Abstract
Background
Plasma expander (PE) based on polyethylene glycol (PEG) conjugated to albumin has shown positive results maintaining blood volume (BV) during hemodilution and restoring BV during resuscitation from hemorrhagic shock. PEG conjugation to human serum albumin (HSA), PEG-HSA, increasing size, weigh and colloidal osmotic pressure (COP), with minor effects on solution viscosity.
Methods
This study was designed to test the hypothesis that PEG-HSA (2 g/dL) produced by direct PEGylation chemistry improves cardiac function during two experimental models, i) moderate hemodilution and ii) resuscitation from hemorrhagic shock, compared to a conventional colloidal plasma expander (dextran 70 kDa, D×70, 6 g/dL). Cardiac function was studied using a miniaturized pressure volume (PV) conductance catheter implanted in the left ventricle (LV) and evaluated in terms of cardiac indices derived from the PV measurements.
Results
PEG-HSA increased cardiac output (CO), stroke volume (SV) and stroke work (SW), and decreased systemic vascular resistance (SVR) compared to D×70, in both experimental models. The improvements induced by PEG-HSA in cardiac function were sustained over the observation time. PEG-HSA cardiac mechanoenergetics changes are the result of increased energy transferred per stroke, and decreased resistance of the vasculature connecting the heart. In summary, PEG-HSA decreased LV ejection impedance.
Conclusion
Ejection of blood diluted with PEG-HSA presented a reduced load to the heart, increased contractile function, and lowered the energy consumed per unit volume compared to D×70. Our results emphasize the importance of heart function as a parameter to be included in the evaluation changes induced by new PEs.
Keywords: cardiac performance, hemodilution, resuscitation from hemorrhagic shock, conductance catheter
INTRODUCTION
Plasma expanders (PEs) are used to maintain and/or restore blood volume. PEs are advantageous compared to blood transfusion due to the absence of immunologic reactions, longer shelf-life, cost-effectiveness and reduced risk of infection. Albumin is considered a near optimal resuscitation fluid, since it is a naturally occurring plasma protein, whose unique molecular size, shape and electrical charge ensures its intravascular retention by the capillary wall of most tissues. Albumin is also the most abundant plasma protein, and principally responsible of the colloid osmotic pressure (COP) of plasma. Polyethylene glycol (PEG) conjugation (PEGylation) has recently been introduced to increase the efficacy of proteins as therapeutic agents.[1, 2] PEGylation enhances albumin PE properties by creating a dispersion of molecules with an uniform molecular size held in solution by polar covalent bonds that attract water molecules.[3-5] PEGylated albumin has higher COP than unPEGylated albumin. In addition, the PEGylation process prevents extravasation, extends intravascular retention time, increases plasma COP, and brings extravascular fluid into the circulation.[6-8]
Microcirculation studies using the hamster window chamber model have demonstrated PEGylated albumin (PEG-Alb) potential as PE during extreme hemodilution and fluid resuscitation from severe hemorrhagic shock.[9-12] Given that these studies showed maintenance and recovery of microvascular function, we studied their systemic and cardiac function during acute hemodilution compared to dextrans with high molecular weight and solution viscosity.[13, 14] A miniaturized conductance catheter implantable in the left ventricle (LV) of small experimental animal models made available a technique to study real-time pressure and volume measurements.[15-18] This system provides measurements of cardiac performance independently of load conditions and heart rate (HR). Consequently, we have implemented the miniaturized conductance catheter in our hamster window chamber model, thus acquiring the necessary information to establish the link between microvascular observations and cardiac function.
Using an acute hemodilution protocol, we have studied the effects of PEG-Alb produced by extension arm mediated chemistry to attach PEG chains to the albumin (Figure 1).[13, 14] PEG-Alb produced by extension arm mediated chemical synthesis, requires an additional molecule (2- iminothiolane) between the PEG chain and the albumin, increasing the number of chemical steps and cost. Lately, we have experimented with a direct PEGylation of human serum albumin (HSA), PEG-HSA, where a succinimide functionalized PEG chain is directly reacted on the HSA, simplifying production steps and reducing cost (Figure 1). Moreover, PEG-HSA at 2 g/dL produced by direct PEGylation has similar biophysical properties, COP and viscosity, that PEG-Alb at 4 g/dL produced by extension arm mediated PEGylation. This suggests that the use of PEG-HSA as PE would lead to the introduction of less PEG and HSA into the circulation and reducing cost. The present study was carried out to evaluate the hypothesis that PEG-HSA biophysical properties provide improved heart performance compared to a conventional colloidal PE, explaining the positive results observed at the microcirculation and mimicking the improvements attained with PEG-Alb at high concentration (4 g/dL). This hypothesis is based on the premise that the heart is the pump responsible for the microvascular blood flow and obtains the energy to eject blood from its own myocardial microcirculation, and that PEG-HSA biophysical properties lower blood viscosity in the central circulation while increasing vascular endothelial shear stress and promoting vasodilatation.[19] Therefore, the changes in cardiac function induced by PEs reflect the balance between the peripheral circulation connected to the heart (after-load) and the amount of energy available to eject the blood (contractile state). To complete this objective, we studied cardiac function with PEG-HSA (2 g HSA per dL) and Dextran 70 kDa (6 g/dL B. Braun Medical, Irvine, CA), using two experimental models, moderate hemodilution and resuscitation from hemorrhagic shock. Changes in heart function were established in terms of indices derived from LV pressure-volume measurements.
