Abstract
Introduction
Traditional clinical trials in psoriasis exclude a significant proportion of patients with complex disease and comorbidities. A consensus panel of 14 experts in the field of psoriasis was formed to conduct a Delphi method exercise to identify difficult-to-treat psoriasis clinical scenarios and to rank treatment approaches.
Methods
The exercise consisted of both survey questionnaires and a live meeting to review and discuss current data (as of 2009, when the exercise was conducted) and arrive at a consensus for optimal treatment options. Seventy difficult treatment scenarios were identified, and the top 24 were selected for discussion at the live meeting.
Results
Six of the 24 discussed case scenarios are presented in this article (another five are presented in Part 2): (1) psoriasis with human papilloma virus-induced cervical or anogenital dysplasia; (2) concomitant psoriasis and systemic lupus erythematosus; (3) severe psoriatic nail disease causing functional or emotional impairment; (4) psoriasis therapies that potentially reduce cardiovascular morbidity and mortality; (5) older patients (≥65 years of age) with psoriasis; and (6) severe scalp psoriasis that is unresponsive to topical therapy.
Conclusion
The Delphi exercise resulted in guidelines for practicing physicians to utilize when confronted with challenging patients with psoriasis.
Keywords: Acitretin, Biologics, Methotrexate, Psoriasis, Psoriatic nail disease, Severe scalp psoriasis, TNF-α inhibitor
Introduction
In an effort to provide treatment guidance regarding a selected group of complex psoriasis scenarios, individuals with a specific interest in psoriatic disease formed a consensus panel and carried out a Delphi exercise. Because the published clinical trials for psoriasis therapeutics typically enroll healthy patients without comorbidity or other complex clinical features (e.g., concomitant lupus erythematosus, significant cardiovascular disease, human papilloma virus [HPV]-induced cervical or anogenital dysplasia, or human immunodeficiency virus [HIV] infection), other sources are needed to guide therapy for these patient types.
The Delphi method is particularly well suited for addressing healthcare-related issues because the outcome represents the collective judgment of the panel of experts on selected topics. The Delphi method includes three basic characteristics: (1) repeated individual questioning of the experts; (2) the avoidance of direct confrontation among the experts (e.g., anonymity); and (3) interspersed controlled opinion and feedback. Importantly, the Delphi method seeks to achieve consensus on complex scenarios where rigorous data are lacking. Available data on a given topic are reviewed extensively, presented, and discussed among the panelists. More importantly, by employing only anonymous voting by the panelists, the Delphi method settles controversy by eliminating the effects of either reputation or “personality.” Consequently, anonymous voting after thorough review of the data allows the panelists to vote for what they truly believe, thus avoiding “groupthink” and sentiment guided more by “eminence,” charisma, and dogmatism.
What follows is an application of the Delphi method for difficult-to-treat clinical scenarios in patients with moderate-to-severe psoriasis. This process occurred in the following three steps over approximately 5 months: (1) selection of difficult-to-treat psoriasis clinical scenarios; (2) selection of potential psoriasis treatment modalities; and (3) the matching, through systematic, iterative rounds of voting, of the clinical scenarios with the most appropriate treatments based on informed assessment of the peer-reviewed literature. At all times, the votes of the individual panelists were kept anonymous; thus, at no point was a single individual able to direct the outcome of any aspect of this process. What follows is a reiteration of a process first implemented in 2008 [1].
Method Overview
This Delphi exercise began with the identification of 14 experts in the field of psoriasis by virtue of their publication record, participation in national meetings, interest in treating psoriasis and psoriatic arthritis, and participation in clinical trials and basic research on psoriatic disease. All experts come from the United States (US). Next, without guidance, the individual panelists were asked to list both challenging clinical scenarios and therapeutic options for psoriasis. Through a series of survey questionnaires and anonymous feedback by the participants, clinical scenarios were selected and ranked, and the treatment options were listed. The top-ranked 24 clinical scenarios were then assigned to the panelists for the preparation of a PowerPoint review of the available peer-reviewed data that supported the use of specific treatment approaches. Each panelist presented his or her assigned clinical scenarios along with the supporting data for the various treatments at a live meeting held on January 22, 2009. After each presentation, a structured and time-limited discussion and debate occurred. A final round of anonymous, electronic voting determined the ultimate ranking of treatment choices for each clinical scenario.
Identification Process
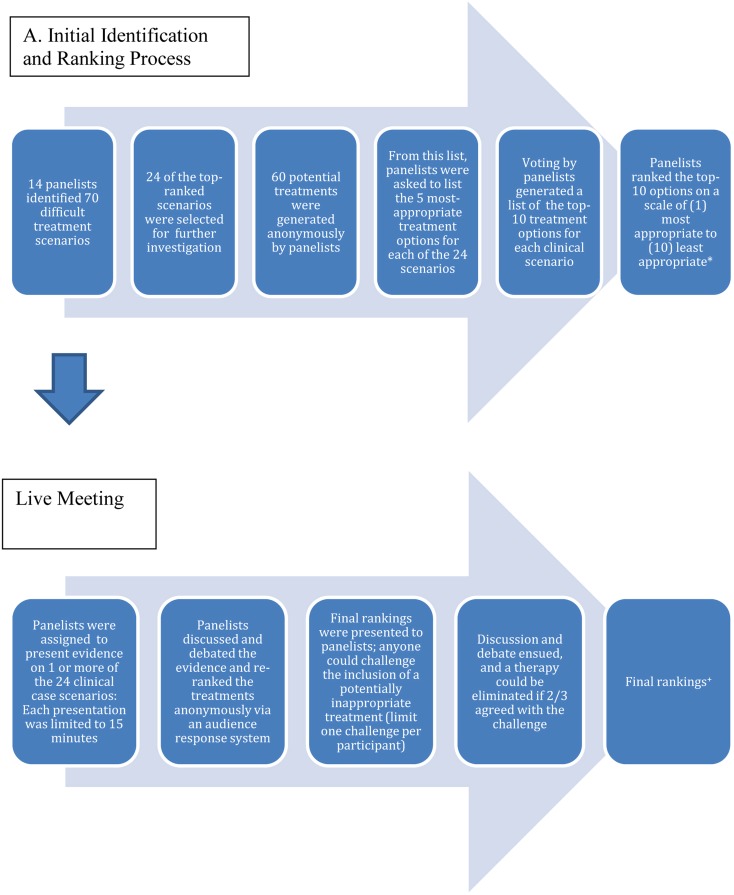
The panelists generated a preliminary set of 70 difficult treatment scenarios based on their clinical expertise and experience (Fig. 1). Next, each scenario was anonymously ranked on a five-point scale ranging from “very important” to “unimportant.” From this process, 24 top-ranked scenarios (by virtue of being ranked more important) were selected (Table 1).
Fig. 1.
The Delphi process used to identify and rank treatment options for challenging psoriasis case scenarios. *These discussions occurred before the voluntary withdrawal of efalizumab from the US market. In cases in which efalizumab ranked among the top treatment options, it has been eliminated. +Six of the cases are presented in this article
Table 1.
The 24 selected topics from the 2009 Psoriasis Consensus Conference
| Psoriasis therapies that potentially reduce cardiovascular morbidity and mortality |
| Palmoplantar psoriasis, unresponsive to topical therapy and phototherapy |
| Preferred therapeutic choice when combining with low-dose methotrexate |
| Prior history of nonmelanoma skin cancer (SCC and BCC) |
| Obese patients with psoriasis and/or psoriatic arthritis |
| Older patients (≥65 years of age) |
| Patients with severe psoriasis and psoriatic arthritis who have recurrent infections on any anti-TNF therapy |
| Newly pregnant women who are currently receiving and responding well to a TNF inhibitor |
| Psoriasis plus the metabolic syndrome |
| Severe psoriatic nail disease causing functional or emotional impairment |
| Erythrodermic psoriasis |
| Severe scalp psoriasis, unresponsive to topical therapy |
| Psoriatic arthritis |
| Treatment for new-onset psoriasis (of any morphology) in patients undergoing anti-TNF therapy for another immune-mediated inflammatory disease (e.g., inflammatory bowel disease or RA) |
| Pustular psoriasis |
| Patients who are already receiving potentially immunosuppressive therapy other than methotrexate |
| Moderate-to-severe psoriasis that has failed to respond to all currently approved therapies for psoriasis in patients who cannot receive methotrexate or cyclosporine A |
| Patients with psoriasis and/or psoriatic arthritis who develop neurologic symptoms or signs after starting a TNF-inhibitor, and then fail appropriate trials of methotrexate and T-cell inhibitors (alefacept and efalizumab) |
| Patients who never respond to or initially respond to then fail all available TNF inhibitors (essentially, treatment for TNF-resistant or -unresponsive patients) |
| Patients with a history of a solid tumor malignancy |
| Inverse psoriasis, unresponsive to topical therapy |
| Psoriasis plus HPV-induced cervical or anogenital dysplasia |
| Psoriasis with concomitant systemic lupus erythematosus |
| Psoriasis in patients ≤16 years of age |
BCC basel cell carcinoma, HPV human papilloma virus, RA rheumatoid arthritis, SCC squamous cell carcinoma, TNF tumor necrosis factor
Each panelist was asked to anonymously submit a list of treatments for psoriasis and/or psoriatic arthritis (Food and Drug Administration [FDA] approved, off-label, or investigational yet near likely approval). From this effort, a list of 60 potential treatments was generated (Table 2). Next, the panelists were asked to anonymously list, according to their knowledge of the literature and personal experience, the five most appropriate treatment options (chosen from the “master” list of 60 treatments) for each of the 24 challenging clinical scenarios. The results of a statistical compilation of the voting from all 14 panelists generated a list of the top-10 treatment options for each clinical scenario. These top-10 options were presented to the panelists, who then ranked each of the 10 options on a scale of 1 (most appropriate) to 10 (least appropriate). This second round of voting thus created an updated, ranked list of treatments for each clinical scenario for the live meeting. Of note, this discussion occurred prior to the voluntary withdrawal of efalizumab from the market, so in cases where efalizumab ranked in the top-10 treatment options it was removed.
