Abstract
Purpose
To assess levels of matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) in follicular fluids and sera of female patients undergoing in vitro fertilization (IVF) treatment, and discover the role of MMPs in IVF outcome prediction.
Methods
Sera and follicular fluids were obtained from 58 female patients treated for infertility by IVF. Twenty-nine of them became pregnant after the embryo-transfer; another 29 were not successful in IVF and did not conceive. Forty female non-pregnant blood donors and 38 healthy pregnant patients in the first trimester after the physiological conception were examined as control groups. MMP-2 and MMP-9 were quantitively assessed using enzyme-linked immunosorbent assay.
Results
IVF females successfully conceiving after the IVF have shown the highest MMP-9 concentrations in sera (833.5 {686.0;958.7} ng/mL) and follicular fluids (9.6 {6.0; 17.0} ng/mL) compared to all other examined cohorts. Different behavior of MMP-2 and MMP-9 during the artificial ovulation was confirmed, because only MMP-9 has shown a vast difference between serum and follicular concentrations.
Conclusions
MMP-9 could be a good predictor of the successful IVF outcome (pregnancy), which was proven for serum as well as follicular MMP-9 levels.
Keywords: Matrix metalloproteinase, MMP-2, MMP-9, In-vitro fertilization, Pregnancy, Follicular fluid
Introduction
The main function of the human ovary is to produce fertilizable oocytes. Follicular development, the rupture of the follicular wall, corpus luteum formation, and luteolysis require extensive tissue and extracellular matrix remodeling, cell migration, and rapid angiogenesis [26]. A large number of studies have implied that a critical role in these processes belongs to matrix metalloproteinases (MMPs) [5,18,26].
The MMP family proteinases are zinc dependent and degrade extracellular matrix at a physiological pH. Currently, 26 human MMPs have been identified, and these enzymes are classified according to their substrate specificity and structural similarities [21]. Numerous studies have documented the important role of the MMP-2 and MMP-9 in promoting follicles and in the ovulatory process. MMP-2 and MMP-9 are localized to the theca of developing follicles and in the stroma of the ovary. Gelatinolytic activity corresponded with the localization of MMP-2 and MMP-9 around the developing follicles and at the apex of pre-ovulatory follicles. In animal models, decreased concentrations of MMPs in pre-ovulatory fluid have been correlated with poor oocyte release and abnormal follicular involution [20,23].
Since the role of MMPs in human female infertility and in artificial ovarian hyperstimulation during the in vitro fertilization (IVF) cycles in humans is not yet fully understood, in the present study we attempted to assess levels of two MMPs, MMP-2 and MMP-9, in follicular fluid as well as the serum of female patients in IVF treatment at the day of the ovum pick-up.
Materials and methods
Subjects
The study group was composed of 136 female patients ages 20 to 44 years (median age 32.0, mean age 33.5 years, interquartile range {IQR} 29.0–34.0).
Fifty-eight (58) women from our cohort were treated for infertility by IVF. Twenty-nine (29) of these became pregnant after the embryo transfer; another 29 were not successful in IVF and did not conceive.
Two control groups were included; first, 40 female non-pregnant blood donors with a median age of 30.5 and IQR (27.5;31.0). For another control group, 38 healthy pregnant patients in the first trimester after physiological conception were recruited, with a median age of 32.5 and IQR (29.0;35.0). Individual subgroups did not differ significantly by age (p = 0.115).
According to power calculation (Statistica CZ 9.0, Statsoft Inc, Tulsa, USA), this sample size was enough to assess differences between serum and follicular concentrations of MMPs.
This study was reviewed and approved by the Institutional Review Board of the Charles University in Prague. All participants were informed about the subject of the study and gave informed consent.
Sample collection
From all subjects studied, blood was collected via puncture of the cubital vein in tubes without additives. In patients undergoing IVF, blood was collected on the day of follicular puncture and oocyte pick-up. A sample of follicular fluid was obtained by trans-vaginal follicular puncture after the oocyte retrieval.