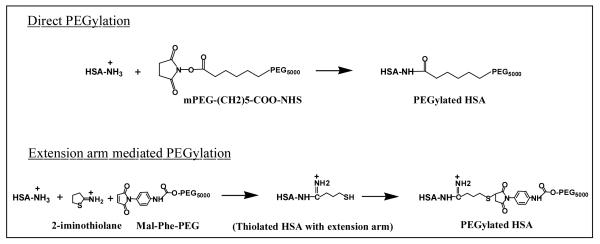
FIGURE 1.
PEGylation of HSA. In Direct PEGylation, succinimide ester of the PEG reagent, mPEG-(CH2)5-COO-NHS reacts directly with protein amino groups by acylation and forms a stable amide bond between the protein and PEG and neutralizes the positive charge of amino group. The 6-carbon alkyl chain of mPEG-(CH2)5-COO-NHS acts as a built-in extension arm and keeps the PEG core away from the protein molecular surface. In Extension arm mediated PEGylation, a thiol reagent, 2-iminothiolane reacts with protein amino groups first and adds an extension arm carrying a thiol group. The added thiol group will be derivatized with a maleimide-PEG reagent. The positive charge of amino group is retained in this method. PEG-HSA used in the present study has been prepared by Direct PEGylation using a PEG-5000 reagent.
METHODS
Human serum albumin pegyation
PEG-HSA was prepared by derivatizing the surface amino groups of HSA by a succinimide ester based PEG reagent; mPEG-(CH2)5-COO-NHS (SUNBRIGHT ME-050HS, MW 5,000, NOF America Corporation). Succinimide esters are highly reactive and modify protein amino groups by acylation (Figure 1). HSA (0.25 mM) was incubated with 10 mM mPEG-(CH2)5-COO-NHS in phosphate buffer saline (PBS) overnight at 4°C. After the incubation, the unreacted PEG reagent was removed by tangential flow filtration using the Minim System (Pall Life Sciences, Ann Arbor, MI) and a 50 kDa membrane (Sartorius mechatronics). After filtration, the product, PEG-HSA was dialyzed to a protein concentration of 2 g of HSA per dL.
Animal preparation
Studies were performed in male Golden Syrian hamsters (Charles River Laboratories; Boston, MA) weighing 60-70g. Animal handling and care followed the NIH Guide for Care and Use of Laboratory Animals. The experimental protocol was approved by the University of California San Diego Institutional Animal Care and Use Committee (protocol S04052). Anesthesia was induced by intraperitoneal (IP) injection of sodium pentobarbital (50 mg/kg) and core body temperature was maintained using a heating pad. Animal preparation included: i) left jugular vein and left femoral artery catheterization, ii) tracheotomy (polyethylene-90 tube to facilitate spontaneous breathing), and iii) left ventricle conductance catheter introduction through the right carotid artery. Animals were placed in the supine position on the heating pad, prior to the experimental procedure. Toe pinching test was performed at least every 5 min, and animals who responded received a small dose of sodium pentobarbital (10-15mg/kg, IP) to prevent animal discomfort.
Inclusion criteria
Animals were suitable for the experiments if: i) mean blood arterial pressure (MAP) > 80 mmHg, ii) heart rate (HR) > 320 beats/minute, and iii) systemic hematocrit (Hct) > 45%.
Systemic parameters
MAP and HR were monitored continuously (MP150, Biopac System Inc.; Santa Barbara, CA), except when: i) blood was sampled, and ii) during blood exchange/withdrawal. The Hct was determined from centrifuged arterial blood samples taken in heparinized capillary tubes.
Blood and plasma expander biophysical properties
Viscosity was measured at a shear rate 160s−1 (Brookfield Engineering Laboratories; Middleboro, MA). Colloidal osmotic pressure (COP) was determined using a membrane colloid osmometer (model 4420, Wescor; Logan, UT).
Cardiac function
The closed chest method was used to study cardiac function. Right common carotid artery was exposed to insert a 1.4F pressure-volume conductance catheter (PV catheter; SPR-839, Millar Instruments; Houston, TX). The pressure-volume catheter was advanced passing through the aortic valve into the LV.[20] At baseline and the end of the experiment, a bolus of 15% hypertonic saline (10 μl) was intravenously injected to determine parallel volume.[21] The pressure and volume signals were continuously acquired (MPVS300, Millar Instruments; Houston, TX and PowerLab 8/30, AD Instruments; Colorado Springs, CO). Left ventricular volume was measured continuously in conductance units (RVU; relative volume unit) and converted to actual blood volume (μl) at the end of the experiment.
Cardiac pressure-volume indices
Cardiac function data were analyzed with PVAN software (Millar Instruments, TX). All cardiac function parameters were averaged from 8-12 cardiac cycles at each time point. End systolic pressure (Pes) was directly measured. Maximum rate of pressure change (dP/dtmax), minimum rate of pressure change (dP/dtmin), maximum filling volume rate (dV/dtmax), cardiac output (CO), stroke work (SW) and stroke volume (SV) were calculated. Systemic vascular resistance (SVR) was calculated as using driving pressure divided flow, SVR = MAP / CO. Systemic vascular hindrance (SVH) reflects the contribution of vascular geometry to SVR, and was calculated dividing SVR by the blood viscosity.
