Abstract
Purpose
The purpose of this study is to study lipid metabolism in oocytes and embryos that is a neglected parameter in human IVF.
Methods
We have tested the total carnitine content (TC) in the follicular fluid of 278 patients (217 non pregnant, 61 pregnant) undergoing IVF.
Results
The follicular fluid TC is neither correlated with the circulating estradiol content in serum nor with the outcome the IVF attempt. Carnitine, through the carnitine shuttle, is a major partner in lipid beta oxidation, metabolic pathway involved in the acquisition of oocyte competence. The expression of carnitine synthesis enzymes and lipid beta oxidation was studied in cumulus cells collected at the time of ovum retrieval and in oocyte. Surprisingly the expression for carnitine synthesis is not detectable in oocytes whereas the enzymes involved in lipid beta oxidation are rather strongly expressed.
Conclusions
The addition of carnitine in oocyte maturation and embryo culture media should not be overlooked.
Keywords: Human follicular fluid, Oocyte, Carnitine, Lipid betaoxidation, Mitochondria
Introduction
(L)Carnitine is a molecule synthesized from lysine and methionine containing a quaternary ammonium function. It plays a major role in the catabolism of lipids, allowing the transport of fatty acids from the cytosol to the mitochondria, where they are metabolized through beta oxidation (carnitine shuttle). Long chain fatty acid oxidation is of paramount importance during human early development [1]. The mitochondria have a preeminent role in oocyte competence and early embryo developmental capacity [2-4]. Even if the lipid content of the mouse oocyte is weak (like in human), when compared to the pig and the cow, beta oxidation seems essential for meiotic resumption [5] and oocyte competence [6, 7]. Its role in early pre-implantation development seems relevant in mouse [6-8] and bovine [9]. In terms of energy yields, one molecule of glucose forms in theory (maximum value) 38 moles of ATP for 106 ATP formed with palmitate, but with a higher formation of Reactive oxygen species (ROS). For Van Blerkom et al. [4], oocyte content of ATP is of major importance for early embryonic development. In human, in the era of in vitro maturation, fertilization and embryo culture, little is known about the metabolism of lipids by the oocyte and early embryo. In this study we have determined the environment of the maturing oocyte, in terms of access to lipid beta oxidation. The concentration of total carnitine (free+esterified) has been determined in follicular fluid of patients undergoing IVF/ICSI, in order to find out if carnitine concentration could be a marker of oocyte quality and further a predictor of pregnancy. The total carnitine concentration was chosen because the esterification process is highly versatile in follicular fluid [10]. We have also tried to determine if a correlation could be established between blood estradiol level and total carnitine concentration in the follicular fluid. Moreover, the expression of the enzymes involved in the carnitine shuttle of the cumulus cells and the oocyte was determined, in order to define the importance of these pathways in human oocytes during the first stages of embryo development from maternal oocyte to zygotic transition. The expression of carnitine acyl transferase, carnitine-acylcarnitine translocase and carnitine acyltransferase II was checked as well. Furthermore, we tried to determine if the addition of carnitine could be important during the final stages of oocyte maturation and early embryo development. For this purpose, the expression of 2 enzymes involved in carnitine biosynthesis, N6-trimethyllysine hydrolase and gamma-butyrobetaïne hydroxylase, was tested in oocyte and cumulus cells.
Material and methods
Patients
278 female patients were included in the study, in two assisted reproduction centers: Clinique Pierre Cherest (Neuilly, France), Clinique du Cotentin (Equeurdreville, 50120 France), for the collect of follicular fluid, the testing of total carnitine and serum estradiol. As the follicular fluid is discarded after oocyte isolation, no ethical committee advice is needed.
The mRNA oocyte content was analyzed from a library established in our center, according to a protocol previously described [11, 12]. The cumulus cells mRNA content and the expression of the pathways involved in lipid beta oxidation were analyzed in the Gurgan Clinic, Ankara [13]. The stimulation protocols were as classically described: long-term desensitization followed by ovarian stimulation with recombinant FSH or human menopausal gonadotrophin (Fostimon: Genevrier, Sophia Antipolis, France). Oocyte retrieval was performed 35–36 h post hCG injection. In a first approach 2 samples were collected in each patient in order to determine if a significant difference can be observed between follicles. The follicular fluids of 278 patients were immediately centrifuged after oocyte isolation. The fluids containing solid residues and/or blood cells were discarded. The supernatants were frozen in liquid nitrogen. Blood samples were retrieved before local anesthesia for oocyte pick up.