Follicular and serum samples were centrifuged (1,600 g, 20 min), divided into aliquot parts, coded, and frozen at −20 °C. Samples were processed within 3 months of freezing.
Determination of MMPs
The concentrations of MMP-2 and MMP-9 in serum and in follicular fluid were measured by enzyme-linked immunosorbent assays (Quantikine®, R&D Systems, USA).
The MMP-2 assay recognizes recombinant (pro- and mature) and natural human MMP-2, and the MMP-9 assay measures recombinant and natural 92 kDa pro-MMP-9 and the 82 kDa active MMP-9, and does not measure the 65 kDa form. MMP concentrations were expressed in ng/mL.
Statistical analysis
The values of MMP concentrations were expressed as median and interquartile ranges (IQR). Given a non-parametric distribution of results, concentrations of markers were compared using the Kruskal-Wallis test. Correlations between levels of markers were examined with Spearman’s rank correlation coefficient via the online statistical software VassarStats (Vassar College, NY, USA) and STATISTICA data analysis software system version 9.0 (StatSoft, Inc., OK, USA). All tests were 2-tailed, and p values <0.05 were considered statistically significant. Receiver operating characteristic (ROC) curve was calculated by the web-based calculator of ROC curves from Johns Hopkins University, Baltimore, Maryland, USA (Eng, [8]).
Results
The general characteristics of the IVF cohort are shown in Table 1.
Table 1.
Descriptive characteristics of IVF female patients
| Total number of IVF patients | 58 |
|---|---|
| - sterility with anovulatory cycles | 10 (17 %) |
| - sterility with Fallopian tube damage or blockage | 7 (12 %) |
| - male factor of sterility | 27 (46 %) |
| - endometriosis | 2 (4 %) |
| - idiopathic sterility | 10 (17 %) |
| - others | 2 (4 %) |
| Age, years (mean ± SD) | 32 ± 3 |
| Number of stimulated follicles in the IVF cycles (mean ± SD) | 14 ± 8 |
| Number of retrieved oocytes (mean ± SD) | 8 ± 6 |
| Clinical pregnancy rate | 29/58 (50 %) |
IVF in vitro fertilization, SD standard deviation.
Table 2 shows the comparison of characteristics between pregnant and non-pregnant women in IVF group. Pregnant women were not significantly younger than of those who failed to conceive. Number of retrieved oocytes and embryos also did not differ between pregnant and non-pregnant patients; however, pregnant patients received significantly more embryos.
-
Serum concentrations of MMP-2
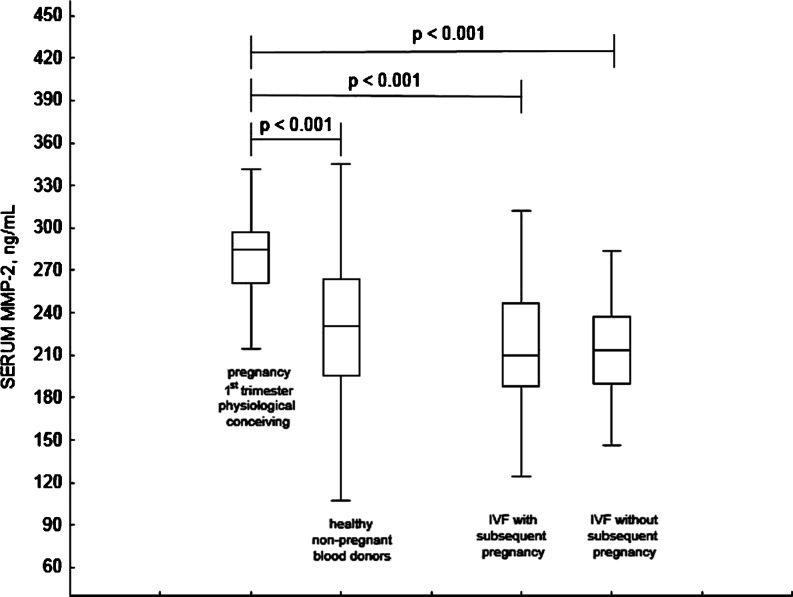
Serum concentrations of MMP-2 were significantly different between groups (p < 0.0001). In women undergoing IVF (irrespective of whether or not they became pregnant after the IVF) as well as in non-pregnant blood donors, low serum MMP-2 levels were seen. Pregnant females have shown significantly higher MMP-2 concentrations in sera; see Fig. 1.
