Abstract
Background
Recurrent pregnancy loss (RPL) had said to be related to the angiotensin converting enzyme insertion/deletion polymorphisms (ACE I/D) gene polymorphisms. But the conclusions were controversial. This meta-analysis was conducted to investigate the real association in ACE I/D polymorphisms and RPL firstly.
Methods
Combine Pubmed Embase and HuGENet database in data analysis for this meta-analysis from October 2000 to November 2011. The metagen system was used to select the models and effects. Odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of this association.
Results
9 studies from six countries with 1264 RPL and 845 controls were included according to our criterion. Following the metagen system, we used the dominant model with random effects. The summary OR =1.61 (95% CI: 1.10-2.36, I2 = 59.0%), which suggested the ACE D allele might increase the RPL risk in Asia (OR=1.97, 95% CI: 1.31-2.98, I2 = 44.4%), among Asians (OR=1.69, 95% CI: 1.06-2.36, I2 =32.7%). In additional, after conducting sensitivity analysis, the results had no differences except for Caucasian subgroup reached to the significance (OR=2.059, 95% CI: 1.455-2.914), so we couldn’t ignore the relationship between the polymorphisms of ACE D/I gene and Caucasians yet. There seemed no publication bias in our eligible studies with Begg’s test (P = 0.867).
Conclusions
Results in this meta-analysis presented the positive function of the ACE I/D polymorphism in increasing the RPL risk. Furfure prospective studies were needed to confirm the precise relationship between the ACE I/D and RPL.
Keywords: Meta-analysis, Angiotensin-converting enzyme insertion/deletion, Gene, Polymorphism, Recurrent pregnancy loss
Back group
Description and variations of the gene
ACE, which generates angiotensin II from angiotensin I as a potent vasopressor [1–3], is a key component of the rennin–angiotensin system (RAS) that affects homeostasis [4]. In addition, the relationship between ACE and the I/D polymorphism of the ACE gene is close [5]. There is evidence that the presence of the D allele or D/D genotype is correlated with elevated plasma and tissue-specific ACE activity. In 1990, a polymorphism was defined in the ACE gene (rs4646994) by Rigat et al. [6] as either the presence (insertion, I) or absence (deletion, D) of a 287bp fragment in intron 16, leading to three genotypes: I/I, I/D, and D/D. Moreover, it was shown that the ACE D allele leads to increased expression of plasminogen activator inhibitor-1 (PAI-1), which can increase the risk of thrombotic events and enhances the production of angiotensin II from angiotensin I [7, 8]. On the account of the thrombophilic defects in gravidas, the risks for pregnancy-associated thromboembolism, and other vascular complications, such as pre-eclampsia and abortion, will be improved [1].
Disease
As is known, women are presented a hypercoagulable state during pregnancy, which may be important in impairing placental flow and foetal growth, but may also predispose patients to develop venous thrombosis at the challenge of delivery.
RPL is defined as two or more consecutive pregnancy losses before 20 weeks gestational age. Up to 5% of women of reproductive age suffer from this, which is actually one of the most common causes of female sterility [9], and approximately 1% of patients have three or more pregnancy losses [10]. The known aetiologic factors for RPL include parental chromosome abnormalities, endocrinological disorders, hereditary thrombophilia, immunologic factors, male factors, and environmental factors. In addition, the ACE I/D polymorphism has been found to be associated with the disease and the hypofibrinolytic disorders [11, 12]. Although the exact factors for RPL are not confirmed, the inherited predisposition to thrombophilia may be one of the main causes according to some studies [13–17].
Recently, a number of genetic polymorphisms have been investigated by meta-analysis for association with RPL, in genes such as the sex hormone receptor gene [18], the vascular endothelial growth factor (VEGF) gene, the p53 gene, endothelial nitric oxide synthase (eNOS) gene [19], 1082/interleukin-10 (IL-10) gene [20], and the MTHFR gene [21]. However, there have been no meta-analyses describing the ACE I/D polymorphism association with RPL. This study aimed to evaluate the influence of ACE I/D polymorphism on RPL with the data from 9 studies published between October 2000 and November 2011.
