% at weeks 4 and 8, and 50% at week 12. All patients experienced an initial decrease from baseline of ≥1 log, continued decreases with treatment were seen in approximately 60% of G1/4/6 and 90% of G2/3.