Abstract
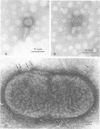
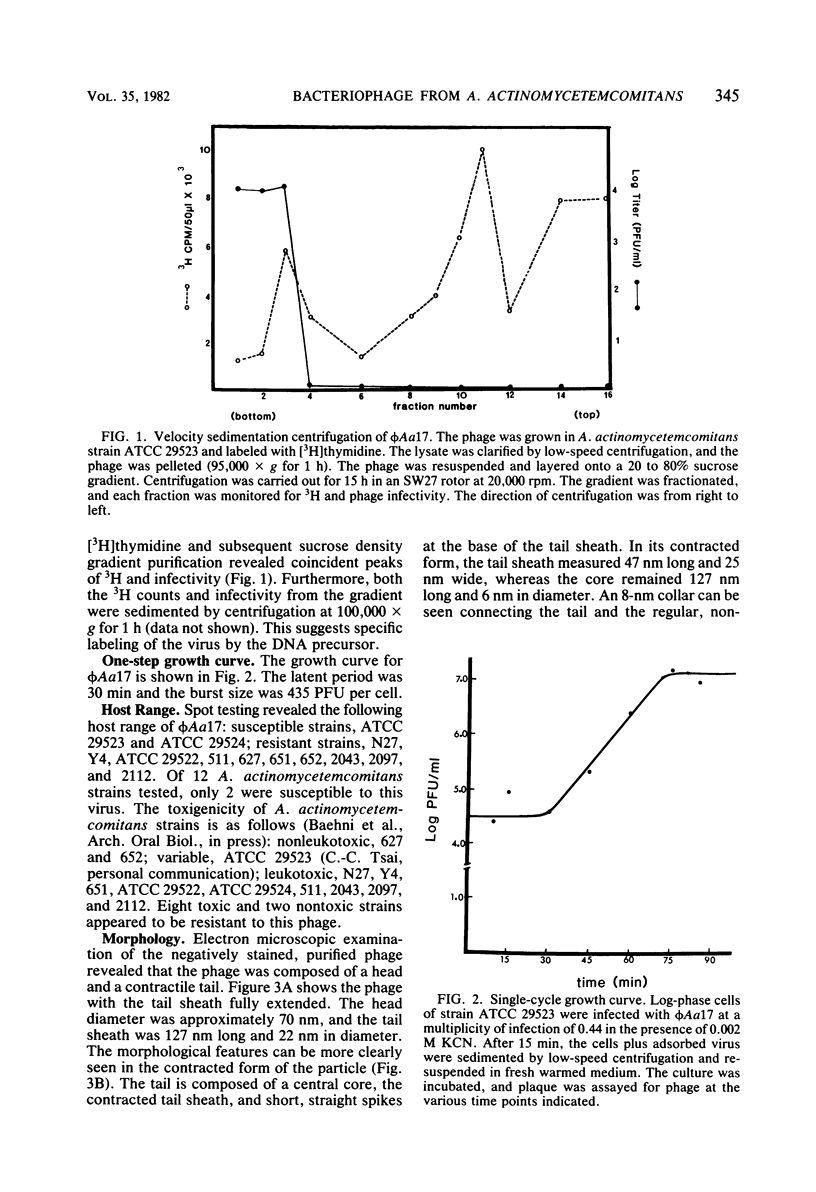
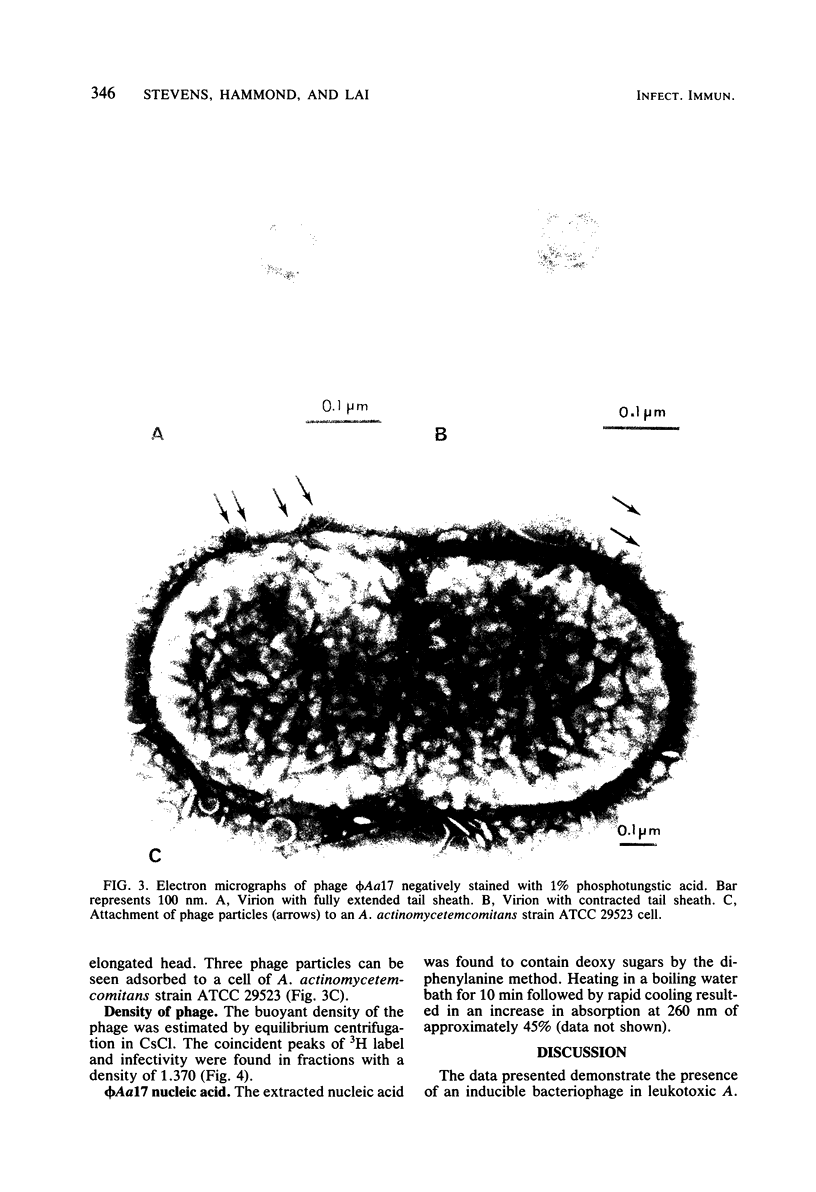
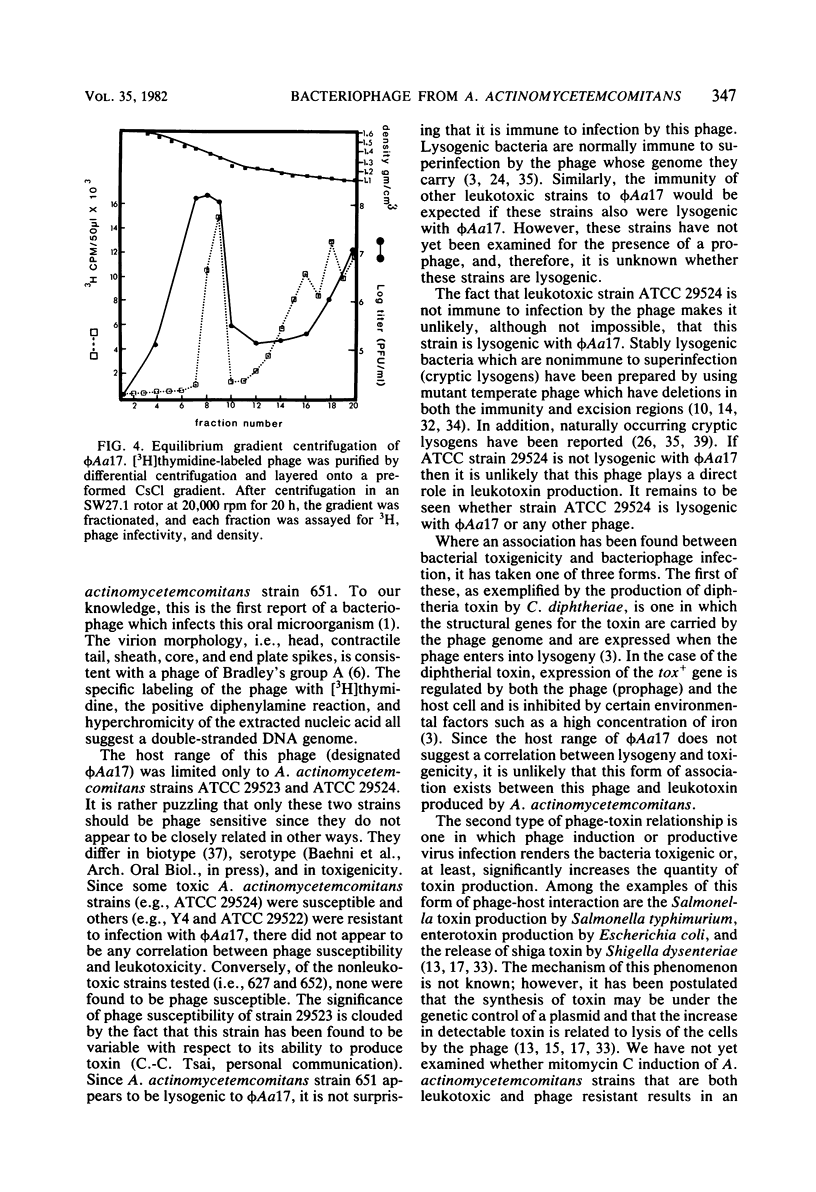
A bacteriophage, designated phi Aa17, was isolated by mitomycin C induction from leukotoxic Actinobacillus actinomycetemcomitans strains 651. Electron microscopy of the virus revealed particles with regular, nonelongated, polyhedral heads, and tails consisting of a contractile sheath and core. Spikes emanated from the base of the tail. The head had a diameter of 70 nm. The fully extended tail sheath had a length of 127 nm and a diameter of 22 nm. In its contracted form, the tail sheath measured 47 nm in length and 25 nm in diameter. The phage had a buoyant density of 1.370 in CsCl, and its genome was found to be double-stranded DNA. A single-cycle growth curve revealed that the phage had a latent period of 30 min and a burst size of 435 PFU per cell. The host range of the phage was examined, and A. actinomycetemcomitans strains ATCC 29523 and ATCC 29524 were found to be phage sensitive, whereas strains Y4, ATCC 29522, 2043, 652, 651, 627, 2097, N27, 2112, and 511 were resistant. The host range of this virus does not suggest any association between the phage and leukotoxin production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W. Bactériophages: propriétés et premièes étapes d'une classification. Pathol Biol (Paris) 1969 Nov;17(21):1003–1024. [PubMed] [Google Scholar]
- Adhya S., Campbell A. Crypticogenicity of bacteriophage lambda. J Mol Biol. 1970 Jun 14;50(2):481–490. doi: 10.1016/0022-2836(70)90206-8. [DOI] [PubMed] [Google Scholar]
- BARKSDALE L., GARMISE L., RIVERA R. Toxinogeny in Corynebacterium diphtheriae. J Bacteriol. 1961 Apr;81:527–540. doi: 10.1128/jb.81.4.527-540.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAIR J. E., CARR M. Lysogeny in staphylococci. J Bacteriol. 1961 Dec;82:984–993. doi: 10.1128/jb.82.6.984-993.