Materials and methods
Search criteria
The Pubmed, Embase and HuGENet databases were combined in this search, with the key words: ‘ACE’, ‘angiotensin-converting enzyme’, ‘thrombophilia’, ‘thrombophilic’, ‘polymorphism’, ‘recurrent spontaneous abortion’, ‘recurrent pregnancy loss’, ‘foetal loss’ and ‘recurrent spontaneous miscarriages’. The latest search was performed in March 2012. The languages of articles were restricted to English. All articles in the meta-analysis were published in the primary literature and were not replicated in other studies. The inclusion criteria were as follows: (1) the studies were performed as case–control or cohort studies of the association between the ACE I/D polymorphism and the risk of recurrent pregnancy loss; (2) the samples that each had more than two pregnancy losses, with no essential diseases inducing the losses were directly classified into case group, and healthy patients or those with one or no losses ones were defined as controls; (3) studies contained recurrent pregnancy loss cases and free controls; (4) studies satisfied Hardy–Weinberg equilibrium. In addition, the studies were restricted it as follows for exclusion criteria: (1) the classifications in studies made it hard to screen cases and controls; (2) studies did not have raw data.
The following data was recorded for each study: author; year of publication; country; continent of the country; number of cases and controls for each ACE I/D and the racial descent of the study population (categorised as Caucasian or Asian) (Table 1).
Table 1.
The essential characteristics of the studies we included
| Authors | Year | Country | Continent | Race | Eligible | Subjects | Method |
|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||
| C. Fatini et al. | 2000 | Italy | Europe | Caucasian | 59 | 70 | PCR |
| T.Buchholz et al. | 2003 | Germany | Europe | Caucasian | 184 | 127 | PCR |
| Chelsi Goodman et al. | 2009 | USA | North America | Caucasian | 120 | 84 | PCR |
| Rami J. Al Sallout et al. | 2010 | Gaza Strip | Asia | Asian | 100 | 100 | PCR |
| MORTEZA BAGHERI et al. | 2010 | Iran | Asia | Asian | 50 | 63 | PCR |
| R. M. Corbo et al. | 2011 | Italy | Europe | Caucasian | 18 | 69 | PCR |
| MAHMOUD AARABI et al. | 2011 | Iran | Asia | Asian | 63 | 94 | PCR |
| Shufang Zhang, MS et al. | 2011 | China | Asia | Asian | 127 | 132 | PCR |
| Ozturk Ozdemir et al. | 2011 | Turkey | Asia | Caucasian | 543 | 106 | PCR |
Statistical analysis
In this meta-analysis, statistics were used to test Hardy–Weinberg equilibrium (HWE; P>0.05) in the control group [22]. In the stage of selecting the genotypic models, the metagen system was used [23], which showed that the dominant model (D/D + D/I versus I/I) would be the appropriate one to describe the relationship between the ACE I/D polymorphism and RPL when the statistical heterogeneity across studies was observed. The odds ratio (OR) with a corresponding 95% confidence interval (CI) was used to reveal the effect of the association. Based on the individual ORs, the pooled OR was estimated. Using this calculation, the I2 would show the criterion to select the fixed effects and the random effects: the fixed effect would be used when I2 <50%, otherwise random effects would be reserved. In addition, when the results of the constituent studies differed among themselves, the effects incorporated an estimate of the inter-study variance and provided wider 95% confidence intervals (95% CI). At the same time, the I2 based Q statistic was used, which described the weighted sum of the squared difference between the overall effect size and the effect size from each study, in order to assess heterogeneity (P<0.10 as the standard) [24]. In order to define the resources of heterogeneity, subgroup analysis was performed. The subgroups were collected together with similar characteristics, such as country, continent and ethnicity. There were seven countries in total: Italy, India, USA, Gaza Strip, Iran, China and Turkey, which were categorised into three continents: Europe, Asia, and North America. With regards to ethnic subgroups, there were two groups: Caucasian and Asian.
After the above procedures were completed, the susceptibility analysis was carried out to estimate the function of every heterogeneity study, and to extract the most heterogeneous study to recalculate the pooled OR, in order to evaluate the effect of the article to the results in total. Using Begg’s unweighted regression test, publication bias was diagnosed both visually by using a funnel plot and statistically. All of the allele frequencies were calculated for studies reporting only genotype data. The analyses were conducted using Stata 9.0 (Stata Corporation, USA), and all P values were two-tailed.
