Abstract
Retinoids are essential in the proper establishment and maintenance of immunity. Although retinoids are implicated in immune related processes, their role in immune cell adhesion has not been well established. In this study, the effect of 9-cis-retinoic acid (9-cis-RA) on human hematopoietic cell adhesion was investigated. 9-cis-RA treatment specifically induced cell adhesion of the human immune cell lines HuT-78, NB4, RPMI 8866, and U937. Due to the prominent role of integrin receptors in mediating immune cell adhesion, we sought to evaluate if cell adhesion was integrin-dependent. By employing a variety of integrin antagonist including function-blocking antibodies and EDTA, we establish that 9-cis-RA prompts immune cell adhesion through established integrin receptors in addition to a novel integrin-independent process. The novel integrin-independent adhesion required the presence of retinoid and was attenuated by treatment with synthetic corticosteroids. Finally, we demonstrate that 9-cis-RA treatment of primary murine B-cells induces ex vivo adhesion that persists in the absence of integrin function. Our study is the first to demonstrate that 9-cis-retinoic acid influences immune cell adhesion through at least two functionally distinct mechanisms.
Keywords: vitamin A, lymphocyte, monocyte, VCAM, disintegrin-like
1. Introduction
The anti-infective attributes and necessity of vitamin A for proper immunity has been reported since the early 1900s, yet the exact role of vitamin A in the establishment and maintenance of immunity remains poorly defined. Retinoids, biologically active oxidative metabolites of vitamin A, profoundly impact immune function by regulating differentiation, proliferation, antibody production, and transmigration of leukocytes. Retinoids and parental vitamin A are obligatory in the formation and population of secondary lymphoid structures within gut mucosa. Current models posit retinoids govern the locale of immune cell trafficking and homing by prompting the expression of defined adhesion or homing molecules such as integrin and chemokine receptors.
Only within the past three decades have the molecular mechanisms by which retinoids elicit their effects been elucidated. Ultimately, retinoids exert transcriptional regulation through the activation of isoform specific transcription factors. The retinoic acid receptor (RAR) and retinoid X receptor (RXR) family of nuclear receptors homo or heterodimerize upon retinoid binding. Nuclear receptor activation by two major bioactive retinoids, all-trans-retinoic acid (at-RA) and 9-cis-retinoic acid (9-cis-RA), facilitate recognition of retinoic acid response elements (RARE) in the promoter of target genes. The cellular responses generated by RXR family members are more pleiotropic as compared to RAR activity, a feature attributed to the promiscuous pairing nature of RXRs. Unlike the RAR family members, RXRs comprise heterodimers that include but are not limited to: thyroid hormone, vitamin D receptors, pregname X receptor, and peroxisome proliferator-activated receptor gamma. Classically, 9-cis-RA can bind and subsequently activate RAR or RXR members while at-RA is an RAR ligand. This difference in nuclear receptor specificity between at-RA and 9-cis-RA may account for distinct efficacies, physiological outcomes, or sensitivity to particular retinoid isomers.
Cell adhesion is paramount in mounting an effective immune response and is an integral component of the immune cell trafficking paradigm. Integrins are a well-characterized superfamily of cell-cell and cell-matrix receptors comprised of α and β noncovalently linked subunits. The α4/α9 integrin subclass that includes α4β1 and α4β7 exhibit an expression pattern, ligand specificity, and functionality that encompass various aspects of immunity, including immune cell adhesion. Engagement of vascular cell adhesion molecule-1 (VCAM-1) by the integrin α4β1, allows cells to firmly arrest to the intravascular wall mediating diapedesis and subsequent infiltration to the infected interstitium. Similarly, α4β7 recognition of mucosal address in cell adhesion molecule-1 (MAdCAM-1) facilitates proper homing of lymphocyte subtypes to various secondary lymphoid sites including those that comprise gut associated lymphoid tissue (GALT). The role of retinoids in influencing integrin function has been evaluated in various cell types, including immune lineages. For example, at-RA can increase or decrease expression of integrin receptors including α4β1 and α4β7. Interestingly, at-RA can also influence the expression of well-established integrin ligands such as VCAM-1, fibronectin, as well as the novel integrin ligand class, ADAMs (a disintegrin and metalloprotease). Studies evaluating the potential involvement of retinoids in cellular adhesion have been predominantly limited to classical integrin-counter receptor interactions. However, we have recently shown that at-RA can also prompt integrin-independent cell adhesion in a particular human B-cell line.
The contribution and influence of at-RA to a variety of immune processes including leukocyte differentiation, proliferation, antibody secretion, and adhesion is addressed in the literature, but there is a paucity of information evaluating if 9-cis-RA or other isomers contribute to immune processes. Given the promiscuous nature of RXRs and the ability of 9-cis-RA to activate both RARs and RXRs, 9-cis-RA may serves as an immuno-modulating compound that influences a spectrum of immune events including cell adhesion. Although we have previously characterized a novel integrin-independent adhesion event prompted by at-RA in the human RPMI 8866 B-cell line, here we present evidence for the first time that exposure to 9-cis-RA induces human immune cell adhesion through both classic integrin-mediated and novel integrin-independent processes by extensively evaluating a spectrum of human hematopoietic cell lineages. Of note, we report that 9-cis-RA exposure of primary B-cells biases adhesion to occur independent of integrin function. We also provide the first insight into how retinoic acid induces immune cell adhesion by mitigating adhesion with either retinoid removal or corticosteroid administration. These data collectively demonstrate that 9-cis-RA induces immune cell adhesion through at least two functionally distinct modes and comprise an unexplored aspect of how retinoids plausibly contribute to immunity.
2. Materials and Methods
2.1 Reagents and Chemicals
Chinese hamster ovary cell derived recombinant human VCAM-1 was purchased from R&D systems (Minneapolis, MN). The fibronectin CS-1 peptide was obtained from American Peptide Company (Sunnyvale, CA). The function-blocking anti-integrin monoclonal antibodies (mAb) P1H4 (anti-α4), 6S6 (anti-β1), YFC118.3 (anti-β2)were purchased from Chemicon International (Temecula, CA). The anti-β7 mAb FIB27 was obtained from BD Biosciences (San Jose, CA). The secondary goat anti-human IgG (Fc-specific) FITC-conjugated antibody was commercially purchased from Caltag/Invitrogen (Carlsbad, CA). The colorimetric phosphatase substrate, retinoids, and various vitamins were all purchased from Sigma (St. Louis, MO). Retinoids and all other vitamins were dissolved at the desired concentration in ethanol and handled accordingly to minimize light exposure. The synthetic corticosteroids 6 α-methylprednisolone 21-hemi succinate sodium salt (MPSS) and dexamethasone (Dex) were obtained from Sigma and dissolved at the desired concentration in water or ethanol, respectively.
2.2 Cell Culture
The mature human B-cell line RPMI 8866 was the kind gift of Dr. John Wilkins (University of Manitoba, Manitoba, Canada). The MOLT-4 and NB4 lines were the generous gift of Dr. Ted Bertrand (East Carolina University, Greenville, NC). The established human lines:\Daudi, HL-60, HuT-78, Jurkat, K562, Raji, RAMOS, and U937 were obtained from ATCC (Manassas, VA). All hematopoietic cell lines were maintained in a 5% CO2 environment with RPMI 1640 media supplemented with 10 mM HEPES, 1mM sodium pyruvate, 10% (v/v) fetal bovine serum, 1% L-glutamine and 1% penicillin-streptomycin.
2.3 Isolation and culture of murine primary B-cells
Primary murine B220+ B cells were isolated from C57BL/6 mice as previously described. Briefly, spleens from mice were used to generate a single cells suspension. B cells were then isolated from the single cell suspension using negative selection (Miltenyi Biotec). Cell viability and purity were routinely checked, respectively, using trypan blue exclusion and anti-B220 antibodies with flow cytometry. To promote activation, B cells were stimulated with lipopolysaccharide (Sigma).
2.4 Recombinant Fc-fusion proteins
Methods for the production and purification of Fc-fusion proteins were described previously. Briefly, DNA constructs were generated by PCR overlap extension to anneal the 5′ GP67 insect secretion signal and the 3′ human IgG3 Fc affinity tag onto regions encoding for the entire disintegrin-like domains of human ADAM28 (Thr407-Gly500), ADAM12 (Asn414-Gly519), or the first two ecto domains of VCAM-1 (Gln23-Leu217). ADAM disintegrin-like domain constructs terminated within 5 residues of the disintegrin-like/cysteine rich domain boundary. The IgG3 Fc fusion-tag consisted of residues C-terminal of Ala130 to exclude the hinge region thereby preventing dimerization of the purified ligands. PCR products were cloned into the TOPO pIB/V5-His vector (Invitrogen, Carlsbad, CA). After sequence verification, High 5 insect cells were transfected with DNA constructs and selected for expression by eukaryotic blasticidin resistance. Conditioned media was harvested, polyethylene glycol concentrated, and applied to a protein-G affinity resin. After extensive washing, bound proteins were eluted with 100 mM citric acid pH 3.0 into a reservoir of 1 M Tris at pH 9.0 for immediate neutralization of the eluant. Recombinant ligands were evaluated for purity and consistency by SDS-PAGE under reducing and nonreducing conditions (supplementary data).