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehni P., Tsai C. C., McArthur W. P., Hammond B. F., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979 Apr;24(1):233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M. Bacteriophage and the toxigenicity of Clostridium botulinum type D. Nat New Biol. 1972 Jan 5;235(53):16–17. doi: 10.1038/newbio235016a0. [DOI] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M., Smith C. A. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971 Apr 30;172(3982):480–482. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- FISCHER-FANTUZZI L., CALEF E. A TYPE OF LAMBDA PROPHAGE UNABLE TO CONFER IMMUNITY. Virology. 1964 Jun;23:209–216. doi: 10.1016/0042-6822(64)90284-3. [DOI] [PubMed] [Google Scholar]
- FREEMAN V. J., MORSE I. U. Further observations on the change to virulence of bacteriophage-infected a virulent strains of Corynebacterium diphtheria. J Bacteriol. 1952 Mar;63(3):407–414. doi: 10.1128/jb.63.3.407-414.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., O'Brien A. D., Wohlhieter J. A. Cellular release of heat-labile enterotoxin of Escherichia coli by bacteriophage induction. Infect Immun. 1978 Mar;19(3):1076–1082. doi: 10.1128/iai.19.3.1076-1082.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Zwenigorodsky V. I. Defective thermal induction of a noninducible bacteriophage. Virology. 1969 Dec;39(4):919–921. doi: 10.1016/0042-6822(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Palchaudhuri S., Maas W. K. Naturally occurring plasmid carrying genes for enterotoxin production and drug resistance. Science. 1977 Oct 14;198(4313):198–199. doi: 10.1126/science.333581. [DOI] [PubMed] [Google Scholar]
- Hariharan H., Mitchell W. R. Observations on bacteriophages of Clostridium botulinum type C isolates from different sources and the role of certain phages in toxigenicity. Appl Environ Microbiol. 1976 Jul;32(1):145–158. doi: 10.1128/aem.32.1.145-158.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston C. W., Koo F. C., Peterson J. W. Characterization of Salmonella toxin released by mitomycin C-treated cells. Infect Immun. 1981 May;32(2):916–926. doi: 10.1128/iai.32.2.916-926.