Transcripts analysis
The mRNA oocyte content was analyzed from a library established in our center, according to a previously described protocol [11, 12]. The work on cumulus cells (mRNA content) and expression of the pathways involved in lipid beta oxidation has been performed in Gurgan Clinic, Ankara [13], on 45 patients. Cumulus cells were removed before ICSI, then washed one time and centrifuged before storage in liquid nitrogen. The expression was determined using microarrays (affimetrix U 133 plus 2.0). No amplification was usually necessary. Data were normalized using the algorithm MAS5, in order to generate a signal proportional to the level of expression for each of the 54,613 chip transcripts (probe set ID). A call signal is indicated and specified if the gene is expressed at a level above the background. Housekeeping and spike controls were used for each determination.
Statistical analysis
Correlation coefficients (R2) were calculated using spearman correlation two-sided test. We compared follicular fluid carnitine concentration in pregnant patients versus non-pregnant patients using a two-sided t-test. Statistical significance was considered when P-value < 0.05.
Results
Follicular fluid carnitine content
The mean age of the patients was 31.6+/−4.6 yr (range 18–43), with no difference between the pregnant and not pregnant group, the mean number of oocytes retrieved was 9.0, with no statistical difference between the 2 groups. The mean size of the follicles on the day of hCG was 20 mm and the average volume of follicular fluid collected was 1.2 ml. Only the samples free of blood cells were studies. In the first series of experiments, in 18 patients 2 samples of FF were analyzed at random. The paired values were analyzed in order to determine if a there was a variation in Total carnitine (TC) content. The technique was validated by showing no existing difference between carnitine concentration in 2 samples originating from the same patient (R2 = 0.92).
The total carnitine content in follicular fluid has been found to be highly variable: from 0.6 μmol/L to 45.8 μ/L. There was no statistically significant difference between the follicular fluid carnitine concentration of pregnant women (N = 61) as compared to non pregnant women (N = 217) (p-value = 0.14): Pregnant =15.02 μmol/L(+/−9.92) and non pregnant =17.03 μmol/L(+/−10.41).
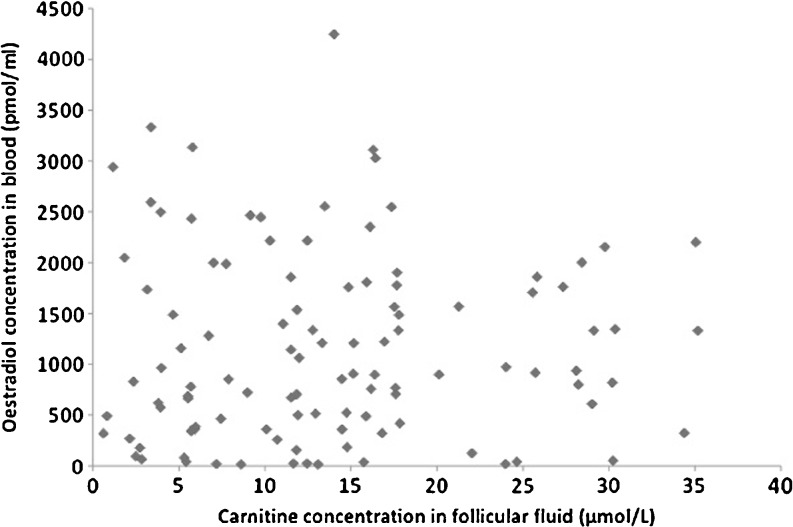
No correlation was found between the follicular fluid TC content and serum estradiol R2 = 0.001 (Fig. 1), the number of oocytes retrieved and the volume of follicular fluid collected
Fig. 1.
