Abstract
Objective
To evaluate the relationship between sperm nuclear vacuoles and sperm morphology and to investigate the influence of the rate of spermatozoa with head vacuolization (SVR) in a seminal sample on the clinical outcomes in couples undergoing intracytoplasmic sperm injection.
Materials
26 patients undergoing infertility investigations were included and were divided in two groups according to an SVR ≤ 20,28 % (Group A) or > 20,28 % (Group B), and were investigated to verify the influence of SVR on the fertilization rate, embryo quality, pregnancy and implantation rates.
Results
Abnormal spermatozoa with nuclear vacuoles were significantly higher (p < 0.001) than the percentage of normal spermatozoa with nuclear vacuoles. Patients in group A had a percentage of abnormal sperm with nuclear vacuole significantly lower compared to group B (p < 0,001), but there was no difference in the percentage of normal sperm with nuclear vacuoles. Fertilization rates and the number of top quality embryos did not differ between the two groups. The pregnancy and implantation rates were significantly higher in Group A compared to Group B (respectively p < 0,05 and p < 0.001).
Conclusions
For the first time, we propose a cut off value in the proportion of sperms with nuclear vacuolization on the total of sperm in seminal samples, and demonstrate a relationship between SNV and clinical outcomes after ICSI. The SNV rate could be introduced as an easy diagnostic evaluation prior to perform an ICSI cycle.
Keywords: Sperm nuclear vacuoles, Sperm morphology, ICSI outcome, High magnification
Introduction
In recent years, increased interest to better understand the concept of “sperm competence” has provided new knowledge about the relationship between the quality of the sperm and clinical outcome in terms of pregnancy and implantation rate, this has lead many researchers to investigate new methods for the selection of sperm in IVF/ICSI treatments [16, 24, 25]. The conventional evaluation of a semen sample does not provide clear indications of the competence of sperm in producing good quality embryos with high implantation potentiality. But, although none of the conventional semen parameters can be considered definitive, sperm morphology seems to be a potential marker of sperm competence, helping, also, in the case of the single sperm morphological analysis to give interesting information about the intrinsic quality of sperm, in particular when ICSI is performed. Several studies have demonstrated that sperm morphology seems to be the best predictor of outcome in spontaneous pregnancy [2, 3], intra-uterine insemination (IUI) [1, 7] and conventional IVF [21]. Other papers considered sperm morphology as a prognostic factor of male fertility [12, 21, 28, 29].
Berkoviz et al. [9] have observed that the presence of nuclear vacuoles in spermatozoa is related to poor outcomes.
The origin of these vacuoles as well as their effect on embryo development and implantation remains unclear, but if sperm morphology is associated in IVF/ICSI cycles with the clinical outcome in terms of pregnancy and fertilization rates, it is possible to hypothesis a relationship between sperm head morphology and chromatin quality.
This field has been investigated by several researchers. Some of these have reported a relationship between the presence of a large sperm-head vacuole and failure of chromatin condensation [10, 14, 15, 23] and/or aberrant chromosome number in spermatozoa [15, 23].
The published data regarding the association between vacuoles and DNA damage are divergent. In original semen samples, nuclear vacuoles have been related to an increase in DNA fragmentation and denaturation [13, 14]. Watanabe et al. [32] did not found significative difference in the percentage of DNA fragmentation between spermatozoa with large vacuoles and those without large vacuoles, and suggested that large vacuoles were not responsible for DNA fragmentation.
The aim of the present study was to investigate the relationship between sperm nuclear vacuoles and sperm morphology in semen sample from patient undergoing ICSI cycles and to understand the influence of the rate of spermatozoa with head vacuolization in a seminal sample (SVR) on the clinical outcomes, evaluating pregnancy rate, fertilisation rate, implantation rate and embryo quality.
Materials and methods
Study participants
The study was conducted at the Centro di Biologia della Riproduzione, Palermo, Italy, from February 2012 to April 2012 on a total of 26 patients underwent ICSI (average age was 38,52 ± 5,1 years).
Semen samples from these patients were investigated to verify the influence of the rate of sperm nuclear vacuolization (SVR) on the biological and clinical outcomes in terms of fertilization rate, embryo quality, pregnancy and implantation rates. The female partners had an average age of 35,5 ± 4,68 and were comparable for demographic characteristics and for ovarian reserve.