2.5 Static cell adhesion assay
Assays were adapted from established techniques. Briefly, recombinant proteins were immobilized at the desired concentration on Immulon-2 HB microtiter wells (Thermo Scientific, Waltham, MA) in a total volume of 100 μl of 0.1 M NaHCO3. For maximal coating, plates were incubated overnight at 4°C. Nonspecific adhesion was minimized by blocking wells with 2% (w/v) bovine serum albumin (BSA) in modified HEPES-Tyrodes buffer (5 mM HEPES, pH 7.4, 150 mM NaCl, 12 mM NaHCO3, 2.6 mM KCl, 0.2 mg/mL BSA, 0.5 mM MgCl2, and 1 mM CaCl2) at room temperature for 30 min. Cells were cultured for the defined times in the presence of the indicated amount of retinoid, vitamin, drug or an equimolar concentration of vehicle (ethanol). The initial time period of 72 hrs was chosen based upon sufficient time for genetic alterations by retinoid action and previous work. For synthetic corticosteroid experiments, cells were cultured for 48 hrs with 0.5 μM of 9-cis-RA for 48 hrs and then co-cultured with specified concentrations of MPSS or Dex for an additional 24 hrs. Before addition to wells, cells were washed twice in HEPES-Tyrodes buffer, manually enumerated, and resuspended to the desired concentration. All cell types analyzed were added to wells (2 × 105/well) in 100 μl HEPES-Tyrodes with or without 1 mM Mn++. When appropriate, inhibitors were added simultaneously to cells at desired concentrations in HEPES-Tyrodes buffer. Function-blocking monoclonal antibodies were used at a final concentration of 5μg/mL and EDTA was used at 5 mM. Cells were incubated with ligands in the presence or absence of inhibitors for 1 hour at 37°C in 5% CO2. After three consecutive washes with HEPES-Tyrodes, wells were analyzed for bound cells by determining the relative cellular acid phosphatase activity within each well. Phosphatase assay buffer (1% v/v Triton X-100, 50 mM sodium acetate at pH 5.0 and 6 mg/mL p-nitrophenyl phosphate) was added to wells, and wells were incubated with the colorimetric substrate for 30 min at 37°C. Color was disclosed by addition of 50 μl/well of 1 N NaOH. Absorbance values were obtained at 405 nm with a plate reader. Specific numbers of adherent cells/well were obtained by correlating experimental absorbance values to a standard curve. Adhesion values obtained with wells coated exclusively with BSA were considered as background values for each experimental condition and were subtracted before reporting final values. Adherent cells/well = adherent cells(recombinant protein) − adherent cells(BSA).
2.6 Flow Cytometry
U937 cells were treated with vehicle or 9-cis-RA for 96 hrs and a total of 2×105 cells/condition were resuspended in HEPES-Tyrode’s buffer. Cells were incubated with ADAM28 Dis-Fc, ADAM12 Dis-Fc or sVCAM-Fc, at the indicated concentrations for 30 min at room temperature in the presence of 5 mM EDTA, or in the presence or absence of CS-1 (500 μg/mL). Following initial binding, cells were fixed in 0.37% formalin/1 × PBS, pH 7.4 for 15 min. Cells were washed twice in FACS buffer (1 × PBS, 2.5% (v/v) fetal calf serum, and 0.02% (v/v) NaN3, pH 7.4) and stained with anti-human IgG-FITC (Invitrogen, Carlsbad, CA) antibody for 15 min. For analysis of mean fluorescence intensity, cells were collected and resuspended in 300 μl of FACS buffer and analyzed using a FACS Calibur flow cytometer and Cell Quest-Pro software.
2.7 Statistical Analyses
All error bars represent stand deviation values. Where appropriate a two-tailed Student’s t-test was employed to determine significant differences utilizing a threshold of 0.01.
3. Results
3.1 9-cis-Retinoic Acid Induces Human B-cell Adhesion to Select Immune Ligands
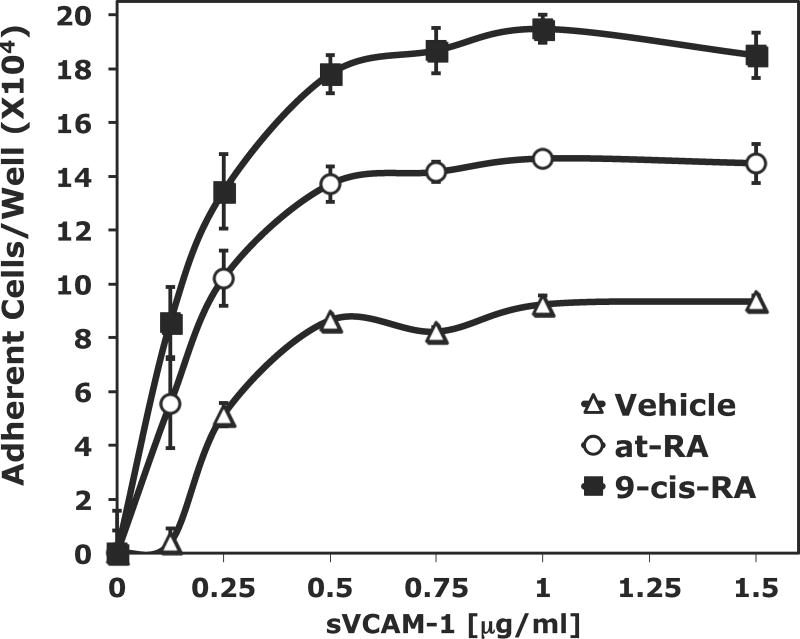
The role of 9-cis-RA in immune cell adhesion remains poorly defined. To determine if 9-cis-RA can prompt immune cell adhesion, we utilized a static cell adhesion assay with well-defined integrin ligands. Our studies employed various recombinant forms of human VCAM-1 due to its specificity for leukocyte integrins and role in diapedesis. First, we assessed adhesion with sVCAM-1, a recombinant protein encompassing the entire ectodomain of human VCAM-1. Additionally, we generated a recombinant Fc fusion protein containing only the first two ectodomains of human VCAM-1 (sVCAM-Fc) that traveled as a highly purified monomer under reducing and nonreducing conditions (supplemental data). As shown in Figure 1A, adhesion to sVCAM-1 obtained with RPMI 8866 cells cultured in the presence of 1 μM 9-cis-RA exceed the levels obtained with un-activated vehicle cells at all ligand concentrations tested. As a positive control, cells were maintained in media containing 1 μM at-RA. At sVCAM-1 concentrations exceeding 0.5 μg/mL, 9-cis-RA exposed cells produced greater than 90 percent adhesion of input cells. In contrast, the same ligand concentration yielded less than 50 percent adhesion of input cells cultured with vehicle alone.
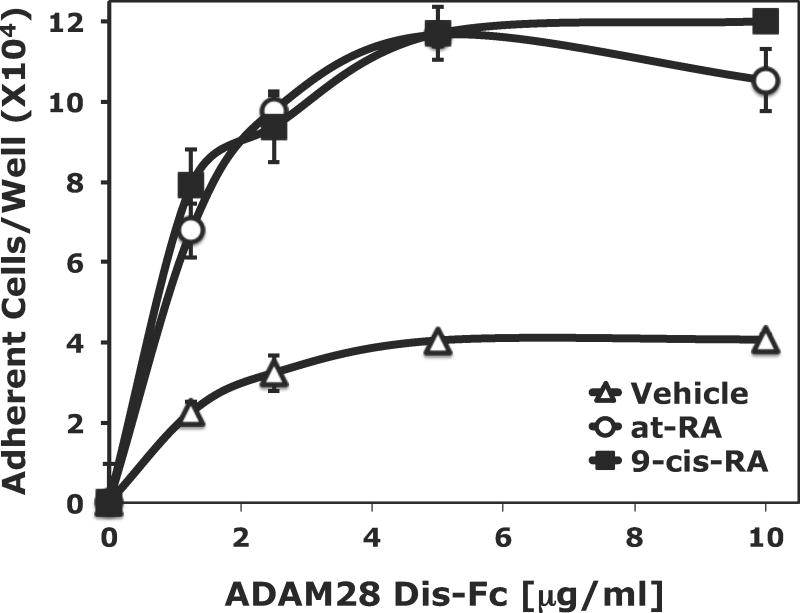
Figure 1.
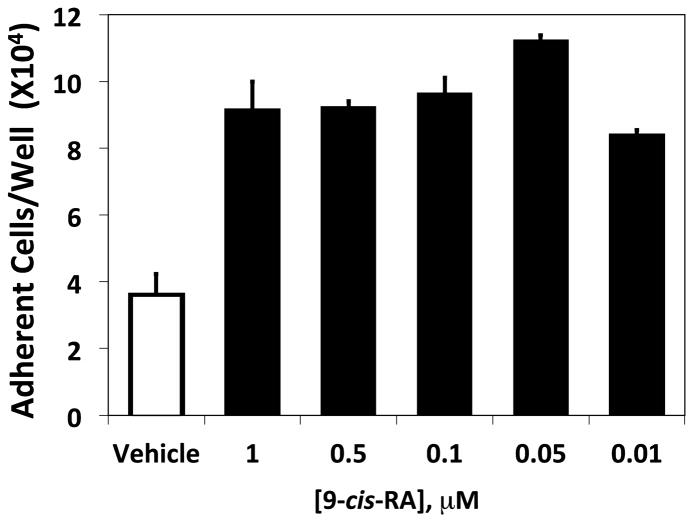
9-cis-RA exposure promotes human RPMI 8866 B-cell adhesion to multiple integrin ligands. (A) Microtiter wells were coated with various concentrations of recombinant sVCAM-1 protein. RPMI 8866 cells cultured for 72 hrs in the presence of vehicle (△), 1 μMt-RA (○) or 1 μM 9-cis-RA (■) were added to wells (2×105 cells/well) (B) Microtiter wells were coated with various concentrations of recombinant ADAM28 Dis-Fc protein. As in (A), RPMI 8866 cells cultured for 72 hrs in the presence of vehicle (△), 1 μM t-RA (○) or 1 μM 9-cis-RA (■). (C) RPMI 8866 cells cultured for 24 hrs with vehicle or 1 μM 9-cis-RA. Vehicle treated cells in buffer containing no integrin activator (shaded bars), or in buffer containing the exogenous integrin activator Mn++ (white bars) and 9-cis-RA treated cells (black bars) were added to wells as previously described. Cells were incubated with 5μg/mL of human sVCAM-Fc, human ADAM28, or human ADAM12 Dis-Fc recombinant proteins. (D) RPMI 8866 cells were exposed to the indicated concentration of 9-cis-RA for 72 hrs. Adhesion assays were repeated as in (A) with wells coated with 3 μg/mL sVCAM-Fc. All results shown are the average ± SD of triplicate determinations.