-
Serum concentrations of MMP-9
In serum MMP-9, a meaningfully different picture was seen: IVF females successfully conceiving after IVF have shown the highest concentrations compared to all other examined cohorts; see Fig. 2.
-
MMP-2 and MMP-9 during IVF
In women undergoing IVF treatment, MMP-9 levels were higher in sera and follicular fluids of women who successfully conceived after IVF, whereas MMP-2 levels did not show such association.
Table 2.
IVF patients with and without pregnancy
| Pregnant, n = 29 | Non-pregnant n = 29 | P value* | |
|---|---|---|---|
| Age, years (mean ± SD) | 33 ± 2 | 34 ± 4 | 0.524, NS |
| D2 FSH, IU/L (median; IQR) | 6.52 (4.31;8.95) | 6.12 (5.02; 9.47) | 0.672, NS |
| rFSH treatment duration, days (mean ± SD) | 11 ± 2 | 12 ± 1.50 | 0.641, NS |
| Units of rFSH required to achieve follicular maturity (total rFSH dose, IU, mean ± SD) | 2,548 ± 874 | 2,497 ± 739 | 0.394, NS |
| 17-beta-estradiol, nMol/L (median; IQR) | 11.82 (5.98;13.21) | 14.71 (7.44;16.93) | 0.102, NS |
| Number of retrieved oocytes, (mean ± SD) | 8 ± 4 | 7 ± 5 | 0.690, NS |
| Fertilization rate per retrieved oocyte (% ± SD) | 69.54 ± 18.41 | 71.25 ± 20.18 | 0.487, NS |
| Number of obtained embryos per patient (mean ± SD) | 5 ± 2 | 5 ± 3 | 0.608, NS |
| Cleavage rate (% ± SD) | 81.45 ± 25.51 | 83.78 ± 27.54 | 0.740, NS |
| Embryos transferred, (mean ± SD) | 1.85 ± 0.35 | 1.15 ± 0.72 | 0.041 |
IVF in vitro fertilization, SD standard deviation, D2 Day 2 of the menstrual cycle, FSH follicle-stimulating hormone, rFSH recombinant follicle-stimulating hormone, IQR interquartile range, NS non-significant.
*Mann–Whitney U-test
Fig. 1.
Serum concentrations of MMP-2 in examined cohorts of patients. The boxplot represents lower and upper quartile, the horizontal line represents median, the whiskers represent sample minimum and maximum
Fig. 2.
Serum concentrations of MMP-9 in examined cohorts of patients. The boxplot represents lower and upper quartile, the horizontal line represents median, the whiskers represent sample minimum and maximum
At the time of ovulation, IVF women have approximately the same concentrations of MMP-2 in follicular fluid and in serum; however, these women have multifold lower concentrations of MMP-9 in follicular fluid compared to serum (p < 0.0001). In serum and follicular MMP-2 levels, significant positive correlation was shown (r = 0.287, p = 0.043). In MMP-9, no relationship between high serum and low follicular levels was observed.
A summary of the results and a comparison of the concentrations of MMPs in sera and follicular fluids with respect to the IVF outcome are presented in Table 3.
Table 3.
