Abstract
Purpose
This research proposed to study the changes in lipid composition in cumulus cells (CCs) from women who achieved pregnancy compared with women who did not, after in vitro fertilization treatment. This approach has the potential to provide novel information on the lipid metabolism of the CCs and as an additional method to predict pregnancy.
Method
Fifty-four samples from couples with tubal and male factor infertility and where the female partner was age 35 or younger were divided in two groups according to their level of hCG 14 days after embryo transfer as follows: (1) 23 samples in pregnant group and (2) 31 samples in non-pregnant group. Lipid extraction was performed by the Bligh-Dyer protocol, and lipid profiles were obtained by MALDI-TOF MS. Mass spectra data were processed with MassLynx, and statistical analysis was performed using MarkerLynx extended statistic. OPLS-DA model was built.
Results
S-plot Analysis revealed three ions as potential markers in the pregnant group, and five ions in the non-pregnant group. These ions were identified in the human metabolome database (HMDB) as phosphatidylcholine in the pregnant group and as phosphatidylethanolamine, phosphatidylserine and phosphatidylinositol species in the non-pregnant group. These lipids might be involved in cell proliferation and differentiation, apoptosis and GAP junction regulation.
Conclusion
We conclude that MALDI-TOF MS can be used as an informative and fast analytical strategy to obtain and study the lipid profile of cumulus cells and can potentially be used as a supporting tool to predict pregnancy based on the metabolic state of the CCs.
Keywords: Cumulus cell, Lipid profile, MALDI-TOF MS, Pregnancy outcome
Introduction
Aspects of embryo morphology have been used to predict pregnancy during in vitro fertilization (IVF) treatment. These include the number of blastomeres in a given stage of development, the regularity of cell division and the degree of fragmentation [2]. Although embryo morphology has been correlated with implantation potential [6], selecting embryos with the appropriate morphological appearance alone is not sufficient to guarantee embryo viability and improve the chances of pregnancy. Several methods, such as removing the polar body [34] and one blastomere from the embryo on the third day of culture [28], were proposed to evaluate oocyte and embryo development, although it is not known what impact these techniques have on embryonic development. Therefore, new non-invasive approaches in oocyte and embryo metabolomics appear to be an alternative for gamete selection and have been proposed for assessing the potential of oocytes and embryos using embryo culture media, follicular fluid and cells [6].
The ability to achieve good embryo development is reported to be dependent on oocyte competence, which is acquired during folliculogenesis [40]. Thus, follicle maturation is crucial for an oocyte to become a good quality embryo and consequently achieve a successful pregnancy [19].
During follicular development, the granulosa cells differentiate into two layers; the first layer, which is composed of theca cells, coats the follicular antrum and is responsible for estrogen production and follicular rupture, and the second layer, which consists of cumulus cell (CCs), involves the oocyte. The function of these cells is partially regulated by oocyte factors, and therefore CCs assist with oocyte maturation and further development [14].
The interaction of the oocyte and the CCs occurs at gap junctions [41], through which nutrients and maturation-enabling factors are provided and where the exchange of metabolites and ions between the oocyte and the CCs is facilitated [13]. Therefore, reciprocal and optimal exchange between the oocyte and the CCs is essential for the acquisition of oocyte competence. It has been hypothesized that studying these cells can elucidate the physiological importance of these processes and may lead to optimization of IVF [41]; the lipid composition of the CCs may reflect and determine oocyte maturation, fertilization and early embryo development potential.
With the rise of new technologies based on mass spectrometry such as the -omics approaches (proteomics, metabolomics, lipidomics), which provide detailed molecular information, novel analytical tools can be developed and applied to IVF to discover new biomarkers to predict pregnancy [21]. Indeed, the lipidomic approach based on mass spectrometry coupled with a lipid database search can investigate the potential interactions of lipid signaling and their impact in modulating physiology [5].
Lipids are essential cellular constituents. They have many functions within cells and regulate several biological processes. Lipids are the building blocks of bilayer membranes and regulate key cellular functions by acting as signaling molecules and precursors for second messengers and by regulating signal transduction pathways, cell proliferation and apoptosis [20].
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been successfully employed as a tool for the analysis of lipid composition because it provides results within a few minutes with a minimal degree of analyte fragmentation, which allows for the analysis of highly complex structures [37].
The introduction of more complex data treatments from a mathematical and statistical standpoint has been carried out in multivariate analysis. This allows the analysis of multiple variables at the same time and draws a smaller number of variables, which allow for the visualization of data [33]. The two most common multivariate analyses are the principal component analysis (PCA), which is an unsupervised test, and the orthogonal partial least square discriminate analysis (OPLS-DA), which is a supervised test employed to build a predictive model [23].