Since 9-cis-RA exposure induces robust cell adhesion to the classic integrin counter receptor VCAM-1, we expanded our studies to include the ADAM family of proteins. ADAMs are a novel class of integrin ligand capable of mediating proteolytic and signaling events. Of the known human ADAMs, nearly a third are implicated in immune function with respect to their proteolytic substrates, integrin ligand properties, or expression pattern. Specifically, retinoid mediated adhesion to ADAM28 was assessed since this ADAM is recognized by the α4/α9 immune integrin subclass, predominantly expressed by mature human B-cells, and expression at the mRNA level is enhanced upon retinoid treatment. We tested the ability of 9-cis-retinoic acid to modulate adhesion to a purified recombinant ADAM28 disintegrin-like domain (ADAM28 Dis-Fc). As with sVCAM-1, culturing RPMI 8866 cells in the presence of 1 μM 9-cis-RA for 72 hrs bestowed cellular adhesion to the ADAM28 Dis-Fc in a dose-dependent and saturable manner (Fig. 1B). At a ligand concentration greater than 2 μg/mL, more than 50 percent adhesion of input cells was obtained when cells were exposed to 9-cis-RA. At the same level of ligand concentration, only 20 percent adhesion of vehicle cells was observed.
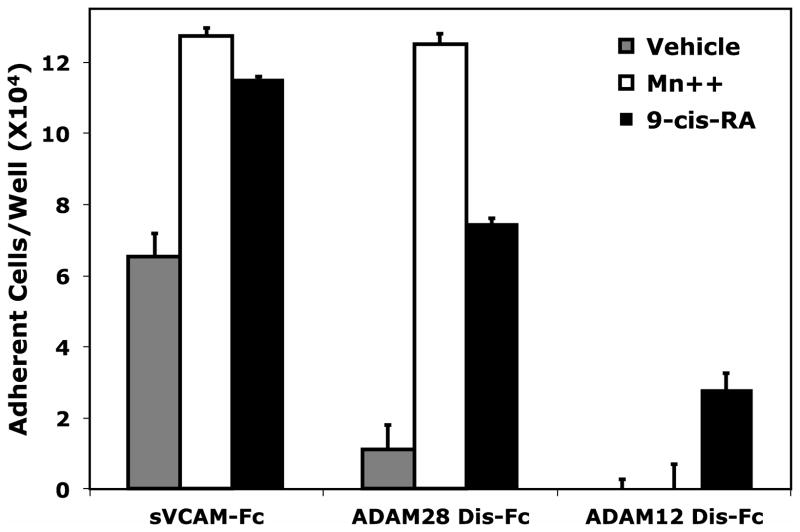
To address if the augmented adhesion to 9-cis-RA is observed upon an acute exposure of retinoic acid, cell adhesion assays were repeated with multiple ligands using RPMI 8866 cells cultured in the presence of vehicle or 9-cis-RA for 24 hrs. Higher levels of cell adhesion were observed when RPMI 8866 cells were maintained in 1 μM 9-cis-RA and added to wells coated with the immune ligands, sVCAM-Fc and ADAM28 Dis-Fc, at 5 μg/mL as compared to vehicle (Fig. 1C). As a positive control, the exogenous integrin activator Mn++ was added to vehicle cells to insure measurable adhesion at 24 hrs. To demonstrate adhesion specificity to immune ligands, RPMI 8866 cells exposed to vehicle or 9-cis-RA were added to wells coated 5 μg/mL of ADAM12 Dis-Fc, a non-immune ADAM. Although retinoid exposed cells did demonstrate a detectable increase in cell adhesion on the ADAM12 Dis-Fc, selected immune ligands consistently produced higher levels of cell adhesion. These data demonstrate that 9-cis-RA induces RPMI 8866 selective cell adhesion to physiologically relevant substrates. Titration experiments demonstrated that 9-cis-RA was capable of inducing RPMI 8866 B-cell adhesion at a variety of clinically relevant retinoid concentrations (Fig. 1D).
3.2 9-cis-Retinoic Acid Stimulates B-Cell Adhesion Independent of Integrin Function
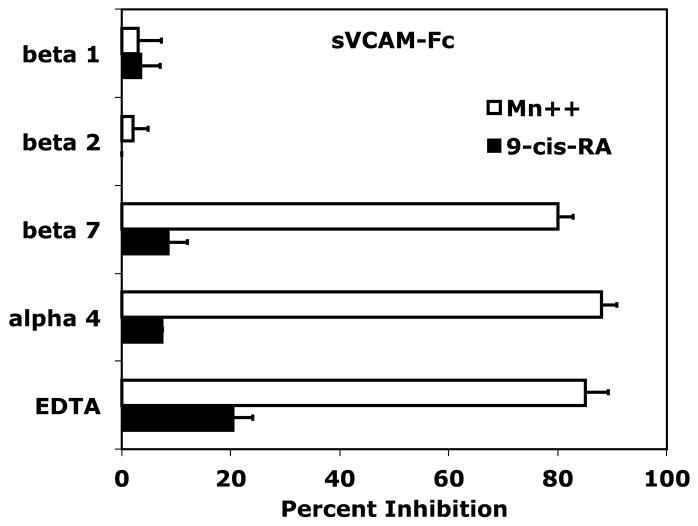
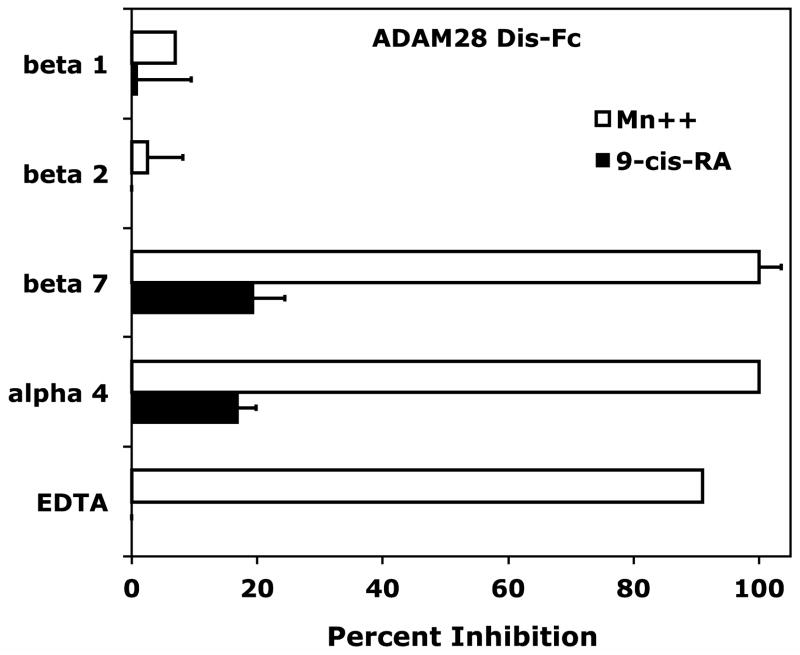
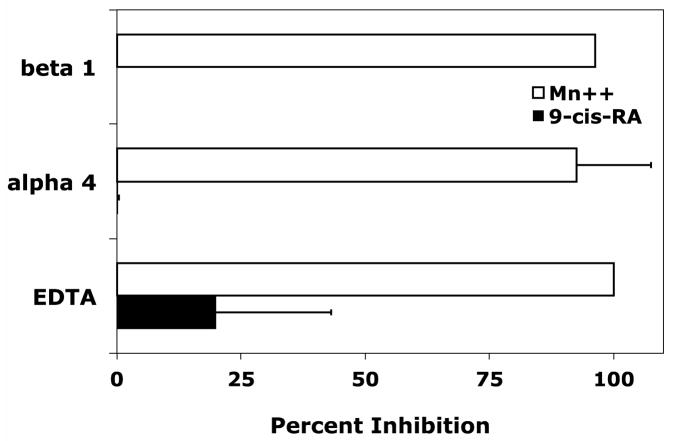
Classical mechanisms for leukocyte trafficking are predominantly governed by the integrin superfamily of adhesion receptors. Each member of the α4/α9 subclass contributes to leukocyte adhesion or transmigration by recognizing various members of the cell adhesion molecule family. Retinoids have the distinct ability to govern cell adhesion by modulating both integrin and integrin ligand expression. To determine if B-cell adhesion observed in the presence of 9-cis-RA was integrin-dependent, we assessed RPMI 8866 cell adhesion to immobilized sVCAM-Fc in the presence of various integrin inhibitors. Consistent with literature findings, RPMI 8866 cell adhesion to wells coated with sVCAM-Fc was strongly inhibited with function blocking antibodies to either the integrin α4 (P1H4) or β7 (FIB 27) subunits when cells were incubated in the presence of 1 mMMn++, an exogenous integrin activator (Fig. 2A). In addition, the pan-integrin antagonist EDTA potently blocked adhesion of vehicle treated cells in buffer containing Mn++ to sVCAM-Fc. In contrast, 9-cis-RA treated RPMI 8866 cells added to wells in buffer lacking Mn++ maintained strong adhesion in presence of all inhibitors tested (Fig. 2A). Similar inhibition results were obtained for full-length sVCAM-1 (data not shown). Comparable results were obtained with ADAM28Dis-Fc substrate, an established α4β1/α4β7 ligand (Fig. 2B). These results demonstrate 9-cis-RA is capable of inducing novel integrin-independent adhesion to immune ligands in a particular human B-cell lineage.