Relationship between Follicular fluid total carnitine (Micromolar) and estradiol concentration in blood (pmoles/mL)
Expression of beta oxidation pathway and carnitine synthesis in oocytes and cumulus cells
Carnitine synthesis
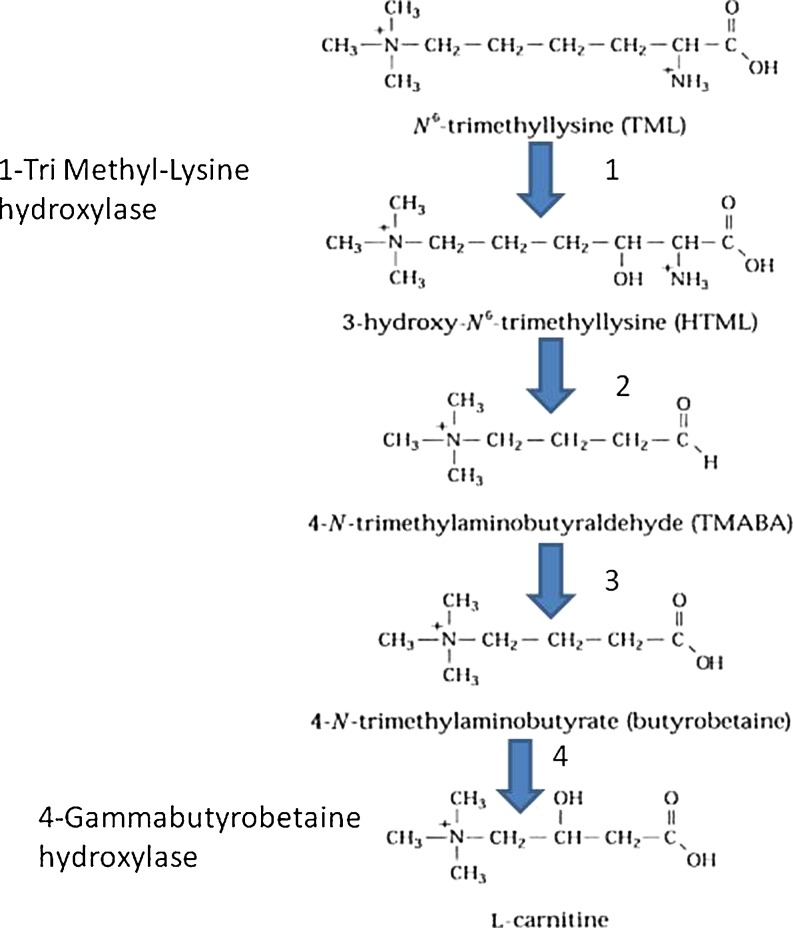
We did not find any expression in the 2 steps of carnitine synthesis tested here either in oocytes or in CC cells (Fig. 2, Table 1).
Fig. 2.
The four more important steps in carnitine synthesis. Only the tri methyl Lysine hydroxylase and the gammabutyrobetaine hydoxylase have been tested
Table 1.
Expression of carnitine synthesis pathway
| Enzyme/Signal | Oocytes | CC cells |
|---|---|---|
| Carnitine synthesis | ||
| Trimethyl lysine hydroxylase | 23 | 230 |
| Gamma butyrobetaine hydroxylase | 16.9 | 434 |
Detection limits for positive expression S = 50 for oocytes, S = 500 for CC cells)
Lipid beta oxidation (Table 2, Fig. 3)
Table 2.
Expression of lipid beta oxidation and carnitine shuttle pathway and carnitine shuttle
| Oocytes | Cumulus cells | ||||
|---|---|---|---|---|---|
| Step 1: Activation of fatty acids: AcylCoA synthesis | |||||
| AcylCoA synthetase | Long chain 1 | 608(200)a | 1784 (144) | ||
| Long chain 2 | 447 (127) | ND | |||
| Long chain 3 | 5636(1852) | 1520 (173) | |||
| Long chain 4 | NDa | 1729 (188) | |||
| Long chain 5 | NDa | NDa | |||
| Long chain 6 | 393 (88) | ND | |||
| Step2: Penetration of activated fatty acids in mitochondria, Carnitine shuttle | |||||
| Carnitine PalmitoYl transferase | I | 627 (285) | ND | ||
| “” | “ | “ | II | 1060 (133) | ND |
| Translocase | 886 (277) | 1396 (132) | |||
| Step 3: Dehydrogenation of activated fatty acids | |||||
| Acyl coA dehydrogenase (FAD dependant) | |||||
| Very long chain (VL) | 1186(186) | 1807(651) | |||
| Long Chain (L) | ND | ND | |||
| C4–C12 straight chain (M) | 342(110) | 1957(410) | |||
| Short chain (S) | 345(183) | ND | |||
| Short branched (SB) | ND | ND | |||
| Step 4: Hydratation of dehydroacylCoA | |||||
| Enoyl CoA hydratase (crotonase) | 4694 (1985) | 4552 (732) | |||
| Step 5: Shortening of the fatty acid chain and release of Acetyl CoA | |||||
| 3hydroxyacyl Coenzyme A dehydrogenase 3-ketoacyl-Coenzyme A thiolase/ | |||||
| Enoyl-Coenzyme A hydratase (Trifunctional) | 5015(1642) | 4706 (532) | |||
Detection limits for positive expression S = 50 for oocytes, S = 500 for CC cells)
aNot detected in all the samples; ND below background level: not detected
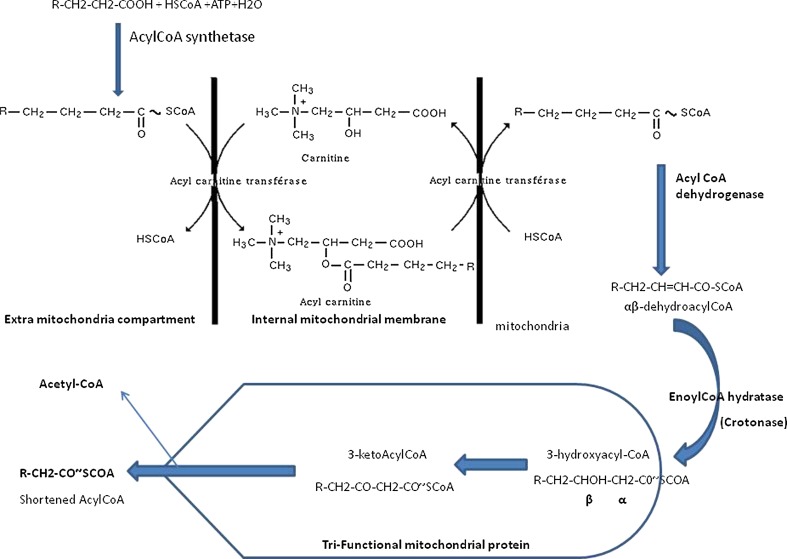
Fig. 3.