Lipid profile analysis of CCs, which are usually discarded during oocyte retrieval, may indirectly reflect the competence of the oocyte. Because of the importance of CCs for the oocyte viability, the present study compared the lipid MALDI-TOF MS profile of CCs from women who did and did not achieve pregnancy following IVF treatment. The goal of the study was to investigate the metabolism of the CCs and to evaluate whether changes in the lipid profile of the CCs can be used as a prognostic tool to identify lipids that are indicative of oocyte competence. To our knowledge, this is the first time that MALDI-TOF MS has been used to study the lipid composition of CCs, which has a promising application for assisted reproductive technologies (ART).
Materials and methods
Study group
An observational case-control study was carried out among patients seeking IVF treatment at the Human Reproduction Section of Sao Paulo Hospital. This study received approval from the Institutional Review Board of the São Paulo Federal University Research Ethics Committee (protocol: 1299/10).
Samples of CCs were obtained from 54 women up to 35 years old without endometriosis, polycystic ovarian syndrome or ovarian dysfunction, who underwent in vitro fertilization (IVF) by intracytoplasmic sperm injection (ICSI) after referral to the IVF program at São Paulo Federal University. To ensure that male factor infertility did not affect the model, sperm with less than 3 million sperm ml−1 and above 4 % strict morphology according to the Kruger classification [24] were excluded. Patients were subdivided into two groups. The first group consisted of 23 samples from patients who achieved pregnancy (age 30.61 ± 3.88 years, mean ± SD). The second group consisted of 31 samples from women referred to the IVF program who did not achieve pregnancy in that treatment cycle (age 30.97 ± 3.48 years, mean ± SD).
All patients underwent IVF because of tubal factor female infertility and/or mild male factor infertility. For both groups, only couples in which the female partner was 35 years or younger and had a serum follicle-stimulating hormone (FSH) level of between 3 and 9 μg/ml on Day 3 of the menstrual cycle previous to the treatment cycle were included.
All women received a similar ovarian stimulation protocol for IVF. Controlled ovarian stimulation was performed with the use of exogenous recombinant gonadotropins (225 IU/day of Gonal-F, Merck-Serono, Darmstadt, Germany) starting on cycle day 2. When the leading follicle reached 14 mm in diameter, endogenous LH release was suppressed with the use of a GnRH antagonist analog (Cetrorelix—Cetrotide; Merk-Serono) until the day of hCG administration. When the leading follicle reached 17 mm in diameter, a total dose of 250 μg of hCG was administered. Ultrasound-guided transvaginal oocyte retrieval was performed 36 h after hCG administration. For all patients, the excess of CCs were collected prior to fertilization, after follicle pick-up from the group of oocytes present in both ovaries. Removal of excess of cumulus cells is routinely performed prior to oocytes incubation in our laboratory. This procedure is only performed in oocytes with expanded cumulus cells and in patients who will be submitted to in vitro fertilization by ICSI. Thus, the samples of cumulus cells in the present study were collected prior to fertilization, which was performed by ICSI in mature oocytes (MII). Samples of CCs were stored in microtubes at −20 °C until lipid extraction.
Lipid extraction
Lipids were extracted based on the protocol by Bligh and Dyer (Bligh et al. 1959) with some modifications. Briefly, 50 μL of distilled water was placed in a microtube with the CCs, and they were mixed with a pipette to break the cells by osmotic lysis. The next step was to add 125 μL of chloroform and 250 μL of methanol. The mixture was homogenized and vortexed for 1 min. The polar and apolar phases were separated by the addition of 100 μL of water and 125 mL of chloroform. The mixture was vortexed for 1 min and centrifuged at 3,000× g for 1 min. The lower phase containing the lipids was recovered and transferred to a clean microtube, which was left open at room temperature for 1 h until the solvent evaporated.
MS data acquisition
A volume of 10 μL of chloroform was added to each sample to dissolve the lipids contained in the microtube. For MALDI-TOF MS analysis, 2 μL of each sample were deposited on the MALDI target plate and covered with 1 μL of 2,5-dihydroxybenzoicacid (DHB 0.5 M) dissolved in 90 % methanol. Mass spectra were acquired in the positive ion mode using a Q-ToF Premier (Synapt HDMS) mass spectrometer (Waters, Manchester, UK) equipped with a 200 Hz solid-state laser in the m/z 700–1,200 range in the reflectron mode. Typical operating conditions were: laser energy 250 a.u., sample plate 20 V and the Trap and Transfer collision energies were 6 and 4 V, respectively (QTOF-MS mode).
