Abstract
Carcinoids are neuroendocrine malignancies, often characterized by their potential to hypersecrete bioactive hormones that contribute to the carcinoid syndrome. Previously, we reported that AKT serves as a central regulator of carcinoid growth and phenotypic expression, demonstrated by genetic silencing of AKT. However, until only recently, no small-molecule inhibitor of AKT kinase activity has been developed. MK-2206, a novel allosteric inhibitor of AKT, is currently undergoing clinical trials for the treatment of solid tumors. In this study, we explored the effect of MK-2206 on carcinoid cell proliferation and bioactive hormone production using two carcinoid cell lines of pancreatic (BON) and bronchopulmonary (H727) origin. Treatment with MK-2206 effectively suppressed AKT phosphorylation at the Serine 473 site, while significantly reducing cell proliferation in a dose-dependent manner. Most notably, MK-2206 treatment inhibited ASCL1, CgA, and NSE expression, collectively recognized as markers of neuroendocrine tumor malignancy. Furthermore, MK-2206-treated cells exhibited an increase in levels of cleaved PARP and cleaved caspase-3, alongside a concomitant reduction in Mcl-1 and XIAP, indicating that the anti-proliferative effect of this agent occurred through the induction of apoptosis. In conclusion, MK-2206 suppresses carcinoid tumor growth and alters its neuroendocrine phenotype, suggesting this drug may possess therapeutic potential for patients with carcinoid syndrome. These studies merit further investigation.
Keywords: carcinoids, Akt pathway, chromogranin A, neuroendocrine markers, ASCL1
Introduction
Carcinoids are slow-growing neuroendocrine (NE) malignancies, with a reported incidence of approximately 5:100,000 [1,2]. These cancers primarily arise from the enterochromaffin cells of the gastrointestinal or pulmonary system, and follow a typically indolent clinical course. As a result, carcinoids are often diagnosed incidentally, which can delay appropriate treatment. Therefore, these cancers frequently lead to hepatic metastasis. This occurrence generally results in the carcinoid syndrome, a disease which entails the hypersecretion of an array of bioactive hormones, including serotonin, growth hormone, and gastrin [1,2]. However, the carcinoid syndrome may occur in the setting of bronchopulmonary metastasis even in the absence of hepatic involvement. Patients with carcinoid syndrome generally present with debilitating symptoms including flushing, diarrhea, vomiting, abdominal pain, and tricuspid insufficiency. Localized carcinoid tumors are typically curable with surgery [3,4]. However, systemic therapies for treating metastatic carcinoids have only shown limited antitumor efficacy. Mainstay approaches to managing disseminated disease primarily focus on symptomatic control and chronic care [5,6]. This underscores the urgency for identifying novel targeted therapies for treating patients with unresectable conditions.
Recent studies have identified the phosphoinositide 3-kinase (PI3K)/AKT pathway to be hyperactive in human carcinoid tumors. Of note, it has been demonstrated that suppressing AKT expression, either pharmacologically, using the PI3K-inhibitor LY294002, or directly, using a gene-specific siRNA against AKT, markedly reduces cell growth while altering the malignant phenotype of both pancreatic and bronchopulmonary, human-derived carcinoid cell lines [7–9]. Given these findings, carcinoids may be highly susceptible to selective inhibition of AKT. Therefore, AKT may pose as a potential pharmacotherapeutic target in carcinoids.
Because competitive ATP-inhibitors of AKT often bind to protein sites that are biochemically homologous and nonspecific, they have been reported to exhibit a high incidence of off-target effects. In contrast, allosteric, non-competitive ATP-inhibitors of AKT kinase activity appear to display a much higher level of target specificity. Recently, Merck® reported results of a phase I clinical trial characterizing its newly developed compound MK-2206, an allosteric, small-molecule inhibitor of AKT, proposed for the treatment of solid tumors [10]. Similar to other non-competitive ATP inhibitors, MK-2206 binds to a site other than the ATP-binding domain, causing conformational changes that prevent AKT localization and subsequent kinase activity [10,11]. Along with MK-2206, several classes of small-molecule inhibitors of AKT have been described, each with varying potencies and specificities to their respective AKT isoforms [12].