Schematic representation of beta oxidation and the enzymes involved
The large majority of the enzymes involved in lipid beta oxidation are expressed in the oocyte. For the activation of the fatty acid (FA) chains ACS 3 is the most expressed in CC cells and oocyte. The enzymes of the carnitine shuttle are expressed in the oocyte, but transferases are poorly expressed in CC cells. The dehydrogenase are more specifically oriented towards the FA with long chains. The crotonase is well expressed in CC cells and oocyte, as well as the key protein, the tri-functionnal mitochondrial protein that has an expression reaching 100 times the background level in the oocyte and 10 times the background level in CC cells.
Discussion and conclusion
We found carnitine in all the samples tested. However we were unable to determine a correlation between follicular fluid TC and serum estradiol. In the group of 278 patients a highly versatile level of TC was found (varying from 1 to 50) without any correlation between the TC level, the number of oocytes retrieved and the capacity of the oocytes to lead to a pregnancy. The mean volume collected was similar in the 2 groups of patients (1.2 mL): in any case the volume cannot explain at all the wide variations observed for TC concentration between patients. The concentration in serum was shown to be higher than in follicular fluid: 90 μM in serum for [14], or 50 μM [15] vs around 15 to 20 μM in our case. Follicular fluid TC is probably not only originating from a serum transudate.
In fine we observed that the TC content was ovary dependant and not follicle dependant. The absence of expression of the enzymes involved in carnitine synthesis in CC cells is rather a confirmation of this aspect. But it can be also argued that the cumulus cells, at this stage, have reached the end of their life’s journey and they have no longer any metabolic back up for the oocyte. However, during the late stages of oocyte maturation, follicular fluid, the surrounding milieu is rich in lipids even if at a lower level than in serum and there is a slight correlation between follicular and serum free fatty acids [16, 17].
More surprising was the absence of a significant expression for carnitine synthesis in oocyte. The mitochondria are the epicenter of oocyte and the early embryo metabolism [2-4]. Post fertilization paternal mt DNA cannot survive in the early embryo. Maternal mitochondria (around 220.000) [18] contribute extensively to energy metabolism and especially ATP formation that positively impacts further embryo development. Lipid metabolism is rather a neglected parameter in early embryonic development: all the enzymes involved in beta oxidation are expressed in the oocyte. Metabolism of lipids via beta oxidation needs carnitine for entry into the mitochondria i.e. carnitine shuttle. It is important in mouse and bovine for oocyte competence and early embryonic development [5-7, 9, 19]. The mouse preimplantation embryo is fully competent for metabolism of exogenous fatty acids [20]. In human very little is known concerning lipid metabolism, and the absence of carnitine synthesis by the oocyte and the early embryo should not be overlooked for the definition of oocyte in vitro maturation media and even for early embryo culture. The source of fatty acids can be the lipids bound to albumin. The absence of exogenous carnitine may lead to developmental anomalies in the early human embryo, which may be linked to deficiencies in lipid metabolism: this aspect can be relevant especially in women over 37. A lower level of glucose metabolism, associated with mitochondria aging [18-21] and an absence of lipid metabolism may lead to a lack of ATP and developmental arrest before blastocyst formation, as mitochondria replication only starts at this stage. Preliminary experiments have shown that in mouse, addition of carnitine can improve blastocyst development in vitro [22]: a high concentration does not seem important but its “catalytical” role seems relevant.