Figure 2.
9-cis-RA stimulates integrin-independent RPMI 8866 B-cell adhesion. (A) Cell adhesion assays were repeated as previously described. Function-blocking monoclonal antibodies to the individual integrin subunits listed on the ordinate were added at a final concentration of 5 μg/mL. The pan-integrin blocker EDTA was added at a final concentration of 5 mM. RPMI 8866 cells cultured for 72 hrs in the presence of vehicle (white bars) or 1μM9-cis-RA (black bars) were added to wells coated with 3μg/mL of recombinant sVCAM-Fc. As a positive control for integrin-dependent adhesion, vehicle cells were resuspended and added to wells in buffer containing the exogenous integrin activator Mn++. (B) The cells from a 72 hr time point and integrin inhibitors described in (A) were repeated with wells coated with 5μg/mL of ADAM28 Dis-Fc. Percent inhibition = (1−[adherent cells(inhibitor)−adherent cells(BSA)]/[adherent cells(no inhibitor) − adherent cells(BSA)])×100. Data displayed are the average inhibition values ± SD from at least two experiments each done in triplicate.
3.3 The presence of 9-cis-RA is required for RPMI 8866 B-cell Adhesion
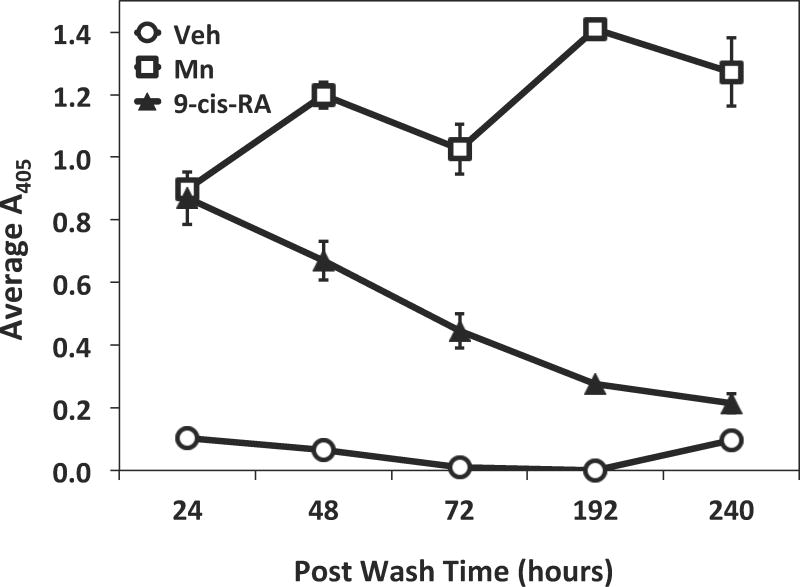
Retinoids are well characterized as differentiation agents that can permanently influence the expression pattern of certain molecules including adhesion receptors. To determine if retinoid exposure permanently imprints cellular adhesiveness, RPMI 8866 cells were initially exposed to 9-cis-RA for 72 hrs. The cells were washed and cultured in the absence of retinoid for various times sufficient for propagation. As a positive control, the exogenous integrin activator Mn++ was added to vehicle cells to insure measurable adhesion at all time points. Adhesion returned to basal levels on the ADAM substrate within 240 hrs of retinoid removal (Fig. 3).
Figure 3.
9-cis-RA exposure does not imprint adhesion to RPMI 8866 progeny cells. RPMI 8866 cells were initially exposed to 1 μM 9-cis-RA for 72 hrs. After 72 hrs, cells were pelleted, conditioned media containing retinoid was removed, and cells were further propagated in culture media lacking 9-cis-RA. Adhesion assays were performed with the ADAM28 Dis-Fc substrate as previously described at the indicated times post-wash(abscissa). Shown are data from a representative experiment done in triplicate ± SD.
3.4 9-cis-RA Stimulated RPMI 8866 B-cell Adhesion is Attenuated by Corticosteroids
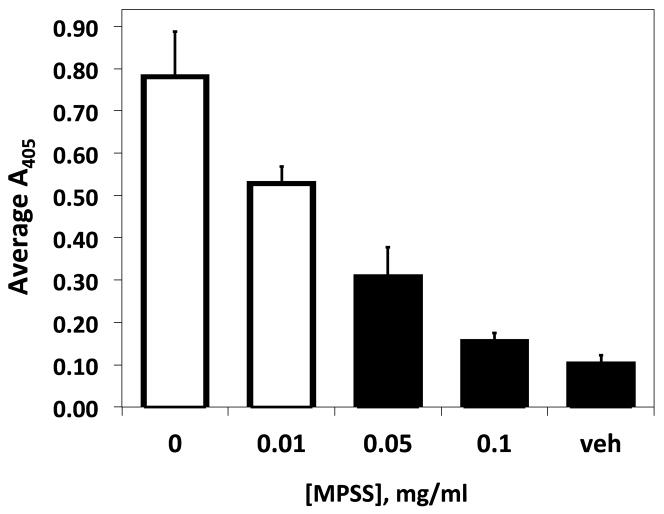
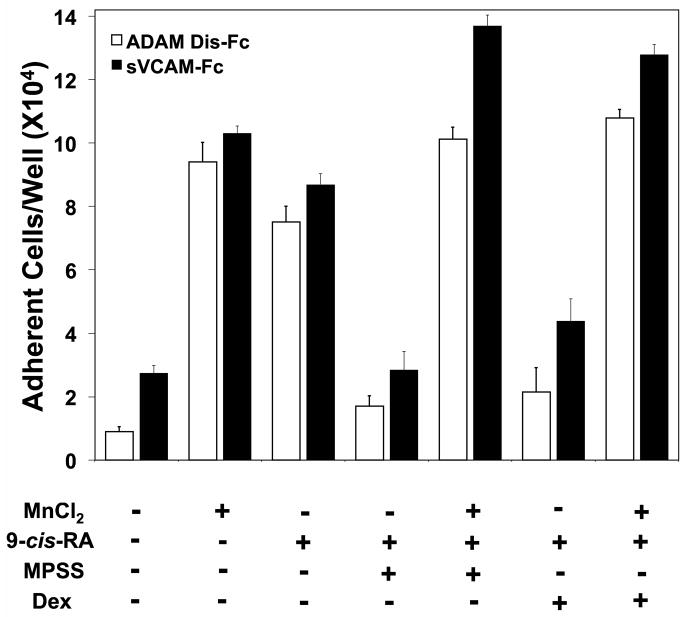
Retinoids promote inflammatory processes with respect to trans-endothelial migration, tissue infiltration, and cytokine production. Our current results suggest that retinoids may additionally contribute to the inflammatory response by stimulating cell adhesion to specific substrates involved in the immune response such as VCAM-1. In our study, we tested the adhesive effect of co-culturing cells with 9-cis-RA with two anti-inflammatory corticosteroids. As shown in Figure 4A, the presence of methylprednisolone (MPSS) potently attenuated cell adhesion to sVCAM-Fc within a 24 hour time period of administration in a dose-dependent manner. The dampened adhesion was not due to lower levels of integrin expression (data not shown) or function since cells treated with MPSS and retinoid were adherent upon integrin activation (Fig. 4B). Dexamethasone, a structurally distinct synthetic corticosteroid, elicited similar dampening of cell adhesion as MPSS (Fig. 4B).
Figure 4.
Synthetic corticosteroids potently dampen retinoid induced B-cell adhesion without altering integrin function. RPMI 8866 cells were cultured in the presence of 0.5 μM 9-cis-RA (white bars) or equimolar concentration of ethanol (black bars) for 72 hrs. MPSS was administered to retinoid-exposed cells 24 hrs before assaying at the indicated concentrations. Adhesion assays were repeated with 3 μg/mL VCAM-Fc as described previously. (B) RPMI 8866 cells cultured in the presence of 0.5μM 9-cis-RA or equimolar concentration of ethanol for 72 hrs. Where indicated (+) MPSS or Dex was added for the final 24 hrs of culture at 0.1 mg/mL. Cell adhesion assays were repeated with wells coated sVCAM-Fc (■) or ADAM Dis-Fc (□). Mn++ was added where indicated to exogenously activate integrin function. Results shown are triplicate determinations of a representative experiment ± SD.