Data analysis
The mass spectra of each sample were accumulated using MarkerLynx 4.1 software (Waters, Manchester, UK) and exported for principal component analysis (PCA) and orthogonal partial least square discriminant analysis (OPLS-DA) by MarkerLynxTM XS (Waters, Manchester, UK). The method parameters were as follows: mass tolerance = 0,5 Da, baseline noise = 50 and intensity threshold (count) = 1,000 with deisotope data.
The S-plot of the OPLS-DA analysis provided a list of ions responsible for the differences between the groups, and the lipid subclasses of these ions were searched in the Human Molecular Database (HMDB) (http://www.hmdb.ca). A mass tolerance of 0.1 Da was adopted. Mass error in ppm was calculated for all of the ions found and was considered only when the mass error was ≤50 ppm.
Results
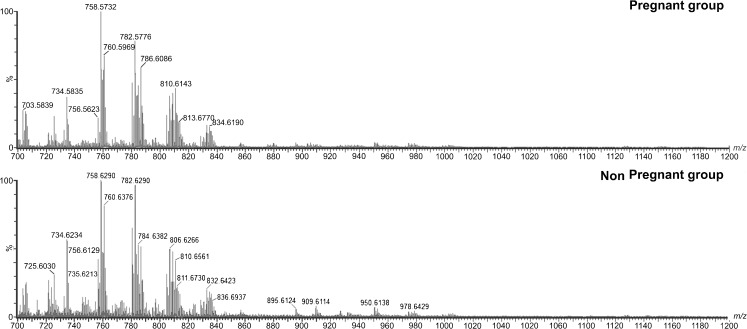
A total of 54 samples of CCs were obtained from women undergoing IVF, of which 23 samples were from woman who achieved pregnancy and 31 samples were from woman who did not achieve pregnancy. Clinical data including the patients’ number of follicles, the number of oocytes retrieved and the rate of mature oocytes (MII) are presented in Table 1, and the representative spectra of each group are shown in Fig. 1.
Table 1.
Clinical characteristics of patients from the pregnant group and the non-pregnant group
| Pregnant group | Non-pregnant group | P-value | |
|---|---|---|---|
| Age (years) | |||
| Mean; SD | 30.61; 3.88 | 30.97; 3.48 | 0.72 |
| 95 % CI | 26.73–34.49 | 27.49–34.45 | |
| FSH (μg ml−1) | |||
| Mean; SD | 6.17; 1.89 | 6.96; 2.20 | 0.17 |
| 95 % CI | 4.29–8.06 | 4.77–9.16 | |
| LH (μg ml−1) | |||
| Mean; SD | 4.39; 1.77 | 4.72; 2.07 | 0.54 |
| 95 % CI | 2.61–6.16 | 2.65–6.78 | |
| Follicles (n) | |||
| Mean; SD | 20.39; 12.58 | 14.29; 6.59 | 0.02 |
| 95 % CI | 7.81–32.97 | 7.70–20.88 | |
| Oocytes (n) | |||
| Mean; SD | 12.70; 8.11 | 8.10; 4.39 | 0.01 |
| 95 % CI | 4.59–20.81 | 3.70–12.49 | |
| Recovery rate (%) | |||
| Mean; SD | 0.64; 0.21 | 0.57; 0.17 | 0.22 |
| 95 % CI | 0.43–0.85 | 0.40–0.75 | |
| MII oocytes (%) | |||
| Mean; SD | 0.66; 0.24 | 0.82; 0.19 | 0.009 |
| 95 % CI | 0.42–0.91 | 0.63–1.01 | |
| Fertilization rate (%) | |||
| Mean; SD | 0.78; 0.24 | 0.70; 0.27 | 0.22 |
| 95 % CI | 0.54–1.02 | 0.43–0.97 | |
Bold P-values indicate statistical significance
Fig. 1.
