Abstract
Background/Aims
Women with diabetes experience a disproportionately greater burden of diabetic kidney disease (DKD) risk factors compared to men, however sex-specific differences in DKD are not well defined. The effect of age on sex differences in DKD is unknown.
Methods
We performed a cross-sectional analysis of the prevalence of DKD (eGFR <60 ml/min/1.73m2 or microalbuminuria), advanced DKD (eGFR <30 ml/min/1.73 m2), and common DKD risk factors in the Pathways Study (N = 4,839), a prospective cohort study of diabetic patients from a managed care setting. Subjects were stratified by age <60 and ≥60 years to examine for differences by age. Logistic regression models examined the association between sex and prevalence of DKD and risk factors.
Results
Women of all ages had 28% decreased odds of DKD (OR 0.72, 95% CI 0.62–0.83); however, they had a greater prevalence of advanced DKD (OR 1.67, 95% CI 1.05–2.64), dyslipidemia (OR 1.42 95% CI 1.16–1.74), and obesity (OR 1.87, 95% CI 1.60–2.20) compared to men. Women had similar odds of hypertension and poor glycemic control as men. Women ≥60 years had increased odds of advanced DKD, hypertension, dyslipidemia, and obesity compared to similarly aged men. Women <60 years had increased odds of obesity compared to their male counterparts.
Conclusion
Women with diabetes had an increased prevalence of advanced DKD and common DKD risk factors compared to men and these disparities were most prominent amongst the elderly.
Keywords: sex difference, gender difference, diabetic kidney disease
Introduction
Diabetes mellitus is the leading cause of kidney failure in the United States and is associated with substantial morbidity, mortality, and health care [1]. The lifetime risk of diabetes is greater in women than in men [2] and women with diabetes have a greater prevalence of diabetic kidney disease (DKD) risk factors including hypertension [3, 4], hyperglycemia [4, 5], dyslipidemia [3–7], and obesity [4]; however, whether women have a greater prevalence of DKD is unclear. Age appears to modify the association between sex and nondiabetic kidney disease. In a large meta-analysis of nondiabetic kidney disease, Neugarten et al. found that men had more rapid renal decline compared to women [8], however a subsequent study of older subjects of postmenopausal age found that women had a faster progression of renal disease compared to men [9]. Whether there are analogous differences by age in DKD remains unknown.
Female sex has been associated with a higher risk of retinopathy and neuropathy in type 1 diabetes [10]. There effect of sex on nephropathy is not well established, however estrogen has several effects on the kidney that may be renoprotective [11]. Estrogen deficiency accelerates the progression of glomerulosclerosis in ovariectomized mice [12] and conversely, estrogen supplementation in postmenopausal women has been shown to reduce albuminuria [13]. Furthermore, fibroblast growth factor-23 (FGF-23) levels, which predicts progression to chronic kidney disease, are higher in postmenopausal women who are not on hormone replacement compared to postmenopausal women on hormone replacement or men [14]. Women with diabetes have lower levels of estradiol compared to nondiabetic women [15] and postmenopausal women with diabetes may represent a high-risk group for DKD.
In the current study, we examined the association of sex and age on the prevalence of DKD and DKD risk factors in a large cohort of outpatient diabetic patients from a vertically integrated managed care setting. We hypothesized that DKD would be more prevalent in women with diabetes compared to men and that these sex differences in DKD would be most prominent in elderly women. Additional, we hypothesized that women with diabetes would have a higher frequency of DKD risk factors such as hypertension, dyslipidemia, and obesity compared to men with diabetes.
Methods
Study Population
We performed a cross-sectional analysis of baseline data from the Pathways Study, which has been described previously [16, 17]. In brief, the Pathways Study is a prospective epidemiologic survey of the prevalence and impact of depression on patients with diabetes at Group Health (GH), a large nonprofit health maintenance organization (HMO) in Washington and Idaho, USA, currently with over 500,000 enrollees. The study protocol was reviewed and approved by the institutional review boards at GH and the University of Washington. Nine primary care clinics were chosen for patient recruitment based on clinic size, racial/ethnic diversity, and proximity to Seattle, WA. From these selected clinics, 9,064 potential candidates for the study were identified from the GH diabetes registry. Between 2001–2002, surveys were sent to these patients regarding demographic information, diabetes history, and diabetes complications (retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular disease, or metabolic). Of those identified, 1,222 patients were excluded from the study for the following reasons: no diabetes, gestational diabetes, cognitive impairment, severe illness, deceased, disenrollment from GH, or language/hearing problems. Of the remaining 7,842 patients eligible for the study, 4,839 (61.7%) returned the survey of which 4,467 (92.3%) gave permission to link survey data with automated data from GH. Patients with a history of end-stage renal disease (ESRD) (n = 67) were excluded from these analyses, leaving a total of 4,400 subjects for this study.