Estimation of left ventricular blood volume
LV blood volume was measured continuously in conductance units (RVU; relative volume unit) and converted to actual blood volume (μl) at the end of the experiment.[20]
Equivalent wall shear stress
Wall shear stress (WSS) was calculated from blood viscosity and wall shear rate (WSR), assuming blood as a Newtonian fluid and using Poiseuille’s law, WSR=4Q/πr3 , where Q is volumetric flow rate or cardiac output (CO) and r is equivalent vessel radius for the entire circulation in front of the heart. Since this radius is hypothetical value, we compared the ratio of radius between PEs, which is also proportional to the ratio of SVH (SVH ∝ L/r4 where L is assumed constant vessel length). Therefore, the ratio of WSR between the two PEs was expressed by
And, the ratio of WSS between the two PEs used in the study was estimated by:
Moderate hemodilution protocol
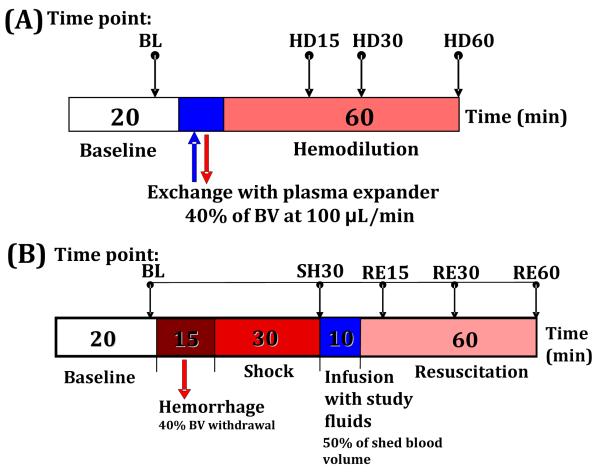
Anesthetized hamsters were exchanged by 40% of estimated blood volume (BV) with the test solutions. Total BV was estimated as 7% of body weight. Test solutions were infused into the left jugular vein catheter at a rate of 100μl/min, and blood was simultaneously withdrawn at the same rate from the left femoral artery catheter using a dual syringe pump (33 syringe pump, Harvard Apparatus, Holliston, MA). Animals were allowed to recover for 10 min after the exchange, and they were followed for 60min after the exchange completion. Blood samples were collected at the end of experiment for subsequent measurement of viscosity, plasma colloid osmotic pressure and blood conductance. Cardiac function indices were measured and analyzed at baseline and 15 min, 30 min and 60 min after hemodilution. Figure 2A illustrates this experimental protocol.
FIGURE 2.
(A) Diagram of moderate isovolemic hemodilution protocol. Baseline characterization was performed before hemodilution. Animals were exchanged for 40% of their blood volume (estimated as 7% of body weight) with dextran 70 or PEG-HSA at a rate of 100μl/min. The exchange period changed depending on the animal’s body weight. (B) Diagram of resuscitation from hemorrhagic shock protocol. Blood volume was withdrawn for 40% of blood volume. 30 minutes after the hemorrhage, they were resuscitated with test plasma expanders. The volume infused was equal to 50% of shed blood volume. RBC, red blood cell, Hct; hematocrit, BL; baseline, HD; hemodilution, SH; shock, RE; resuscitation.
Hemorrhagic shock resuscitation protocol
Anesthetized hamsters were hemorrhaged by withdrawing 40% of the animal’s BV (estimated as 7% of body weight) via the femoral artery catheter within 15 min. The hypovolemic shock condition was maintained for 30 minutes. Resuscitation was implemented by infusion of 50% of the shed BV (20% of BV) of test solutions via jugular vein catheter within 10 min. Only fifty percent of shed volume was used during the volume resuscitation, since auto-transfusion (fluid from extravascular space moves into intravascular space) restores about half of the shed volume during the shock period. Animals were allowed to recover for 10 min after the hemorrhagic shock and after the resuscitation before data collection. The monitored parameters were recorded and analyzed at before hemorrhage (baseline), 30 min after hemorrhage (shock), and 15 min, 30 min and 60 min after volume resuscitation, as schematically shown in Figure 2B.
Test solutions
The PEs evaluated were: D×70, 6% dextran 70 kDa (B. Braun Medical, Irvine, CA); and PEG-HSA, 2 g/dL. Table 1 presents physical properties of test solutions.
TABLE 1. Physical properties of test solutions.
| Solution | Type | Viscosity# (cP) |
COP (mmHg) |
Composition |
|---|---|---|---|---|
| PEG-HSA | LVPE | 2.2 | 58 | PEG+ 2 g/dl HSA |
| Dextran 70 kDa | MVPE | 2.9 | 52 | 6% D×70 + 0.9% NaCl |
Shear rate of 160 s−1 at 37°C.
Experimental groups
In each experimental model and prior baseline assessment, the animals were randomly assigned into an experimental groups based on the PE. Hemodilution model: i) D×70, animals hemodiluted with dextran; and ii) PEG-HSA, animals hemodiluted with PEG-HSA. Hemorrhagic shock resuscitation model: i) D×70, animals resuscitated with dextran, and ii) PEG-HSA, animals resuscitated with PEG-HSA.