3.5 Integrin-independent RPMI 8866 Cell Adhesion is Induced Specifically by Vitamin A Derivatives
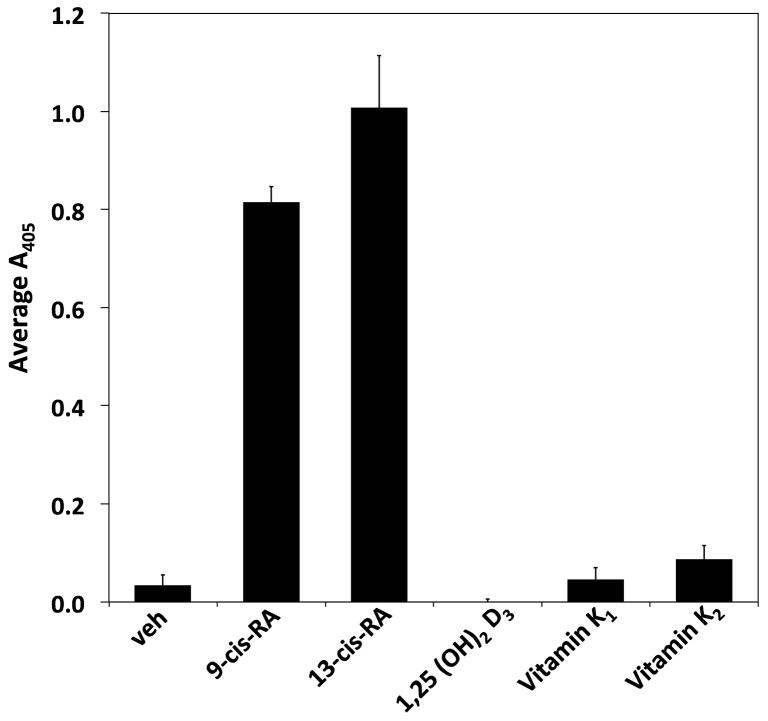
Since at-RA and 9-cis-RA induce RPMI 8866 B-cell adhesion, we investigated if 13-cis-RA, another biologically relevant retinoid isomer, also prompts adhesion. As shown in Figure 5A, 13-cis-RA stimulated RPMI 8866 B-cell adhesion to immune ligands at levels comparable to 9-cis-RA. Due to its emergence as a mediator of immune function and its capacity to bind receptors known to pair with RXRs, the potentially adhesive effects of 1,25-dihydroxy vitamin D3 (calcitriol) was also examined. The two predominate forms of vitamin K have not been reported to contribute directly to immunity and were employed as negative controls. The reported RPMI 8866 cell adhesion is specific to vitamin A derivatives as 1,25-dihydroxy vitamin D3 or vitamin K did not induce adhesion above levels obtained with vehicle alone (Fig. 5A).
Figure 5.
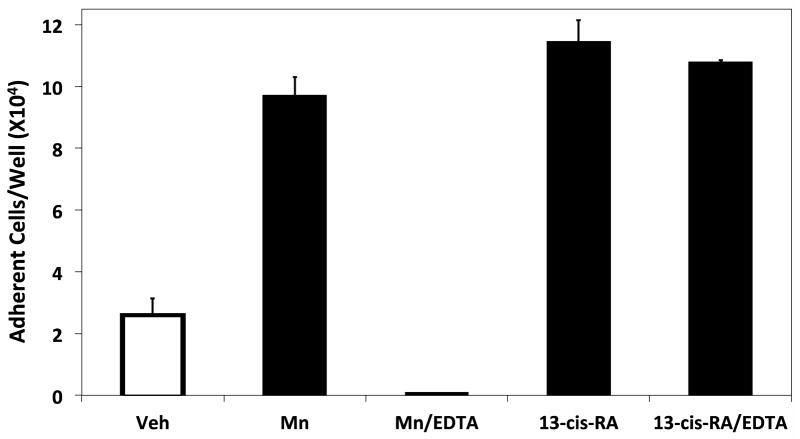
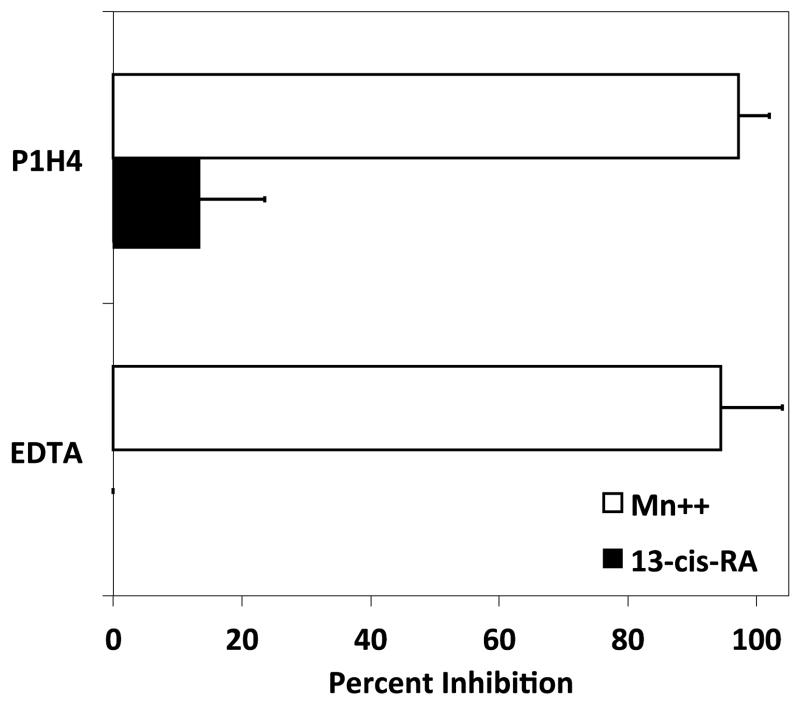
RPMI 8866 integrin-independent cell adhesion is specifically induced by retinoids. (A) Various stimuli were cultured with RPMI 8866 tested to determine if their presence augments human B-cell adhesion to ADAM28 Dis-Fc. All vitamin or vitamin derivatives were incubated with RPMI 8866 cells for 72 hrs at 1 μM (B) RPMI 8866 B-cells were cultured in the presence of 1 μM 13-cis-RA or equimolar concentration of vehicle for 72 hrs and evaluated for adhesion on 3μg/mL sVCAM-Fc in the presence or absence of EDTA. For a positive control of integrin-dependent adhesion, 1 mM of Mn++ was added to the buffer. (C) Adhesion assays were repeated with RPMI 8866 cells stimulated with 13-cis-RA in the presence of the integrin inhibitors P1H4 (function blocking anti-α4) or EDTA. Displayed are the results obtained from three separate inhibition experiments each done in triplicate with 5 μg/mL of ADAM28 Dis-Fc ± SD.
To ensure the adhesion obtained with 13-cis-RA treatment is analogous to that prompted by 9-cis-RA, adhesion assays were repeated with RPMI 8866 cells treated with 13-cis-RA and select integrin inhibitors. In contrast to cells activated with Mn++, integrin inhibition did not adversely impact the extent of cell adhesion with cells treated with 13-cis-RA (Fig. 5B and 5C). These results suggest that retinoids are activating a common, novel adhesion event in RPMI 8866 cells.
3.6 Human Immune Lineages Employ Divergent Adhesion Mechanisms Upon 9-cis-RA exposure
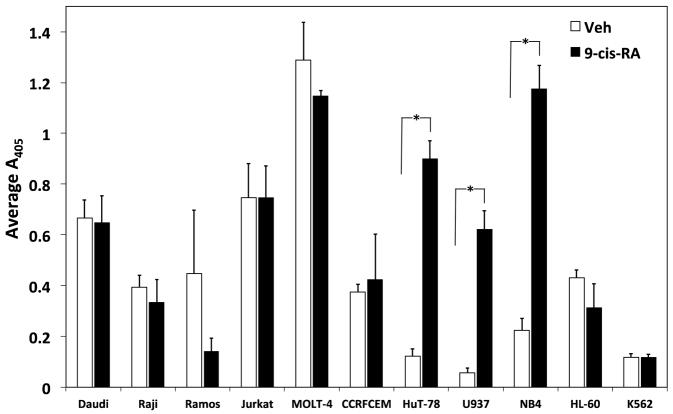
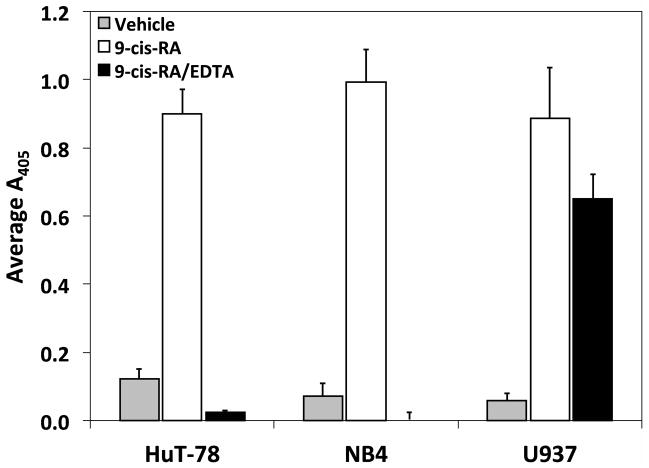
To elucidate if the observed adhesion induced by 9-cis-RA is a ubiquitous response, a variety of established human hematopoietic immortal cell lineages were assessed for their ability to augment cellular adhesion upon retinoid exposure. The blood lineages tested were comprised of three Burkitt’s B-cell lines (Daudi, Raji, Ramos), four T-cell lineages (Jurkat, MOLT-4, CCRF-CEM, HuT-78), one monocytic line (U937), two acute promyelocytic leukemia (APL) derived lines (NB4, HL-60), and one erythroleukemic line (K562). Among the diverse cells evaluated, the cutaneous T-lymphoma cell line HuT78, the APL cell line NB4, and the monocytic lineage U937 exclusively generated adhesion levels significantly above vehicle when cultured with 9-cis-RA (Fig. 6A). To assess integrin contribution to the retinoid mediated augmented adhesion, adhesion assays were repeated with the three responsive cell lines with and without EDTA. As evident in Figure 6B, the adhesion of HuT78 and NB4 cells induced by 9-cis-RA is reliant upon the presence of divalent cations. These findings are indicative of integrin contribution and are consistent with literature reports of at-RA treated NB4 cells. In contrast, the U937 monocyticline maintained cell adhesion in the absence of divalent cations; a trend similar to that observed with the RPMI 8866 cells.