Sperm preparation
Fresh semen samples were obtained from the 26 patients by masturbation after a sexual abstinence period of 2–5 days, collected in a sterile container, and liquefied for 20 min at 37 °C.
Semen analysis was performed according to World Health Organization [33] guidelines, and the following parameters were recorded: volume, sperm concentration, head and neck morphology, motility and vitality.
The liquefied semen sample was processed by swim-up. The sample was diluted with hepes buffered medium (G-MOPS-V1, Vitrolife, Sweden) and centrifuged at 300 g x for 7 min. The supernatant was discarded and the pellet was recovered and pipetted in the bottom of a 5 mL tube (Falcon, USA) containing fertilization medium (G-IVF, Vitrolife, Sweden).
After incubation, the upper phase was recovered for high magnification sperm analysis and ICSI.
High-magnification nuclear-vacuole analysis
For high magnification analysis 0,1 ml aliquot of prepared sample was smeared on a glass slide, air-dried at room temperature, rehydrated in HEPES-buffed medium (G-MOPS, Vitrolife, Sweden) and covered with a glass slide.
The sperm analyses were performed by the same embryologist. At least 200 spermatozoa per sample were analysed for the morphological state acrosome, post-acrosomal lamina, neck, tail and nucleus, using x100 objective with immersion oil.
The criteria for the morphologic normalcy of sperm nucleus were defined by both shape and chromatin content, according to Bartoov et al. [5]. Sperm nuclei exhibiting smooth, symmetric and oval configurations were considered morphologically normal. The nuclear content was defined abnormal if it contained one or more vacuoles that occupied more than 4 % of the normal nuclear area.
A sperm cell exhibiting a normal nucleus as well as a normal acrosome, post-acrosomal lamina, neck, tail, mitochondria, and no cytoplasmic droplet or cytoplasm around the head was classified as morphologically normal [5].
Spermatozoa were divided in 4 groups: according to morphology and presence of nuclear vacuole, normal spermatozoa with nuclear-vacuole, normal spermatozoa without nuclear-vacuole, abnormal spermatozoa with nuclear-vacuole, abnormal spermatozoa without nuclear-vacuole. Percentage of spermatozoa with nuclear vacuole was determined.
Controlled ovarian stimulation
Ovarian stimulation was achieved by pituitary downregulation using a gonadotropin-releasing hormone agonist (Buserelin, Sanofi-Aventis, Germany) followed by ovarian stimulation with recombinant follicle-stimulating hormone (Gonal-F, Serono, Genevra, Switzerland). Follicular growth was monitored by ultrasound and measurement of peripheral estradiol levels. When adequate follicular growth and serum estradiol level were observed, recombinant human chorionic gonadotropin (Gonasi, AMSA SRL, Rome, Italy) was administrated to trigger final follicular maturation. Oocyte retrieval was performed by transvaginal ultrasound guided follicle aspiration, after 34 to 36 h from hCG injection. The oocytes containing cumulus cells were collected from clear follicular fluid.
ICSI procedure
After retrieval, oocyte were incubated in culture medium (G-IVF, Vitrolife, Sweden) covered by mineral oil (Ovoil, Vitrolife, Sweden) at 37 °C, 6 % CO2 and 5 % O2 for 2–4 h until denudation. Cumulus cells were removed with a 30 seconds exposure to HEPES-buffed medium containing 40 IU/mL hyaluronidase (SAGE, USA), after which cumulus cells were manually removed using a flambe glass Pasteur pipette.
Denuded oocytes were then assessed for nuclear status and cultured in G1 medium (Vitrolife, Sweden) covered by mineral oil (Ovoil, Vitrolife, Sweden) at 37 °C, 6 % CO2 and 5 % O2; those that were observed to have released the first polar body were considered mature (MII) and used for ICSI procedure.
MII oocytes were placed individually in 2 μL droplet of HEPES-buffed medium (G-MOPS-V1, Vitrolife, Sweden) and 2 μL aliquot of prepared sperm were pipetted into a 5 μL droplet of polyvinylpyrrolidone (PVP SAGE, USA) in a culture dish (Nunc 3001, Hereford, UK) covered by warm mineral oil.