Statistical analysis
Results are presented as mean ± standard deviation. The values are presented relative to the baseline in the moderate hemodilution model and relative to shock 30 min (time point prior to resuscitation). A ratio of 1.0 signifies no change from the baseline or shock 30 min, whereas lower or higher ratios are indicative of changes proportionally lower or higher than the baseline or shock 30 min. The Grubbs’ method was used to assess closeness for all measured parameters values at baseline. This method quantifies how far each parameter value is from the other values obtained, computing a P value supposing that all the values were really sampled from a Gaussian population. Data between time points in the same group were analyzed using analysis of variance for repeated measures (ANOVA) and followed by post hoc analyses with the Dunnett’s multiple comparison tests when appropriate. An unpaired t-test with two-tailed comparison was performed between groups at the time point of interest. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, San Diego, CA). Results were considered statistically significant if P < 0.05.
RESULTS
Moderate hemodilution
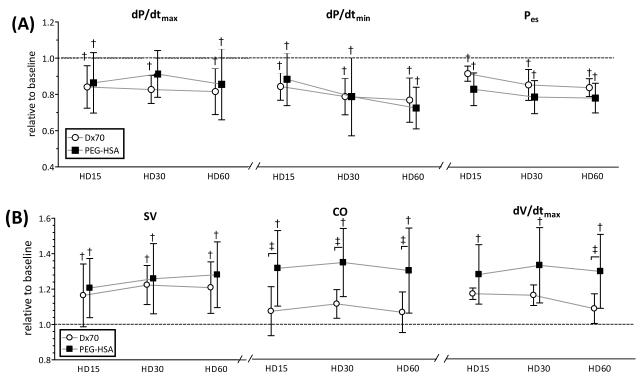
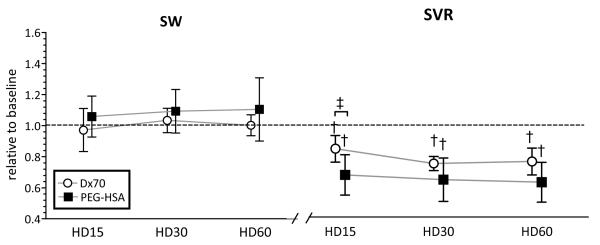
Twelve animals were included in the hemodilution study, D×70 (n=6) and PEG-HSA (n=6). All animals passed the Grubbs’ test ensuring that all the measured parameter values at baseline were within a similar population. Summarized blood rheological properties at 60 min after hemodilution are presented in Table 2. After hemodilution, both groups presented a statistical decrease in Hct and blood viscosity (P<0.05). However, plasma viscosities after hemodilution with PEG-HSA and D×70 increased to 111% and 121% of the baseline (P<0.05), respectively. MAP was statistically decreased after hemodilution with PEG-HSA and D×70 compared to baseline (P<0.05). Cardiac function indices after hemodilution relative to the baseline are shown in Figure 3 and Figure 4 at several time points (15min, 30min and 60min after hemodilution). LV pressure parameters (e.g. Pes, dP/dtmax and dP/dtmin) after hemodilution with PEG-HSA and D×70 statistically decreased compared to baseline (P<0.05). CO after hemodilution with PEG-HSA statistically increased compared to baseline and to hemodilution with D×70. CO after hemodilution with D×70 was not different from baseline. dV/dtmax after hemodilution with PEG-HSA statistically increased compared to baseline and D×70 (P<0.05). SVR after hemodilution with PEG-HSA was statistically lower compared to hemodilution with D×70. The mean equivalent WSS after hemodilution with PEG-HSA was statistically higher compared to hemodiluted with D×70.
TABLE 2. Rheological properties at 60 min after hemodilution.
| 60 min after hemodilution |
Hct (%) |
Blood viscosity# (cP) |
Plasma viscosity# (cP) |
Plasma COP (mmHg) |
|---|---|---|---|---|
| Blood (Baseline) | 52±1 | 4.71±0.62 | 1.05±0.04 | 16±2 |
| D×70 | 28±2* | 3.09±0.09* | 1.27±0.03* | 16±1 |
| PEG-HSA | 27±2* | 2.84±0.24* | 1.17±0.03* | 18±2 |
Shear rate of 160 s−1 at 37°C.
P<0.05 compared to baseline
FIGURE 3.
Left ventricular cardiac function indices derived by PV conductance catheter at each time point after hemodilution. (A) indices related to left ventricular pressure and (B) indices related to left ventricular volume. Broken lines represent the values at baseline. Values are presented as means ± SD. †, P<0.05 compared with baseline; ‡, P<0.05 between groups. dP/dtmax; maximum rate of pressure change, dP/dtmin; minimum rate of pressure change, Pes; end systolic pressure, SV; stroke volume, CO; cardiac output, dV/dtmax; maximum filling volume rate. Data was collected at marked time points and artificially spaced for clarity in the plot.
FIGURE 4.
Left ventricular stroke work (SW) and calculated systemic vascular resistance (SVR) from left ventricle pressure and volume, heart rate and blood pressure at each time point after hemodilution. Broken lines represent the values at baseline. Values are presented as means ± SD. †, P<0.05 compared with baseline; ‡, P<0.05 between groups. Data was collected at marked time points and artificially spaced for clarity in the plot.