Table 2.
Potential psoriasis treatment options generated during the Delphi process
| 6-mercaptopurine | 6-thioguanine | 6-thioguanine plus biologic therapy |
| 6-Thioguanine plus phototherapy | Abatacept | Acitretin |
| Acitretin + biologic therapy | Acitretin + hydroxyurea | Adalimumab |
| Alefacept | Anthralin | Azathioprine |
| Certolizumab (pegylated fab fragment against TNF) | COX-2 inhibitors | Crude coal tar |
| Cyclosporine | Efalizumaba | Etanercept |
| Excimer laser | Hydroxychloroquine | Hydroxyurea |
| Infliximab | Intra-articular corticosteroids | Intralesional corticosteroids |
| Leflunomide | LCD | Methotrexate plus alefacept |
| Methotrexate plus efalizumab | MTX | MTX plus a TNF inhibitor |
| MTX plus cyclosporine | Mycophenolate mofetil | Natural UV light |
| NSAID | Sulfasalazine | Systemic corticosteroids |
| TACR olimus (oral formulation) | TNF inhibitor (adalimumab preferred more than the other two available drugs of this class) | TNF inhibitor (etanercept preferred more than the other two available drugs of this class) |
| TNF inhibitor (infliximab preferred more than the other two available drugs of this class) | TNF inhibitor (without concern for any individual drug) | TNF inhibitor plus cyclosporine |
| Tocilizumab (humanized monoclonal antibody against IL-6r) | Topical calcipotriene | Topical calcitriol |
| Topical corticosteroid in combination with calcipotriene | Topical corticosteroid plus topical calcipotriene | Topical corticosteroids |
| Topical immunomodulator (tACR olimus or pimecrolimus) | Topical tazarotene | Topical tazarotene + topical corticosteroid |
| UV phototherapy—broadband b | UV phototherapy—narrow band b | UV phototherapy—PUVA |
| UV phototherapy plus acitretin | UV phototherapy plus biologic | UV phototherapy plus LCD, crude coal tar, or anthralin (Goeckerman, Ingram) |
| UV therapy plus oral systemic therapy (other than acitretin) | Ustekinumab (monoclonal antibody against IL-12/23) | As a first choice, no therapy should be given in this scenario |
COX-2 cyclooxygenase 2, IL interleukin, LCD liquid carbonis detergens, MTX methotrexate, NSAID nonsteroidal anti-inflammatory drug, PUVA psoralen + UVA phototherapy, TNF tumor necrosis factor, UV ultraviolet
aRemoved from the US market in 2009
Live Meeting
A live meeting was scheduled to discuss the 24 top-ranked difficult treatment scenarios. Each panelist was assigned one or more clinical scenarios to present. Panelists were assembled in a conference room around a horseshoe-shaped table.
During each 15-min presentation, the panelist offered evidence-based data regarding the safety and efficacy of most, if not all, of the top-10 ranked treatment options determined by the previous round of voting. A 15-min discussion and debate with the other participants followed, during which each of the non-presenting participants were given 1 min to make concise points regarding their views of the presented data and treatment preferences. Using an anonymous audience response system (ARS; TurningPoint Technologies, Youngstown, OH, USA), all panelists participated in the final round of voting, re-ranking the 10 choices based on their final assessment of the data. Importantly, treatments that were previously not listed in the top 10 could not be added during the live meeting. Real-time tabulation and viewing of the final rankings occurred. Panelists also recorded their rankings in a workbook, which allowed confirmation of the electronically acquired data.
After the final ranking, if a treatment option was thought to be medically inappropriate for a given scenario, individual panelists were allowed to “challenge” the inclusion of the potentially inappropriate treatment. The challenging panelist was given 1 min to support his or her case for elimination of a therapy. Subsequently, two opposing arguments presented by two different panelists against the challenge were allowed, each 1 min in length. In these scenarios, another round of anonymous voting occurred. A listed therapy was eliminated as a treatment option only if a two-thirds majority (e.g., 10 panelists) agreed with the challenge. Each panelist was limited to one challenge during the live meeting.
In the interest of brevity and relevance, six of the 24 considered scenarios are presented in Part 1 of this article. Part 2 of the article will present another five scenarios that were discussed. These selected scenarios were chosen by the first author (B.E.S.).
Statistical Analysis
For comparing three or more groups, Friedman’s test was used to rank the data in each set from high to low. Each set of data was ranked separately. The value of the Friedman statistic was calculated from the sums of the ranks and sample sizes. Dunn’s multiple comparison post-test was utilized to compare the difference in the sum of ranks between data columns. The calculation of the P-value takes into account the number of comparisons. Statistical analysis was performed with GraphPadPrism version 5.00 for Windows (GraphPad Software, San Diego CA, USA).
Classification of Experimental Evidence Supporting a Therapeutic Option
Recommendations from the Agency for Health Care Policy Research (AHCPR) [2] were used to grade the experimental evidence as it relates to therapeutic recommendations in each case study. The categories of evidence include: level 1a: evidence from meta-analysis of randomized controlled trials (RCTs); level 1b: evidence from one or more RCT; level 2a: evidence from one or more controlled trials (without randomization); level 2b: evidence obtained through other well-designed studies (quasi-experimental); level 3: evidence from nonexperimental studies (descriptive studies such as comparative or correlation studies or case–control studies); level 4: expert committee opinions, clinical experience.
Preliminary recommendations for treatments were made using the best available evidence extracted from published literature. The strengths of recommendations were graded as follows: grade A: category 1 evidence; grade B: category 2 evidence or extrapolation from category 1 evidence; grade C: category 3 evidence or extrapolation from category 1 or category 2 evidence; grade D: category 4 evidence or extrapolation from category 2 or category 3 evidence.
Where definitive scientific evidence was lacking, “expert opinion” and consensus (e.g., the community standard) were used for suggested recommendations for key practical issues.
Results
Case Scenario 1: Psoriasis and HPV-Induced Cervical or Anogenital Dysplasia
The HPV has a role in many diseases, but the most infamous is cervical cancer. In a study of 3,607 women with cervical cancer, HPV DNA was detectable in 92.5% (grade C evidence) [3]; however, genotypes 16 and 18 comprise 71% of those cases. In addition, HPV is widely prevalent in the worldwide population.
The presence of HPV DNA has been demonstrated in up to 90% of the lesional skin scrapings of psoriasis patients, and it has been suggested that HPV produces antigens that stimulate psoriatic disease (grade D evidence) [4]. In a case study, Rust et al. suggested a link between psoriasis, light exposure, and HPV in the development of cutaneous squamous cell carcinoma (SCC), as a patient with psoriasis treated extensively with ultraviolet (UV) therapy was found to have HPV serotypes 12 and 17 in some of her cutaneous SCCs (grade D evidence) [5]. Additional patients with psoriasis who were treated with psoralen UVA (PUVA) therapy and methotrexate were found to have HPV-positive SCCs, but the overall copy number and replication activity was low (grade D evidence) [6]. The cumulative effects of UV exposure and immunosuppression must also be considered.
As many of the current psoriasis therapies are immunosuppressants or immunomodulators, selection of an agent for a patient with psoriasis with cervical or anogenital dysplasia is complicated, but there are no specific data addressing this scenario. Retinoids have been tested in cervical intra-epithelial neoplasia (CIN) and were not effective in preventing disease progression (grade A evidence) [7]. A recurrence of condylomata acuminata and a flare of pre-existing genital lesions have been reported with etanercept and infliximab, respectively (grade D evidence) [8]. A randomized, placebo-controlled clinical trial evaluating etanercept for pediatric psoriasis also found a higher incidence of skin papillomas/warts; a total of 16 lesions in the treatment group as compared to 0 in the placebo group (grade A evidence) [9].