Results
Characteristics
After searching the PubMed, Embase and HuGENet database with the associated key words, there were 244 articles about the ACE gene polymorphism. 15 relevant studies were identified that described the association between the ACE I/D polymorphism and RPL. While reading the full texts, the following articles were removed: two studies did not meet Hardy–Weinberg equilibrium (P = 0.0003 and P = 0.0217, respectively) [25, 26]; one study could not extract the allele data (ACE I/D in RPL) from its groups [27]; one study was not only focused on RPL, but also on pre-eclampsia and foetal growth restriction which made it hard to get the data required [28]; and the other two studies included the data of case and control groups, but little information about the ACE I/D polymorphism [16, 29]. Finally, there were 9 studies meeting the inclusion criteria that were included [11, 30–37]. Among the collected studies, five investigated Caucasian populations [11, 30, 31, 34, 37], and the others investigated Asian patients [33, 34, 36, 37]. They were located in Italy, China, USA, Gaza Strip, Iran and Turkey. The case groups were defined as experiencing at least two unexplained consecutive spontaneous miscarriages without other factors influencing the miscarriages directly. The case populations had no history of chronic infections, thromboses, autoimmune diseases, endocrinologic disorders, or congenital anomalies [11, 30–37]. All of the cases came from the hospital, and the controls were limited to patients having delivered at least one healthy, term infant with no history of pregnancy loss or healthy volunteers.
Subgroup analyses and study quality
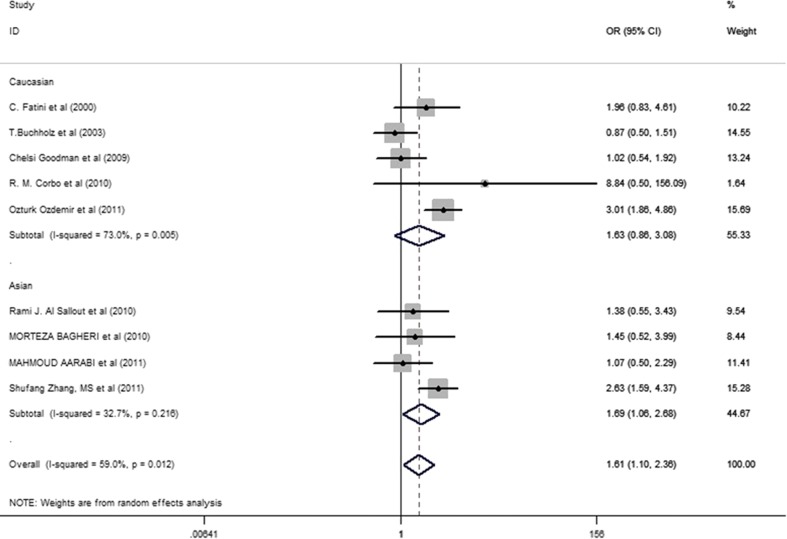
After analyzing samples using the metagen system, the dominant model was chosen with random effects. The summary OR = 1.61 (95% CI: 1.10-2.36, I2 = 59.0%). When calculating the entire data grouping by continent, via random effects, the D allele of the ACE I/D polymorphism was shown to be the risk factor for the group from Asia, in comparison to other continents (Asia: OR = 1.97, 95% CI: 1.31-2.98, I2 = 44.4%; Europe: OR = 1.45, 95% CI: 0.61-3.44, I2 = 54.6%). With regards to ethnic groups, the same result was found for Asians but not in Caucasians with an OR of 1.69 (95% CI: 1.06-2.36, I2 = 32.7%) for the Asian group and an OR of 1.63 (95% CI: 0.86-3.08, I2 = 73.0%) for the Caucasian group (Fig. 1).
Fig. 1.