Resuscitation from hemorrhagic shock
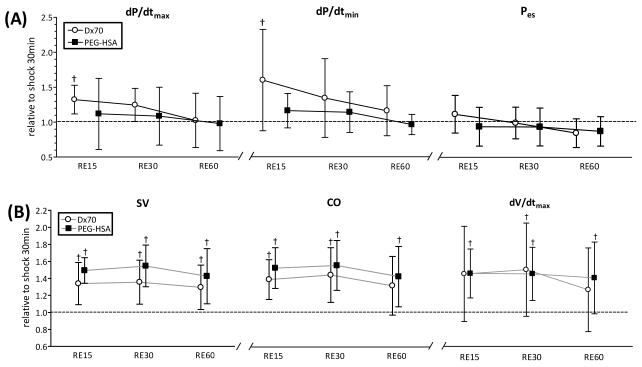
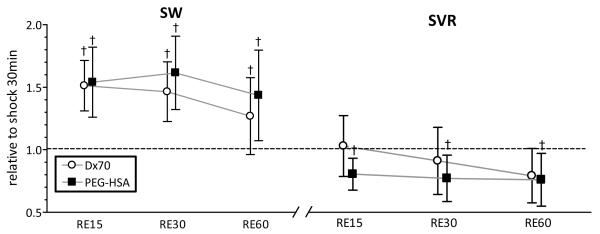
Ten animals were included in the hemorrhagic shock-resuscitation study, D×70 (n=5) and PEG-HSA (n=5). All animals included in the study passed the Grubbs’ test for parameter values at baseline, ensuring that all the measured parameter values at baseline were within a similar population. Table 3 summarizes blood rheological properties at 60 min after resuscitation. Plasma viscosity after resuscitation with PEG-HSA and D×70 statistically increased compared to baseline (P<0.05). Hemorrhage statistically decreased MAP in both groups compared to baseline (P<0.05). Resuscitation with PEG-HSA and D×70 statistically increased MAP compared to shock, however in both groups pressure decreased during the resuscitation phase to the shock level. Cardiac function indices after resuscitation relative to shock (SH30) are presented in Figure 5 and Figure 6. CO, SW, SV and dV/dtmax after resuscitation with PEG-HSA and D×70 statistically increased compared to shock (P<0.05). Additionally, SW after resuscitation with PEG-HSA statistically increased compared to shock, whereas resuscitation with D×70 did not recover SW from the shock level. SVR after resuscitation with PEG-HSA statistically decreased compared to shock, whereas SVR after resuscitation with D×70 remained at the shock level. The mean equivalent WSS after resuscitation with PEG-HAS was lower compared to resuscitation with D×70, although not statistically different.
TABLE 3. Rheological properties at 60 min after resuscitation.
| 60 min after resuscitation |
Hct (%) |
Blood viscosity# (cP) |
Plasma viscosity# (cP) |
|---|---|---|---|
| Blood (Baseline) | 52±1 | 4.71±0.62 | 1.05±0.04 |
| Blood (Shock) | 30±4 | 2.58±0.23 | - |
| D×70 | 22±3‡ | 2.88±0.61 | 1.32±0.03 |
| PEG-HSA | 24±3‡ | 2.39±0.31 | 1.13±0.06 |
Shear rate of 160 s−1 at 37°C.
P<0.05 compared to shock.
FIGURE 5.
Left ventricular cardiac function indices derived by PV conductance catheter at each time point after resuscitation. (A) indices related to left ventricular pressure and (B) indices related to left ventricular volume Broken lines represent the values at shock (30 min after hemorrhage, SH30). Values are presented as means ± SD. †, P<0.05 compared to hypovolemic shock (SH30); ‡, P<0.05 between groups. dP/dtmax; maximum rate of pressure change, dP/dtmin; minimum rate of pressure change, Pes; end systolic pressure, SV; stroke volume, CO; cardiac output, dV/dtmax; maximum filling volume rate. Data was collected at marked time points and artificially spaced for clarity in the plot.
FIGURE 6.
Left ventricular stroke work (SW) and calculated systemic vascular resistance (SVR) from left ventricle pressure and volume, heart rate and blood pressure at each time point after resuscitation. Broken lines represent the values at shock (30 min after hemorrhage, SH30). Values are presented as means ± SD. †, P<0.05 compared to hypovolemic shock (SH30). Data was collected at marked time points and artificially spaced for clarity in the plot.
DISCUSSION
This study demonstrated that the biophysical properties of PEG conjugated human serum albumin (PEG-HSA) produce beneficial effects on cardiac function compared to dextran 70 kDa (D×70), a conventional PE, when used during hemodilution and resuscitation from hemorrhagic shock. In both experimental models, PEG-HSA increased CO and SW compared to D×70 over the observation time. The responses were determined by the interaction between LV preload, myocardium contractile state and the after-load. Therefore to optimize the work performed by the heart with a PE, the biophysical properties of the PE need to systemically reduce vascular resistance and maintain pressure and at microvascular level and sustain perfusion and oxygenation, since the oxygen carrying capacity is limited by the dilution induced by the PE. Mechanistically, the benefits observed with PEG-HSA during hemodilution and hemorrhagic shock resuscitation, are explained by the decrease in peripheral vascular resistance and an improvement of perfusion and oxygenation to the heart. Since there was no difference in MAP between PEG-HSA and D×70, and CO increased only with PEG-HSA after hemodilution, we calculated a decrease in SVR with PEG-HSA compared to D×70. The combination of decrease in SVR and higher dP/dtmax when PEG-HSA is used as a PE compared to D×70, indicates a reduction in the forces that oppose left ventricular contraction. Consequently, PEG-HSA decreased the left ventricular ejection impedance explaining the increased stroke volume together with the decrease in SVR.