Typical MALDI-MS data for the pregnant and non-pregnant groups. The y-axis shows relative abundances whereas the x-axis shows m/z values
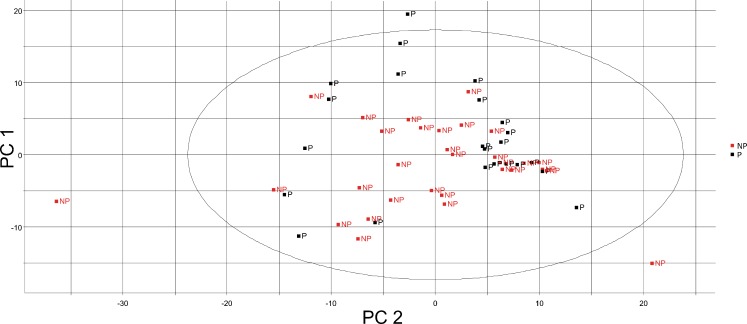
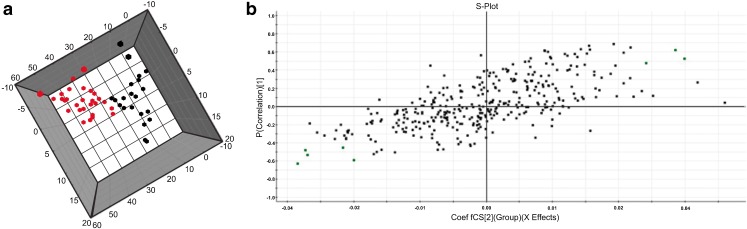
Multivariate statistical analyses, such as PCA and OPLS-DA, were carried out on preprocessed MALDI-TOF-MS data, including the relative ion intensities and m/z values, to understand the difference in lipid profiles. The PCA 2D plot of the samples from the pregnant and non-pregnant groups showed that the groups could be differentiated (Fig. 2). Because individual variation, PCA was not satisfactorily separate the experimental groups. The explained variance of each principal component is shown in Table 2. The OPLS-DA (Fig. 3a) was also performed, and the pregnant and non-pregnant groups were clearly separated, demonstrating that the OPLS-DA was more effective for predicting pregnancy using the CCs lipid profile.
Fig. 2.
PCA 2D shows the variance between groups by scores of PC1 x PC2. The black points show the pregnant group by individual PCs; the red points show the non-pregnant groups
Table 2.
Percentage of variance explained by principal component analysis (PCA)
| Number of component | Individual variance explained (%) | Cumulative variance explained (%) |
|---|---|---|
| 1 | 23.38 % | 23.38 % |
| 2 | 12.32 % | 35.70 % |
| 3 | 9.58 % | 45.27 % |
| 4 | 6.56 % | 51.83 % |
| 5 | 5.63 % | 57.47 % |
| 6 | 5.25 % | 62.72 % |
| 7 | 3.67 % | 66.39 % |
| 8 | 3.31 % | 69.70 % |
| 9 | 2.79 % | 72.50 % |
Fig. 3.
OPLS-DA 3D Plot (a) shows scores of individual samples x CP1 x CP2 of pregnant (black squares) and non-pregnant (red squares) groups and the S-Plot (b). Lipids denoted by the red square could be selected as the lipids important for the separation of the two groups (Table 3)
To detect the differential lipids related to the separation of the pregnant and non-pregnant groups, three and five ions, respectively, with the highest covariance between groups were selected from the S-plot (Fig. 3b) of the OPLS-DA as potential biomarker candidates.. From the three ions selected from the CCs samples of the pregnant group, and only one was identified as phosphatidylcholine. In the non-pregnant group, of the five ions selected, four were identified as phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI). Therefore, all lipids identified in this study belong to the phospholipid subclass. Ions (m/z), mass error (ppm) and the lipid subclasses identified are shown in Table 3.
Table 3.
Ions identified by the S-Plot in both groups. The ions were related to the lipid subclass and mass error
| Mass (m/z) | Group | Chemical Formula | Lipid subclass | Adducts | Mass error (ppm) |
|---|---|---|---|---|---|
| 750.4191 | NP | C38H66NO10P | PS(18:3(9Z,12Z,15Z)/14:1(9Z)) | M+Na [1+] | 16.67 |
| PS(14:1(9Z)/18:3(9Z,12Z,15Z)) | M+Na [1+] | ||||
| 807.4818 | NP | C41H75O13P | PI(16:2(9Z,12Z)/16:0) | M+H [1+] | 24.72 |
| PI(16:1(9Z)/16:1(9Z)) | M+H [1+] | ||||
| PI(16:0/16:2(9Z,12Z)) | M+H [1+] | ||||
| 806.4842 | NP | C42H74NO10P | PS(20:4(5Z,8Z,11Z,14Z)/16:0) | M+Na [1+] | 12.41 |
| PS(20:3(8Z,11Z,14Z)/16:1(9Z)) | M+Na [1+] | ||||
| PS(18:3(9Z,12Z,15Z)/18:1(9Z)) | M+Na [1+] | ||||
| PS(18:2(9Z,12Z)/18:2(9Z,12Z)) | M+Na [1+] | ||||
| PS(18:1(9Z)/18:3(9Z,12Z,15Z)) | M+Na [1+] | ||||
| PS(16:1(9Z)/20:3(8Z,11Z,14Z)) | M+Na [1+] | ||||
| PS(16:0/20:4(5Z,8Z,11Z,14Z)) | M+Na [1+] | ||||
| 822.4423 | NP | C45H70NO8P | PE(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:4(6Z,9Z,12Z,15Z)) | M+K [1+] | 0.001 |
| PE(20:5(5Z,8Z,11Z,14Z,17Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | M+K [1+] | ||||