Serum and follicular levels of MMP-2 and MMP-9 in of women undergoing IVF
| Result of IVF | p-value | |||
|---|---|---|---|---|
| Pregnant | Non-pregnant | |||
| MMP-2 [ng/mL] | Follicular fluid | 179.1 (154.2;200.1) | 161.2 (152.0;179.4) | 0.106, NS |
| Serum | 209.8 (187.6;246.4) | 213.2 (190.2;237.2) | 0.476, NS | |
| MMP-9 [ng/mL] | Follicular fluid | 9.6 (6.0;17.0) | 7.1 (4.6;10.7) | 0.003 |
| Serum | 833.5 (686.0;9 58.7) | 729.1 (544.7;922.7) | 0.035 | |
For serum and follicular MMP-9 concentrations at the day of ovum pick-up in the IVF cohort, ROC curves were constructed and areas under curves (AUCs) were calculated. Upon calculated values, serum MMP-9 levels have better diagnostic performance compared to MMP-9 concentrations in follicular fluid. See Fig. 3.
Fig. 3.
ROC curve analysis for serum and follicular fluid concentrations of MMP-9
Discussion
In the present study, it is demonstrated that MMP-9 serum as well as follicular concentrations are related to a successful IVF resulting in pregnancy. Moreover, different behaviors of MMP-2 and MMP-9 during the artificial ovulation were confirmed; only MMP-9 has shown a vast difference between serum and follicular concentrations.
Currently, a limited number of studies have investigated intrafollicular and serum MMPs, with inconsistent conclusions [1,2,6,10,16,22]. However, MMPs theoretically could be important players in IVF processes. These enzymes are key for follicular development, ovulation, and the formation of corpus luteum and its regression, and involve extensive tissue remodeling. The development of ovarian follicles and the breakdown of the follicular wall to release a mature oocyte at the time of ovulation require extensive angiogenesis and remodeling [6].
MMP-2 is constitutively produced in the body; it is, therefore, not surprising that concentrations of MMP-2 in serum and follicular fluid were in reciprocal correlation. The study by Gottsch et al. [11] observed a regulatory role of MMP-2 relevant to the folliculo–luteal transformation in ewes. The results of this intrafollicular immunoblockade research indicate that MMP-2 plays a critical role in the biomechanics of ovulation and the formation of the corpus luteum in ewes. The study showed that MMP-2 degrades the fabric of basement membranes, which are necessary for ovulatory ovarian rupture to occur, and contributes to the reorganization of the thecal connective tissue foundation into a web-like mesh, which forms inroads into the granulosa compartment for endothelial and thecal cell infiltration and provides a supportive structure for capillary sprouting. Ovarian surface contiguous with follicles injected with anti-MMP-2 was smooth and undisturbed, which is indicative of a failure of ovulation (that is, there was no morphological evidence of ovarian rupture). The walls of unruptured follicles were luteinized. It is proposed that MMP-2 is a mediator of ovulation and luteal development. Our study has shown that the concentration of MMP-2 in pregnant and non-pregnant post-IVF groups does not differ, which is in line with research by Lee et al. This workgroup studied the serum activity of MMP-2 in women undergoing IVF treatment, and MMP-2 activity was proven in the follicular fluids and culture media of all women. Furthermore, no difference in its expressions was found between the pregnant and non-pregnant groups [15].
The highest serum concentrations of MMP-2 were observed in healthy pregnant women. It seems that MMP-2 is involved in pregnancy, which corresponds with studies by Bischof et al. This fact was explained by the placental trophoblast’s proliferative and invasive properties with MMP secretion [3]. Moreover, the importance of higher concentrations of MMP-2 in the serum of pregnant women was shown by Koucky et al., who confirmed that low serum concentrations of MMP-2 in pregnant women lead to preterm labor and are connected with funisitis and elevated umbilical concentration of IL-6 [14].
There is substantial evidence that the metalloproteinases and their inhibitors play a role in the events associated with the periovulatory and luteal periods [4]; however, little is known about the cellular localization of this exquisite proteolytic system. The increase in MMP-2 is involved in degradation of the granulosa cell basement membrane or in the development of the extensive vascularization of the forming corpus luteum. An additional role for the gelatinases is associated with follicular luteinization and atresia.