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Marmur J. Characterization of inducible bacteriophages in Bacillus licheniformis. J Virol. 1970 Feb;5(2):237–246. doi: 10.1128/jvi.5.2.237-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Iida H. Conversion of toxigenicity in Clostridium botulinum type C. Jpn J Microbiol. 1970 Jan;14(1):87–89. doi: 10.1111/j.1348-0421.1970.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Inoue K., Iida H. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1971 Feb;24(1):53–56. [PubMed] [Google Scholar]
- Irving J. T., Newman M. G., Socransky S. S., Heely J. D. Histological changes in experimental periodontal disease in rats mono-infected with a gram-negative organism. Arch Oral Biol. 1975 Mar;20(3):219–220. doi: 10.1016/0003-9969(75)90013-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Ohishi I., Sakaguchi G. Evidence that botulinum C2 toxin has two dissimilar components. Infect Immun. 1980 Aug;29(2):390–394. doi: 10.1128/iai.29.2.390-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. P., Schlievert P. M., Watson D. W. Transfer of group A streptococcal pyrogenic exotoxin production to nontoxigenic strains of lysogenic conversion. Infect Immun. 1980 Apr;28(1):254–257. doi: 10.1128/iai.28.1.254-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- Kiley P., Holt S. C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980 Dec;30(3):862–873. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsanovich K. Cryptic lysogeny in Proteus mirabilis. J Gen Virol. 1973 Jun;19(3):311–320. doi: 10.1099/0022-1317-19-3-311. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Lai C. H., Evian C. I. Comparative antibody titers to Actinobacillus actinomycetemcomitans in juvenile periodontitis, chronic periodontitis and periodontally healthy subjects. J Clin Periodontol. 1981 Jun;8(3):155–164. doi: 10.1111/j.1600-051x.1981.tb02027.x. [DOI] [PubMed] [Google Scholar]