Current studies on MK-2206 indicate it is indeed safe, with some associated but tolerable side-effects including reports of a grade 1–4 rash. These investigations have also established MK-2206’s pharmacologic parameters including dose-limiting toxicities, pharmacokinetics, pharmacodynamics, and alternative-day dosing regimens for phase II clinical trials [10]. This drug has also has been purported to possess exceptional antitumor efficacy both in vitro and in vivo as a single agent, in addition to being a potent sensitizer to a number of other chemotherapeutic agents [10,13–15].
Herein, we investigated the effect of MK-2206 treatment on tumor growth, NE biomarker expression, and the apoptotic cascade in both pancreatic and bronchopulmonary carcinoid cell lines.
Materials and Methods
Cell Culture and Treatment
Human pancreatic carcinoid cells (BON) were provided by Drs. B. Mark Evers and Courtney M. Townsend Jr. (University of Texas Medical Branch, Galveston, TX, USA), and bronchopulmonary carcinoid cells (H727) were purchased from the American Type Culture Collection (Manassas, VA, USA). BON cells were maintained in DMEM/F-12 (Life Technologies, Grand Island, NY, USA), and H727 cells were maintained in RPMI1640 (Life Technologies, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Grand Island, NY, USA). Both cell lines were grown in a humidified atmosphere of 5% CO2 at 37°C. MK-2206 was dissolved in dimethyl-sulfoxide (DMSO, Fischer Scientific, Pittsburgh, PA, USA), and cells were treated with varying doses as indicated.
Cell Viability
Carcinoid cell viability was determined using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) rapid colorimetric assay. BON and H727 cells were plated in 24-well plates and allowed to adhere overnight. Cells were then treated with MK-2206 in quadruplicate at each dose. Treatments lasted for up to 6 days, and were replenished after 48 hours. On the day of cell viability determination, media was replaced with 250 μL of serum-free medium containing 0.5 mg/mL MTT. Plates were then incubated at 37°C for 3.5 hours, followed by the addition of 750 μL of DMSO, and measured at 540 nm using a spectrophotometer (μQuant, Bio-Tek Instruments, Winooski, VT, USA).
Immunoblot Analysis
Following treatment, cells were washed in 1X PBS, lysed in lysis buffer (50 mM Tris, 0.15 M NaCl, 0.5% Na/deoxycholate, 0.1% SDS, 1% Nonidet P-40, 0.1% protease inhibitor cocktail and 0.6 mM phenylmethanesulfonyl fluoride), and then prepared into lysates as previously described [16]. A bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA) was used to determine the concentration of total cellular protein. Subsequently, these samples were denatured and resolved on 7%, 10%, or 12% SDS-PAGE gels (Invitrogen, Grand Island, NY, USA). Proteins were then transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA), after which these protein-bound membranes were blocked for at least 30 minutes in PBS-T containing milk (1x PBS, 5% dry milk, 0.05% Tween-20), and then incubated overnight at 4°C in their respective primary antibodies. Each antibody was diluted as follows: 1:2000 for mammalian achaete-scute complex-like1 (BD PharMingen, San Diego, CA, USA), 1:3000 for chromogranin A (Zymed Laboratories, San Francisco, CA, USA), and 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Trevigen, Gaithersburg, MD, USA). Phosphorylated AKTSer473, phosphorylated AKTThr308, total AKT, total caspase-3, cleaved caspase-3, cleaved caspase-7, total-PARP, cleaved-PARP, X-linked Inhibitor of Apoptosis (XIAP), Mcl-1, Survivin, and β-Actin (Cell Signaling Technology, Beverly, MA, USA) were all diluted to 1:1000. Following incubation in primary antibody, membranes were washed 3×5 minutes in PBS-T. Blots were then incubated in either horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (Cell Signaling Technology), depending on the source of the primary antibody. Membranes were then washed for 3×5 or 3×10 minutes in PBS-T. SuperSignal West Pico, Femto (Pierce, Rockford, IL, USA) or Immunstar (Bio-Rad Laboratories, Hercules, CA, USA) kits were then used for membrane development, according to manufacturers’ instructions.