Measurements
Automated data from GH provided information regarding laboratory tests, hospitalizations, and outpatient visits up to 18 months prior as well as pharmacy records regarding medication use up to 12 months prior to study enrollment. The average creatinine or albuminuria result was used if subjects had multiple measurements within this time frame. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equations [18]. Microalbuminuria was defined as urine albumin to creatinine ratio ≥17 mg/g for men and ≥25 mg/g for women, using previously defined sex-specific cutoffs [19]. Patients with hypertension were identified by International Classification of Diseases, Ninth Revision diagnosis codes [20]. Body mass index (BMI) was calculated from self-reported height and weight (kg/m2).
Outcomes
The primary outcomes were sex differences in the prevalence of DKD or advanced DKD at study enrollment, amongst the subset of subjects with serum creatinine testing in the 18 months prior to study enrollment (n = 3,024) We defined DKD as the presence of either an eGFR <60 mL/min/1.73 m2 or microalbuminuria. Since a moderate decline in eGFR is normal in the elderly, we also looked at sex differences in the prevalence of advanced DKD as defined by an eGFR <30 mL/min/1.73 m2. Secondary outcomes were sex differences in the baseline prevalence of the following DKD risk factors: (1) hypertension (n = 4,400), (2) poor glycemic control (hemoglobin A1c ≥8.5%, n = 4,140), (3) dyslipidemia (low-density lipoprotein (LDL) >130 mg/dL, n = 3,043), and (4) obesity (BMI ≥30 kg/m2, n = 4,690). The number of subjects in each analysis differs due to missing laboratory data.
Statistical Analyses
Statistical analyses were performed using Stata version 12 (StataCorp, College Station, TX, USA). Significant sex differences in primary and secondary outcomes were determined using independent t tests allowing for unequal variances and χ2 tests. Logistic regression models were used to determine if there were associations between sex and DKD prevalence and risk factors. Given the high frequency missing LDL values (30.8%), imputed LDL (using age, sex, race, education, chronic kidney disease (CKD) stage, hypertension, hemoglobin A1c, and BMI) was used only for the purposes of adjustment in regression models. A priori, all models were adjusted for age, race/ethnicity, education, and smoking. Models for DKD were additionally adjusted for hemoglobin A1c, imputed LDL, and BMI; hypertension was not included as a covariate because of its collinearity with the outcome. Models for DKD risk factors were additionally adjusted for CKD stage and each of the other DKD risk factors. An interaction term between sex and age was examined in exploratory analyses of unstratified data and found to be significant (P <0.05) in the models for hypertension and dyslipidemia, therefore these effects are only presented in age-stratified models. To further examine differences by age, stratified analyses by age <60 and ≥60 years were conducted. Age 60 was used as a cutoff since prior studies in the literature found that >99% of women were post-menopausal by the age of 57 [14]. Age-stratified analyses were adjusted for the same covariates as the unstratified analyses, with the exception of age, which was felt to be overadjustment as addition of age into these models resulted in similar point estimates but wider confidence intervals. All analyses were also repeated in the subset of patients with type 2 diabetes, which resulted in similar results as in the entire cohort; therefore, results are presented combined for all outcomes.
Results
Pathways Cohort Characteristics
Of the 4,400 subjects in the final cohort, 49.0% were women (Table 1). Men tended to be older, married, and had higher levels of education and income than women. Among enrollees, racial composition was similar between sexes and was comprised of approximately 23.3% ethnic minorities. The prevalence of smoking was similar by sex. Overall, 11.6% of participants had type 1 diabetes. Mean duration of diabetes, hemoglobin A1c, and insulin use did not differ between men and women. Men had a higher mean number of diabetic complications compared to women (1.4 vs. 1.2, P <0.001).
Table 1.