In light of these data, there is some consensus that tumor necrosis factor alpha (TNF-α)-inhibitors may augment the risk of HPV-induced verruca vulgaris and perhaps condylomata acuminata. There are no reports on methotrexate-induced HPV disease. Further, there are no data regarding the effect of ustekinumab on HPV. Another consideration is the use of the recombinant HPV vaccine that has selective efficacy against HPV types 6, 11, 16, and 18 (grade A evidence) [10]. Some practitioners suggest that prior to treating psoriasis or psoriatic arthritis with an immunosuppressive medication, it may be prudent to administer this vaccine to younger patients without a history of demonstrated HPV infection, but the data supporting this contention are completely lacking.
Discussion
The panel recognized that genital HPV disease typically will be detected by gynecologist/obstetricians rather than dermatologists, but these clinicians may not recognize or report associations with psoriasis and its therapy. Therefore, the literature may not be sufficient at this point. For most psoriasis therapies, an annual examination by a gynecologist is not even recommended. Many of the panelists agreed that there is an increase in warts, molluscum contagiosum, and herpes zoster in patients treated with TNF-α inhibitors, although this also could apply to other immunosuppressant therapies.
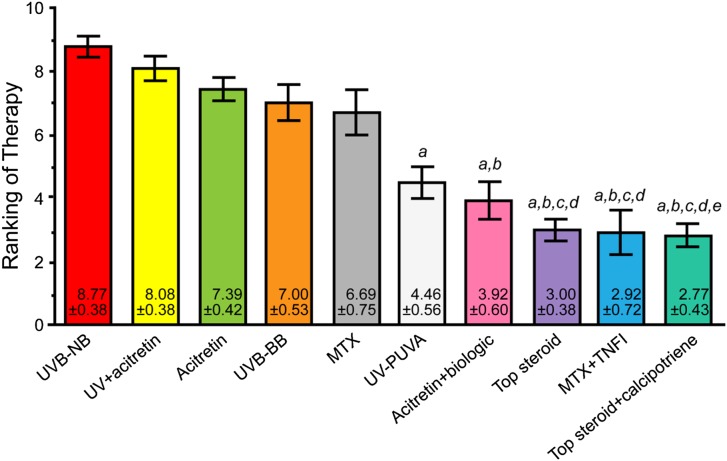
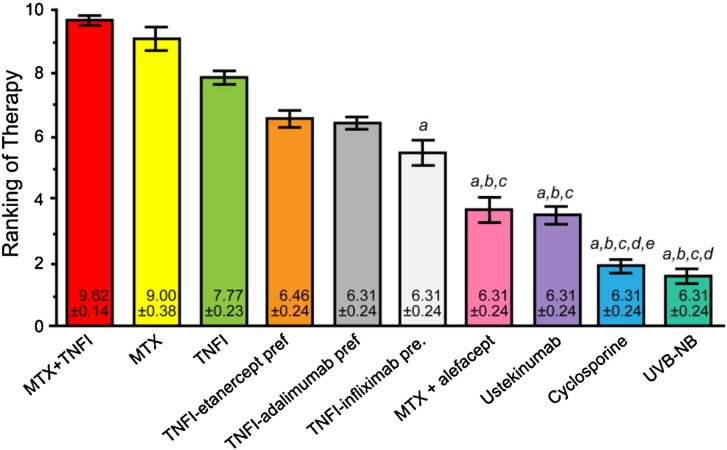
The panelists ranked the following as top treatments for HPV-induced cervical or anogenital dysplasia: narrowband UVB therapy, UV phototherapy + acitretin, acitretin alone, broadband UVB therapy, methotrexate, PUVA, acitretin + a biologic agent, a topical steroid, methotrexate + a TNF-α inhibitor, and a topical steroid + calcipotriene. While some of these choices represent a higher risk proposition (e.g., methotrexate + a TNF-α inhibitor), each remains a viable option in the right setting. Figure 2 presents the final results of the voting process.
Fig. 2.
Final results of the voting on case scenario 1, psoriasis with HPV-induced cervical or anogenital dysplasia. a denotes P < 0.05 compared with UVB-NB therapy; b denotes P < 0.05 compared with UV + acitretin therapy; c denotes P < 0.05 compared with acitretin therapy; d denotes P < 0.05 compared with UVB-BB therapy; e denotes P < 0.05 compared with MTX therapy. HPV human papilloma virus; MTX methotrexate; TNFI tumor necrosis factor inhibitor; Top topical; UVB-BB broadband ultraviolet B therapy; UVB-NB narrowband ultraviolet B therapy
Treatment Challenges: None.
Case Scenario 2: Concomitant Psoriasis and Systemic Lupus Erythematosus
Cases of concomitant psoriasis with systemic lupus erythematosus (SLE) are rare, occurring in only 0.69% of patients with psoriasis and 1.1% of patients with SLE (grade C evidence) [11]. However, subacute cutaneous lupus erythematosus (SCLE) may be mistaken for psoriasis and can coexist with SLE (grade C evidence) [12]. In either case, despite its therapeutic uses in psoriasis, phototherapy is a major concern. Regardless, no published studies exist to guide therapeutic recommendations.
For psoriasis, methotrexate has well-documented efficacy (grade A evidence) [13]. In SLE, methotrexate decreases the need for steroids and is therapeutic in reducing revised Systemic Lupus Activity Measure (SLAM-R) scores (grade A evidence) [14].
Acitretin is a psoriasis therapy that has been tested in SLE. For patients with predominantly cutaneous SLE, acitretin has been successful in completely clearing psoriasis in 15 of 20 patients (grade B evidence) [15]. In a separate study, acitretin was found to improve cutaneous lesions in 46% of patients (grade B evidence) [16].
Cyclosporine has an established role in psoriasis, but it is rarely utilized in SLE due to potential renal toxicity. This agent may be of limited use as a second-line therapy (grade B evidence) [17] or in the settings of lupus-related thrombocytopenia (grade C evidence) [18] and hemolytic anemia (grade D evidence) [19].
All of the TNF-α agents have significant roles in the treatment of psoriasis and there are a few case reports of benefit in SLE and lupus nephritis (grade D evidence) [20]. However, these therapies have an accepted risk of drug-induced SLE (grade D evidence) [21]. Ustekinumab presents another option given its success in psoriasis, but there are no published studies describing its use in SLE.
For SLE, azathioprine has a long history of use, especially for lupus nephritis (grade B evidence) [22, 23]. Azathioprine also has efficacy in psoriasis, with 55% of patients clearing at least 75% of their psoriasis over a mean of 12 months of therapy, although the supporting literature is over 30 years old (grade B evidence) [24, 25]. Mycophenolate mofetil has growing support as an alternative to azathioprine or as a supplementary medication in SLE and lupus nephritis (grade C evidence) [26]. In addition, there is one open-label study demonstrating modest benefit in psoriasis with 22% of patients reaching a 75% reduction in the Psoriasis Area and Severity Index (PASI) score after 12 weeks (grade B evidence) [27]. Hydroxychloroquine also has efficacy in cutaneous lupus, with 50% of patients experiencing improvement (grade B evidence), but its utility in psoriasis is questionable [16]. There is one report of the successful treatment of psoriasis and SCLE with hydroxychloroquine (grade D evidence) [28]. Yet, there are other reports of hydroxychloroquine causing worsening of psoriasis or pustular flares (grade D evidence) [29, 30].
Discussion
Panelists shared their individual experience with these rare cases. One stated that he uses TNF-α inhibitors in patients with SLE without difficulty and that the combination with hydroxychloroquine could be considered to reduce the formation of antibodies against the drug, although there are no published data to support this contention. Another shared the experience that the TNF-α-induced lupus-like syndromes typically have the skin findings of SLE, but are less likely to produce renal or central nervous system (CNS) manifestations. While a specific antibody pattern for TNF-α-inhibitor-induced lupus has not been identified, another panelist noted higher anti-DNA antibodies in patients treated with infliximab. These antibodies have been noted in patients treated with anti-TNF-α agents without any clinical manifestations suggestive of SLE. Another suggested the potential of abatacept as a crossover therapy, as it has been used in rheumatoid arthritis (RA) and is being evaluated for the treatment of psoriatic arthritis.