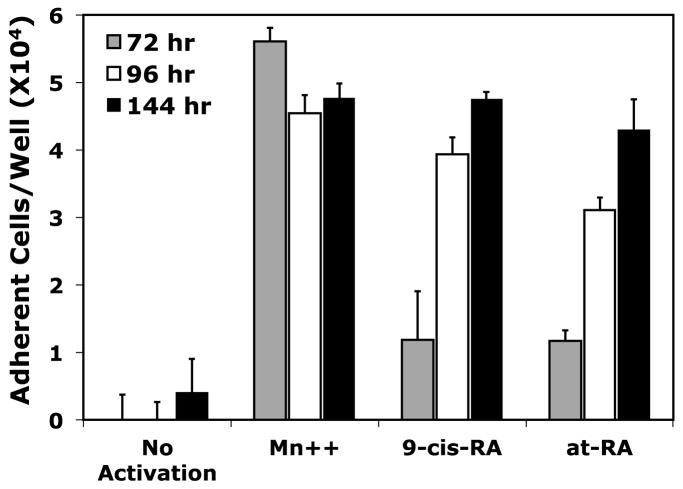
Figure 6.
9-cis-RA dependent cell adhesion is cell specific. (A) Assorted human immortal blood cell lineages cultured for 72 hrs in the presence of vehicle (white bars) or 1 μM 9-cis-RA (black bars) were assessed for adhesion to immobilized sVCAM-Fc or ADAM28 Dis-Fc. (B) HuT-78, NB4, and U937 cells exposed to 1 μM 9-cis-RA for at least 72 hrs and subsequently evaluated for adhesion to of 3 μg/mL sVCAM-Fc in the presence or absence of 5 mM EDTA. (C) U937 cells were cultured for 72 (shaded bars), 96 (white bars) or 144 hrs (black bars) in the presence of vehicle (ethanol), or 1 μM of indicated retinoid. Adhesion assay were conducted as previously described with wells coated with 5 μg/mL of sVCAM-Fc. (D) U937 cells cultured for 96 hrs in the presence of vehicle or 1 μM 9-cis-RA were added to wells coated with 5μg/mL of sVCAM-Fc in the presence of various integrin inhibitors. Data shown are the average percent inhibition values obtained from three experiments each done in triplicate ± SD. * signifies a significant difference between adhesion values obtained with vehicle versus 9-cis-RA treated cells (Student’s t-test, p<0.01).
3.7 The Human Monocytic Cell Line U937 Augments Integrin-Independent Adhesion in Response to 9-cis-Retinoic Acid
Although 9-cis-RA is capable of inducing a robust adhesion response, our data suggest that this response is cell-type specific. To evaluate if 9-cis-RA impacts the adhesion of immune lineages other than lymphocytes, we tested the ability of U937 cells, a human monocytic cell line, to mediate integrin independent cell adhesion. A number of studies have reported that U937 cells are retinoid responsive with respect to differentiation, proliferation and cytokine production. U937 cells were cultured for various times in the presence of 1 μM at-RA or 9-cis-RA and cell adhesion assays were repeated as previously described. As shown in Figure 6C, U937 cell adhesion to immobilized sVCAM-Fc was dependent upon both the presence of the retinoid and exposure time. The relative number of adherent cells reported at the 144 hr retinoid exposure is increased 3-fold compared to the 72 hr treatment. 9-cis-RA adhesion values obtained at the 144 hr treatment are similar to values obtained with vehicle treated U937 cells in buffer containing the exogenous integrin activator Mn++. Similar results were obtained with at-RA (Fig. 6C). To assess if the observed 9-cis-RA induced adhesion was attributable to integrin recognition, we employed function-blocking mAb to the α4 and β1 integrin subunits. These particular integrin subunits were targeted since the α4β1 integrin is the predominant adhesion receptor for VCAM-1 and is highly expressed on U937 cells. None of the inhibitors tested blocked 9-cis-RA treated U937 cell adhesion to sVCAM-Fc (Fig. 6D). In contrast, U937 cells exposed to vehicle and activated with Mn++ demonstrated greater than 90% reduction for all three inhibitors. These results suggest that 9-cis-RA exposure to U937 cells induces adhesion to a classic immune substrate through a novel mechanism that is not contingent on integrin function and mimics that reported earlier for the RPMI 8866 B-cell line.
3.8 9-cis-Retinoic Acid promotes soluble binding of select immune ligands
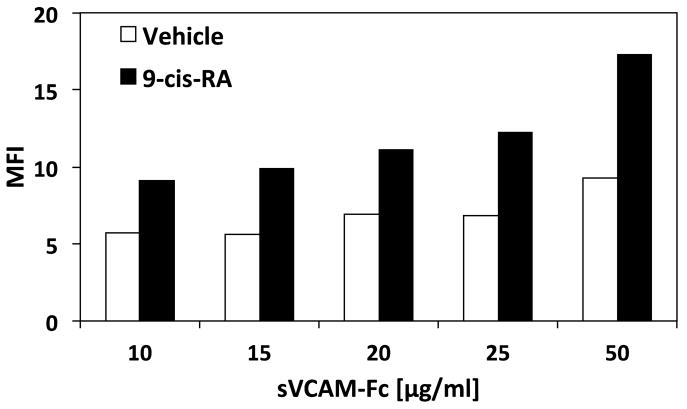
We have demonstrated 9-cis-RA-mediated integrin-independent adhesion of specific immune cell lineages to immobilized ligands via static cell adhesion assays. Since cell adhesion is facilitated by cell surface interactions, the ability of 9-cis-RA to mediate soluble ligand binding merits addressing. To establish whether retinoid-exposed cells were capable of binding immune ligands in solution, flow cytometric analysis of U937 cells incubated with increasing concentrations of sVCAM-Fc was performed (Fig. 7A). To hinder integrin-facilitated cell surface binding, EDTA was included in the binding buffer. U937 cells cultured with 9-cis-RA demonstrated a dose-dependent increase in sVCAM-Fc binding as compared to vehicle treated cells. Similar binding trends were observed with ADAM28 Dis-Fc (data not shown).
Figure 7.
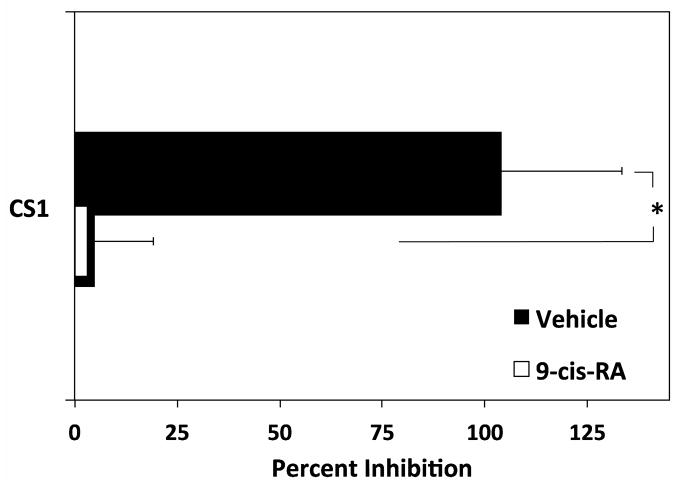
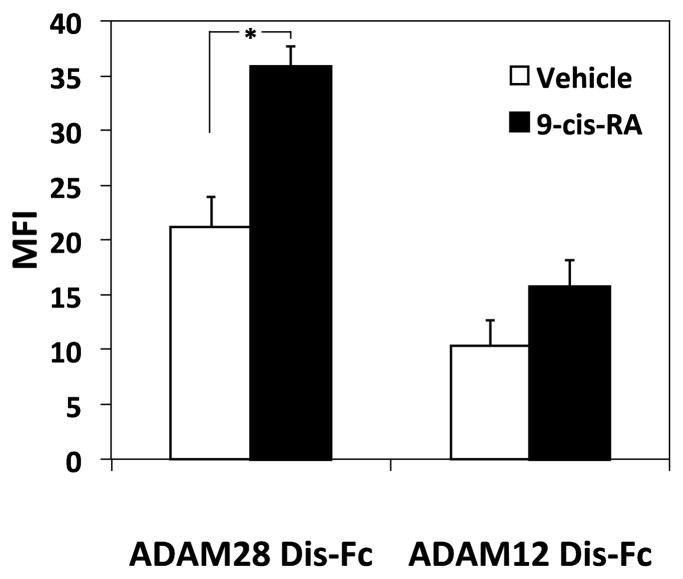
9-cis-RA induces soluble binding of immune ligands to the U937 cell surface. (A) U937 cells were treated with vehicle (white bars)or 1 μM 9-cis-RA (black bars) for 96 hrs prior to incubation with the indicated concentrations of sVCAM-Fc (μg/mL). Mean fluorescence intensity (MFI) was assessed via flow cytometric analysis. (B) Cells were treated as in (A). Incubation with sVCAM-Fc (50 μg/mL) was done in the presence of the α4β1 ligand mimetic, CS-1 peptide (500 μg/mL). Data shown are the average percent inhibition done in triplicate ± SD. * signifies a significant difference between MFI values obtained with vehicle versus 9-cis-RA treated cells (Student’s t-test, p<0.01). (C) Cells were treated as described with vehicle (white bars) or 9-cis-RA (black bars) followed by incubation with 50 μg/mL of ADAM 28 Dis-Fc or ADAM 12 Dis-Fc, MFI was assessed via flow cytometric analysis. Experiments were performed in triplicate ± SD (Student’s t-test, p<0.01).
In a set of independent experiments, U937 cells were treated with the α4β1 ligand mimetic connecting segment-1 (CS-1) peptide (Fig. 7B). The CS-1 peptide is derived from an alternatively spliced region of fibronectin recognized by the integrin α4β1. Inclusion of the CS-1 derived peptide with vehicle treated U937 cells completely abolished sVCAM-Fc binding. In contrast, the CS-1 region had minimal effect on sVCAM-Fc cell surface binding when U937 were exposed to 9-cis-RA.