Meta-analysis with a dominant model (DD + ID versus II allele) and random-effects for the association between RPL risk and the ACE D/I polymorphism. The name of the first author and year of publication for each study was shown. The race subgroups were divided into Caucasian and Asian. For each continent and race comparison the OR and accompanying 95% CI are revealed to show the association of the ACE D/I polymorphism with RPL
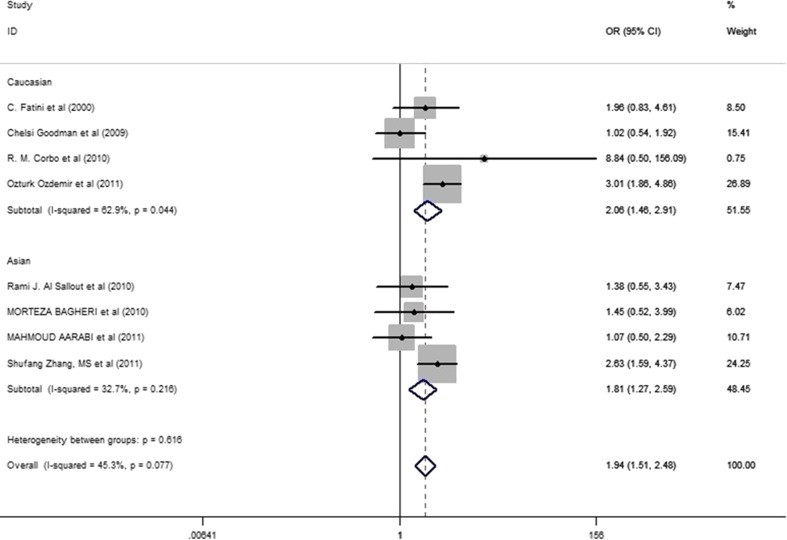
Because of the I2 > 50% there seems to be high heterogeneity. So we conduct the sensitivity analysis which can reveal the studies influencing the heterogeneity of results tremendously. After the sensitive analysis, we remove the article with high heterogeneity. [30]. surprisingly, the value of I2 dropped to 45.3%, so the fixed effects were used. In addition, there were no differences, except for the Caucasian subgroup, that reached significance (OR=2.059, 95% CI: 1.455-2.914) (Fig. 2). This suggested that the D allele might be a protective factor not only in Asians but also among Caucasians.
Fig. 2.
Meta-analysis with a dominant model (DD + ID versus II allele) and fixed-effects for the association between RPL risk and the ACE D/I polymorphism. The name of the first author and year of publication for each study was shown. The race subgroups were divided into Caucasian and Asian. For each continent and race comparison the OR and accompanying 95% CI are revealed to show the association of the ACE D/I polymorphism with RPL. The result was showed after sensitivity analysis
Publication bias
For the assessment of publication bias, the inverted funnel plot was assessed and shown to be symmetrical by analysis using the Begg’s test (P = 0.867). Above all, the association between the ACE I/D polymorphism and RPL is revealed in Table 2.
Table 2.
The data of meta-analysis about models and subgroups
| Race | Comparisons | Genotype cases | Genotype controls | OR | 95% CI | I2 | P for heterogeneity |
|---|---|---|---|---|---|---|---|
| Europe | 3 | 261 | 266 | 1.45 | 0.61-3.44 | 54.6% | 0.110 |
| Asia | 5 | 883 | 495 | 1.97 | 1.31-2.98 | 44.4% | 0.126 |
| Caucasian | 5 | 924 | 456 | 1.63 | 0.86-3.08 | 73.0% | 0.005 |
| Asian | 4 | 340 | 389 | 1.61 | 1.06-2.36 | 32.7% | 0.216 |
| aEurope | 2 | 77 | 193 | 2.22 | 0.98-5.03 | 0.0% | 0.324 |
| aAsia | 5 | 883 | 495 | 2.17 | 1.63-2.89 | 44.4% | 0.126 |
| aCaucasian | 4 | 740 | 329 | 2.06 | 1.46-2.91 | 62.9% | 0.044 |
| aAsian | 4 | 340 | 389 | 1.81 | 1.27-2.59 | 32.7% | 0.216 |
a After the sensitive analysis, remove the article of heterogeneity. [30]
Discussion
Description
As with thrombophilia genes, the ACE gene does not only reduce brinolysis and restrict bleeding during pregnancy, but also increases the risk for other vascular complications, such as pre-eclampsia and abortion. Recently, however, some studies have reported an association between RPL and the ACE I/D polymorphism, with incompatible results, but there is no meta-analysis describing the association between the ACE I/D polymorphism and RPL. In addition, with the large amount of samples in the similar studies investigating the association between RPL and the ACE I/D polymorphism, the conclusion of this meta-analysis will be more powerful.