Pathways cohort (n = 4,400) patient characteristics by sex.
| Characteristic | Women n = 2,154 | Men n = 2,246 | p |
|---|---|---|---|
| Age (years) | 62.6 ± 14.0 | 64.0 ± 12.6 | <0.001 |
| Age ≥60 years | 1,245 (57.8) | 1,419 (63.2) | <0.001 |
| Race | 0.45 | ||
| Non-Hispanic white | 1,642 (76.2) | 1,733 (77.2) | |
| Non-Hispanic black | 183 (8.5) | 165 (7.4) | |
| Asian | 199 (9.2) | 200 (8.9) | |
| Other | 130 (6.0) | 148 (6.6) | |
| Married | 1,130 (53.0) | 1,750 (78.3) | <0.001 |
| >High school education | 1,525 (71.9) | 1,758 (79.1) | <0.001 |
| Income ≥$20,000/year | 872 (52.2) | 1,174 (62.2) | <0.001 |
| Smoker | 170 (8.1) | 207 (9.3) | 0.14 |
| DM history | |||
| Type 1 DM | 242 (11.3) | 270 (12.0) | 0.44 |
| Duration of DM (years) | 9.3 ± 9.0 | 9.5 ± 9.3 | 0.50 |
| Number of DM complications | 1.2 ± 1.2 | 1.4 ± 1.3 | <0.001 |
| Hemoglobin A1c (%) | 7.8 ± 1.5 | 7.8 ± 1.6 | 0.44 |
| Hemoglobin A1c ≥8.5% | 510 (25.2) | 522 (24.7) | 0.67 |
| Insulin | 658 (30.6) | 648 (28.9) | 0.22 |
| Oral diabetic medications | 1,251 (58.1) | 1,378 (61.4) | 0.03 |
| Renal function | |||
| Creatinine (mg/dL) | 0.9 ± 0.4 | 1.2 ± 0.4 | <0.001 |
| eGFR (ml/min/1.73 m2) | 74.4 ± 23.3 | 71.5 ± 20.4 | <0.001 |
| Microalbuminuria | 496 (33.9) | 686 (46.5) | <0.001 |
| DKD (eGFR <60 ml/min/1.73 m2 or microalbuminuria) | 799 (49.6) | 992 (56.9) | <0.001 |
| Advanced DKD (eGFR <30 ml/min/1.73 m2) | 53 (3.6) | 36 (2.3) | 0.04 |
| Hypertension | 959 (44.5) | 904 (40.3) | 0.004 |
| Dyslipidemia | |||
| LDL (mg/dL) | 116.0 ± 36.1 | 108.1 ± 33.4 | <0.001 |
| LDL >130 mg/dL | 449 (32.1) | 386 (23.5) | <0.001 |
| Obesity | |||
| BMI (kg/m2) | 32.4 ± 8.2 | 29.7 ± 5.7 | <0.001 |
| BMI ≥30 kg/m2 | 1,192 (56.6) | 932 (41.9) | <0.001 |
Data are mean ±standard deviation or n (%). DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; DKD = diabetic kidney disease; LDL = low-density lipoprotein; BMI = body mass index.
Prevalence of DKD and Advanced DKD
Compared to women, men had higher mean serum creatinine (1.17 vs. 0.92 mg/dL, P <0.001), lower mean eGFR (71.5 vs. 74.4 mL/min/1.73 m2, P <0.001), and a greater prevalence of microalbuminuria (46.5% vs. 33.9%, P <0001). DKD was more prevalent in men compared to women (56.9% vs. 49.6%, P <0.001). However, advanced DKD was more prevalent in women compared to men (3.6% vs. 2.3%, P = 0.04). In adjusted logistic regression models, women had 28% decreased odds of DKD (OR 0.72, 95% CI 0.62–0.83) but 67% increased odds of advanced DKD (95% CI 1.05–2.64) compared to men (Table 3).
Table 3.
Unadjusted and adjusted odds of diabetic kidney disease (DKD) prevalence and risk factors for women compared to men.
| Outcome | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| DKD (eGFR <60 ml/min/1.73 m2 or microalbuminuria) | 0.74 (0.65–0.85) | 0.72 (0.62–0.83) |
| Advanced DKD (eGFR <30 ml/min/1.73 m2) | 1.55 (1.01–2.39) | 1.67 (1.05–2.64) |
| Hemoglobin A1c ≥8.5% | 1.03 (0.90–1.19) | 1.00 (0.83–1.19) |
| BMI ≥30 kg/m2 | 1.81 (1.60–2.04) | 1.87 (1.60–2.20) |
DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; LDL = low-density lipoprotein; BMI = body mass index; OR = odds ratio; CI = confidence interval.