| PE(18:4(6Z,9Z,12Z,15Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | M+K [1+] | ||||
| 1041.54 | NP | UNIDENTIFIED | >50 | ||
| 886.6428 | P | C52H88NO8P | PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:2(13Z,16Z)) | M+H [1+] | 12.18 |
| PC(22:4(7Z,10Z,13Z,16Z)/22:4(7Z,10Z,13Z,16Z)) | M+H [1+] | ||||
| PC(22:2(13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | M+H [1+] | ||||
| C50H90NO8P | PC(20:0/22:5(7Z,10Z,13Z,16Z,19Z)) | M+Na [1+] | 14.94 | ||
| PC(20:0/22:5(4Z,7Z,10Z,13Z,16Z)) | M+Na [1+] | ||||
| PC(18:4(6Z,9Z,12Z,15Z)/24:1(15Z)) | M+Na [1+] | ||||
| PC(24:1(15Z)/18:4(6Z,9Z,12Z,15Z)) | M+Na [1+] | ||||
| PC(22:5(7Z,10Z,13Z,16Z,19Z)/20:0) | M+Na [1+] | ||||
| PC(22:5(4Z,7Z,10Z,13Z,16Z)/20:0) | M+Na [1+] | ||||
| PC(22:4(7Z,10Z,13Z,16Z)/20:1(11Z)) | M+Na [1+] | ||||
| PC(22:2(13Z,16Z)/20:3(8Z,11Z,14Z)) | M+Na [1+] | ||||
| PC(22:2(13Z,16Z)/20:3(5Z,8Z,11Z)) | M+Na [1+] | ||||
| PC(22:1(13Z)/20:4(8Z,11Z,14Z,17Z)) | M+Na [1+] | ||||
| PC(22:1(13Z)/20:4(5Z,8Z,11Z,14Z)) | M+Na [1+] | ||||
| PC(22:0/20:5(5Z,8Z,11Z,14Z,17Z)) | M+Na [1+] | ||||
| PC(20:5(5Z,8Z,11Z,14Z,17Z)/22:0) | M+Na [1+] | ||||
| PC(20:4(8Z,11Z,14Z,17Z)/22:1(13Z)) | M+Na [1+] | ||||
| PC(20:4(5Z,8Z,11Z,14Z)/22:1(13Z)) | M+Na [1+] | ||||
| PC(20:3(8Z,11Z,14Z)/22:2(13Z,16Z)) | M+Na [1+] | ||||
| PC(20:3(5Z,8Z,11Z)/22:2(13Z,16Z)) | M+Na [1+] | ||||
| PC(20:1(11Z)/22:4(7Z,10Z,13Z,16Z)) | M+Na [1+] | ||||
| 858.9356 | P | UNIDENTIFIED | >50 | ||
| 917.06 | P | UNIDENTIFIED | >50 |
NP Non-pregnant group; P Pregnant group; PS Phosphatidylserine; PI Phosphatidylinositol; PE Phosphatidylethanolamine; PC Phosphatidylcholine
Discussion
Aspects of embryo morphology have prognostic importance for pregnancy following ART [18,45]. Given the role of CCs in oocyte maturation support, that they actively influence the oocyte’s acquisition of developmental competence, and that many cumulus function are regulated by the oocyte [14], the chemical analysis of these cells may be a promising approach for the non-invasive assessment of oocyte competence, embryo development potential and future pregnancy. Several groups have been searching for new markers of oocyte competence and pregnancy outcome in these cells [1,3]. However, to our knowledge, this work is the first time that analysis of the lipid profile of CCs by MALDI-TOF MS has been studied.
The clinical data analysis revealed that the number of follicles and number of oocytes were higher in pregnant women than in non-pregnant women, which did not influence the retrieval rate. However, the MII rate was higher in the group of women who did not achieve pregnancy. The increase in the number of follicles and the number of oocytes retrieved in the pregnant group only suggests that these women had higher follicular recruitment compared to the group that did not achieve pregnancy, but it does not necessarily indicate that all of the oocytes were completely maturated. On the other hand, the analysis of clinical data has shown that the non-pregnant group had a higher average rate of retrieval of mature oocytes. Our findings regarding the lipid profile in the group with unsuccessful treatment indicate that the retrieval of mature oocytes in this group may have been inadequate. Even though we collected only CCs expanded, eventually we might have been collected CCs from immature oocytes, like oocytes in metaphase I (MI), which also show expanded DC. However, were not observed statistical difference in percentage of mature and immature oocytes (Table 1) indicates that the oocyte maturity was has not been influence in the results of lipid profile.