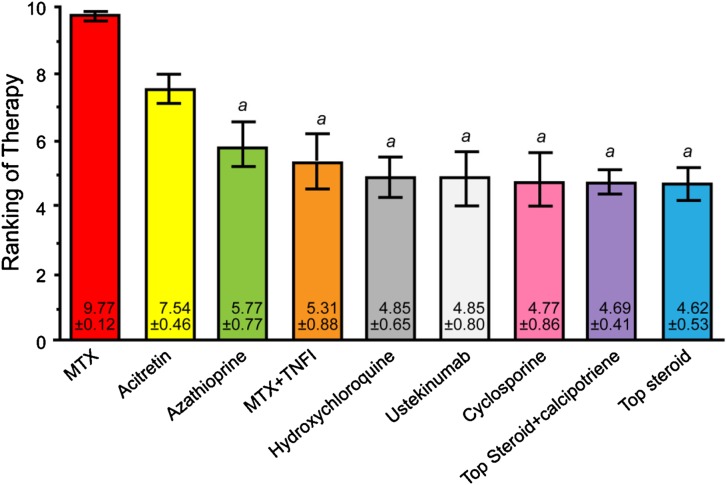
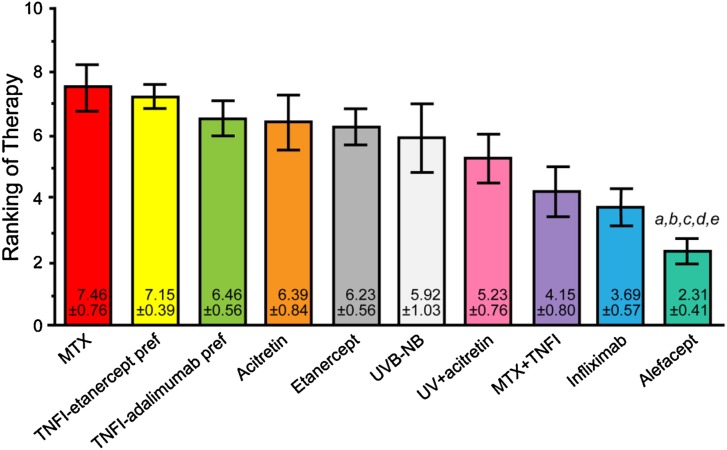
The agents ranked highest for psoriasis and concomitant SLE were methotrexate, acitretin, azathioprine, methotrexate + a TNF-α inhibitor, hydroxychloroquine, ustekinumab, cyclosporine, topical steroids + calcipotriene, and topical steroids. Figure 3 presents the final results of voting on this case.
Fig. 3.
Final results of the voting on case scenario 2, concomitant psoriasis and systemic lupus erythematosus. a denotes P < 0.05 compared with MTX therapy. MTX methotrexate; TNFI tumor necrosis factor inhibitor
Treatment Challenges: None.
Case Scenario 3: Severe Psoriatic Nail Disease Causing Functional or Emotional Impairment
In a group of patients hospitalized for psoriasis, up to 78% had psoriatic nail disease. Those with nail findings were significantly older and also a higher incidence of psoriatic arthropathy (grade C evidence) [31]. The majority of patients had both fingernail and toenail involvement with subungual hyperkeratosis as the most common abnormality regardless of site. Some of the features of psoriatic nail disease resemble onychomycosis and 18% of patients with psoriatic nail disease may also have positive mycological cultures. While psoriatic nail disease may be the sole manifestation of psoriasis, it may still result in significant impairment to patients.
For limited nail disease, topical approaches such as corticosteroids, 5-fluorouracil, calcipotriene, keratolytics, retinoids, and anthralin produce variable responses and may require long treatment periods and great patient adherence to therapy (grade C evidence) [32]. The requirement for consistent long-term use may deter some patients. Intralesional corticosteroids also have been implemented through needle or needle-less methods, but remain a difficult therapy for patients.
Many patients with nail disease also have arthritis; thus, treatments are often chosen for their ability to improve the manifestations of psoriatic arthritis. Both methotrexate (grade D evidence) [33, 34] and adalimumab (grade D evidence) [35] have successfully treated psoriatic onycho-pachydermoperiostitis (POPP), a condition that leads to psoriatic onychodystrophy (connective-tissue thickening of the distal phalanx and a periostial reaction). With therapy, both the nail and joint findings improve significantly, but there is no evidence for efficacy in isolated nail disease outside of POPP. Low-dose cyclosporine led to significant improvement of nail dystrophy in a small set of patients with psoriatic onychodystrophy, although the measure for quantifying nail severity was not described (grade D evidence) [36]. A larger study supported the utility of cyclosporine in psoriatic nail disease alone and in combination with topical calcipotriene under occlusion (grade B evidence) [37]. Cyclosporine and etretinate have been compared, with each showing very modest efficacy, but the 10-week treatment period examined would be too short to fully evaluate an adequate response (grade A evidence) [38]. A large trial using photo-derived nail psoriasis severity index (NAPSI) scores found significant reductions with etanercept treatment after a 12-week period as compared with controls (grade A evidence) [39]. Infliximab also effectively treats psoriatic nail disease, with 44.7% of patients demonstrating marked improvement at week 50 (grade A evidence) [40]. Ustekinumab also improves nail psoriasis, reducing the NAPSI score by 50% after 24 weeks of continuous therapy [41].
Discussion
Panelists shared that many of their patients are able to clear their nail disease or have a significant improvement with either conventional systemic or effective biologic therapies. Some recommended fungal evaluation for nonresponders to psoriatic medications. General conclusions were that the TNF-α inhibitors had the most rigorous efficacy data, but that many other systemic medications have the potential to normalize nails. Concerns for elevated blood pressure or renal disease may limit cyclosporine exposure to a limited time period. Given the slow growth of nails, eventual toxicity was raised as a potential hindrance to long-term cyclosporine usage. While the majority of discussion focused on the use of systemic medications, topical therapies were recommended as an adjuvant directed to cuticular disease.
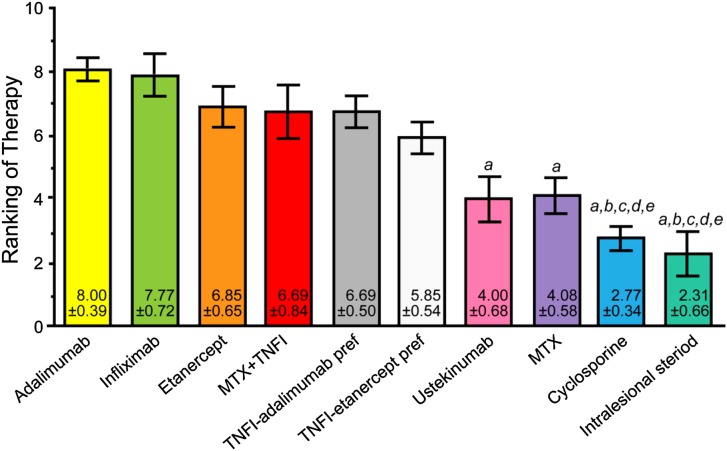
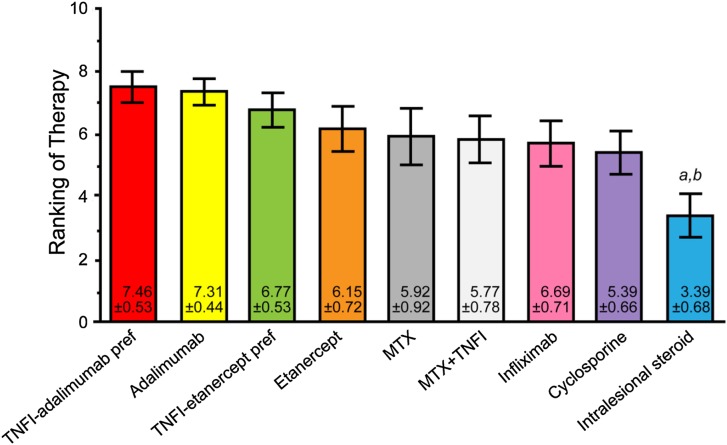
The top-ranked treatments for severe psoriatic nail disease causing functional or emotional impairment were adalimumab, infliximab, etanercept, methotrexate + a TNF-α inhibitor, any TNF-α inhibitor with adalimumab preferred, any TNF-α inhibitor with etanercept preferred, ustekinumab, methotrexate, cyclosporine, and intralesional steroids. Figure 4 presents the final results of voting on this case.
Fig. 4.
Final results of the voting on case scenario 3, severe psoriatic nail disease causing functional or emotional impairment. a denotes P < 0.05 compared with adalimumab therapy; b denotes P < 0.01 compared with infliximab therapy; c denotes P < 0.05 compared with etanercept therapy; d denotes P < 0.05 compared with MTX + TNFI therapy; e denotes P < 0.05 compared with TNFI-adalimumab preferred therapy. MTX methotrexate; pref preferred; TNFI tumor necrosis factor inhibitor
Treatment Challenges: None.
Case Scenario 4: Psoriasis Therapies That Potentially Reduce Cardiovascular Morbidity and Mortality
Hypertension, diabetes, obesity, smoking, and dyslipidemia are commonly accepted risk factors for cardiovascular disease (grade B evidence) [42, 43]. As compared to their age and sex-matched counterparts, psoriasis patients are more frequently diagnosed with many of those conditions (grade C evidence) [44]. In general, obesity, hypertension, diabetes, hyperlipidemia, and smoking are found at a higher prevalence in the psoriatic population and the prevalence of each risk factor increases as the severity of psoriasis increases (grade B evidence) [45].