This meta-analysis is based on 9 studies containing 1264 cases and 845 controls. The major finding of this study demonstrates that the D allele of the ACE I/D polymorphism is likely to be a major risk factor for susceptibility to RPL among gravidas especially in Asia and Asian populations.
Subgroups results and sensitivity analysis
In the special continent subgroup, in Asia, the summary OR was 1.97 (95% CI: 1.31-2.98, I2 = 44.4%), and in the race subgroup, the D allele was also a risk factor in Asians (OR=1.69, 95% CI: 1.06-2.36), but showed no association in other subgroups. This suggests a possible role for ethnic differences as a result of genetic backgrounds and the environment. The same results were proposed by Shufang Zhang et al. [36] and Ozturk Ozdemir et al. [37] in their recent work, even though the incompatible viewpoints were revealed by other studies [32, 33, 35]. In addition, because the frequency of allele could affect the results of genetic association studies, the genetic background of RPL was evaluated. The frequency of the D allele in the positive studies was 18.9%, while the higher ratio was shown in other studies of Asian populations (71.0%, 59.5% and 51.6% respectively). The same results were shown for Caucasian populations (Table 3). Significantly, the frequency of the D allele, which was suggested as the potential risk allele, had a lower frequency in the controls than among the cases.
Table 3.
the frequency of the allele and the P value of Hardy–Weinberg equilibrium
| Year | Country | Racial descent | Case | Control | HWE(P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DD | ID | II | D | I | DD | ID | II | D | I | ||||
| 2000 | Italy | Caucasian | 28 (47.5%) | 21 (35.6%) | 10 (16.9%) | 77 | 41 | 20 (28.6%) | 30 (42.9%) | 20 (28.6%) | 70 (50.0%) | 70 (50.0%) | 0.2320 |
| 2003 | Italy | Caucasian | 59 (32.1%) | 83 (45.1%) | 42 (22.8%) | 201 | 167 | 30 (23.6%) | 71 (55.9%) | 26 (20.5%) | 131 (51.6%) | 123 (48.4%) | 0.1791 |
| 2009 | USA | Caucasian | 34 (28.3%) | 55 (45.8%) | 31 (25.8%) | 123 | 117 | 28 (33.3%) | 34 (40.8%) | 22 (26.2%) | 90 (53.6%) | 78 (46.4%) | 0.0877 |
| 2010 | Gaza Strip | Asian | 49 (49.0%) | 42 (42.0%) | 9 (9.0%) | 140 | 60 | 54 (54.0%) | 34 (34.0%) | 12 (12.0%) | 142 (71.0%) | 58 (29.0%) | 0.0812 |
| 2010 | Iran | Asian | 17 (34.0%) | 26 (52.0%) | 7 (14.0%) | 60 | 40 | 24 (38.1%) | 27 (42.9%) | 12 (19.0%) | 75 (59.5%) | 51 (40.5%) | 0.3801 |
| 2011 | Italy | Caucasian | 12 (66.7%) | 6 (33.3%) | 0 (0.0%) | 30 | 6 | 22 (31.9%) | 34 (49.3%) | 13 (18.8%) | 78 (56.5%) | 60 (43.5%) | 0.9830 |
| 2011 | Iran | Asian | 19 (30.2%) | 30 (47.6%) | 14 (22.2%) | 68 | 58 | 25 (26.6%) | 47 (50.0%) | 22 (23.4%) | 97 (51.6%) | 91 (48.4%) | 0.9921 |
| 2011 | China | Asian | 21 (16.5%) | 49 (38.6%) | 57 (44.9%) | 91 | 163 | 8 (6.1%) | 34 (25.8%) | 90 (68.2%) | 50 (18.9%) | 214 (81.1%) | 0.0641 |
| 2011 | Turkey | Caucasian | 212 (39.0%) | 260 (47.9%) | 71 (13.1%) | 684 | 402 | 19 (17.9%) | 54 (50.9%) | 33 (31.1%) | 92 (43.4%) | 120 (56.5%) | 0.7036 |
A sensitivity analysis was also performed, after excluding the studies influenced the by results the most [30]. The overall I2 dropped to 45.3% by analyzing fixed effects. The results reveal substantial changes: in the race analysis, the Caucasian subgroup was also shown to have a relationship with the RPL (OR = 2.06, 95% CI: 1.46-2.91). Comparing the five articles about Caucasians, no prominent differences were found among them. We infer the reason may be the gene differences in the Germany comparing to other countries.