The intensity of ions found were above the set detection limit; however, the different ions observed between the groups were not the most intense on the spectrum (Fig. 1). Therefore, it would not be possible differentiate them without the use of statistical tools.
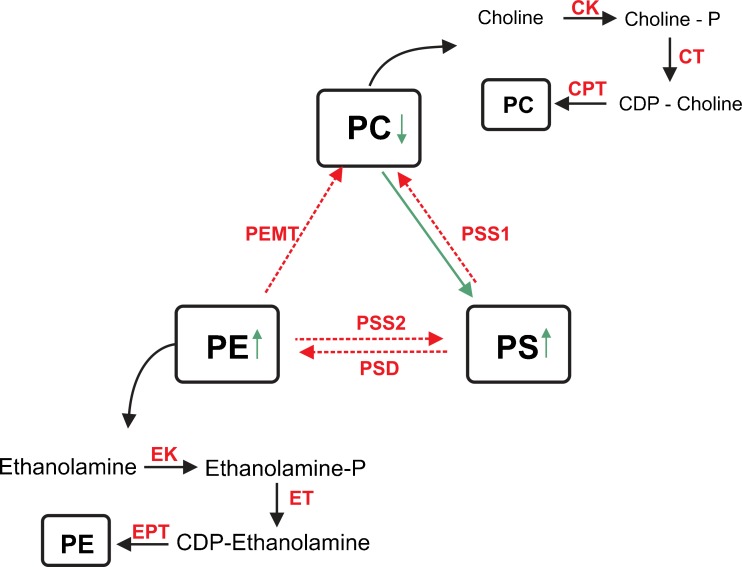
Phosphatidylcholine, identified as ions with m/z 886.6428 in the pregnant group, is the lipid subclass that is most abundant in cell membranes, and lipids of this class constitute 40–50 % of total phospholipids. PC roles have been well established in differentiation and cell proliferation [43]. CCs undergo proliferation before the LH surge due to the action of growth factors secreted by the oocyte to regulate the function of the CCs [17]. During the pre-ovulatory period, the action of LH begins a cascade of events leading to extensive proliferation and expansion of the CCs [10,27]. The competence to undergo expansion is a unique characteristic of the differentiation of CCs that is essential for normal oocyte development, ovulation and fertilization [11]. Therefore, PC is important for maintaining cellular integrity and cell growth. Furthermore, several studies have clearly demonstrated that PC deficiency alone may induce apoptosis [8]. Although we identified PC only in the pregnant group, as shown in Table 2, this result does not mean that it is absent in the non-pregnant group. It is possible that in the non-pregnant group, PC synthesis is decreased or is converted into the two other lipid subclasses found in this group (PE and PS), as demonstrated in Fig. 4.
Fig. 4.
Metabolic correlation between phosphatidylserine (PS) and phosphatidylethanolamine (PE), found in the non-pregnant group, and phosphatidylcholine (PC), found in the pregnant group. PSS1 PS synthase-1; PSS2 PS synthase-2; PSD phosphatidylserine decarboxylase; PEMT phosphatidylethanolamine N-methyltransferase; ET ethanolamine transferase; EK ethanolamine kinase; CPT phosphoethanolamine cytidylyltransferase; EPT ethanolamine phosphotransferase; CT ethanolamine transferase; CK choline kinase
Phosphatidylethanolamine (PE) is the second most abundant component of phospholipid membranes, constituting 20–50 % of total phospholipid, and was identified in the non-pregnant group as the ion with m/z 822.4423. PE performs several biological functions in addition to being a primary constituent of the inner leaflet of the plasma membrane. Among the biological functions described in previous studies of PE redistribution and translocation between the bilayer membranes, there are two principal functions of PE that are of interest in our study. First, PE is redistributed and transiently externalized during mitotic cell division [12], which may occur during proliferation of the CCs. PE is also redistributed and exposed to the extracellular environment that signals cell death when the membrane asymmetry is compromised [32]. Li et al. described that the decrease in the ratio of PC to PE was associated with a reduction in membrane integrity (Caballero et al.2010;[25]). However, a more recent study attributed the loss of membrane integrity to PC deficiency alone [31]. Otherwise, low PC intensity and high PE intensity in our study may indicate that the CC in non-pregnant women could be undergoing apoptosis. Nevertheless, more structural and functional studies of PE are needed to understand the mechanism that is occurring.