The ICSI procedure was performed using the Narishighe Micromanipulation System (Narishighe, Japan) and sperm morphology selection was assessed with an inverted microscope (Olympus, Japan), according to [22]. A single spermatozoon was chosen at x200 following to routine rough morphologic criteria for ICSI and whenever possible, were excluded spermatozoa with obvious defects, such as elongated or tapered head, macrocephalic or microcephalic heads, amorphous head, broken neck, or presence of cytoplasmic droplet [12].
Each microinjected oocyte was immediately transferred in 12 μL droplet of G1 medium (Vitrolife, Sweden) in a culture dish (Nunc 3001, Hereford, UK) covered by oil and incubated at 37 °C, 6 % CO2 and 5 % O2.
Assessment of fertilization, embryo quality and embryo transfer
Fertilization was assessed 18 h after ICSI. Normal fertilization was confirmed when two clearly distinct pronuclei were present. Fertilization rate was defined as the percentage of pronuleate oocytes to the number of oocytes injected. Embryo quality was evaluated under inverted microscope on the second day of development according to Veeck [31]. The following parameters were recorded: number of blastomere, fragmentation percentage and variation in blastomere symmetry. The embryos were transferred at 48 h after ICSI. An embryo was considered to be a good-quality embryo if there were four or five blastomeres on day 2, and less than 10 % anucleated fragments and the total absence of multinucleated blastomeres.
Pregnancy and implantation
Serum β-hCG concentration was measured 14 days after oocyte retrieval to verify pregnancy. Follow-up inspection was continuously conducted for women whose serum β-hCG concentration was positive. Clinical pregnancy was judged by observation of the gestational sac on vaginal ultrasonography after 6–7 weeks of gestation. A pregnancy with fetal heartbeat for longer than 10 weeks was also defined as an ongoing pregnancy. The implantation rate was calculated as the percentage of implanted embryos to the total number of transferred embryos.
Pregnancy rate was defined as the percentage of cycles resulted in pregnancy to the total of ICSI cycles.
Statistical analysis
Statistical analysis was performed with MedCalc program. Data were presented as mean ± standard deviation. For comparison of the continuous variables, including the numbers of oocyte injected, fertilization rate, percentage of top quality embryos and number of transferred embryos, the Student’s t-test was used. The discrete variables included ICSI pregnancy per cycle and implantation rate per pregnancy obtained. Comparison between the matched groups in the discrete variables was made by Chi-square tests. Results were considered statistically significant if p < 0.05.
To examine the performance of SVR to predict a pregnancy after an ICSI cycle, receiver operating characteristic curve (ROC) were constructed, using SAS 9.3 software. An optimized threshold was determined. The discriminative performance of the model was assessed by the area under ROC curve. Sensitivity was defined as the fraction of cycles that resulted in a pregnancy that was predicted correctly, and specificity was defined as the fraction of cycle not resulting in a pregnancy that was predicted correctly.
Results
The seminal characteristics in the study population were: volume 2,98 ± 1,26; total sperm count (10^6/mL) 31,85 ± 19,98; motility: rapid + slow progression (% spermatozoa) 51,35 ± 21,19; morphology: normal forms (% spermatozoa) 2,1 ± 1,82.
Moreover the 26 seminal samples studied, we found that the average SVR was 24,83 ± 7,08 and the percentage of abnormal spermatozoa with nuclear vacuoles was (22,07 ± 8,58), significantly higher (p < 0.001) than the percentage of normal spermatozoa with nuclear vacuoles (2,2 ± 1,82).