The use of systemic therapies or multiple uses of potent topical corticosteroids have been correlated with higher rates of diabetes and atherosclerosis (grade C evidence) [46]. However, psoriasis may be an independent risk factor for cardiovascular events, specifically myocardial infarction (grade B evidence) [45]. This risk may arise from multiple different factors. Patients with psoriasis have an increased incidence of conventional cardiovascular risk factors such as diabetes, hypertension, obesity, and smoking, but they are also treated with multiple medications that may be dyslipidemic (e.g., corticosteroids, acitretin, or cyclosporine) or have other cardiovascular implications. In addition, chronic and uncontrolled inflammation from psoriatic disease may be related to endothelial dysfunction that increases cardiovascular risk. With this new perspective on psoriasis, this discussion examined the role of psoriasis treatments in altering psoriasis comorbidities or overall cardiovascular risk.
Many studies in the RA population have delved into the assessment of cardiovascular risk. C-reactive protein (CRP), a measure of systemic inflammation, has been the most predictive marker for cardiovascular-related mortality in patients with RA (grade C evidence) [47]. There is evidence for the role of systemic inflammation in cardiovascular risk as these patients have more unrecognized myocardial infarctions as compared to controls, and significantly more events in the 2 years immediately preceding the diagnosis of RA (grade B evidence) [48]. This suggests that systemic inflammation can damage the vascular endothelium and have cardiac consequences well before the joint manifestations of RA. In treating RA, methotrexate has been associated with a decreased risk for acute myocardial infarction, but both oral corticosteroids and biologic agents (etanercept, infliximab, and anakinra) have significantly increased the risk (grade C evidence) [49]. However, another study found that biologics (adalimumab, etanercept, infliximab, and anakinra) were only associated with increased risks when used in combination with other immunosuppressants, and that cytotoxic immunosuppressive agents (e.g., azathioprine, cyclosporine) and oral corticosteroids maintained a significantly elevated risk for cardiovascular events (grade C evidence) [50]. The Consortium of Rheumatology Researchers of North America (CORRONA) has developed a RA registry of over 10,000 patients. From this cohort, the incidence of cardiovascular events in patients exposed specifically to TNF-α inhibitors is 0.51 events/1,000 patient years, but TNF-naive patients have a higher incidence with 0.75 events/1,000 patient years. Duration of exposure is another contributing factor, as a longer period of exposure to TNF-inhibitors (>1 year) correlates with the greatest protection (grade B evidence) [51].
For individual therapies, methotrexate has the most evidence in psoriasis and may be able to reduce the incidence of vascular disease. A study of patients with psoriasis on methotrexate therapy demonstrated a significantly reduced risk of vascular disease compared to subjects who were not prescribed methotrexate (grade C evidence) [52]. This effect was most notable when methotrexate was given at a low cumulative dose and was further enhanced by the concomitant use of folic acid. The proposed mechanism of risk reduction is the ability of methotrexate to decrease chronic inflammation. Those requiring higher doses of methotrexate were also found to receive a benefit, but they may represent long-standing and more severe systemic inflammation, accounting for the difference in benefit as compared with patients receiving lower doses. As those with lower doses benefit most, early treatment with methotrexate may be effective in decreasing cardiovascular mortality. In addition, the use of folic acid may act to reduce hyperhomocysteinemia, which may be a separate contributor to vascular disease risk.
Etanercept treatment in patients who are obese and diabetic led to significant reductions in systemic inflammatory markers, such as CRP and interleukin-6 (IL-6), but was unable to affect vascular reactivity or insulin sensitivity (grade A evidence) [53]. In patients with the metabolic syndrome, etanercept showed similar effects with its reduction of CRP, IL-6, and fibronectin, but did not alter insulin sensitivity or body composition (grade A evidence) [54]. Etanercept also has been shown to lower the CRP of prospectively treated patients with moderate-to-severe psoriasis [55].
In patients with RA refractory to infliximab, short-term adalimumab therapy significantly improved endothelial vasodilatory response and reduced CRP levels (grade C evidence) [56]. In a recent study, both adalimumab and infliximab were shown to improve endothelial function, although common carotid artery intima-medial thickness did not change (grade B evidence) [57]. However, these patients were also on other disease-modifying antirheumatic drugs (DMARDs), which does not allow for conclusions regarding an isolated TNF-α-inhibitor effect. Adalimumab has also been shown to increase high-density lipoprotein (HDL) levels while decreasing low-density lipoprotein (LDL), thus creating a favorable cardiovascular risk profile in patients with RA (grade A evidence) [58].
Infliximab alone exerts a rapid and significant reduction in serum insulin levels and the insulin/glucose index of patients with RA immediately following infusion (grade C evidence) [56]. Long-term infliximab treatment has also been demonstrated to improve insulin sensitivity (grade C evidence) [59]. For lipid regulation, infliximab therapy has been shown to increase total cholesterol, LDL, and HDL levels without altering the atherogenic ratio (grade B evidence) [60]. However, a separate study demonstrated elevated LDL/HDL and total/HDL-cholesterol ratios, creating a pro-atherogenic lipid profile despite the antiinflammatory effect of infliximab (grade B evidence) [61].
A cohort of patients with RA was studied to determine the incidence of the first cardiovascular event in those treated with either etanercept or adalimumab. There was a significant decrease in the age-sex adjusted incidence rate in the TNF-α-treated patient group as compared with controls: 14.0/1,000 person years and 35.4/1,000 person years, respectively (grade B evidence) [62]. These findings suggest that TNF-α treatments may have the potential to decrease the risk of a patient developing a cardiovascular event.
Discussion
Many panelists expressed concern for the lack of data with various agents on cardiovascular risks inpatients with psoriasis. The majority of the data stems from patients with RA.
Regarding the available choices, the panel agreed that all methotrexate options should include concomitant folic acid. At the time the meeting was held, there were no published data for ustekinumab and its effect on cardiovascular risk. Since the meeting, pooled data from the phase 2 and 3 trials of ustekinumab have shown the drug to have neither a positive or negative effect on major cardiovascular risks [62]. Additional data were presented at the meeting showing that ustekinumab was able to decrease CRP values in patients with psoriasis and had improved efficacy for joint symptoms in those with CRP levels elevated to >0.4 mg/dL prior to therapy (grade A evidence) [63, 64]. However, a poster presentation for another IL-12/23 inhibitor that is structurally similar to ustekinumab (ABT-874 or briakinumab), suggested that drugs that target the p40 subunit of IL-12/23 may increase the risk of major adverse cardiovascular events [65]. Many choices do not have any data for cardiovascular risk (e.g., phototherapy, alefacept alone or in combination with methotrexate); a recognized limitation to the voting.
The panelists voted to include the following treatments as psoriasis therapies that potentially reduce cardiovascular morbidity and mortality: methotrexate + TNF-α inhibitor, methotrexate, TNF-α-inhibitor alone, any TNF-α inhibitor with etanercept preferred, any TNF-α inhibitor with adalimumab preferred, any TNF-α inhibitor with infliximab preferred, methotrexate + alefacept, ustekinumab, cyclosporine, narrowband UVB phototherapy. It should be noted that cyclosporine may alter lipid profiles unfavorably, and therefore remains a more complex treatment decision in this setting. Figure 5 shows the final results of voting.
Fig. 5.
Final results of the voting on case scenario 4, psoriasis therapies that potentially reduce cardiovascular morbidity and mortality. a denotes P < 0.05 compared with MTX-TNFI therapy; b denotes P < 0.01 compared with MTX therapy; c denotes P < 0.05 compared with TNFI therapy; d denotes P < 0.01 compared with TNFI (etanercept preferred) therapy; e denotes P < 0.01 compared with TNFI (adalimumab preferred) therapy. MTX methotrexate; pref preferred; TNFI tumor necrosis factor inhibitor; UVB-NB narrowband ultraviolet B therapy
Treatment Challenges
(1) Removal of ustekinumab. At the time of this meeting in 2009, ustekinumab was not approved or available in the US for the treatment of psoriasis and panelists expressed concern that there was extremely limited data on its use. Documented cardiovascular events had also occurred in the treatment group in the early trials (two myocardial infarctions and one stroke; grade A evidence) [66], although there was only one patient who experienced a stroke in the phase 3 trials (grade A evidence) [67]. The only beneficial cardiovascular evidence was a potential decrease in CRP.
However, the group argued that many other choices voted on had even less data or no data regarding cardiovascular risk factors, and ustekinumab remained on the list as a therapeutic option, with 11 participants voting to keep it as opposed to two voting to remove it.