Phosphatidylserine (PS) is also observed in the non-pregnant group at relatively low levels and constitutes 2–10 % of the total phospholipid membrane. It was identified through the two ions found in this group with m/z 750.4191 and 806.4842. In cells, PS is located exclusively in the intracellular environment (Caballero et al.), which plays an important role because many intracellular proteins, such as protein kinases, require PS for proper activation and/or location [7,25,31]. Moreover, PS has also been described as a marker of apoptosis [4,15,16]. In contrast, other studies have been suggested that an increase in PS protects cells against apoptosis [22,30,36]. The postulated mechanism is that an increase in PS synthesis increases translocation of Raf-1, a kinase involved in apoptosis, to the membrane. In both mechanisms, PS is exposed on the cell surface and/or in the extracellular environment. Regardless of whether PS was increased in the non-pregnant group as a marker of apoptosis or as a marker of anti-apoptosis mechanisms, it is clear that these CCs were damaged, and their altered roles may leads to injury during oogenesis in these patients.
Finally, PI was identified in the non-pregnant group (m/z 807.4818) and is found relatively less often in the phospholipid cell membranes of mammals [42]. PI is a glycerophospholipid component in eukaryotic cells [36]. PI and its derivatives have been described as precursors for cellular signaling molecules [4] and in the biosynthesis of the glycerophosphotidylinositol anchor [30,36], besides being a structural component of the membrane [15]. PI and its phosphorylated forms, together with a phosphoinositide-specific phospholipase C (PI-PLC), generate diacylglycerol (DAG) [22], which is another lipid subclass. This pathway was described as being responsible for stimulating protein kinase (PK) activation, including mitogen-activated kinase protein (MAPK), which has been previously reported as an attendee in the regulation of oocyte meiotic resumption, in which it may act directly or indirectly [9]. Recent studies have shown that MAPK is required in gonadotropin-induced meiotic resumption [35,38,39,44]. Other studies have also established that MAPK as well as protein kinase A (PKA) interrupts the communication between the CCs and the oocyte, thus decreasing the cyclic adenosine monophosphate (cAMP) level in the female gamete, which initiates its meiotic resumption [26,29,35]. Therefore, we propose that the high PI intensity in the NP group indicates that the degradation of DAG has been not occurring or that it has been occurring at low activity, due to the reduced efficiency of meiotic resumption in this group.
Conclusion
Our study compared the lipid profile of cumulus cells from women who achieved pregnancy and woman who did not achieve pregnancy following IVF treatment by MALDI-TOF MS. These results suggest, through the analysis of lipid subclasses, that it is possible to predict pregnancy occurrence or failure and thus contributes to a better understanding of the putative mechanisms involved in oocyte development. Our results provide the possibility for studies of the structural characterization of these molecules and also provide opportunities to investigate the possible relations between gene and protein expression and the lipids subclasses observed in this pioneering study.
Acknowledgements
Funding for the study was provided by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process no 2010/14732-5.
Footnotes
Capsule Lipid profile by MALDI-TOF MS in cumulus cells as a predictor of pregnancy.
References
- 1.Adriaenssens T, Wathlet S, Segers I, Verheyen G, Vos A, Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–70. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R, Sciorio R, Kinnell H, Bayne R, Thong K, DeSousa P, et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138:629–37. doi: 10.1530/REP-09-0144. [DOI] [PubMed] [Google Scholar]
- 3.Assidi M, Montag M, Ven K, Ma S. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet. 2011;28:173–88. doi: 10.1007/s10815-010-9491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–64. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 5.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284:21599–612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brison DR, Houghton FD, Falconer D, Roberts SA, Hawkhead J, Humpherson PG, et al. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod. 2004;19:2319–24. doi: 10.1093/humrep/deh409. [DOI] [PubMed] [Google Scholar]
- 7.Caballero F, Fernandez A, Matias N, et al. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–36. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Z, Houweling M, Chen MH, Record M, Chap H, Vance DE, et al. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J Biol Chem. 1996;271:14668–71. doi: 10.1074/jbc.271.3.1732. [DOI] [PubMed] [Google Scholar]
- 9.Paolo G, Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 10.Diaz FJ, O'brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–48. doi: 10.1210/me.13.6.1035. [DOI] [PubMed] [Google Scholar]