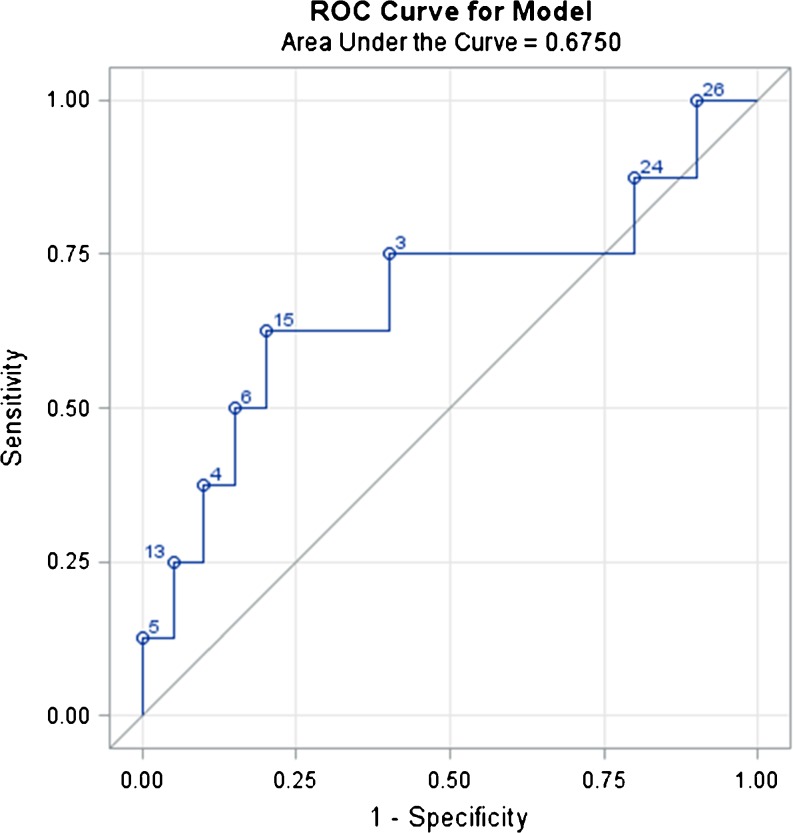
Using the ROC curve, SVR shows the best discriminatory performance to distinguish patients who obtained pregnancy between the others that is 20.28.
However the AUC obtained is 0,675, showing a weak reliability of the event, that is the pregnancy (Fig. 1).
Fig. 1.
ROC Curve. ROC Curve analysis for the percentage of sperm vacuolization using 100 x magnification as a prognostic factor regarding clinical pregnancy after ICSI. The area under the curve is 0,675. The best discriminating percentage is 20,28 %
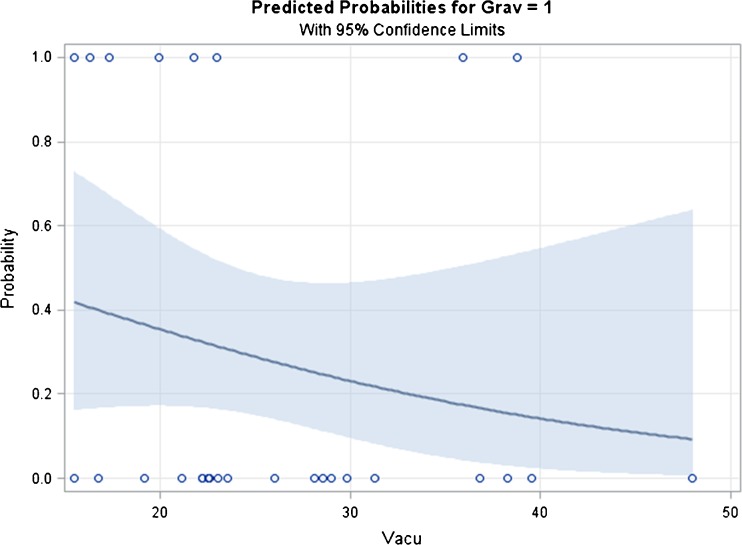
Figure 2 shows the predicted probability for pregnancy with 95 % confidence limit, indicating that the probability of pregnancy decreases with the increasing percentage of SVR. A percentage of 20,28 of SVR was assumed as a cut off to evaluate the ICSI outcome. According to this cut off value the study population was divided in two groups (A and B). The Group A consisted of 7 couples, with male partner showing an incidence of sperm nuclear-vacuole lower or equal to 20,28 %; the Group B included 19 couples, with a nuclear-vacuole rate of sperm higher than 20,28 %.
Fig. 2.