(2) Removal of narrowband UVB therapy. The suggestion to remove narrowband UVB therapy was based on a lack of evidence for reduction in cardiovascular mortality. However, many panelists argued that it does not have documented increases in cardiovascular risk and that it can function as a psoriasis treatment without cardiovascular harm. The panel voted to allow phototherapy to remain on the list, with seven voting to allow it as a treatment option and six voting to remove it.
Case Scenario 5: Older Patients (≥65 Years of Age) with Psoriasis
Geriatric medicine identifies itself as serving patients typically ≥65 years of age, a fast-growing subset of the population (grade C evidence) [68]. From 1950 to 2006, the average annual population growth rate in the US was 1.2%, but 1.5% for those ages 65–74 years and 2.8% for those >75 years. By 2050, 20.7% of the population is expected to be >65 years of age (grade C evidence) [69].
Polypharmacy is a significant consideration in this group, as it increases the risk for adverse drug events and overall mortality (grade C evidence) [70]. In addition, the incidence of multiple medical problems such as mobility issues, arthritis, stroke, congestive heart failure, glucose intolerance, hypertension, osteoporosis, and malignancy increases with age, thereby influencing which medicines one may prescribe. Importantly, too, the elderly often live on fixed incomes and may find the affordability of the various treatments prohibitive.
Multiple age-related changes affect the pharmacokinetics of topical and systemic medications. With age, shifts in body composition may affect drug distribution and half-life, while decreases in renal and liver function may alter medication metabolism and clearance. In addition, skin surface changes, such as reduced hydration of the stratum corneum, a decreased lipid component, and lowered microcirculation, may all impact percutaneous absorption of topical medications (grade C evidence) [71].
Psoriasis therapy in particular has specific considerations in the aging population. With the high incidence of polypharmacy, drug-induced or drug-exacerbated psoriasis becomes more prominent. Older patients also face a range of medical, social, psychological, and financial stressors that will affect the availability and efficacy of medications or their practical use and patient adherence. With these considerations, one group suggests the use of topical medications as a first-line therapy, followed by narrowband UVB therapy. They reserve the use of methotrexate, acitretin, or cyclosporine only for patients with severe psoriasis, as there is a diminished therapeutic index in the elderly (grade D evidence) [72].
Few therapies have been studied in an elderly psoriasis population. Pooled data from the clinical trials of alefacept found similar efficacy and adverse event profiles in the elderly as compared to all enrolled psoriasis patients (grade A evidence) [73]. In patients with RA, methotrexate metabolic clearance is inversely proportional to age and further decreases with declines in creatinine clearance (grade B evidence) [74]. For psoriasis, methotrexate-associated myelosuppression may be fatal and is more likely to occur in the elderly (grade C evidence) [75]. The pharmacokinetics of cyclosporine in organ-transplant recipients does not differ significantly with age, but the elderly are still at risk for adverse drug events or the consequences of polypharmacy (grade B evidence) [76]. In elderly patients with RA, the use of TNF-α inhibitors has been associated with an increased risk of new-onset congestive heart failure (CHF) or exacerbation of pre-existing CHF. In those with prior CHF, the risk of death was fourfold higher in TNF-α users as compared to methotrexate users (grade B evidence) [77].
Discussion
Overall, the data for specific therapies in the elderly psoriatic population are limited. In many of the psoriasis trials, there is an underrepresentation of elderly patients as they are often excluded by comorbidities. The elderly have also experienced a longer duration of disease, which may affect their health prior to therapy and alter their response to treatment. Many panelists agreed that the therapeutic index is significantly reduced in the elderly. However, if the age-related concerns or risk factors are known, then panelists felt that systemic medications could still be of use in this population. Methotrexate was offered as an example, as it is typically efficacious at a lower dose in the elderly. While this may be due to age-related reductions in creatinine clearance, if this is known prior to the start of therapy, overdoses or adverse events may be avoided. Attention to polypharmacy is a requirement, as drug interactions may be more likely.
In contrast to methotrexate, other panelists supported the use of TNF-α agents as they may present a safer option when used in the appropriate subset of patients. Of the TNF-α agents, etanercept was highlighted for having the shortest half-life. Acitretin was also discussed as an option at low doses. In respect to disease severity, some proposed having the same treatment modalities available regardless of age. In addition, as many young patients have multiple conditions, comorbidities may dictate treatment rather than age.
Specific vaccinations are also recommended for elderly patients. The efficacy of these vaccines in the context of systemic psoriasis therapies remains debatable. Regardless, the panelists agreed that live vaccination should be avoided while a patient receives immunosuppressive therapy.
Top-ranked treatments for older patients with psoriasis include methotrexate, any TNF-α inhibitor with etanercept preferred, any TNF-α inhibitor with adalimumab preferred, acitretin, etanercept, narrowband UVB phototherapy, UV phototherapy + acitretin, methotrexate + TNF-α inhibitor, infliximab, and alefacept. Figure 6 presents the final results of voting.
Fig. 6.
Final results of the voting on case scenario 5, older patients (≥65 years of age) with psoriasis. a denotes P < 0.01 compared with MTX therapy; b denotes P < 0.01 compared with TNFI-etanercept preferred therapy; c denotes P < 0.05 compared with TNFI-adalimumab preferred therapy; d denotes P < 0.05 compared with acitretin therapy; e denotes P < 0.05 compared with etanercept therapy. MTX methotrexate; pref preferred; TNFI tumor necrosis factor inhibitor; UV ultraviolet; UVB-NB narrowband ultraviolet B therapy
Treatment Challenges: None.
Case Scenario 6: Severe Scalp Psoriasis that is Unresponsive to Topical Therapy
Psoriasis has a well-known impact on both mental health and quality of life (grade C evidence) [78, 79]. Scalp psoriasis does not differ, as up to 57% of patients experience psychological and social distress relating to sensations of scalp itch and scaling (grade C evidence) [80]. Many patients will face involvement of the majority of the scalp surface, and up to 86% will have other bodily areas of involvement.
For the TNF-α inhibitor class of therapeutics, both etanercept and infliximab have scalp-specific data. In one study, etanercept improved the Physician’s Global Assessment (PGA) scalp psoriasis score by 58% at 12 and 24 weeks (grade B evidence) [81]. Scalp improvement correlated with a similar response in the skin; the total body surface area affected improved by 50–60%. A randomized, double-blind, placebo-controlled trial that was underway for etanercept in the treatment of moderate-to-severe plaque psoriasis in patients with scalp disease at the time of the live meeting in 2009 has since been completed [82]. It showed that at a dose of 50 mg twice weekly, etanercept improved the Psoriasis Scalp Severity Index (PSSI) score by 87% by week 12 compared with a 20% improvement in the placebo group (grade A evidence) [83]. Even when the dose was reduced to 50 mg once weekly from weeks 12–24, the PSSI score improvement was maintained, while patients who were switched from placebo to etanercept 50 mg twice weekly had a 79% mean improvement in their PSSI score. For infliximab, subanalysis of three RCTs demonstrated consistently high efficacy for scalp psoriasis with up to 79–85% of patients reaching a PASI 75 in the head and neck region (grade A evidence) [84]. The efficacy on the trunk was nearly equivalent, but slightly lower on the extremities. Therapy with alefacept induced 16.7% of patients to reach a scalp PGA of “clear/almost clear” after 16 weeks of therapy (grade B evidence) [85]. A second course of therapy was able to increase the proportion of responders to 26.7%.
Other systemic agents such as methotrexate, cyclosporine, and intralesional corticosteroids do not have studies dedicated to scalp treatment. From the nonsystemic options, the excimer laser has produced significant responses when used in combination with manual hair separation or a hair blower device to increase the visible scalp surface area (grade B evidence) [86, 87]. Forty-nine percent of patients were able to clear greater than 95% of their scalp disease in an average of 21 treatments [87]. However, phototoxicity around the ears and neck was a common adverse event of excimer laser therapy.
Grenz ray therapy has also been shown to clear scalp psoriasis in up to 78% of patients and a combination with topical corticosteroids induced a longer remission period (grade B evidence) [88, 89].
Discussion
Many panelists stated that the scalp and the rest of the integument should be considered equivalent and agreed that no modifications in the therapeutic regimen were necessary for scalp-specific treatment. One panelist noted that for the few patients with scalp-only involvement, it might be more difficult to attain health insurance approval for costlier systemic medications based on the relatively low body-surface area involvement.
Some panelists noted that they had seen improvement with hair removal on the scalp or the beard area, which may be secondary to the increased exposure to solar-derived UV light.
Top-ranked treatments for severe scalp psoriasis unresponsive to topical therapies were any TNF-α inhibitor with adalimumab preferred, adalimumab alone, any TNF-α inhibitor with etanercept preferred, etanercept, methotrexate, methotrexate + TNF-α inhibitor, infliximab, cyclosporine, and intralesional steroids. The final results of the voting are presented in Fig. 7.
Fig. 7.