Statistical Analysis
All results shown represent the mean ± SEM, unless specifically noted. Comparisons between treatments were analyzed using Analysis of Variance testing (SPSS software ver10.0; SPSS Inc., Chicago, IL). A p value less than 0.05 was considered significant.
Results
MK-2206 treatment inhibits cell proliferation of carcinoid cell lines in a dose-dependent manner'
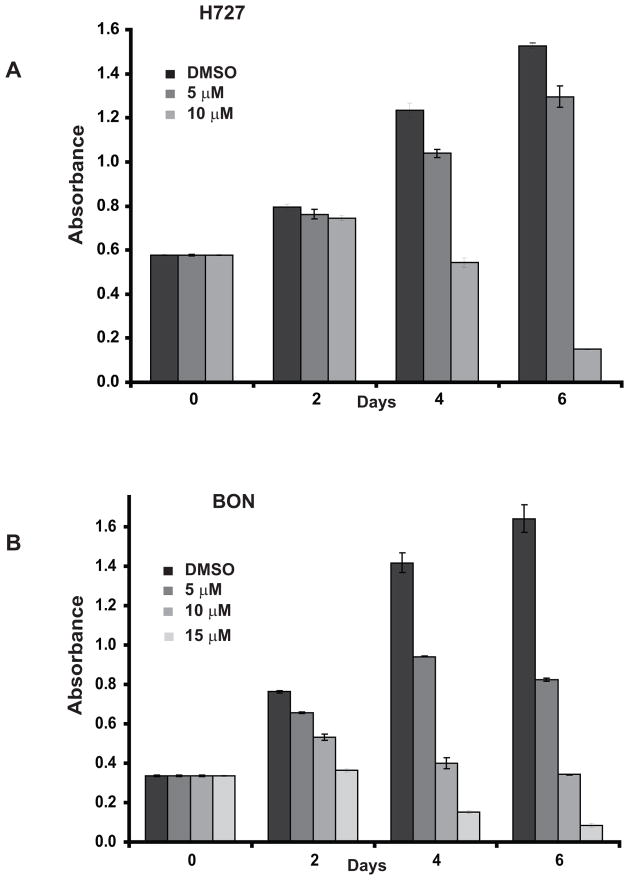
We began by investigating the effect of MK-2206 treatment on cell proliferation in two human carcinoid cell lines: pancreatic (BON) and bronchopulmonary (H727). Cell proliferation was assessed using an MTT assay for up to 6 days following MK-2206 treatment, with results indicating that carcinoid cell growth had dose-dependently decreased in both cell lines, compared to control (DMSO) treatment. Though only a modest reduction in H727 cell growth occurred following 2 days of MK-2206 treatment, BON cells appeared to respond more favorably, with a statistically significant and dose-dependent suppression of viability within the same treatment period. Nevertheless, by the 6th day of MK-2206 treatment, H727 cell proliferation was reduced by 90% following 10 μM of MK-2206 (Figure 1A), while BON cells, following 15 μM of MK-2206 treatment, experienced a comparable response with an 87% decrease in viability (Figure 1B).
Figure 1.
MK-2206 exerts an anti-proliferative effect on carcinoids. The effect of MK-2206 on carcinoid cell growth was determined using an MTT assay in H727 (A) and BON (B) cell lines. MK-2206 treatment dose-dependently suppressed cell viability. Values are represented as a mean ± SEM. The effect of treatment at each time point was statistically significant (P<0.05) relative to controls.