All models are adjusted for age, race/ethnicity, education, and smoking. DKD models are additionally adjusted for hemoglobin A1c, LDL, and BMI. DKD risk factor models are additionally adjusted for chronic kidney disease stage and each of the other risk factors.
Prevalence of DKD Risk Factors
Women had a greater prevalence of hypertension compared to men (44.5% vs. 40.3%, P = 0.004). Glycemic control was similar between men and women; approximately 25% of participants had a hemoglobin A1c ≥8.5%. Compared to men, women had higher mean LDL (116.0 vs. 108.1 mg/dL, P <0.001) and a greater prevalence of LDL >130 mg/dL (32.1% vs. 23.5% P <0.001). Moreover, women were more obese than men (mean BMI 32.4 vs. 29.7 kg/m2, P <0.001). In multivariable logistic regression models for DKD risk factors, women had 87% greater odds of obesity (95% CI 1.60–2.20) but similar odds of poor glycemic control compared to men (OR 1.00, 95% CI 0.83–1.19).
DKD and Risk Factor Prevalence Stratified by Sex and Age
Men had a greater prevalence of DKD compared to women in both age groups (Table 2). Women ≥60 years old had a greater prevalence of advanced DKD (5.2% vs. 3.3%, P = 0.04), hypertension (54.0% vs. 46.8%, P <0.001), and dyslipidemia (29.6% vs. 19.5%, P <0.001) compared to men of the same age; younger subjects had no sex differences in these factors. Women were significantly more obese than men in both age groups. Glycemic control was similar between men and women in both age groups.
Table 2.
Diabetic kidney disease and risk factor prevalence in the Pathways cohort by age and sex.
| Outcome | Women | Men | p |
|---|---|---|---|
| DKD (eGFR <60 ml/min/1.73 m2 or microalbuminuria) | |||
| <60 years | 268 (41.6) | 294 (48.4) | 0.01 |
| ≥60 years | 531 (54.9) | 698 (61.4) | 0.002 |
| Advanced DKD (eGFR <30 ml/min/1.73 m2) | |||
| <60 years | 6 (1.0) | 2 (0.4) | 0.20 |
| ≥60 years | 47 (5.2) | 34 (3.3) | 0.04 |
| Hypertension | |||
| <60 years | 287 (31.6) | 240 (29.0) | 0.25 |
| ≥60 years | 672 (54.0) | 664 (46.8) | <0.001 |
| Hemoglobin A1c ≥8.5% | |||
| <60 years | 268 (31.6) | 237 (30.9) | 0.75 |
| ≥60 years | 242 (20.6) | 285 (21.1) | 0.76 |
| LDL >130 mg/dL | |||
| <60 years | 201 (35.8) | 182 (30.5) | 0.05 |
| ≥60 years | 248 (29.6) | 204 (19.5) | <0.001 |
| BMI ≥30 kg/m2 | |||
| <60 years | 613 (69.0) | 442 (53.8) | <0.001 |
| ≥60 years | 579 (47.5) | 490 (34.9) | <0.001 |
Data are number (%). DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; LDL = low-density lipoprotein; BMI = body mass index.
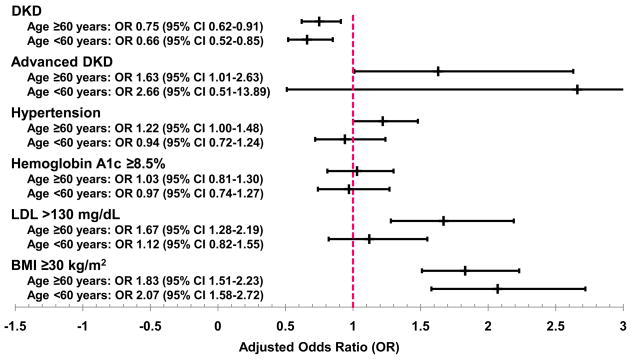
In multivariable logistic regression models, women had decreased odds of DKD compared to men of the same age (<60 years: OR 0.66, 95% CI 0.52–0.85; ≥60 years: OR 0.75, 95% CI 0.62–0.91) (Figure 1). Women ≥60 years had 63% greater odds of advanced DKD (95% CI 1.01–2.63), 22% greater odds of hypertension (95% CI 1.00–1.48), and 67% greater odds of dyslipidemia (95% CI 1.28–2.19) compared to men of the same age; there were no sex-specific differences in these factors in subjects <60 years. Women in both age groups had approximately 2-fold greater odds of obesity compared their male counterparts. There were no age-stratified differences in glycemic control in the regression models.