A study by Ogiwara et al. [19] found that follicle rupture in the medaka ovary involves the cooperation of at least three matrix metalloproteinases (MMPs), together with the tissue inhibitor of metalloproteinase-2b protein. We determined the discrete roles of each of these proteins during follicle rupture. The results indicated that gelatinase A induces the hydrolysis of type IV collagen constituting the basement membrane, membrane-type 2 MMP degrades type I collagen present in the theca cell layer, and MT1-MMP and the tissue inhibitor of metalloproteinase-2b are involved in the production and regulation of gelatinase A. In a study by Liu et al. (1998), in situ hybridization was used to study the regulation and distribution of mRNA coding for MMP-2 (gelatinase A) and its cell surface activator membrane-type MMP1 (MT1-MMP) during gonadotropin-induced ovulation in the rat. The expression kinetics and tissue distribution support the notion that MT1-MMP may have dual functions in the ovary. Initially, MT1-MMP may act as a matrix-degrading protease inside the follicle during follicular development, and later, just prior to ovulation, as an activator of pro-MMP-2 in theca-interstitial cells surrounding pre-ovulatory follicles. Naruse [17] demonstrated in situ activity and localization of the MMPs, MMP-2 and MMP-9, and their inhibitors in the placental bed during early pregnancy. This is the first comprehensive characterization of the gelatinases and their inhibitors in the placental bed during early pregnancy. MMP-2 and TIMP-2 were localized strongly to uNK cells in the placental bed throughout early pregnancy. Seval [24] observed that MMP-2, particularly in the fourth week, appeared to be expressed more strongly in extravillous trophoblasts (EVTs) and vascular endothelial cells in the first trimester of pregnancy. Therefore, MMP-2 is likely to be the primary mediator in invasion of the trophoblast into the decidual endometrium, as well as in vascular remodeling and angiogenesis in the first trimester of pregnancy. The high expression of TIMP-1 and TIMP-2 in EVTs and glandular epithelium suggests that a restricted and balanced expression of these molecules is important for matrix remodeling and controlled trophoblast invasion during placentation. Seval et al. conclude that MMP-2 and MMP-9 and their inhibitors determine the invasive behavior of trophoblast into the endometrium and that MMP-2 may be the key regulator of trophoblast invasion in early human pregnancy. Isaka et al. [12] used purified cytotrophoblasts (CTs) to examine the expression and activity of MMP-2 and MMP-9, and their invasive ability. In first-trimester placental tissue, the MMP-2 expression was observed in extravillous trophoblasts (EVTs), and MMP-9 mainly in villous cytotrophoblasts (VCTs). In full-term placental tissue, the MMP-2 expression was observed in the EVTs similar to that in the first trimester, whereas the gelatinase activity in these cells was decreased or completely lost. Using purified CTs, the gelatinase activity was marked in early CTs, but not term CTs. Invasive ability of early CTs was inhibited by tissue inhibitor of metalloproteinase (TIMP)-2 and MMP-2 antibody in a dose-dependent manner. These suggested that the invasive ability of trophoblasts may be regulated by the enzyme activity of gelatinases, especially MMP-2.
The presence of MMP-9 in follicular fluid, which has substrate specificities that include the basement membrane constituent collagen, indicates that it is likely to be required for tissue remodeling during follicle growth and development. MMP-9 production must be induced in the body [25], and there was no correlation between its concentrations in serum and follicular fluid. Interestingly, the production of MMP-9 in follicular fluid is significantly lower than in serum; most important, in women who have managed to become pregnant after the IVF, the follicular fluid and the serum should have higher concentrations of MMP-9 starting on the day of the ovum pick-up. This fact could supplement the findings of Dubois et al. in animal models. The authors of this study conducted experiments on gelatinase B–deficient mice, and found that these mice had much smaller litters and that the percentage of infertile breeding pairs was elevated [7]. MMP-9 is likely to play a role in the breakdown in extracellular matrix during the follicle growth and development, in follicle migration in ovulation, and in controlling other cell functions, including the cell cycle [13].