Limitations
Although powerful studies and tests have been identified in the meta-analysis, which indicates the potential association of the risk of RPL and the ACE I/D polymorphism, there are still some limitations of the eligible studies.
(1) For the cases and controls, the studies have some differences from each other. Even though the studies have similar inclusion criteria, there are also some differences such as cases lacking some factors which could induce RPL directly, but these factors are not exactly the same. Moreover, with regards to the resources of the samples, some controls came from the hospitals, others were healthy and the other studies included the mixed groups. In addition, the definitions of the maternal ages in every study have some potential differences. However, it is said that maternal age is one of the factors that can enhance the effect of genetic polymorphisms [18]. Therefore, the factors mentioned above might lead to the selection bias, which was a possible major source of heterogeneity resulting from non-systemic samples, creating some deviation. (2) Only studies published in English from the three selected databases were included for the data analysis. Therefore, some potential studies which were included in other databases, those published in other languages or unpublished studies could be missed. As a result, data in the selected articles could not be extracted, which could lead to the data deletion. (3) In this meta-analysis, the I2 seems to be high, as does the pooled P value, which can lead to result bias. In addition, only focusing on the relationship between the genetic polymorphism and the function of the environment is also a source of limitation.
Conclusions
RPL is a serious disease influencing the reproduction of humans. To forecast it by detecting ACE I/D polymorphisms may be a new way to decrease the incidences. Based on the studies available, this meta-analysis demonstrated that the ACE I/D polymorphisms is associated with RPL susceptibility, especially the number of D allele, which is the main risk factor for RPL in Asia and Asian populations, as well as in Caucasians.
Acknowledgments
This study was partially supported by grants from the Guangxi major disease prevention and control of new technology research and development (1298003-6-4), Guangxi Population Research Foundation (1104) from Guangxi family planning commission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Capsule ACE D/I polymorphism increase the RPL risk.
Chen Yang, Wu Fangfang and Li Jie-These authors contributed equally to this work.
Contributor Information
Zhang Xuerong, Email: zxrsv@sina.com.
Hu Yanling, Email: ylhupost@163.com.
References
- 1.Kempf Haber M, Klimek M. Thrombophilia in pregnancy and its influence on venous thromboembolism and recurrent miscarriages. Przegl Lek. 2005;62:164–168. [PubMed] [Google Scholar]
- 2.Sayed-Tabatabaei FA, Oostra BA, Isaacs A, Duijn CM, Witteman JC. ACE polymorphisms. Circ Res. 2006;98:1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 3.Ueda S, Elliott HL, Morton JJ, Connell JM. Enhanced pressor response to angiotensin I in normotensive men with the ACE deletion allele. Hypertension. 1995;25:1266–1269. doi: 10.1161/01.HYP.25.6.1266. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz T, Thaler CJ. Inherited thrombophilia: impact on human reproduction. Am J Reprod Immunol. 2003;49:1–13. doi: 10.1034/j.1600-0897.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiwanitkit V. Angiotensin-converting enzyme gene polymorphism: I and D alleles from some different countries. Clin Appl Thromb Hemost. 2004;10:179–182. doi: 10.1177/107602960401000209. [DOI] [PubMed] [Google Scholar]