- 12.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232:430–4. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 13.Eppig J. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45:824–30. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- 14.Eppig J. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–38. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 15.Fadok VA, Cathelineau A, Daleke DL, Henson PM, Dl B. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–7. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 16.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 17.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 18.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 19.Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, et al. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod. 2008;23:1118–27. doi: 10.1093/humrep/den048. [DOI] [PubMed] [Google Scholar]
- 20.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 21.Hillier S. Research challenge: what is the best non-invasive test of oocyte/embryo competence? Mol Hum Reprod. 2008;14:665. doi: 10.1093/molehr/gan068. [DOI] [PubMed] [Google Scholar]
- 22.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Park SY, Na JK, Seong ES, Yu CY. Metabolite profiling based on lipophilic compounds for quality assessment of perilla (Perilla frutescens) cultivars. J Agric Food Chem. 2012;60:2257–63. doi: 10.1021/jf204977x. [DOI] [PubMed] [Google Scholar]
- 24.Kruger T, Menkveld R, Stander F, Lombard C, Merwe V, Jp VZ, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–31. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Liang CG, Huo LJ, Zhong ZS, Chen DY, Schatten H, Sun QY. Cyclic adenosine 3',5'-monophosphate-dependent activation of mitogen-activated protein kinase in cumulus cells is essential for germinal vesicle breakdown of porcine cumulus-enclosed oocytes. Endocrinology. 2005;146:4437–44. doi: 10.1210/en.2005-0309. [DOI] [PubMed] [Google Scholar]
- 27.Lin YH, Hwang JL, Seow KM, Huang LW, Chen HJ, Tzeng CR. Effects of growth factors and granulosa cell co-culture on in-vitro maturation of oocytes. Reprod Biomed Online. 2009;19:165–70. doi: 10.1016/S1472-6483(10)60068-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Zhou C, Xu Y, Fang C, Zhang M. Pregnancy outcome in preimplantation genetic diagnosis cycle by blastomere biopsy is related to both quality and quantity of embryos on day 3. Fertil Steril. 2009;91(4 Suppl):1355–7. doi: 10.1016/j.fertnstert.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi T, Otsuka F, Inagaki K, Otani H, Takeda M, Suzuki J, et al. Differential regulation of steroidogenesis by bone morphogenetic proteins in granulosa cells: involvement of extracellularly regulated kinase signaling and oocyte actions in follicle-stimulating hormone-induced estrogen production. Endocrinology. 2007;148:337–45. doi: 10.1210/en.2006-0966. [DOI] [PubMed] [Google Scholar]
- 30.Neshat MS, Raitano AB, Wang HG, Reed JC, Sawyers CL. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol Cell Biol. 2000;20:1179–86.32. doi: 10.1128/MCB.20.4.1179-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niebergall L, Vance D. The ratio of phosphatidylcholine to phosphatidylethanolamine does not predict integrity of growing MT58 Chinese hamster ovary cells. Biochim Biophys Acta. 2012;1821:324–34. doi: 10.1016/j.bbalip.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Post JA, Bijvelt JJ, Aj V. Phosphatidylethanolamine and sarcolemmal damage during ischemia or metabolic inhibition of heart myocytes. Am J Physiol. 1995;268:H773–80. doi: 10.1152/ajpheart.1995.268.2.H773. [DOI] [PubMed] [Google Scholar]
- 33.Rantalainen M, Cloarec O, Ebbels TM, Lundstedt T, Nicholson JK, Holmes E, et al. Piecewise multivariate modelling of sequential metabolic profiling data. BMC Bioinformatics. 2008;9:105. doi: 10.1186/1471-2105-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich A, Klatsky P, Carson S, Wessel G. The transcriptome of a human polar body accurately reflects its sibling oocyte. J Biol Chem. 2011;286:40743–9. doi: 10.1074/jbc.M111.289868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2010;90:259–89. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- 36.Salomoni P, Wasik MA, Riedel RF, Reiss K, Choi JK, Skorski T, et al. Expression of constitutively active Raf-1 in the mitochondria restores antiapoptotic and leukemogenic potential of a transformation-deficient BCR/ABL mutant. J Exp Med. 1998;187:1995–2007. doi: 10.1084/jem.187.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiller J, Arnhold J, Benard S, Muller M, Reichl S, Arnold K. Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: A methodological approach. Anal Biochem. 1999;267:46–56. doi: 10.1006/abio.1998.3001. [DOI] [PubMed] [Google Scholar]
- 38.Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–44. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- 39.Shewan A, Eastburn DJ, Mostov K. Phosphoinositides in cell architecture. Cold Spring Harb Perspect Biol. 2011;3:a004796. doi: 10.1101/cshperspect.a004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirard MA, Dufort I, Coenen K, Tremblay K, Massicotte L, Robert C. The use of genomics and proteomics to understand oocyte and early embryo functions in farm animals. Reprod Suppl. 2003;61:117–29. [PubMed] [Google Scholar]
- 41.Tanghe S, Soom A, Nauwynck H, Coryn M, Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61:414–24. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 42.Vance J. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–87. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H, Fukami K, Watanabe Y, Ozaki C, Takenawa T. Phosphatidylinositol 4,5-bisphosphate reverses the inhibition of RNA transcription caused by histone H1. Eur J Biochem. 1998;251:281–7. doi: 10.1046/j.1432-1327.1998.2510281.x. [DOI] [PubMed] [Google Scholar]
- 45.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]