Final results of the voting on case scenario 6, severe scalp psoriasis that is unresponsive to topical therapy. a denotes P < 0.05 compared with TNFI-adalimumab preferred therapy; b denotes P < 0.05 compared with adalimumab therapy. MTX methotrexate; pref preferred; TNFI tumor necrosis factor inhibitor
Treatment Challenges: None.
Discussion
Clinical trials of traditional systemic and biologic treatments for psoriasis exclude a significant proportion of patients with either comorbidities or clinical states that present risk. The Delphi method was used to clarify the therapeutic approach to 24 important and complex clinical scenarios, which were assigned to a group of expert panelists for detailed review prior to presentation at a live meeting. Six of these scenarios are presented here; another five scenarios are presented in Part 2. The iterative and anonymous voting process of this method depends on an unbiased view of the available clinical data and leads to more objective consensus. This exercise is not meant to be the “final word” with regard to therapy for these types of patients, and the numerical “precision” of the rankings should not mislead the reader into believing we have created a completely valid “top-10 list.” In fact, for example, with reference to any specific patient, the treatment option ranked first might not be “better” than those ranked either 5th or 10th. Instead, the final rankings should be viewed as guidance for practical, potentially effective, and likely safe treatment in a majority of instances. Of course, medical appropriateness of any given therapeutic modality will vary from patient to patient. Because the Delphi method does not introduce better data for a given topic, it cannot produce an idealized outcome. In this vein, the process we have utilized selects rational treatment choices for each clinical scenario, but these choices often are not supported by rigorous studies. At the very least, this evidence-based approach relying on anonymous consensus is a more objective tool for reaching consensus.
An important limitation of this process is that new highly relevant information may be published subsequent to the exercise, which in this case was conducted in 2009. Since that time, for example, efalizumab was voluntarily removed from the US market due to the risk of progressive multifocal leukoencephalopathy (PML) [90, 91].
Another limitation is that some treatment options, such as UV phototherapy, are performed in different settings and guided by different protocols. Specifically, UV phototherapy may be conducted in an office, at a hospital, or at home. Some practitioners might consider tanning beds at commercial facilities a viable alternative to a practice or home-based approach. Regardless, we assumed that the choice for “UV phototherapy” encompasses the generic approach of treating psoriasis with UV light. The reader is encouraged to apply his or her knowledge, style, and experience in determining the generalizability of recommendations for the broader term “UV phototherapy” to the specific types of this modality.
Another potential limitation is conflict of interest among the panelists. Our selection of panelists was guided by their extensive experience with the use of all modalities for the treatment of psoriasis. Undeniably, this group has an extraordinarily large base of patients, and thus experience, on which to guide their opinions. Importantly, the results of the voting for many of the scenarios indicated a great deal of objectivity in the analysis. In fact, many of the biologic agents (e.g., adalimumab, alefacept, etanercept, infliximab, ustekinumab) were rated lowly. On the other hand, for certain scenarios, older conventional modalities such as UV phototherapy, methotrexate, acitretin, and cyclosporine were selected as the most appropriate therapeutic choices. The conflicts of interests for this large panel are extensive, but also are quite broad and diverse both with regard to each individual panelist and across the entire group. The authors think this resulted in a very fair process that, in fact, treated biologic therapies quite appropriately.
Finally, the panel of experts is derived entirely from the US. The treatment options are based on what is locally available in the US, and therefore are sometimes not relevant to the rest of the world.
The goal in performing this Delphi exercise was to help clinicians in practice benefit from these consensus opinions, offering guidance when presented with patients displaying similar challenging clinical scenarios, and allowing for the use of specific treatment approaches that are effective and safe.
Acknowledgments
The Delphi exercise and meeting was supported by educational grants from Abbott Laboratories, Amgen, Astellas, Galderma, and Genentech, and held in association with Millennium CME Institute, Inc. and Advances in Cosmetic and Medical Dermatology: Maui Derm.
Conflict of interest
All of the authors disclose that they received support for travel and an honorarium for participating in the Delphi exercise. J.C.C. discloses that she has received honoraria from Abbott, Amgen, Centocor, Eisai, and Genentech for speaking and consulting. D.C. discloses that he has received honoraria from Abbott, Amgen, Galderma, Johnson & Johnson, Steifel, and Viewpoint Securities for speaking and consulting. J.J.C. discloses that he has received honoraria from Abbott, Amgen, and Genentech for speaking and consulting. K.B.G. discloses that he has received honoraria and grants from Abbott, Amgen, Centocor, and Gladerma for grants and consulting. A.G. discloses that she has received honoraria from Acetelion, Almirall, Amgen, Beiersdorf, Bristol Myers Squibb, Can-Fite, Celera, Celgene, Centocor, Corgentech, Cytokine, Dermipsor, Immune Control, Incyte, Kemia, Magen Biosciences, Medarex, Novo Nordisk, PureTech, RxClinical, Roche, Sankyo, Teva, UCB, Warner Chilcott, Wyeth for speaking and consulting; almost all income from these activities was paid directly to her employer. She has received research/educational grants from Abbott, Amgen, Celgene, Centocor, Immune Control, Incyte, and Wyeth. A.F.K. discloses that he has received honoraria from Abbott, Amgen, Biogen Idec, Centocor, and UCB to support research studies. N.J.K. discloses that he has received honoraria from Abbott, Amgen, Astellas, Celggene, Centocor, Genentech, Genmab, Kemia, Novartis, Peplin, and Watson for speaking, consulting, and for research studies. G.G.K. discloses that he has received honoraria from Abbott, Almirall, Alza, Amgen, Anacor, Astellas, Barrier Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Centocor, CombinationRx, Exelixis, Genentech, Genzyme, Isis, L’Oreal, Lupin Limited, Magen Biosciences, MedaCorp, Medicis, Novartis, Nova Nordisc, Schering Plough, Somagenics, theDerm.org, Synvista, Warner Chilcott, UCB, USANA Health Sciences, and ZARS for speaking and consulting. He owns equities and stock options in ZARS. He has received partial stipend support for a clinical research fellowship from Abbott, Amgen, and Centocor. C.L.L. discloses that he has received honoraria from Abbott and Amgen for speaking and from Abbott, Amgen, Centocor, Eli Lilly, and Pfizer, Inc., for consulting. He has received research grant support from Abbott, Amgen, Anacor, Celgene, Centocor, Eli Lilly, Galderma, GlazoSmithKline, Incyte, Maruho, Novartis, Novo Nordisk, Pfizer, Inc., Schering Plough, Sirtris, Stiefel, Vascular Biogenic, and Wyeth. S.S. discloses that he has received honoraria from Abbott, Centocor, Genentech, and UCB for speaking and consulting. J.M.S. discloses that he has received honoraria from Abbott, Amgen, Centocor, and Genentech for speaking and consulting. G.E.S. has no disclosures other than that cited above for all participants. M.Y. discloses that she has received honoraria from Abbott, Amgen, Astellas, Centocor, and Genentech for speaking and consulting. B.E.S. discloses that he has received honoraria for consulting from Abbott, Amgen, Celgene, Centocor/Johnson & Johnson, Galderma, Leo, Maruho, and Novartis. Editorial assistance in the preparation of this article was provided by an independent medical editor, Nancy Monson. B.E.S. is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Strober B, Berger E, Cather J, et al. A series of critically challenging case scenarios in moderate to severe psoriasis: a Delphi consensus approach. J Am Acad Derm. 2009;61:S1–S46. doi: 10.1016/j.jaad.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality. Available at: www.ahrq.gov. Accessed 29 Nov 2011. [DOI] [PubMed]
- 3.Munoz N, Bosch FX, Castellsaque X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 4.Majewski S, Favre M, Ortho G, Jablonska S. Is human papillomavirus type 5 the putative autoantigen involved in psoriasis? J Invest Dermatol. 1998;111:541–542. doi: 10.1046/j.1523-1747.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 5.Rust A, McGovern RN, Gostout BS, Pershing DH, Pittelkow MR. Human papillomavirus in cutaneous squamous cell carcinoma and cervix of a patient with psoriasis and extensive ultraviolet radiation exposure. J Am Acad Dermatol. 2001;44:681–686. doi: 10.1067/mjd.2001.112359. [DOI] [PubMed] [Google Scholar]