Figure 1.
Adjusted odds of diabetic kidney disease (DKD) prevalence and risk factors for women compared to men, stratified by age. All models are adjusted for race/ethnicity, education, and smoking. DKD models are additionally adjusted for hemoglobin A1c, LDL, and BMI. DKD risk factor models are additionally adjusted for chronic kidney disease stage and each of the other risk factors. DKD = diabetic kidney disease; LDL = low-density lipoprotein; BMI = body mass index; OR = odds ratio; CI = confidence interval.
Discussion
In this analysis of patients with diabetes from a managed care setting, we found that women had a greater prevalence of advanced DKD, hypertension, dyslipidemia, and obesity than men. These sex-specific differences were primarily accounted for by subjects ≥60 years old. To our knowledge, this is the first study to report the effect of age on sex-associated differences in DKD.
While it is generally accepted that women are at reduced risk of nondiabetic kidney disease, sex differences in DKD are less clear. We found that although men with diabetes had a greater prevalence of microalbuminuria, women with diabetes were at increased odds of advanced renal dysfunction. Other studies have likewise suggested that women with diabetes have a high prevalence of impaired eGFR. In a population-based study of 3,288 patients with diabetes in the United Kingdom, women had a greater prevalence of eGFR <60 ml/min/1.73 m2 compared to men, based on the Modification of Diet in Renal Disease (MDRD) study equation [21]. The UK Prospective Diabetes Study 74 (UKPDS) found that although men with diabetes had a higher risk of microalbuminuria, women with diabetes were at higher risk of Cockcroft-Gault estimated creatinine clearance <60 mL/min/1.73 m2 [22]. In contrast, studies from Hong Kong [23] and Japan [24] reported a greater prevalence of DKD in men versus women, although this may be related to the racial/ethnic differences in the study populations. None of these previous studies used the CKD-EPI equations for estimating GFR, which has been shown to be more accurate than the MDRD equation in patients with diabetes [18].
Our study also found sex-related disparities in the prevalence of common DKD risk factors. Women in our study had a greater prevalence of dyslipidemia and obesity compared to men. Previous studies in academic [5], HMO [3], and population-based [7] settings have reported that women with diabetes were more likely to have hypertension and dyslipidemia compared to men. A cross-sectional multicenter study found that women with diabetes had higher blood pressure and LDL compared to men with diabetes [25]. Although other studies reported worse glycemic control in diabetic women compared to men [4, 5, 7, 25], we did not find any sex differences in this risk factor.
We found that age affected the association between sex and DKD. Women over the age of 60, the majority of whom were presumably post-menopausal, had higher odds of advanced DKD compared to men of the same age. We are not aware of any other studies that examined sex differences in DKD by age. We also found that elderly women had greater odds of common DKD risk factors such as hypertension and dyslipidemia compared to their male counterparts, and these associations were not observed in the younger subjects. This is similar to the findings by Nilsson et al. who reported that women with diabetes had worse blood pressure and glycemic control than men, and these differences were most prominent amongst patients over the age of 60; sex-related differences in eGFR were not examined in this study [6].
The mechanisms behind how age modifies DKD prevalence and risk factors may be related to hormonal changes that occur with aging. Estrogen affects the kidney by reduction of mesangial cell proliferation, increased activity of metalloproteinase enzymes and nitric oxide synthesis, changes in inflammation, decreased renin-angiotensin system activity, and decreased FGF-23 levels [11, 14]. Other potential mechanisms for sex differences in kidney disease may be related to differential renal hemodynamics, diet, kidney/glomerular size, and sex-specific genetic polymorphisms [26].
It is important to note limitations of this study. Our study population is an insured population, therefore generalizability of our results may be limited to those with similar access to health care. We had very few subjects <60 years with advanced DKD and this particular analysis was underpowered to detect sex-specific differences. Still, our study was large enough to identify sex differences in advanced DKD amongst subjects ≥60 years old and to perform age-stratified analyses for other outcomes. While our study did include a small number of type 1 diabetic patients, the vast majority had type 2 diabetes, which is the largest growing population of patients developing DKD and ESRD (2). Although we did not have true GFR measurements, we used the CKD-EPI equations to estimate GFR which should be more accurate than the MDRD equation in this population.