Predicted probability for pregnancy with 95 % confidence limit
As shown in Table 1, there was no difference in male age, percentage of normal forms and motility in the two groups. The mean percentage of sperm with nuclear vacuoles was 17,21 ± 1,74 in Group A and 27,67 ± 6,13 in Group B (p < 0,001). Moreover the percentage of abnormal sperm with nuclear vacuole in Group A was significantly lower than in Group B (p < 0.05), but there was no difference in the percentage of normal sperm with nuclear vacuoles. Fertilization rates did not differ between the two groups (86 ± 21,91 in Group A vs. 75,76 ± 21,76 in Group B). We found that the number of top quality embryos was not statistically different. The pregnancy and implantation rates were significantly higher in Group A compared to Group B, respectively 57,14 vs. 15,79 for pregnancy rate (p < 0,05) and 35,7 and 7,7for implantation rate (p < 0,001).
Table 1.
Comparison between group A and group B in clinical outcome parameters
| Group A (SVR ≤ 20,28) means ± s.d. (total) | Group B (SVR > 20,28) means ± s.d. (total) | P value | |
|---|---|---|---|
| Patient number | 7 | 19 | |
| Male age (y) | 36,71 ± 6,18 | 39 ± 4,67 | n.s. |
| Female age (y) | 34,14 ± 3,68 | 37,17 ± 5,32 | n.s. |
| Total sperm count (10^6/mL) | 43 ± 21,17 | 27,64 ± 18,41 | n.s. |
| Morphology: normal forms (%) | 30,71 ± 10,18 | 28,16 ± 14,83 | n.s. |
| Motility: rapid + slow progression (%) | 52,86 ± 17,99 | 50,79 ± 22,69 | n.s. |
| Sperm nuclear vacuoles rate (%) | 17,21 ± 1,74 | 27,67 ± 6,13 | <0,001 |
| Abnormal spermatozoa with nuclear vacuoles (%) | 14,59 ± 2,33 | 24,83 ± 8,4 | <0,05 |
| Normal spermatozoa with nuclear vacuoles (%) | 2,62 ± 1,23 | 2,04 ± 2 | n.s. |
| N° of MII oocytes injected* | 5,43 ± 2,07 | 4,26 ± 2,33 | n.s. |
| N° of zygotes* | 4,43 ± 1,9 | 3,11 ± 1,79 | n.s. |
| Fertilization rate (%) | 86 ± 21,91 | 75,76 ± 21,76 | n.s. |
| N° of top embryos* | 4,14 ± 1,77 | 3,05 ± 1,78 | n.s. |
| N° of embryos transferred* | 2,29 ± 0,95 | 2,37 ± 0,96 | n.s. |
| Pregnancy rate (%) | 57,14 | 15,79 | <0,05 |
| Implantation rate (%) | 35,7 | 7,7 | <0,001 |
Discussion
The present study focused on a specific sperm abnormality, the large sperm nuclear vacuoles.
The nature and origin of sperm nuclear vacuole are not jet clear. Several researchers have speculated that vacuoles could be a morphological evidence of chromatin alterations [10, 14, 15, 23] that could originate from spermatogenesis impairment or abnormal maturation during male genital tract transit [18].
The first step of the present work was to evaluate the relationship between sperm nuclear vacuoles and sperm morphology in semen sample. This relationship has been investigated by several researchers, but results are controversial: Bartoov et al. [5] did not find any relationship; Oliveira et al. [20] and Perdrix et al. [23] showed a strong association between sperm vacuolization and morphology alterations. In our study, the percentage of abnormal spermatozoa with nuclear vacuoles was significantly higher than the percentage of normal spermatozoa with nuclear vacuoles and the incidence of nuclear vacuoles was extremely lower in normal shaped sperm. The nuclear vacuoles, in our experience, seemed to be associated with a global alteration of spermatozoa morphology.
The second step of the present study was to assess the influence of sperm nuclear vacuoles rate on the biological and clinical outcomes, in couples underwent ICSI.
We identified a cut off of 20,28 % of sperm nuclear vacuolization (SNV) on the total of sperm in a seminal sample as a physiological threshold. In our study we observed that patients undergoing ICSI, grouped according to SNV, showed similar percentages of fertilization and embryo development, but more pregnancies were achieved with a higher implantation rate when SNV was lower or equal to 20,28 %.