MK-2206 alters the neuroendocrine phenotype by suppressing neuroendocrine-specific biomarkers of malignancy
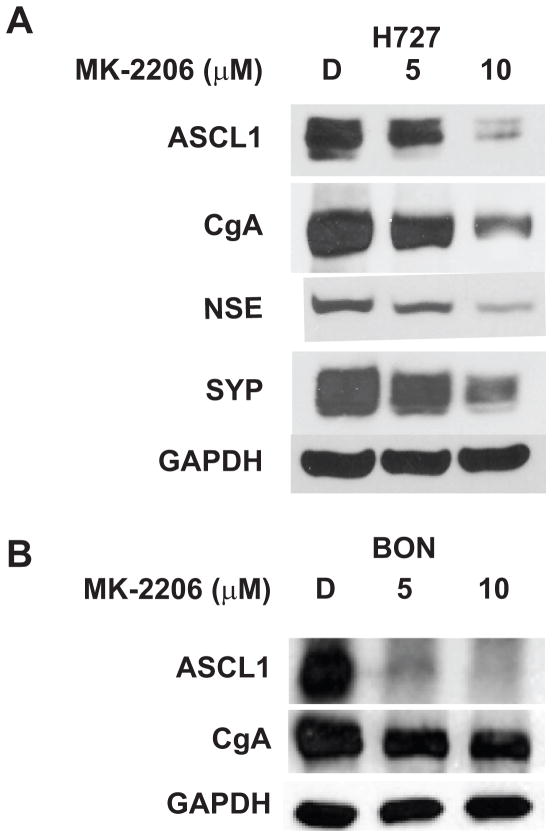
Carcinoid syndrome progression and symptomatology are largely consequent of the expression and co-secretion of a number of bioactive amines, among which include chromogranin A (CgA). These biomarkers along with an elevated expression of the achaete-scute complex-like1 (ASCL1) collectively portend a poor prognosis for carcinoid patients, and are routinely utilized for diagnostic purposes. Given the prognostic significance of ASCL1 and CgA, we analyzed the impact of MK-2206 on the expression of these neuroendocrine phenotypic markers. Levels of ASCL1 and CgA were significantly reduced by MK-2206 treatment in a dose-dependent manner, as shown by Western blot analysis of lysate extracts from MK-2206-treated H727 and BON cells (Figure 2A and 2B). Since over-expression of ASCL1 is clearly implicated in neuroendocrine tumorigenesis, suppression of ASCL1 alongside the co-expressed CgA may collectively represent a possible mechanism by which MK-2206 exerts its anti-cancer effect on carcinoids. Furthermore, there appeared to be a significant reduction in levels of other bioactive neuroendocrine markers such as Neuron-specific Enolase (NSE) and Synaptophysin (SYP) following MK-2206 treatment of H727 cells (Figure 2A). These findings cumulatively suggest that MK-2206 has the ability to alleviate symptoms of the carcinoid syndrome.
Figure 2.
MK-2206 alters the expression of neuroendocrine phenotypic markers in carcinoids. MK-2206 treatment reduced levels of achaete-scute complex-like1 (ASCL1), chromogranin A (CgA), Neuron-specific Enolase (NSE), and Synaptophysin (SYP) in H727 (A) and BON (B) cell lines. GAPDH was used as a loading control.
MK-2206 selectively inhibits the phosphorylation of AKTSer473 in carcinoid cell lines
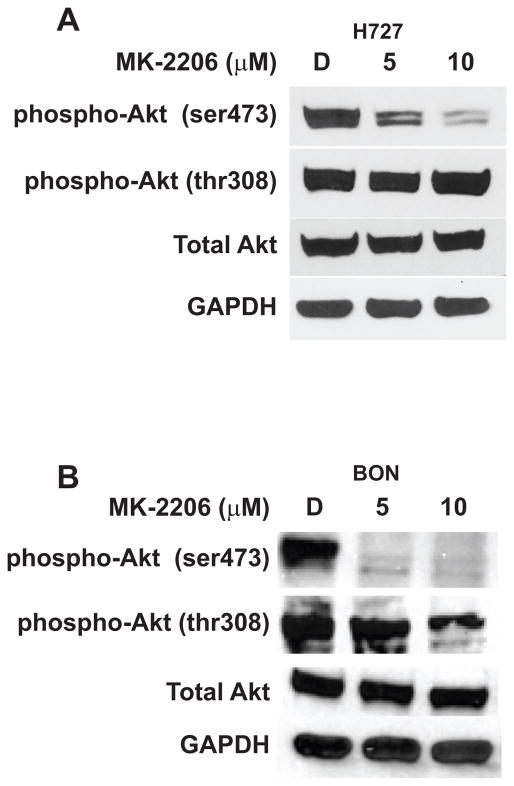
Based on the reported inhibitory activity of MK-2206 on AKT expression in other cancer types, we sought to confirm if this agent also inhibits AKT phosphorylation, specifically at the Serine 473 and Threonine 308 sites, in carcinoid cell lines as well. Both H727 and BON cells were treated with MK-2206 for a given period, and subsequently underwent Western analysis for expression of phosphorylated AKT. We observed that MK-2206 inhibited AKT phosphorylation at the Serine 473 site. Interestingly, there was no significant effect on phosphorylated AKTThr308 in either H727 or BON cells (Figure 3A and 3B), indicating that MK-2206 does not effect upstream components of the PI3K pathway. We have previously reported that silencing AKT expression in carcinoids using AKT-specific siRNA sequences effectively suppresses cell proliferation and reduces NE marker expression [8,9]. Collectively, these data suggest that the antitumor effect of MK-2206 on carcinoids may be due to its targeted suppression of AKT activity.