It is known, that high-quality embryos lead to a high pregnancy ratio [9], but a serum or follicular fluid biomarker that directly indicates embryo quality has not yet been discovered. The role of MMPs in relation to human reproduction remains unclear. However, in our study, serum and follicular MMP-9 levels were significantly different in the successful versus unsuccessful IVF subgroups. An important point of practical importance of our study is that MMP-9 in serum and follicular fluid could be an useful marker for IVF.
Nevertheless, our results have some limitations that need to be addressed. The first of them is the composition of the healthy non-pregnant reference group. Our results are underscored by the need of prospectively recruited control group with the proven information about the fertility of recruited women. Secondly, this is a pilot study, so that the sample size and the restriction of the data to one medical centre only limits the generalisibility of the results. Our findings are associative and cause and effect cannot be determined. However, the results have biological plausibility, and provide a valid answer to the clinical question of whether there are differences in the MMPs-2 and 9 behavior during the assisted reproduction processes.
In conclusion, MMP-9 could be a predictor of the successful IVF outcome (pregnancy), which was proven for serum as well as follicular MMP-9 levels. More studies are needed to determine the biological basis for the impact of MMP-9 on normal and assisted human reproduction.
Acknowledgments
This work was supported by the Research Program P25/LF1/2 and SVV-2012-264512 Projects of Charles University in Prague.
Footnotes
Capsule
We demonstrate that matrix metalloproteinase-9 (MMP-9) serum as well as follicular concentrations are related to a successful IVF resulting in pregnancy.
References
- 1.Baka S, Zourla K, Kouskouni E, Makrakis E, Demeridou S, Tzanakaki D, Hassiakos D, Creatsas G. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo. 2010;24(3):293–6. [PubMed] [Google Scholar]
- 2.Baka S, Zourla K, Malamitsi-Puchner A, Makrakis E, Kaparos G, Demeridou S, Moustakarias T, Tzanakaki D, Hassiakos D, Kouskouni E. Intrafollicular levels of matrix metalloproteinases-2 and -9 in patients with polycystic ovaries are not associated with pregnancy rate during IVF cycle. In Vivo. 2009;23(1):89–92. [PubMed] [Google Scholar]
- 3.Bischof P, Meisser A, Campana A. Control of MMP-9 expression at the maternal-fetal interface. J Reprod Immunol. 2002;55(1–2):3–10. doi: 10.1016/S0165-0378(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 4.Curry TE, Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod. 2001;65(3):855–65. doi: 10.1095/biolreprod65.3.855. [DOI] [PubMed] [Google Scholar]
- 5.Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–65. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 6.D’Ascenzo S, Giusti I, Millimaggi D, Marci R, Tatone C, Colonna RC, Moscarini M, Pavan A, et al. Intrafollicular expression of matrix metalloproteinases and their inhibitors in normally ovulating women compared with patients undergoing in vitro fertilization treatment. Eur J Endocrinol. 2004;151(1):87–91. doi: 10.1530/eje.0.1510087. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B, Arnold B, Opdenakker G. Gelatinase B deficiency impairs reproduction. J Clin Invest. 2000;106(5):627–628. doi: 10.1172/JCI10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng J. ROC analysis: web-based calculator for ROC curves. Baltimore: Johns Hopkins University, 2006. Available from: http://www.jrocfit.org.