According to Berlovitz et al. [9], this sperm malformation seems to be a “pregnancy risk factor” and it may easily be missed by standard selection prior to ICSI.
It is logical to suppose that, in case of sperm with nuclear vacuoles, the time axis of embryonic development is probably normal at the early phases (normal fertilization and development of top-quality embryos), but the embryo survival in the later stages is impaired (lower implantation and pregnancy).
The biochemical mechanism underlying the effect of nuclear vacuole phenomenon on late embryonic development, implantation and pregnancy is not yet clear and is probably genetically determined because the capacity of human spermatozoa to fertilize an oocyte and produce an embryo with a high potential for implantation also depends on DNA integrity [15].
Franco et al. [13] reported a significantly increased incidence in DNA fragmentation in sperm with vacuoles, compared with those without vacuoles. On the other hand Watanabe et al. [32], using the same TUNEL kit as used by [13], did not find any significant difference in the percentage of TUNEL-positive cells between spermatozoa with large vacuoles and those without them and concluded that large vacuoles were not involved in causing DNA fragmentation evaluation. [23] showed that DNA fragmentation was not increased in spermatozoa with nuclear vacuole. To explain this contradiction Franco et al. [14] proposed two hypotheses. First of all, the absence of any selection on native spermatozoa before DNA fragmentation evaluation [23] could introduce a methodology bias because dead sperm were present in original semen sample, but not in vacuolated spermatozoa. Second they used a different nuclear vacuole definition.
Other study investigated the association between nuclear vacuoles and chromatin packaging. According to Boitrelle et al. [10], we consider large vacuole as nuclear “thumbprint” linked to failure of chromatin condensation, and we are going to investigate deeply about the real significance of vacuolization of human sperm chromatin, mainly analysing the markers of chromatin condensation.
In order to understand the molecular basis of nuclear vacuole phenomenon on embryonic development further study should be done, with particular attention to DNA fragmentation and chromatin packaging.
Bartoov et al. [4] have developed a real-time technique to assess detailed morphology of motile spermatozoa, called motile sperm organelle examination (MSOME). MSOME is performed, using an inverted light microscope equipped with high power Normarski optics enhanced by digital imaging, to achieve a magnification of above x6000. This procedure has shown that spermatozoa, which appear morphologically normal at x200 or x400, present defects under high magnification and new abnormal aspects have been defined, especially sperm-head vacuoles, [5].
Several authors tried to establish a standardized sperm MSOME classification [5, 11, 26, 27, 30], but no consensus has been established concerning normal or abnormal MSOME criteria.
Because MSOME is an unstained cytological technique, together with a micromanipulation system, has allowed the development of a modified ICSI procedure called intracytoplasmic morphologically selected sperm injection (IMSI), based on microinjection of selected spermatozoa into oocyte [5].
IMSI brought benefits to ART procedure, resulting in better outcomes in fertilization rates [17], embryo quality [6, 9], development to blastocyst stage [30], implantation and pregnancy rates [6, 8, 9], compared with conventional ICSI.
Given that apparently normal spermatozoa visualized in the low magnification range in conventional ICSI may possess hidden abnormalities, MSOME could be useful to detect anomalies at higher magnification.
To the best of our knowledge the present study is the first that proposes a threshold value of SVR to increase chances of success in ART cycles based on high magnification nuclear vacuoles analysis. The small sample size of the present study limits the reliability of the results. However the present work introduced a simple method for the diagnostic evaluation of sperm prior to perform an ART cycle, providing insights into the correlation between sperm quality and clinical results.
Footnotes
Capsule We propose a cut off value in the proportion of sperm with nuclear vacuolization (SNV) on the total of sperm in seminal samples, and demonstrate a relationship between SNV and clinical outcomes after ICSI. The SNV rate could be introduced as an easy diagnostic evaluation prior to perform an ICSI cycle.