Acknowledgments
Fundings
The work on oocytes microarrays study was funded by Organon France and realized by partnerships (Dr Soularue). The work on cumulus cells mRNA content was sponsored by IBSA, Lugano (Switzerland), thanks to Dr Maurizio Dattilo. The salary of DM was paid by Unilabs, France
Footnotes
Capsule
We have tested the total carnitine content (TC) in the follicular fluid of 278 patients undergoing IVF, as carnitine is involved in lipid beta oxidation, participating to the oocyte competence. The follicular fluid TC is neither correlated with the circulating estradiol content in serum nor with the outcome the IVF attempt. . Surprisingly the expression for carnitine synthesis is not detectable in oocytes whereas the enzymes involved in lipid beta oxidation are rather strongly expressed. In conclusion, we think that the addition of carnitine in oocyte maturation and embryo culture media should not be overlooked.
References
- 1.Oey NA, Boer ME, Wijburg FA, Vekemans M, Augé J, Steiner C, Wanders RJ, Waterham HR, Ruiter JP, Attié-Bitach T. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005;57:755–9. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- 2.Cummins JM. The role of maternal mitochondria during oogenesis, fertilization and embryogenesis. Reprod Biomed Online. 2002;4:176–82. doi: 10.1016/S1472-6483(10)61937-2. [DOI] [PubMed] [Google Scholar]
- 3.Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 5.Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76:844–53. doi: 10.1002/mrd.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–18. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 7.Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol Reprod. 2011;85:548–55. doi: 10.1095/biolreprod.110.090415. [DOI] [PubMed] [Google Scholar]
- 8.Hewitson LC, Martin KL, Leese HJ. Effects of metabolic inhibitors on mouse preimplantation embryo development and the energy metabolism of isolated inner cell masses. Mol Reprod Dev. 1996;43:323–30. doi: 10.1002/(SICI)1098-2795(199603)43:3<323::AID-MRD6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2006;73:1195–201. doi: 10.1002/mrd.20494. [DOI] [PubMed] [Google Scholar]
- 10.Ménézo Y, Testart J, Thebault A, Frydman R, Khatchadourian C. The preovulatory follicular fluid in the human: influence of hormonal pretreatment (clomophene-hCG) on some biochemical and biophysical variables. Int J Fertil. 1982;27:47–51. [PubMed] [Google Scholar]
- 11.Menezo Y, Jr, Russo G, Tosti E, Mouatassim S, Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J Assist Reprod Genet. 2007;24:513–20. doi: 10.1007/s10815-007-9167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benkhalifa M, Montjean D, Cohen-Bacrie P, Ménézo Y. Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertil Steril. 2010;93:1585–90. doi: 10.1016/j.fertnstert.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 13.Ménézo Y, Pluntz L, Chouteau J, Gurgan T, Demirol A, Dalleac A, Benkhalifa M. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod Biomed Online. 2011;22:647–52. doi: 10.1016/j.rbmo.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in nonobese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1602–6. doi: 10.1093/humrep/den109. [DOI] [PubMed] [Google Scholar]
- 15.Takiyama N. Matsumoto K age-and sex-related differences of serum carnitine in a Japanese population. J Am Coll Nutr. 1998;17:71–4. doi: 10.1080/07315724.1998.10720458. [DOI] [PubMed] [Google Scholar]
- 16.Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95:1970–4. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menezo Y, Testart J, Khatchadourian C, Frydman R. Human preovulatory follicular fluid: the lipids. Are they the trigger for capacitation? Int J Fertil. 1984;29:61–4. [PubMed] [Google Scholar]
- 18.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10:23–32. doi: 10.1093/molehr/gah004. [DOI] [PubMed] [Google Scholar]
- 19.Sutton-McDowall ML, Feil D, Robker RL, Thompson JG, Dunning KR. Utilization of endogenous fatty acid stores for energy production in bovine preimplantation embryos. Theriogenology. 2012;77:1632–41. doi: 10.1016/j.theriogenology.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Hillman N, Flynn TJ. The metabolism of exogenous fatty acids by preimplantation mouse embryos developing in vitro. J Embryol Exp Morphol. 1980;56:157–68. [PubMed] [Google Scholar]
- 21.Wilding M, Dale B, Marino M, Matteo L, Alviggi C, Pisaturo ML, Lombardi L, Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 22.Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-Carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril. 2009;91:589–96. doi: 10.1016/j.fertnstert.2007.11.067. [DOI] [PubMed] [Google Scholar]