- 6.Rigat B, Hubert C, Alhenc-Gelas F, Caambien F, Corvol P, Soubrier F. An insertion ⁄ deletion polymorphism in the angiotensin I converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatini C, Gensini F, Sticchi E, Battaglini B, Prisco D, Fedi S, et al. ACE DD genotype: an independent predisposition factor to venous thromboembolism. Eur J Clin Invest. 2003;33:642–647. doi: 10.1046/j.1365-2362.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Kim JW, Kim S, Gwon HC, Ryu JC, Huh JE, et al. Polymorphism of angiotensin converting enzyme gene is associated with circulating levels of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 1997;17:3242–3247. doi: 10.1161/01.ATV.17.11.3242. [DOI] [PubMed] [Google Scholar]
- 9.Sarig G, Younis JS, Hoffman R, Lanir N, Blumenfeld Z, Brenner B. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342–347. doi: 10.1016/S0015-0282(01)02971-5. [DOI] [PubMed] [Google Scholar]
- 10.Younis JS, Ohel G, Brenner B, Ben-Ami M. Familial thrombophilia—the scientific rationale for thrombophylaxis in recurrent pregnancy loss? Hum Reprod. 1997;12:1389–1390. doi: 10.1093/humrep/12.7.1389. [DOI] [PubMed] [Google Scholar]
- 11.Fatini C, Gensini F, Battaglini B, Prisco D, Cellai AP, Fedi S, et al. Angiotensin-converting enzyme DD genotype, angiotensin type 1 receptor CC genotype, and hyperhomocysteinemia increase firsttrimester fetal-loss susceptibility. Blood Coagul Fibrinolysis. 2000;11:657–662. doi: 10.1097/00001721-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Dossenbach-Glaninger A, Trotsenburg M, Schneider B, Oberkanins C, Hopmeier P. ACE I/D polymorphism and recurrent first trimester pregnancy loss: interaction with SERPINE1 4G/5G and F13 Val34Leu polymorphisms. Br J Haematol. 2008;141:269–271. doi: 10.1111/j.1365-2141.2008.07058.x. [DOI] [PubMed] [Google Scholar]
- 13.Middeldorp S. Pregnancy failure and heritable thrombophilia. Semin Hematol. 2007;44:93–97. doi: 10.1053/j.seminhematol.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Eldor A. Thrombophilia, thrombosis and pregnancy. Thromb Haemost. 2001;86:104–111. [PubMed] [Google Scholar]
- 15.Carp H, Salomon O, Seidman D, Dardik R, Rosenberg N, Inbal A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum Reprod. 2002;17:1633–1637. doi: 10.1093/humrep/17.6.1633. [DOI] [PubMed] [Google Scholar]
- 16.Prandoni P, Tormene D, Simioni P, Girolami A. Venous thromboembolism, fetal loss and preeclampsia in pregnant women with congenital thrombophilia. Clin Lab. 2001;47:155–159. [PubMed] [Google Scholar]
- 17.Micco P, D’Uva M, Strina I, Placido G, Fiore R, Quaranta S, et al. Recurrent pregnancy loss and thrombophilia. Clin Lab. 2007;53:309–314. [PubMed] [Google Scholar]
- 18.Su MT, Lin SH, Chen YC. Association of sex hormone receptor gene polymorphisms with recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2011;96:1435–1444. doi: 10.1016/j.fertnstert.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:803–812. doi: 10.1093/humupd/dmr027. [DOI] [PubMed] [Google Scholar]
- 20.Ostojic S, Pereza N, Kastrin A, Peterlin B. Association between genetic polymorphisms in cytokine genes and recurrent miscarriage–a meta-analysis. Reprod Biomed Online. 2009;19:406–414. doi: 10.1016/S1472-6483(10)60176-9. [DOI] [PubMed] [Google Scholar]