References
- 1.Akl LD, Oliveira JB, Petersen CG, Mauri AL, Silva LF, Massaro FC, Baruffi RL, Cavagna M, Franco JG., Jr Efficacy of the motile sperm organelle morphology examination (MSOME) in predicting pregnancy after intrauterine insemination. Reprod Biol Endocrinol. 2011;9:120. doi: 10.1186/1477-7827-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoov B, Eltes F, Pansky M, Lederman H, Caspi E, Soffer Y. Estimating fertility potential via semen analysis data. Hum Reprod. 1993;8(1):65–70. doi: 10.1093/humrep/8.suppl_1.65. [DOI] [PubMed] [Google Scholar]
- 3.Bartoov B, Eltes F, Pansky M, Langzam J, Reichart M, Soffer Y. Improved diagnosis of male fertility potential via a combination of quantitative ultramorphology and routine semen analyses. Hum Reprod. 1994;9(11):2069–75. doi: 10.1093/oxfordjournals.humrep.a138395. [DOI] [PubMed] [Google Scholar]
- 4.Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve pregnancy rate with intracitoplasmatic sperm injection. N Engl J Med. 2001;345:1067–8. doi: 10.1056/NEJM200110043451416. [DOI] [PubMed] [Google Scholar]
- 5.Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile sperm cells is asociated with IVF-ICSI outcome. J Androl. 2002;23:1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartoov B, Berkoviz A, Eltes F, Kogosowski A, Yogoda A, Lederman H, Artzi S, Gross M, Barak Y. Pregnancy rate are higher with intracitoplsmatic morphologically selected sperm injection than with conventional intracytoplasmatic injection. Fertil Steril. 2003;80:1413–9. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Berkovitz A, Eltes F, Soffer Y, Zabludovsky N, Beyth Y, Farhi J, Levran D, Bartoov B. ART success and in vivo sperm cell selection depend on the ultramorphological status of spermatozoa. Andrologia. 1999;31:1–8. [PubMed] [Google Scholar]
- 8.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–90. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 9.Berkoviz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90. doi: 10.1093/humrep/del049. [DOI] [PubMed] [Google Scholar]
- 10.Boitrelle F, Ferfouri F, Petit JM, Segretain D, Tourain C, Bergere M, Bailly M, Vialard F, Albert M, Selva J. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprint linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8. doi: 10.1093/humrep/der129. [DOI] [PubMed] [Google Scholar]
- 11.Cassuto NG, Bouret D, Plouchart JM, Jellard S, Vanderzwalmen P, Balet R, Larue L, Barak Y. A new real-time morphology classification for human spermtozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 12.Vos A, Velde H, Joris H, Verheyen G, Devroey P, Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril. 2003;79(1):42–8. doi: 10.1016/S0015-0282(02)04571-5. [DOI] [PubMed] [Google Scholar]
- 13.Franco JG, Jr, Baruffi RLB, Mauri AL, Petersen CG, Oliveira JBA, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online. 2008;17:42–5. doi: 10.1016/S1472-6483(10)60291-X. [DOI] [PubMed] [Google Scholar]
- 14.Franco JG, Jr, Mauri AL, Petersen CG, Massaro FC, Silva LF, Felipe V, Cavagna M, Pontes A, Baruffi LR, Oliveira JBA, Vagnini LD. Large nuclear vacuoles are indicative of abnormal chromatine packaging in human spermatozoa. Int J Androl. 2012;35(1):46–51. doi: 10.1111/j.1365-2605.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 15.Garolla A, Fortini D, Menegazzo M, Toni L, Nicoletti V, Moretti A, Selice R, Engl B, Foresta C. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online. 2008;17:610–6. doi: 10.1016/S1472-6483(10)60307-0. [DOI] [PubMed] [Google Scholar]
- 16.Gianaroli L, Magli MC, Ferraretti AP, Crippa A, Lappi M, Capitani S, Baccetti B. Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertil Steril. 2010;93(3):807–13. doi: 10.1016/j.fertnstert.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Junca A, Cohen-Bacrie M, Hazout PA.Improvement of fertilization and pregnancy rate after intracytoplasmic fine morphology selected sperm injection Fertil Steril 200482S173. 10.1016/j.fertnstert.2004.07.44915363721 [DOI] [Google Scholar]