- 9.Fu J, Wang XJ, Wang YW, Sun J, Gemzell-Danielsson K, Sun XX. The influence of early cleavage on embryo developmental potential and IVF/ICSI outcome. J Assist Reprod Genet. 2009;26(8):437–41. doi: 10.1007/s10815-009-9342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes VA, Vieira CS, Jacob-Ferreira AL, Belo VA, Soares GM, Fernandes JB, Ferriani RA, Tanus-Santos JE. Imbalanced circulating matrix metalloproteinases in polycystic ovary syndrome. Mol Cell Biochem. 2011;353(1–2):251–7. doi: 10.1007/s11010-011-0793-6. [DOI] [PubMed] [Google Scholar]
- 11.Gottsch ML, Kirk EA, Murdoch WJ. Role of matrix metalloproteinase 2 in the ovulatory folliculo-luteal transition of ewes. Reproduction. 2002;124:347–352. doi: 10.1530/rep.0.1240347. [DOI] [PubMed] [Google Scholar]
- 12.Isaka K, Nishi H, Nakai H, Nakada T, Feng Li Y, Ebihara Y, Takayama M. Matrix metalloproteinase-26 is expressed in human endometrium but not in endometrial carcinoma. Cancer. 2003;97(1):79–89. doi: 10.1002/cncr.11030. [DOI] [PubMed] [Google Scholar]
- 13.Jovanović M, Stefanoska I, Radojcić L, Vićovac L. Interleukin-8 (CXCL8) stimulants trophoblast cell migration and invasion by increasing levels of matrix metalloporteinase (MMP)2 and (MMP)9 and integrin alpha5 and beta1. Reproduction. 2010;139(4):789–798. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- 14.Koucký M, Germanová A, Kalousová M, Hill M, Cindrová-Davies T, Pařízek A, Svarcová J, Zima T. Low maternal serum matrix metalloproteinases (MMP)-2 concentrations are associated with preterm labor and fetal inflammatory response. J Perinat Med. 2010;38(6):589–596. doi: 10.1515/jpm.2010.092. [DOI] [PubMed] [Google Scholar]
- 15.Lee DM, Lee TK, Song HB, Kim CH. The expression of matrix metalloproteinase-9 in human follicular fluid is associated with in vitro fertilization pregnancy. BJOG. 2005;112(7):946–51. doi: 10.1111/j.1471-0528.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Cai LY, Lv HM, Xia L, Zhang YJ, Zhang HX, Guan YM. Raised serum levels of matrix metalloproteinase-9 in women with polycystic ovary syndrome and its association with insulin-like growth factor binding protein-1. Gynecol Endocrinol. 2008;24(5):285–8. doi: 10.1080/09513590802056995. [DOI] [PubMed] [Google Scholar]
- 17.Naruse K, Lash GE, Innes BA, Otun HA, Searle RF, Robson SC, Bulmer JN. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009;24(3):553–61. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- 18.Ny T, Wahlberg P, Brändström IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol. 2002;187(1–2):29–38. doi: 10.1016/S0303-7207(01)00711-0. [DOI] [PubMed] [Google Scholar]
- 19.Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T. Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci U S A. 2005;102(24):8442–7. doi: 10.1073/pnas.0502423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan MJB, Stamoui A, Thomas ES, Richardson MC. Gonadotropin regulation of production of tissue inhibitor of metalliproteinases and by luteinized human granulated cells a potential mechanism for luteal rescue. Mol Hum Reprod. 1997;3:405–14. doi: 10.1093/molehr/3.5.405. [DOI] [PubMed] [Google Scholar]
- 21.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and regulation of tissue remodeling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley SC, Gibson AH, Leask R, Mauchline DJW, Pedersen HG, Watson ED. Secretion of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases into follicular fluid during follicle development in equine ovaries. Reproduction. 2001;121:553–560. doi: 10.1530/rep.0.1210553. [DOI] [PubMed] [Google Scholar]
- 23.Russell L, Salamunsen LA, Findlay LR. Immunization against the N-terminal peptide of the inhibin alphaY3-subunit (Alpha N) disrupts tissue remodeling and the increase in matrix metalloproteinase-2 during ovulation. Endocrinology. 1995;136:3657–3664. doi: 10.1210/en.136.8.3657. [DOI] [PubMed] [Google Scholar]
- 24.Seval Y, Cakmak H, Kayisli UA, Arici A. Estrogen-mediated regulation of p38 mitogen-activated protein kinase in human endometrium. J Clin Endocrinol Metab. 2006;91(6):2349–57. doi: 10.1210/jc.2005-2132. [DOI] [PubMed] [Google Scholar]
- 25.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsafriri A, Reich R. Molecular aspects of mammalian ovulation. Exp Clin Endocrinol Diabetes. 1999;107(1):1–11. doi: 10.1055/s-0029-1212066. [DOI] [PubMed] [Google Scholar]