Blood transfusion generally treats both hypovolemia and insufficient oxygen delivery. However, while volume and oxygen transport capacity are invariably restored by blood transfusion, it brings additional immune, inflammatory and biochemical problems, which has been clinically observed and associated with increased morbidity and mortality.[22] In an anemic condition, CO increases due to reduced blood viscosity and maintenance of tissue oxygenation and organ function at lower blood oxygen-carrying capacity.[23] Our experimental study shows that PEG-HSA increases CO, thus increasing oxygen delivery and compensating for the reduced oxygen carrying capacity. Similar increases on cardiac indexes after hemodilution with PEG-HSA and D×70 were obtained before using a modified thermodilution technique, however this technique did not allow us to establish a functional reason for the enhanced cardiac function with PEG-HSA.[10] Ultimately, the increase in CO benefits from the remaining erythrocytes, by providing oxygen and sustaining tissue metabolic needs, in addition to facilitating the wash out of metabolites and cellular breakdown byproducts that have accumulated during ischemia.
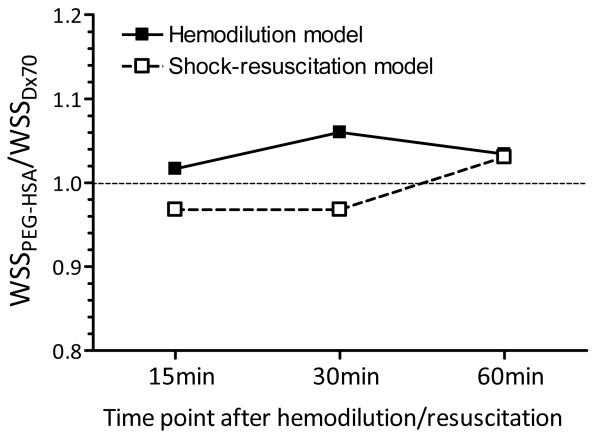
Blood flow or blood perfusion per se has additional effects, especially the mechanical interaction between blood and the vascular endothelium. Mechanistically, the endothelium acts as a signal transducer, sensing changes in intraluminal blood flow. This mechanical signal regulates endothelial nitric oxide (NO) synthase, which generates NO in blood vessels and is involved with regulating vascular tone by inhibiting smooth muscle contraction and platelet aggregation.[9, 24] Endothelial NO synthase permits the rapid adjustments of NO release in response to changes in blood flow and shear stress. In our study, we speculate that the increase in blood flow due to higher CO after hemodilution and resuscitation with PEG-HSA increases shear stress on vascular endothelial cells and stimulates the generation of NO producing vascular relaxation and explaining the decrease in SVR. Mechanistically, we have shown that the dilution of blood with PEG-HSA changes wall shear stress along the circulatory network, explaining the state of supra perfusion. Thus blood diluted with PEG-HSA has a lower blood viscosity in high shear rate zones of the circulation, like the heart and major vessels; while apparent viscosity and shear stress increases in the microcirculation promoting endothelium NO production and vasorelaxation.[19] Alternatively, the reduction in Hct associated with the intrinsic blood dilution decreases NO scavenging capacity of the blood, as well as affect the thickness of the red cell exclusion free layer in the lumen of the vessel.[25] Alternatively, the reduction in Hct associated with the intrinsic blood dilution in both experimental models, could have reduced the NO scavenging capacity of the blood, as well as affect the thickness of the red cell exclusion free layer in the lumen of the vessel.[25] Our calculation of equivalent wall shear stress in both experimental models (Figure 7) indicates that PEG-HSA produced higher estimated wall shear rate and wall shear stress compared to D×70, mostly as a consequence of the higher CO attained with PEG-HSA. In microvascular studies with PEG-HSA, we had calculated local higher wall shear stress in microvessels (40 to 80 μm) and observed the resulting vasodilation compared to other PEs.[10, 26] Therefore, PEG-HSA can be considered a supra-plasma expander, which enhances blood flow at moderate plasma concentrations (0.5 - 1 g/dl), which also increases vascular shear stress at comparatively low viscosity.
Figure 7.
The ratio of estimated equivalent wall shear stress (WSS) between PEG-HSA and D×70 at each time point after hemodilution or resuscitation.
In general, the myocardial oxygen consumption per beat is determined by the ventricular pressure volume area (PVA), which represents the total mechanical energy generated by ventricular contraction. Previous studies have shown that the LV PVA is linearly related to myocardial oxygen consumption per beat at a given heart rate with a stable inotropic background.[27] Therefore, in our study in both experimental models PEG-HSA showed increased PVA and increased SW compared to D×70, which indicates that PEG-HSA provided sufficient oxygen to the heart to allow the myocardium to exert more work. Microvascular studies using the hamster window model during extreme hemodilution (11% Hct) with PEG-HSA have shown an increase in oxygen delivery and extraction compared to other PEs.[10, 11, 28] Therefore, we postulate that the supra-perfusion properties of PEG-HSA have beneficial effects on cardiac mechano-energetic responses, as well on microvascular responses compared to D×70.