Figure 3.
MK-2206 inhibits AKT phosphorylation. MK-2206 treatment reduced expression of phosphorylated AKT at the Serine 473 position, whereas that of the auto-phosphorylation site at the Threonine 308 position did not significantly change, demonstrated in both H727 (A) and BON (B) cell lines. However, MK-2206 had no effect on total AKT levels in either cell line. GAPDH was used as a loading control.
MK-2206 inhibits the growth of carcinoid cell lines via the induction of apoptosis
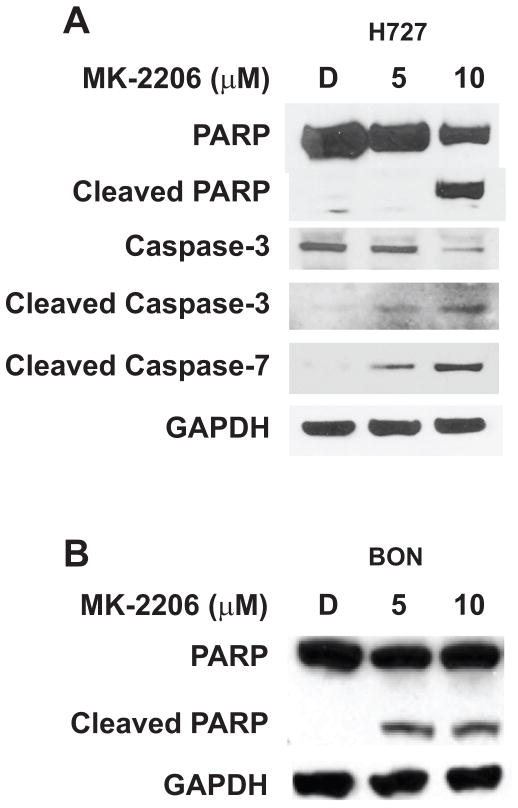
Having observed the growth-suppressive effect of MK-2206 in both H727 and BON cells, we next sought to determine the mechanism of drug-induced cytotoxicity by first assessing the activity of molecular markers of apoptosis in response to MK-2206 treatment. Following treatment with MK-2206, carcinoid cell lines were examined by Western blotting for their expression of cleaved poly (ADP)-ribose polymerase (PARP), cleaved caspase-3, and cleaved caspase-7. It is known that during execution of the apoptotic cascade, cleavage of PARP, a caspase-3 substrate, must precede the cleavage of caspase-3, an ICE-like protease activated in response to various cytotoxic stimuli. Similarly, cleavage of caspase-7 has also been strongly defined as a precedent to PARP cleavage in a number of stress-induced models. Therefore, the cleavage of PARP and various caspases can be reliable indicators of apoptotic induction following external stimuli such as MK-2206 treatment. Indeed, we observed an elevation in levels of cleaved PARP in both H727 and BON cells following MK-2206 treatment (Figure 4A and 4B). Furthermore, we observed an increase in cleaved caspase-3 and -7 following MK-2206 treatment of H727 cells (Figure 4A).
Figure 4.
MK-2206 induces apoptosis in carcinoids. Increasing concentrations of MK-2206 led to the cleavage of poly ADP ribose polymerase (PARP), along with that of caspases-3, and -7 in the H727 cell line (A). The BON cell line also exhibited an increase in PARP cleavage following treatment with MK-2206 (B). GAPDH was used as a loading control.