The integrin-independent cell-surface binding of soluble protein ligands induced by 9-cis-RA was also discriminatory. Consistent with the trend obtained with the static cell adhesion assay (Fig. 1C), ADAM 28 Dis-Fc preferentially bound to U937 cells activated with 9-cis-RA as compared to ADAM 12 Dis-Fc. Cell surface binding of the non-immune ADAM 12 Dis-Fc did not significantly increase upon 9-cis-RA treatment unlike the binding of ADAM 28 Dis-Fc. These data collectively demonstrate that 9-cis-RA induces selective adhesion of immune ligands in solution.
3.9 Integrin-independent adhesion is induced in primary murine B-cells by 9-cis-RA
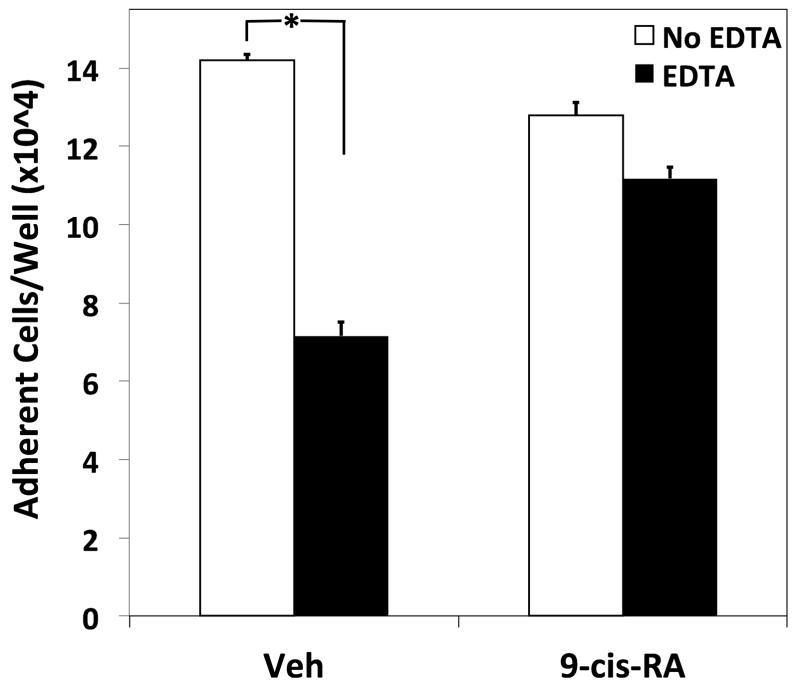
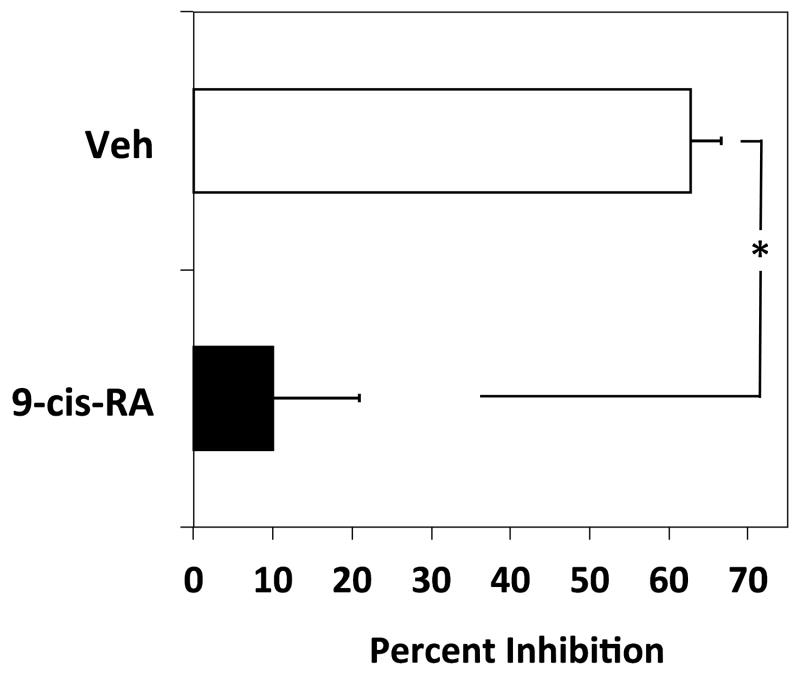
To determine if the novel adhesion observed for the immortal cell lines U937 and RPMI 8866 is exhibited by primary immune cells, we tested if murine B-splenocytes cultured ex vivo in the presence of 9-cis-RA would adhere to sVCAM-1 in the presence of EDTA. As shown in Figure 8A, EDTA significantly reduced the number of vehicle treated primary B-cells adhering to sVCAM-1. However, if the primary cells were cultured in the presence of 1 μM 9-cis-RA for 48 hrs, EDTA did not reduce the level of adhering lymphocytes to a significant extent. A summary of separate experiments established that primary B-cells cultured in the presence of 9-cis-RA transitioned to integrin-independent adhesion as compared to cells cultured with vehicle alone (Fig. 8B).
Figure 8.
Integrin-independent cell adhesion is induced in primary murine B-cells by 9-cis-RA. (A) LPS-stimulated murine splenocytes were cultured for 48 hrs in the presence of vehicle or 1 μM 9-cis-RA. Cells were added to wells coated with 1 μg/mL of sVCAM-1 in the absence (white bars) or presence of 5 mM EDTA (black bars). Representative data shown were done in triplicate ± SD. (B) The average inhibition values obtained from four separate adhesion experiments utilizing 0.5–1.0 μg/mL of sVCAM-1 are shown. * represents a significant difference of adhesion values between wells containing or lacking EDTA (Student’s t-test, p<0.01).
4. Discussion
The current study assesses the role of retinoids in immune cell adhesion and establishes that 9-cis-RA is capable of promoting cell adhesion through dissimilar mechanisms in a variety of established and primary immune cells. Prototypical interactions that facilitate firm immune cell adhesion preceding diapedesis occur between integrin heterodimers and counter-receptors such as VCAM-1. While the cutaneous T-lymphoma line HuT-78 and the APL line NB4 augment cell adhesion in response to 9-cis-RA through established integrin receptors, the selective, robust cellular adhesion response in RPMI 8866 human B-cells, U937 monocytic cells, and primary mouse B-cells occurred through a novel integrin-independent mechanism. Our data demonstrate that the adhesion observed with 9-cis-RA in these three cell types occurred predominantly independent of integrin function with greater than 80% of the observed adhesion persisting in the presence of various integrin neutralizing agents. Collectively, these results suggest that 9-cis-RA can promote immune cell adhesion through a classical integrin-dependent process as well as induction of a novel adhesion receptor or complex capable of promoting firm arrest on established integrin ligands.
The observed 9-cis-RA dependent adhesion is reminiscent of the adhesion previously reported with at-RA and seen in the current study with 13-cis-RA. Cell adhesion occurred independent of integrin function, adhesion was maintained in the absence of divalent cations, and the adhesion was cell type and immune ligand specific. These data suggest that retinoids prompt immune cell adhesion through a related cellular adhesion receptor(s)and/or pathway(s). In addition, these novel cellular adhesion effects are mitigated in the presence of anti-inflammatory synthetic corticosteroids without diminished integrin expression or function. These findings suggest 9-cis-RA transduces novel adhesion events through proinflammatory signaling pathways. Recent studies substantiate such a model by reporting retinoid enhancement of the human cyclooxygenase-2 promoter activity through RAR and RXR action.
More refined isolation and detection methods have introduced debate over in vivo retinoid isomer levels. Indeed, the relative physiological tissue-specific quantities of 9-cis-RA have been speculated to be insufficient to activate RARs and RXRs. The unique integrin-independent immune cell adhesion reported in our current work was also observed with the 13-cis-RA isomer. The 13-cis-RA isomer is readily detected in a variety of tissues including secondary lymphoid tissue and may be the most abundant isomer within serum. In addition, at-RA, the best-characterized isomer, produces adhesion effects comparable to those reported here for 9-cis-RA (Fig. 6C).
Although not all lymphocytes tested were retinoid responsive, it is clear that retinoids impact multiple types of immune cells including monocytes. Similar to lymphocytes, monocytes employ integrins to facilitate firm adhesion to the endothelial cell wall and promote extravasation from the vascular lumen to basement membrane and surrounding stroma. We tested the human monocytic cell line U937 for the ability to modulate cellular adhesion in response to 9-cis-RA exposure. Adhesion obtained with U937 cells at 72 hrs was consistently lower compared to retinoid treated RPMI 8866 cells. Higher adhesion numbers were obtained when U937 cells were exposed to retinoids for 96, 144 or 168 hrs (Fig. 6C and data not shown). Activated U937 cells show an induction of select retinoid X nuclear receptors. We suggest that the delayed retinoid dependent adhesive response reported here may require induction of retinoid receptors.
A defining attribute of retinoid action is cellular differentiation. This feature of retinoid biology is championed by the utilization of at-RA as an intervention for acute promyelocytic leukemia patients. This retinoid-based strategy is characterized as a differentiation therapy and marks a milestone in molecular-based cancer therapies. In addition, retinoids have been shown to be determinates for immune lineage subtypes partly by defining adhesion receptor repertoires. Although retinoids imprint homing phenotypes to immune cells, the integrin-independent adhesion reported currently was contingent upon the presence of the retinoid stimulus. This finding would suggest that the induced adhesion requires de novo protein synthesis of an unidentified protein receptor or activator. It was beyond the scope of the current study to identify and isolate such a receptor.