To interpret the experimental result presented here, it is necessary to understand that each animal specie and anesthetic, affect the outcome of the study.[29] Our studies were carried out using an acute hemodilution and fluid resuscitation from hemorrhage of 40% of BV in anesthetized animals, and found considerable differences in cardiac function with different PEG-HSA and D×70. Therefore, the anesthetized state of the animals did not seem to limit the effects produced by the PEs evaluated in the study. Various animal models of heart failure may be more sensitive to the cardio-depressive effects of injectable agents and each animal specie seems to respond differently.[30] Previous investigations in hamsters have shown that pentobarbital anesthesia produces stable cardiac, lung, and airways mechanics, which allows for long-term studies.[31] In our study, the dose of sodium pentobarbital during surgical preparation was five times higher than during the experiment. Retrospective analysis of the outcomes and anesthesia indicates no correlation between the anesthetic doses and the hemodynamics responses. Therefore, we can conclude that the cardio-depressive effects of pentobarbital, if any, did not influence the results, and that the differences in cardiac function measured, were caused by the PE used in the studies.
PEG-HSA used in the present study has been prepared by acylation of amino groups of HSA using mPEG-(CH2)5-COO-NHS. This chemistry is distinct from the extension arm mediated PEGylation that has been used earlier to prepare PEG-Alb.[9, 13, 14] In extension arm mediated PEGylation, amino groups of albumin were derivatized with 2-iminothiolane that adds thiol groups with an extension arm on amines (Figure 1). The added thiols were reacted with a maleimide-PEG reagent (Mal-Phe-PEG). In the present protocol of acylation, the PEG reagent directly reacts with amino groups of proteins without using any additonal reagent (Direct PEGylation). Direct PEGylation enhanced the molecular size, hydrodynamic volume, COP and viscosity of HSA. PEG-HSA prepared by direct PEGylation exhibited super-plasma expander capability and restored the systemic/microvascular parameters in experimental models. Therefore, the biophysical properties endowed to PEG-HSA by PEGylation are responsible for the super-plasma expander activity of PEG-HSA. The chemistry of PEGylation and addition/length of extension arm on albumin amino groups do not seem to influence the efficiency of PEG-HSA to act as a super-plasma expander, in terms of cardiac function compared to our previous results.[13, 14] PEGylation of proteins by mPEG-(CH2)5-COO-NHS may be more cost effective than by extension arm mediated PEGylation since it uses only the PEG reagent and no additional reagents are required. Moreover, the extension arm mediated PEGylation needs an additional step of blocking free thiols that were not derivatized by maleimide-PEG to prevent crosslinking. The long 6-carbon alkyl chain on mPEG-(CH2)5-COO-NHS not only increases the stability of the succinimide ester but also reduces steric hindrance for the bulky PEG reagent to access protein amino groups. In addition, the long alkyl chain adds spacing between the protein molecular surface and the PEG entity as in extension arm mediated PEGylation. Thus, direct PEGylation by mPEG-(CH2)5-COO-NHS is a simple and effective method to make PEG-HSA with superior PE properties.
In conclusion, PEG-HSA is a novel PE, that when used during hemodilution and shock-resuscitation shows positive effects on cardiac and vascular function compared to dextran 70 kDa. The principal benefit observed with PEG-HSA was the increase in CO, resulting from an increase in SV, which is determined by the myocardium contractile state. Since the LV and the arterial system are connected elastic chambers, when blood is diluted with PEG-HSA, the energy transfer from the LV to the arterial system is facilitated by PEG-HSA’s biophysical properties. Thus, PEG-HSA decreased the ejection impedance, basically improving the coupling between ventricular function and arterial load conditions. In addition to the coupling between heart and arteries, PEG-HSA increased the energy supplied and the SW obtained with the supplied energy, also called mechanical efficiency, which is defined as the ratio of SW to PVA. Our results show the benefits in cardiac function with PEG-HSA and bring heart function as an important parameter to be included in the evaluation and analysis of the effects induced by PEs biophysical properties.
ACKNOWLEDGEMENTS
The authors thank Cynthia Walser for the surgical preparation of the animals. This work was supported by Program project P01-HL071064 and grants R01-HL62354. No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54(4):459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- [2].Yoh Kodera AM, Misao Hiroto, Hiroyuki Nishimura, Asako Ishii. Tomoo Ueno and Yuji Inada: Pegylation of proteins and bioactive substances for medical and technical applications. Progress in Polymer Science. 1998;23(7):1233–71. [Google Scholar]
- [3].Sahu RK, Nacharaju P, Manjula BN, et al. Induced plasma expander-like properties as a function of PEG-chains on extension arm facilitated PEGylation of albumin: “mushroom to brush-like” conformational transition of the PEG-albumin conjugate. Artif Cells Blood Substit Immobil Biotechnol. 2009;37(6):245–56. doi: 10.3109/10731190903356438. [DOI] [PubMed] [Google Scholar]
- [4].Li D, Hu T, Manjula BN, et al. Extension arm facilitated pegylation of alphaalpha-hemoglobin with modifications targeted exclusively to amino groups: functional and structural advantages of free Cys-93(beta) in the PEG-Hb adduct. Bioconjug Chem. 2009;20(11):2062–70. doi: 10.1021/bc900170e. [DOI] [PubMed] [Google Scholar]