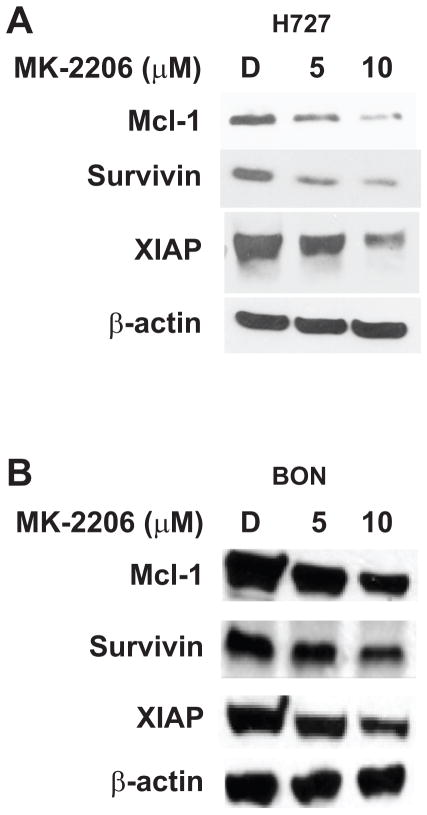
MK-2206 reduces pro-survival markers Mcl-1, Survivin and X-linked Inhibitor of Apoptosis (XIAP) in carcinoid cell lines
To further corroborate our findings suggesting apoptotic induction as a result of MK-2206 treatment in our two carcinoid cell lines, we next assessed the activity of various molecular triggers responsible for evasion from apoptosis. Among these included members of the Inhibitor of Apoptosis (IAP) protein family, which are known for their role in intercepting programmed cell-death triggers by binding to caspases, thereby preventing their cleavage and subsequent activation. Among the IAP members, we chose to investigate the activity of XIAP and Survivin, in addition to levels of the Bcl-2 family member known as the Induced Myeloid Leukemia Cell Differentiation protein, or Mcl-1. We demonstrated a reduction in expression of XIAP, Survivin and Mcl-1 in both MK-2206-treated H727 and BON cells, which, of note, occurred at the same doses that demonstrably induced apoptosis (Figure 5A and 5B). Control cells exhibited relatively higher levels of all pro-survival markers, suggesting that these factors may contribute to the pro-tumorigenic phenotype characteristic of carcinoids.
Figure 5.
MK-2206 reduces the expression of cell-survival markers in carcinoids. Treatment with MK-2206 significantly reduced Mcl-1, Survivin, and XIAP expression in both H727 (A) and BON (B) cell lines. β-actin was used as a loading control.
Discussion
The urgency for new therapies for the treatment of carcinoids is underscored by the complex nature of their management [1,2,6,17]. Carcinoids are generally encountered inadvertently, and are often unresectable at the time of diagnosis. Recent efforts have focused on elucidating signaling pathway derangements that define the carcinoid phenotype with the aim of developing targeted therapies that are more effective [17–21]. Among these pathways is the phosphoinositide 3-kinase (PI3K)/AKT pathway, which regulates such functions as mitogenesis, cellular motility, and protection from apoptosis in normal tissues [22,23]. Hyperactivation of the PI3K-AKT pathway has been commonly implicated in the growth, progression and phenotypic expression of a number of neoplasms including breast, prostate, colon, ovarian, and neuroendocrine cancers [22,23]. The current course of drug development has attempted to exploit the critical nature of the PI3K-AKT pathway in various cancer pathogeneses through the use of small-molecule inhibitors [24].
In this study, we tested the therapeutic potential of the AKT inhibitor MK-2206 in both pancreatic and bronchopulmonary carcinoid cell lines, BON and H727, respectively. We demonstrate that this drug exerts a significant antiproliferative effect on both cell lines. This is strongly aligned with reports which describe the capacity of MK-2206 to inhibit cell growth in other cancer types including thyroid cancer lines that harbor mutations in the PI3K/AKT pathway, in addition to colon cancer, gliomas, non-small cell lung cancers, and melanomas [13,15]. Our study conveyed that MK-2206’s antitumor effect correlated with a reduction in AKT phosphorylation at the Serine 473 residue in both BON and H727 cells. Furthermore, this was associated with an inhibition of ASCL1, CgA, SYP, and NSE, neuroendocrine biomarkers that are clinically utilized to assess the degree of treatment efficacy and one’s clinical course [19,25,26]. These data suggest that MK-2206 may not only inhibit carcinoid cell proliferation, but may also alleviate symptoms associated with bioactive hormone production. Therefore, MK-2206 may be a viable therapeutic option for palliating carcinoid syndrome symptomatology in addition to reducing tumor burden.