- 21.Nelen WL, Blom HJ, Steegers EA, Heijer M, Eskes TK. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril. 2000;74:1196–1199. doi: 10.1016/S0015-0282(00)01595-8. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Ha TC, Tai BC. XRCC1 gene polymorphisms and breast cancer risk in different populations: A meta-analysis. Breast. 2009;18:183–191. doi: 10.1016/j.breast.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Vettriselvi V, Vijayalakshmi K, Paul SF, Venkatachalam P. ACE and MTHFR gene polymorphisms in unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2008;34:301–306. doi: 10.1111/j.1447-0756.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi YS, Kwon H, Kim JH, Shin JE, Choi Y, Yoon TK, et al. Haplotype-based association of ACE I/D, AT1R 1166A>C, and AGT M235T polymorphisms in renin–angiotensin–aldosterone system genes in Korean women with idiopathic recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 2011;158:225–228. doi: 10.1016/j.ejogrb.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Bukreeva L, Grigorov A, Kiesewetter H, Hoppe B. Association of angiotensin-converting enzyme intron 16 insertion/deletion polymorphism with history of foetal loss. J Renin Angiotensin Aldosterone Syst. 2009;10:237–240. doi: 10.1177/1470320309343813. [DOI] [PubMed] [Google Scholar]
- 28.Mello G, Parretti E, Gensini F, Sticchi E, Mecacci F, Scarselli G, et al. Maternal-Fetal Flow, Negative Events, and Preeclampsia Role of ACE I/D Polymorphism. Hypertension. 2003;41:932–937. doi: 10.1161/01.HYP.0000063146.40351.AD. [DOI] [PubMed] [Google Scholar]
- 29.Yenicesu GI, Cetin M, Ozdemir O, Cetin A, Ozen F, Yenicesu C, et al. A Prospective Case–Control Study Analyzes 12 Thrombophilic Gene Mutations in Turkish Couples with Recurrent Pregnancy Loss. Am J Reprod Immunol. 2010;63:126–136. doi: 10.1111/j.1600-0897.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Buchholz T, Lohse P, Rogenhofer N, Kosian E, Pihusch R, Thaler CJ. Polymorphisms in the ACE and PAI-1 genes are associated with recurrent spontaneous miscarriages. Hum Reprod. 2003;18:2473–2477. doi: 10.1093/humrep/deg474. [DOI] [PubMed] [Google Scholar]
- 31.Goodman C, Hur J, Goodman CS, Jeyendran RS, Coulam C. Are Polymorphisms in the ACE and PAI-1 Genes Associated with Recurrent Spontaneous Miscarriages? Am J Reprod Immunol. 2009;62:365–370. doi: 10.1111/j.1600-0897.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Sallout RJ, Sharif FA. Polymorphisms in NOS3, ACE and PAI-1 Genes and Risk of Spontaneous Recurrent Miscarriage in the Gaza Strip. Med Princ Pract. 2010;19:99–104. doi: 10.1159/000273067. [DOI] [PubMed] [Google Scholar]
- 33.Bagheri M. Abdi Rad I, Omrani MD, Nanbaksh F. Polymorphisms of the angiotensin converting enzyme gene in Iranian Azeri Turkish women with unexplained recurrent pregnancy loss. Hum Fertil (Camb) 2010;13:79–82. doi: 10.3109/14647273.2010.484844. [DOI] [PubMed] [Google Scholar]
- 34.Corbo RM, Ulizzi L, Piombo L, Scacchi R. Association of ACE I/D polymorphism and recurrent miscarriages in an Italian population with a pre-modern reproductive pattern. Ann Hum Biol. 2011;38:102–105. doi: 10.3109/03014460.2010.481265. [DOI] [PubMed] [Google Scholar]
- 35.Aarabi M, Memariani T, Arefi S, Aarabi M. Hantoosh Zadeh S, Akhondi MA, Modarressi MH. Polymorphisms of plasminogen activator inhibitor-1, angiotensin converting enzyme and coagulation factor XIII genes in patients with recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:545–548. doi: 10.3109/14767058.2010.511331. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Wang J, Wang B, Ping Y, Ma X. Strong Association Between Angiotensin I-Converting Enzyme I/D Polymorphism and Unexplained Recurrent Miscarriage of Chinese Women—A Case–Control Study. Reprod Sci. 2011;18:743–746. doi: 10.1177/1933719111415865. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir O, Yenicesu GI, Silan F, Köksal B, Atik S, Ozen F et al. Recurrent Pregnancy Loss and Its Relation to Combined Parental Thrombophilic Gene Mutations. Genet Test Mol Biomarkers. 2011; 2: [Epub ahead of print]. [DOI] [PubMed]