- [5].Meng F, Manjula BN, Smith PK, et al. PEGylation of human serum albumin: reaction of PEG-phenyl-isothiocyanate with protein. Bioconjug Chem. 2008;19(7):1352–60. doi: 10.1021/bc7003878. [DOI] [PubMed] [Google Scholar]
- [6].Manjula BN, Tsai A, Upadhya R, et al. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem. 2003;14(2):464–72. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- [7].Li D, Manjula BN, Acharya AS. Extension arm facilitated PEGylation of hemoglobin: correlation of the properties with the extent of PEGylation. Protein J. 2006;25(4):263–74. doi: 10.1007/s10930-006-9010-y. [DOI] [PubMed] [Google Scholar]
- [8].Hu T, Prabhakaran M, Acharya SA, et al. Influence of the chemistry of conjugation of poly(ethylene glycol) to Hb on the oxygen-binding and solution properties of the PEG-Hb conjugate. Biochem J. 2005;392(Pt 3):555–64. doi: 10.1042/BJ20050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cabrales P, Nacharaju P, Manjula BN, et al. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24(1):66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- [10].Cabrales P, Tsai AG, Winslow RM, et al. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol. 2005;289(6):H2392–400. doi: 10.1152/ajpheart.00225.2005. [DOI] [PubMed] [Google Scholar]
- [11].Martini J, Cabrales P, K A, et al. Survival time in severe hemorrhagic shock after perioperative hemodilution is longer with PEG-conjugated human serum albumin than with HES 130/0.4: a microvascular perspective. Crit Care. 2008;12(2):R54. doi: 10.1186/cc6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Winslow RM, Lohman J, Malavalli A, et al. Comparison of PEG-modified albumin and hemoglobin in extreme hemodilution in the rat. J Appl Physiol. 2004;97(4):1527–34. doi: 10.1152/japplphysiol.00404.2004. [DOI] [PubMed] [Google Scholar]
- [13].Chatpun S, Cabrales P. Effects on cardiac function of a novel low viscosity plasma expander based on polyethylene glycol conjugated albumin. Minerva Anestesiol. 2011;77(7):704–14. [PubMed] [Google Scholar]
- [14].Chatpun S, Cabrales P. Effects of plasma viscosity modulation on cardiac function during moderate hemodilution. Asian J Transfus Sci. 2010;4(2):102–8. doi: 10.4103/0973-6247.67034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Georgakopoulos D, Mitzner WA, Chen CH, et al. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274(4 Pt 2):H1416–22. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- [16].Pacher P, Mabley JG, Liaudet L, et al. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287(5):H2132–7. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Westermann D, Mersmann J, Melchior A, et al. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008;117(10):1269–76. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- [18].Nishio R, Sasayama S, Matsumori A. Left ventricular pressure-volume relationship in a murine model of congestive heart failure due to acute viral myocarditis. J Am Coll Cardiol. 2002;40(8):1506–14. doi: 10.1016/s0735-1097(02)02166-6. [DOI] [PubMed] [Google Scholar]
- [19].Sriram K, Tsai AG, Cabrales P, et al. PEG-Albumin supra plasma expansion is due to increased vessel wall shear stress induced by blood viscosity shear thinning. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.01090.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pacher P, Nagayama T, Mukhopadhyay P, et al. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–34. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baan J, van der Velde ET, de Bruin HG, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70(5):812–23. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- [22].Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nat Med. 2010;16(4):381–2. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mirhashemi S, Ertefai S, Messmer K, et al. Model analysis of the enhancement of tissue oxygenation by hemodilution due to increased microvascular flow velocity. Microvasc Res. 1987;34(3):290–301. doi: 10.1016/0026-2862(87)90062-8. [DOI] [PubMed] [Google Scholar]
- [24].Kimura A, Okumura K, Mokuno S, et al. Higher viscosity participates in the regulation of coronary flow via nitric oxide and indomethacin-sensitive contracting factor. Can J Physiol Pharmacol. 2004;82(12):1096–102. doi: 10.1139/y04-127. [DOI] [PubMed] [Google Scholar]
- [25].Hightower CM, Yalcin O, Vazquez BY, et al. Effect of plasma expander viscosity on the cell free layer. Biorheology. 2011;48(2):115–25. doi: 10.3233/BIR-2011-0586. [DOI] [PubMed] [Google Scholar]
- [26].Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287(1):H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- [27].Suga H. Total mechanical energy of a ventricle model and cardiac oxygen consumption. Am J Physiol. 1979;236(3):H498–505. doi: 10.1152/ajpheart.1979.236.3.H498. [DOI] [PubMed] [Google Scholar]
- [28].Cabrales P, Tsai AG, Ananda K, et al. Volume resuscitation from hemorrhagic shock with albumin and hexaPEGylated human serum albumin. Resuscitation. 2008;79(1):139–46. doi: 10.1016/j.resuscitation.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawahara Y, Tanonaka K, Daicho T, et al. Preferable anesthetic conditions for echocardiographic determination of murine cardiac function. J Pharmacol Sci. 2005;99(1):95–104. doi: 10.1254/jphs.fp0050343. [DOI] [PubMed] [Google Scholar]
- [30].Kitagawa H, Kamataki T, Yoshida S. Studies on drug metabolism. IV. Effects of high dose administration of pentobarbital and phenylbutazone on the plasma biologic half lives in various species. Chem Pharm Bull (Tokyo) 1968;16(12):2320–3. doi: 10.1248/cpb.16.2320. [DOI] [PubMed] [Google Scholar]
- [31].Skornik WA, Brain JD. Breathing and lung mechanics in hamsters: effect of pentobarbital anesthesia. J Appl Physiol. 1990;68(6):2536–41. doi: 10.1152/jappl.1990.68.6.2536. [DOI] [PubMed] [Google Scholar]