The antitumor effect of MK-2206 appeared to be due to its ability to initiate apoptosis, while reducing expression of the pro-survival protein Mcl-1 and the Inhibitor of Apoptosis (IAP) proteins XIAP and Survivin. Studies have supported the oncogenic role of Survivin, as it appears highly expressed across several cancer types [27]. The clinicopathological significance of Survivin is inextricably linked to its ability to inhibit the apoptotic cascade in cancer, and in doing so, conferring chemoresistance. In fact, recent evidence has implicated the presence of high Survivin expression in the metastatic potential of carcinoid tumors, suggesting that this protein, among others, is a reliable indicator of carcinoid tumor burden [28]. Similarly, XIAP has also been described as being aberrantly elevated in various malignancies, and furthermore, as a culprit for the development of chemoresistance [29–31]. Finally, Mcl-1 has been characterized as a deterrent of apoptosis in carcinoids, as its expression was found to be significantly elevated across panels of human carcinoid specimens [32]. MK-2206’s ability to exert an antiproliferative effect in carcinoids while simultaneously reducing Survivin, XIAP, and Mcl-1 suggests that part of its antitumor mechanism may involve counteracting survival responses inherent to the cancer phenotype. Often subject to extended periods of chemotherapy, carcinoid patients have an increased likelihood of developing chemoresistance, and thus may stand to benefit from the potential chemosensitizing effects of MK-2206. Further studies are necessary to confirm this hypothesis.
In summary, MK-2206 represents a viable treatment option for mitigating carcinoid tumor burden and symptomatology, given its ability to reduce carcinoid cell proliferation while suppressing NE markers of malignancy. These findings warrant continued exploration into the use of this compound in preclinical models, and subsequent clinical trials on carcinoid patients.
Acknowledgments
Financial Support: This research was supported in part by the Howard Hughes Medical Institute Medical Fellowship Program (Y.S.), a T-35 grant from the NIH (K.S.), an R01 CA121115 grant from the NIH (H.C.), the American Cancer Society Research Scholars Grant (H.C.), the American Cancer Society MEN2 Professorship (H.C.), and an R03 CA155691 from the NIH (M.K.).
We would also like to acknowledge the Merck® Corporation for graciously providing us with MK-2206 in order to carry out our investigation.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Reference List
- 1.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;15:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am. 2006;15:463–478. doi: 10.1016/j.soc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? Journal of the American College of Surgeons. 1998;15:92. doi: 10.1016/s1072-7515(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 4.Lal A, Chen H. Treatment of advanced carcinoid tumors. Curr Opin Oncol. 2006;15:9–15. doi: 10.1097/01.cco.0000198018.53606.62. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother. 2008;15:2617–2626. doi: 10.1517/14656566.9.15.2617. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, Wright NA, Kidd M. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust. 2010;15:46–52. doi: 10.5694/j.1326-5377.2010.tb03742.x. [DOI] [PubMed] [Google Scholar]
- 7.Pitt SC, Davis R, Kunnimalaiyaan M, Chen H. AKT and PTEN expression in human gastrointestinal carcinoid tumors. Am J Transl Res. 2009;15:291–299. [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt SC, Chen H, Kunnimalaiyaan M. Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg. 2009;15:82–88. doi: 10.1016/j.jamcollsurg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;15:2936–2942. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;15:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 11.Bilodeau MT, Balitza AE, Hoffman JM, Manley PJ, Barnett SF, Defeo-Jones D, Haskell K, Jones RE, Leander K, Robinson RG, Smith AM, Huber HE, Hartman GD. Allosteric inhibitors of Akt1 and Akt2: a naphthyridinone with efficacy in an A2780 tumor xenograft model. Bioorg Med Chem Lett. 2008;15:3178–3182. doi: 10.1016/j.bmcl.