- 18.Kacem O, Sifer C, Barraud-Lange V, Ducot B, Ziegler D, Poirot C, Wolf J. Sperm nuclear vacuoles, as assessed by motile sperm organellar morphological examination, are mostly of acrosomal origin. Reprod Biomed Online. 2010;20:132–7. doi: 10.1016/j.rbmo.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Kruger TF, Swanson RJ, Hamilton M, Simmons KF, Acosta AA, Matta JF, Oehinger S, Morshedi M. Abnormal sperm morphology and other semen parameters related to the outcome of the hamster oocyte human sperm penetration assay. Int J Androl. 1988;11(2):107–13. doi: 10.1111/j.1365-2605.1988.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira JB, Massaro FC, Mauri AL, Petersen CG, Nicoletti AP, Baruffi RL, Franco JG., Jr Motile sperm organelle morphology examination is stricter than Tygerberg criteria. Reprod Biomed Online. 2009;18(3):320–6. doi: 10.1016/S1472-6483(10)60088-0. [DOI] [PubMed] [Google Scholar]
- 21.Ombelet W, Bosmans E, Janssen M, Cox A, Vlasselaer J, Gyselaere W, Vandeput H, Gielen J, Pollet H, Maes M, Steeno O, Kruger T. Semen parameters in a fertile versus subfertile population: a need for change in the interpretation of semen testing. Hum Reprod. 1997;12(5):987–93. doi: 10.1093/humrep/12.5.987. [DOI] [PubMed] [Google Scholar]
- 22.Palermo F, Joris H, Devroey P, Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 23.Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, Mousset-Siméon N, Macé B, Rives N. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58. doi: 10.1093/humrep/deq297. [DOI] [PubMed] [Google Scholar]
- 24.Petersen CG, Massaro FC, Mauri AL, Oliveira JB, Baruffi RL, Franco JG., Jr Efficacy of hyaluronic acid binding assay in selecting motile spermatozoa with normal morphology at high magnification. Reprod Biol Endocrinol. 2010;3(8):149. doi: 10.1186/1477-7827-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razavi SH, Nasr-Esfahani MH, Deemeh MR, Shayesteh M, Tavalee M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia. 2010;42(1):13–9. doi: 10.1111/j.1439-0272.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 26.Saïdi R, Rives N, Gruel E, Mazurier S, Mousset-Siméon N, Macé B. Nouvelle classification du spermocytogramme à fort grossissement. Méd Reprod Gyn Endo. 2008;10:315–24. [Google Scholar]
- 27.Sermondade N, Vialard F, Bergère M, Hammound I, Cavelot P, Selva J, Albert M. Evaluation de l’apport de la méthode d’observation des spermatoides à fort groissessement eh ICSI. Andrologie. 2007;17:212–21. doi: 10.1007/BF03040730. [DOI] [Google Scholar]
- 28.Taşdemir I, Taşdemir M, Tavukçuoglu S, Kahraman S, Biberoģlu K. Effect of abnormal sperm head morphology on the outcome of intracytoplasmic sperm injection in humans. Hum Reprod. 1997;12(6):1214–7. doi: 10.1093/humrep/12.6.1214. [DOI] [PubMed] [Google Scholar]
- 29.Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59(2):86–91. doi: 10.1159/000082368. [DOI] [PubMed] [Google Scholar]
- 30.Vanderzwalmet P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, Uher P, Zint M, Lejeune B, Vanderzwalmet S, et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online. 2008;17:617–27. doi: 10.1016/S1472-6483(10)60308-2. [DOI] [PubMed] [Google Scholar]
- 31.Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams & Wilkins; 1986. [Google Scholar]
- 32.Watanabe S, Tanaka A, Fujii S, Mizunuma H, Fukui A, Fukuhara R, Nakamura R, Yamada K, Tanaka I, Awata S, Nagayoshi M. An investigation of the potential effect of vacuoles in human sperm on DNA damage using a chromosome assay and the TUNEL assay. Hum Reprod. 2011;26(5):978–86. doi: 10.1093/humrep/der047. [DOI] [PubMed] [Google Scholar]
- 33.WHO laboratory manual for examination and processing of human semen 5th ed Cambridge. UK: Cambridge University Press; 2010. [Google Scholar]