In summary, our findings suggest that women with diabetes have an increased prevalence of advanced DKD and common DKD risk factors compared to men. These sex-related disparities were most prominent in elderly women, who represent a high-risk group for advanced stage DKD. Further studies are needed to determine whether these age and sex differences in DKD prevalence and risk factors translate into sex-specific differences in renal progression to ESRD.
Acknowledgments
Sources of Support: This work was supported by research grants by the National Institutes of Health National Institute of Diabetes, Digestive, and Kidney Diseases (DK079745, T32 007467) and National Institute of Mental Health (MH41739, MH01643). In addition, Dr. Young’s contribution is supported by resources from the VA Puget Sound Health Care System, Seattle, Washington.
Footnotes
Disclosure: All the authors declared no competing interests.
References
- 1.Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. U.S. Renal Data System, USRDS 2011 Annual Data Report. [Google Scholar]
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara A, Mangione CM, Kim C, Marrero DG, Curb D, Stevens M, et al. Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2008;31(1):69–74. doi: 10.2337/dc07-1244. [DOI] [PubMed] [Google Scholar]
- 4.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63(4):1499–507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 5.Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28(3):514–20. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson PM, Theobald H, Journath G, Fritz T. Gender differences in risk factor control and treatment profile in diabetes: a study in 229 swedish primary health care centres. Scand J Prim Health Care. 2004;22(1):27–31. doi: 10.1080/02813430310003264. [DOI] [PubMed] [Google Scholar]
- 7.Gouni-Berthold I, Berthold HK, Mantzoros CS, Bohm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2008;31(7):1389–91. doi: 10.2337/dc08-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11(2):319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 9.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant. 2003;18(10):2047–53. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 10.Monti MC, Lonsdale JT, Montomoli C, Montross R, Schlag E, Greenberg DA. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J Clin Endocrinol Metab. 2007;92(12):4650–5. doi: 10.1210/jc.2007-1185. [DOI] [PubMed] [Google Scholar]
- 11.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10(2):219–25. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Doublier S, Lupia E, Catanuto P, Elliot SJ. Estrogens and progression of diabetic kidney damage. Curr Diabetes Rev. 2011;7(1):28–34. doi: 10.2174/157339911794273982. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis. 2005;45(6):1019–25. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA. Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: the Heart and Soul Study. Am J Kidney Dis. 2011;58(5):737–45. doi: 10.1053/j.ajkd.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maric C, Sullivan S. Estrogens and the diabetic kidney. Gend Med. 2008;5 (Suppl A):S103–13. doi: 10.1016/j.genm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katon W, Von Korff M, Lin E, Simon G, Ludman E, Bush T, et al. Improving primary care treatment of depression among patients with diabetes mellitus: the design of the pathways study. Gen Hosp Psychiatry. 2003;25(3):158–68. doi: 10.1016/s0163-8343(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 17.Young BA, Katon WJ, Von Korff M, Simon GE, Lin EH, Ciechanowski PS, et al. Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the pathways study. J Am Soc Nephrol. 2005;16(1):219–28. doi: 10.1681/ASN.2004030162. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034–9. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 20.LS, editor. Physician ICD-9-CM. Salt Lake City, UT: Medicode Publications; 1999. [Google Scholar]
- 21.Nag S, Bilous R, Kelly W, Jones S, Roper N, Connolly V. All-cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabet Med. 2007;24(1):10–7. doi: 10.1111/j.1464-5491.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 22.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 23.Luk AO, So WY, Ma RC, Kong AP, Ozaki R, Ng VS, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care. 2008;31(12):2357–61. doi: 10.2337/dc08-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with Type 1 and Type 2 diabetes. Diabet Med. 2010;27(9):1017–23. doi: 10.1111/j.1464-5491.2010.03049.x. [DOI] [PubMed] [Google Scholar]
- 25.McFarlane SI, Castro J, Kaur J, Shin JJ, Kelling D, Jr, Farag A, et al. Control of blood pressure and other cardiovascular risk factors at different practice settings: outcomes of care provided to diabetic women compared to men. J Clin Hypertens (Greenwich) 2005;7(2):73–80. doi: 10.1111/j.1524-6175.2005.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tien KJ, Hsiao JY, Hsu SC, Liang HT, Lin SR, Chen HC, et al. Gender-dependent effect of ACE I/D and AGT M235T polymorphisms on the progression of urinary albumin excretion in Taiwanese with type 2 diabetes. Am J Nephrol. 2009;29(4):299–308. doi: 10.1159/000163592. [DOI] [PubMed] [Google Scholar]