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;15:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, Zhou L, Liao J, Keihack H, Yan L, Rubin E, Yang JM. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther. 2012;15:154–164. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;15:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Liu D, Xing M. The Akt inhibitor MK2206 synergizes, but perifosine antagonizes, the BRAF(V600E) inhibitor PLX4032 and the MEK1/2 inhibitor AZD6244 in the inhibition of thyroid cancer cells. J Clin Endocrinol Metab. 2012;15:E173–E182. doi: 10.1210/jc.2011-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;15:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 17.Carter Y, Jaskula-Sztul R, Chen H, Mazeh H. Signaling Pathways as Specific Pharmacologic Targets for Neuroendocrine Tumor Therapy: RET, PI3K, MEK, Growth Factors, and Notch. Neuroendocrinology. 2012:15. doi: 10.1159/000335136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavel M. Translation of Molecular Pathways into Clinical Trials of Neuroendocrine Tumors. Neuroendocrinology. 2012:15. doi: 10.1159/000336089. [DOI] [PubMed] [Google Scholar]
- 19.Pinchot SN, Pitt SC, Sippel RS, Kunnimalaiyaan M, Chen H. Novel targets for the treatment and palliation of gastrointestinal neuroendocrine tumors. Curr Opin Investig Drugs. 2008;15:576–582. [PMC free article] [PubMed] [Google Scholar]
- 20.Wiedenmann B, Pavel M, Kos-Kudla B. From targets to treatments: a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology. 2011;15:177–190. doi: 10.1159/000329386. [DOI] [PubMed] [Google Scholar]
- 21.Somnay Y, Kunnimalaiyaan M. The phosphatidylinositol 3-kinase/Akt signaling pathway in neuroendocrine tumors. Global J Biochem. 2012;15:3–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;15:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 23.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;15:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 Kinase/AKT/mTOR Signaling in Cancer. Crit Rev Oncog. 2012;15:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;15:111–34. viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;15:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel M, Mahotka C, Krieg A, Bachmann A, Schmitt M, Gabbert HE, Gerharz CD. Novel survivin-related members of the inhibitor of apoptosis (IAP) family. Cell Death Differ. 2000;15:682–683. doi: 10.1038/sj.cdd.4400691. [DOI] [PubMed] [Google Scholar]
- 28.Drozdov I, Kidd M, Nadler B, Camp RL, Mane SM, Hauso O, Gustafsson BI, Modlin IM. Predicting neuroendocrine tumor (carcinoid) neoplasia using gene expression profiling and supervised machine learning. Cancer. 2009;15:1638–1650. doi: 10.1002/cncr.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frenzel LP, Patz M, Pallasch CP, Brinker R, Claasen J, Schulz A, Hallek M, Kashkar H, Wendtner CM. Novel X-linked inhibitor of apoptosis inhibiting compound as sensitizer for TRAIL-mediated apoptosis in chronic lymphocytic leukaemia with poor prognosis. Br J Haematol. 2011;15:191–200. doi: 10.1111/j.1365-2141.2010.08426.x. [DOI] [PubMed] [Google Scholar]
- 30.Kashkar H. X-linked inhibitor of apoptosis: a chemoresistance factor or a hollow promise. Clin Cancer Res. 2010;15:4496–4502. doi: 10.1158/1078-0432.CCR-10-1664. [DOI] [PubMed] [Google Scholar]
- 31.Seeger JM, Brinkmann K, Yazdanpanah B, Haubert D, Pongratz C, Coutelle O, Kronke M, Kashkar H. Elevated XIAP expression alone does not confer chemoresistance. Br J Cancer. 2010;15:1717–1723. doi: 10.1038/sj.bjc.6605704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard DM, Berry D, Przemeck SM, Campbell F, Edwards SW, Varro A. Gastrin increases mcl-1 expression in type I gastric carcinoid tumors and a gastric epithelial cell line that expresses the CCK-2 receptor. Am J Physiol Gastrointest Liver Physiol. 2008;15:G798–G805. doi: 10.1152/ajpgi.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]