The current study provides insight into the role of 9-cis-RA in immune cell adhesion. With the obligate role of retinoids in the occupancy, establishment, and maintenance of lymphoid tissues such as GALT, the current work provides a framework of how retinoids specifically contribute to immune cell trafficking by influencing the propensity of ligand recognition by immune cell types. In addition to the established role of retinoids in integrin biology and function, we have unearthed a non-classical, integrin-independent mechanism whereby retinoids promote immune cell adhesion. The two distinct retinoid-induced adhesion events presently characterized have potential immunological implications into the amount and type of retinoids that an organism consumes and is exposed. Chronic inflammation and immunosuppressed states arise from aberrant immune cell trafficking and our findings demonstrate that retinoids directly influence immune cell adhesion, a key aspect of immune cell circulation. However, further studies are needed to elucidate the specific mechanisms by which retinoids influence immune biology. To advance our understanding of the role of retinoids in immunity, future studies will need to characterize the integrin-independent retinoid induced adhesion receptor/complex, elucidate the signals that sensitize cells to respond to retinoids with respect to adhesion, and determine nutritional parameters of retinoid metabolism that are integral in immunomodulation.
Supplementary Material
Supplemental Data, Figure 1. SDS-PAGE (4–20% gradient) of purified (3 μg/lane) sVCAM-Fc or ADAM 28 Dis-Fc were preformed under reduced (R) and nonreduced (NR) conditions. Protein was visualized with Coomassie Blue.
Acknowledgments
This work was supported by National Institutes of Health Grant from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources (LCB) [#P20 RR16460], a Research Corporation Cottrell College Science Award (LCB) [# 7680], start-up funds from the Brody School of Medicine (LCB), NIH R15AT006122 (SRS)
The authors are grateful to Drs. Phillip Pekala, Fred Bertrand, and William Angus for their insight and valuable suggestions. The authors wish to express their gratitude to Billina Matthews for her unwavering support and unrivaled assistance during the course of this work. Additionally, the authors are indebted to Dr. Jerry Ware for his candid professional mentorship.
Abbreviations
- 9-cis-RA
9-cis-retinoic acid
- 13-cis-RA
13-cis-retinoic acid
- ADAM
a disintegrin and metalloprotease
- at-RA
all-trans-retinoic acid
- FITC
fluorescein isothiocyanate
- MFI
mean fluorescent intensity
- mAb
monoclonal antibody
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- SD
standard deviation
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green HN, Mellanby E. Vitamin a as an Anti-Infective Agent. British medical journal. 1928;2:691–6. doi: 10.1136/bmj.2.3537.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellanby E, Green HN. Vitamin a as an Anti-Infective Agent: Its Use in the Treatment of Puerperal Septigaemia. British medical journal. 1929;1:984–6. doi: 10.1136/bmj.1.3569.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semba RD. Vitamin A as “anti-infective” therapy, 1920–1940. The Journal of nutrition. 1999;129:783–91. doi: 10.1093/jn/129.4.783. [DOI] [PubMed] [Google Scholar]
- 4.Ertesvag A, Naderi S, Blomhoff HK. Regulation of B cell proliferation and differentiation by retinoic acid. Seminars in immunology. 2009;21:36–41. doi: 10.1016/j.smim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Iwata M. The roles of retinoic acid in lymphocyte differentiation. Seminars in immunology. 2009;21:1. doi: 10.1016/j.smim.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Brown DC, Tsuji H, Larson RS. All-trans retinoic acid regulates adhesion mechanism and transmigration of the acute promyelocytic leukaemia cell line NB-4 under physiologic flow. British journal of haematology. 1999;107:86–98. doi: 10.1046/j.1365-2141.1999.01671.x. [DOI] [PubMed] [Google Scholar]
- 7.Ross AC. Vitamin A supplementation and retinoic acid treatment in the regulation of antibody responses in vivo. Vitamins and hormones. 2007;75:197–222. doi: 10.1016/S0083-6729(06)75008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflammatory bowel diseases. 2008;14:275–89. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 10.Sporn MB, Roberts AB, Goodman DS. The Retinoids Biology, Chemistry, and Medicine. New York: Ravens Press; 1994. [Google Scholar]
- 11.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–54. [PubMed] [Google Scholar]
- 12.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacological reviews. 2006;58:760–72. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 13.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 14.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355:359–61. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 15.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 16.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 18.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. The Journal of cell biology. 1999;145:413–20. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annual review of immunology. 2009;27:339–62. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunological reviews. 2007;218:126–34. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 21.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular address in MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 22.Massimi M, Devirgiliis LC. Adhesion to the extracellular matrix is positively regulated by retinoic acid in HepG2 cells. Liver Int. 2007;27:128–36. doi: 10.1111/j.1478-3231.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 23.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Escribese MM, Conde E, Martin A, Saenz-Morales D, Sancho D, Perez de Lema G, et al. Therapeutic effect of all-trans-retinoic acid (at-RA) on an autoimmune nephritis experimental model: role of the VLA-4 integrin. BMC nephrology. 2007;8:3. doi: 10.1186/1471-2369-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medhora MM. Retinoic acid upregulates beta(1)-integrin in vascular smooth muscle cells and alters adhesion to fibronectin. American journal of physiology Heart and circulatory physiology. 2000;279:H382–7. doi: 10.1152/ajpheart.2000.279.1.H382. [DOI] [PubMed] [Google Scholar]
- 26.Worley JR, Baugh MD, Hughes DA, Edwards DR, Hogan A, Sampson MJ, et al. Metalloproteinase expression in PMA-stimulated THP-1 cells. Effects of peroxisome proliferator-activated receptor-gamma (PPAR gamma) agonists and 9-cis-retinoic acid. J Biol Chem. 2003;278:5134–6. doi: 10.1074/jbc.M310865200. [DOI] [PubMed] [Google Scholar]
- 27.Prinzen C, Muller U, Endres K, Fahrenholz F, Postina R. Genomic structure and functional characterization of the human ADAM10 promoter. Faseb J. 2005;19:1522–4. doi: 10.1096/fj.04-3619fje. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti M, Falanga A, Giovanelli S, Oldani E, Barbui T. All-trans-retinoic acid increases adhesion to endothelium of the human promyelocytic leukaemia cell line NB4. British journal of haematology. 1996;93:360–6. doi: 10.1046/j.1365-2141.1996.4911029.x. [DOI] [PubMed] [Google Scholar]
- 29.Bridges LC, Lingo JD, Grandon RA, Kelley MD. All-trans-retinoic acid induces integrin-independent B-cell adhesion to ADAM disintegrin domains. Biochemistry. 2008;47:4544–51. doi: 10.1021/bi702447u. [DOI] [PubMed] [Google Scholar]
- 30.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Seminars in immunology. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AC, Chen Q, Ma Y. Augmentation of antibody responses by retinoic acid and costimulatory molecules. Seminars in immunology. 2009;21:42–50. doi: 10.1016/j.smim.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. Journal of lipid research. 2010;51:1284–97. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridges LC, Sheppard D, Bowditch RD. ADAM disintegrin-like domain recognition by the lymphocyte integrins alpha4beta1 and alpha4beta7. The Biochemical journal. 2005;387:101–8. doi: 10.1042/BJ20041444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges LC, Tani PH, Hanson KR, Roberts CM, Judkins MB, Bowditch RD. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4beta1. J Biol Chem. 2002;277:3784–92. doi: 10.1074/jbc.M109538200. [DOI] [PubMed] [Google Scholar]
- 35.Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin alpha 5 beta 1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–62. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Roberts CM, Tani PH, Bridges LC, Laszik Z, Bowditch RD. MDC-L, a novel metalloprotease disintegrin cysteine-rich protein family member expressed by human lymphocytes. J Biol Chem. 1999;274:29251–9. doi: 10.1074/jbc.274.41.29251. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre P, Thomas G, Gourmel B, Agadir A, Castaigne S, Dreux C, et al. Pharmacokinetics of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1991;5:1054–8. [PubMed] [Google Scholar]
- 39.Mou L, Lankford-Turner P, Leander MV, Bissonnette RP, Donahoe RM, Royal W. RXR-induced TNF-alpha suppression is reversed by morphine in activated U937 cells. J Neuroimmunol. 2004;147:99–105. doi: 10.1016/j.jneuroim.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima H, Kizaki M, Ueno H, Muto A, Takayama N, Matsushita H, et al. All-trans and 9-cis retinoic acid enhance 1,25-dihydroxy vitamin D3-induced monocytic differentiation of U937 cells. Leuk Res. 1996;20:665–76. doi: 10.1016/0145-2126(96)00020-3. [DOI] [PubMed] [Google Scholar]
- 41.Brown TR, Stonehouse TJ, Branch JS, Brickell PM, Katz DR. Stable transfection of U937 cells with sense or antisense RXR-alpha cDNA suggests a role for RXR-alpha in the control of monoblastic differentiation induced by retinoic acid and vitamin D. Exp Cell Res. 1997;236:94–102. doi: 10.1006/excr.1997.3704. [DOI] [PubMed] [Google Scholar]
- 42.Faull RJ, Wang J, Stavros W. Changes in the expression of adhesion molecules as peripheral blood monocytes differentiate into peritoneal macrophages. Nephrol Dial Transplant. 1996;11:2037–44. doi: 10.1093/oxfordjournals.ndt.a027093. [DOI] [PubMed] [Google Scholar]
- 43.Han K, Moon I, Lim HJ. All-trans- and 9-cis-retinoic acids activate the human cyclooxynase-2 gene: a role for DR1 as RARE or RXRE. Molecular biology reports. 2011;38:833–40. doi: 10.1007/s11033-010-0173-4. [DOI] [PubMed] [Google Scholar]
- 44.Kane MA. Analysis, occurrence, and function of 9-cis-retinoic acid. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbalip.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Arnold SL, Amory JK, Walsh TJ, Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. Journal of lipid research. 2012;53:587–98. doi: 10.1194/jlr.D019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 47.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Seminars in immunology. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data, Figure 1. SDS-PAGE (4–20% gradient) of purified (3 μg/lane) sVCAM-Fc or ADAM 28 Dis-Fc were preformed under reduced (R) and nonreduced (NR) conditions. Protein was visualized with Coomassie Blue.