- McConnell M. M., Smith H. R., Willshaw G. A., Field A. M., Rowe B. Plasmids coding for colonization factor antigen I and heat-stable enterotoxin production isolated from enterotoxigenic Escherichia coli: comparison of their properties. Infect Immun. 1981 May;32(2):927–936. doi: 10.1128/iai.32.2.927-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M. M., Smith H. R., Willshaw G. A., Scotland S. M., Rowe B. Plasmids coding for heat-labile enterotoxin production isolated from Escherichia coli O78: comparison of properties. J Bacteriol. 1980 Jul;143(1):158–167. doi: 10.1128/jb.143.1.158-167.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Houston C. W., Koo F. C. Influence of cultural conditions on mitomycin C-mediated bacteriophage induction and release of Salmonella toxin. Infect Immun. 1981 Apr;32(1):232–242. doi: 10.1128/iai.32.1.232-242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Fontana R. Close association between shape alteration and loss of immunity to superinfection in a wild-type Klebsiella pneumoniae stable lysogen which can be both immune and nonimmune to superinfection. J Virol. 1978 Dec;28(3):772–785. doi: 10.1128/jvi.28.3.772-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Reynolds H. S., Genco R. J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980 Sep;29(3):1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- TAKEYA K., SHIMODORI S. "PROPHAGE-TYPING" OF EL TOR VIBRIOS. J Bacteriol. 1963 Apr;85:957–958. doi: 10.1128/jb.85.4.957-958.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Woods D. R. Bacteriophages and cryptic lysogeny in Achromobacter. J Gen Virol. 1974 Jan;22(1):153–157. doi: 10.1099/0022-1317-22-1-153. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZABRISKIE J. B. THE ROLE OF TEMPERATE BACTERIOPHAGE IN THE PRODUCTION OF ERYTHROGENIC TOXIN BY GROUP A STREPTOCOCCI. J Exp Med. 1964 May 1;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. Viral-induced bacterial toxins. Annu Rev Med. 1966;17:337–350. doi: 10.1146/annurev.me.17.020166.002005. [DOI] [PubMed] [Google Scholar]
- van der Vijver J. C., van Es-Boon M., Michel M. F. Lysogenic conversion in Staphylococcus aureus to leucocidin production. J Virol. 1972 Aug;10(2):318–319. doi: 10.1128/jvi.10.2.318-319.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]