- 6.Zumtobel U, Schwarze HP, Favre M, Taieb A, Delaunay M. Widespread cutaneous carcinomas associated with human papillomaviruses 5, 14 and 20 after introduction of methotrexate in two long-term PUVA-treated patients. Dermatology. 2001;202:127–130. doi: 10.1159/000051612. [DOI] [PubMed] [Google Scholar]
- 7.Helm CW, Lorenz DJ, Meyer NJ, Rising WR, Wulff JL. Retinoids for preventing the progression of cervical intra-epithelial neoplasia. Cochrane Database Syst Rev. 2007;CD003296. [DOI] [PubMed]
- 8.Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD. Genital HPV lesions and molluscum contagiosum occurring in patients receiving anti-TNF-alpha therapy. Dermatology. 2008;216:364–365. doi: 10.1159/000117709. [DOI] [PubMed] [Google Scholar]
- 9.Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–251. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- 10.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. [DOI] [PubMed]
- 11.Zalla MJ, Muller SA. The coexistence of psoriasis with lupus erythematosus and other photosensitive disorders. Acta Derm Venereol Suppl (Stockh). 1996;195:1–15. [PubMed] [Google Scholar]
- 12.Lee LA, Roberts CM, Frank MB, McCubbin VR, Reichlin M. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262–1268. doi: 10.1001/archderm.1994.01690100046006. [DOI] [PubMed] [Google Scholar]
- 13.Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 14.Fortin PR, Abrahamowicz M, Ferland D, et al. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2008;59:1796–1804. doi: 10.1002/art.24068. [DOI] [PubMed] [Google Scholar]
- 15.Ruzicka T, Meurer M, Bieber T. Efficiency of acitretin in the treatment of cutaneous lupus erythematosus. Arch Dermatol. 1988;124:897–902. doi: 10.1001/archderm.1988.01670060043013. [DOI] [PubMed] [Google Scholar]
- 16.Ruzicka T, Sommerberg C, Goerz G, Kind P, Mensing H. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br J Dermatol. 1992;127:513–518. doi: 10.1111/j.1365-2133.1992.tb14851.x. [DOI] [PubMed] [Google Scholar]
- 17.Caccavo D, Lagana B, Mitterhofer AP, et al. Long-term treatment of systemic lupus erythematosus with cyclosporin A. Arthritis Rheum. 1997;40:27–35. doi: 10.1002/art.1780400106. [DOI] [PubMed] [Google Scholar]
- 18.Morton SJ, Powell RJ. An audit of cyclosporin for systemic lupus erythematosus and related overlap syndromes: limitations of its use. Ann Rheum Dis. 2000;59:487–489. doi: 10.1136/ard.59.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon K, Korman N, Frankel E, et al. Efficacy of etanercept in an integrated multistudy database of patients with psoriasis. J Am Acad Dermatol. 2006;54:S101–S111. doi: 10.1016/j.jaad.2005.11.1088. [DOI] [PubMed] [Google Scholar]
- 20.Aringer M, Smolen JS. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin Drug Saf. 2008;7:411–419. doi: 10.1517/14740338.7.4.411. [DOI] [PubMed] [Google Scholar]
- 21.Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum. 2008;37:381–387. doi: 10.1016/j.semarthrit.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Berends MA, Snoek J, deJong Em, et al. Liver injury in long-term methotrexate treatment in psoriasis is relatively infrequent. Aliment Pharmacol Ther. 2006;24:805–811. doi: 10.1111/j.1365-2036.2006.03047.x. [DOI] [PubMed] [Google Scholar]
- 23.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 24.Du Vivier A, Munro DD, Verbov J. Treatment of psoriasis with azathioprine. Br Med J. 1974;1:49–51. doi: 10.1136/bmj.1.5897.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro DD. Azathioprine in psoriasis. Proc R Soc Med. 1973;66:747–748. [PMC free article] [PubMed] [Google Scholar]
- 26.Mok CC. Mycophenolate mofetil for non-renal manifestations of systemic lupus erythematosus: a systematic review. Scand J Rheumatol. 2007;36:329–337. doi: 10.1080/03009740701607042. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Rosenthal D, Dutz J, Ho V. Mycophenolate mofetil (CellCept) for psoriasis: a two-center, prospective, open-label clinical trial. J Cutan Med Surg. 2003;7:193–197. doi: 10.1007/s10227-002-0113-6. [DOI] [PubMed] [Google Scholar]
- 28.Sorbara S, Cozzani E, Rebora A, Parodi A. Hydroxychloroquine in psoriasis: is it really harmful? Acta Derm Venereol. 2006;86:450–451. doi: 10.2340/00015555-0141. [DOI] [PubMed] [Google Scholar]
- 29.Gray RG. Hydroxychloroquine provocation of psoriasis. J Rheumatol. 1985;12:391. [PubMed] [Google Scholar]
- 30.Friedman SJ. Pustular psoriasis associated with hydroxychloroquine. J Am Acad Dermatol. 1987;16:1256–1257. doi: 10.1016/S0190-9622(87)80021-X. [DOI] [PubMed] [Google Scholar]
- 31.Salomon J, Szepietowski JC, Proniewicz A. Psoriatic nails: a prospective clinical study. J Cutan Med Surg. 2003;7:317–321. doi: 10.1007/s10227-002-0143-0. [DOI] [PubMed] [Google Scholar]
- 32.Cassell S, Kavanaugh AF. Therapies for psoriatic nail disease. A systematic review. J Rheumatol. 2006;33:1452–1456. [PubMed] [Google Scholar]
- 33.Ochiai T, Washio H, Shiraiwa H, Takei M, Sawada S. Psoriatic onycho-pachydermo-periostitis successfully treated with low-dose methotrexate. Med Sci Monit. 2006;12:CS27–30. [PubMed]
- 34.Bauza A, Redondo P, Aquerreta D. Psoriatic onycho-pachydermo periostitis: treatment with methotrexate. Br J Dermatol. 2000;143:901–902. doi: 10.1046/j.1365-2133.2000.03802.x. [DOI] [PubMed] [Google Scholar]
- 35.Bongartz T, Harle P, Friedrich S, et al. Successful treatment of psoriatic onycho-pachydermo periostitis (POPP) with adalimumab. Arthritis Rheum. 2005;52:280–282. doi: 10.1002/art.20763. [DOI] [PubMed] [Google Scholar]
- 36.Syuto T, Abe M, Ishibuschi H, Ishikawa O. Successful treatment of psoriatic nails with low-dose cyclosporine administration. Eur J Dermatol. 2007;17:248–249. doi: 10.1684/ejd.2007.0163. [DOI] [PubMed] [Google Scholar]
- 37.Feliciani C, Zampetti A, Forleo P, et al. Nail psoriasis: combined therapy with systemic cyclosporin and topical calcipotriol. J Cutan Med Surg. 2004;8:122–125. doi: 10.1007/s10227-004-0114-8. [DOI] [PubMed] [Google Scholar]
- 38.Mahrle G, Schulze HJ, Farber L, Weidinger J, Stiegleder GK. Low-dose short-term cyclosporine versus etretinate in psoriasis: improvement of skin, nail, and joint involvement. J Am Acad Dermatol. 1995;32:78–88. doi: 10.1016/0190-9622(95)90189-2. [DOI] [PubMed] [Google Scholar]
- 39.Rich P, Gupta A, Wang A, Jahreis A. Etanercept improves nail psoriasis. 2006 Annual Meeting of the American Academy of Dermatology. San Francisco, CA. J Am Acad Dermatol. 2006;54(Suppl.):P2751.
- 40.Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 41.Rich P, Langely R, Guzzo C, Wang Y, Rosoph L, Gordon K. Improvement in nail psoriasis with ustekinumab, a new anti-IL-12/23p40 monoclonal antibody: results from a phase 3 trial. Poster present at 17th Congress of the European Academy of Dermatology and Venereology, September 17–21, 2008. Poster FP 1007.
- 42.O’Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol. 2008;61:299–310 (in Spanish). [PubMed]
- 43.Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med. 1990;322:1635–1641. doi: 10.1056/NEJM199006073222304. [DOI] [PubMed] [Google Scholar]
- 44.Kimball AB, Robindson D, Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001–2002. Dermatology. 2008;217:27–37. doi: 10.1159/000121333. [DOI] [PubMed] [Google Scholar]
- 45.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629–634. doi: 10.1016/j.jaad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Goodson NJ, Symmons DP, Scott DG, Bunn D, Lunt M, Silman AJ. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year follow up study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 48.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 49.Suissa S, Bernatsky S, Hudson M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum. 2006;55:531–536. doi: 10.1002/art.22094. [DOI] [PubMed] [Google Scholar]
- 50.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–3798. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 51.Solomon DH, Curtis JR, Kremer JM, et al. TNF blocker use and cardiovascular outcomes. Arthritis Rheum. 2008;58:S544. doi: 10.1002/art.23396. [DOI] [Google Scholar]
- 52.Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262–267. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Dominguez H, Storgaard H, Rask-Madsen C, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strober BE, Teller C, Yamauchi P, et al. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br J Dermatol. 2008;159:322–330. doi: 10.1111/j.1365-2133.2008.08628.x. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Gay MA, DeMatias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–86. [PubMed] [Google Scholar]
- 57.Sidiropoulos PI, Siakka P, Pagonidis K, et al. Sustained improvement of vascular endothelial function during anti-TNF-alpha treatment in rheumatoid arthritis patients. Scand J Rheumatol. 2009;38:6–10. doi: 10.1080/03009740802363768. [DOI] [PubMed] [Google Scholar]
- 58.Popa C, Netea MG, Radstake T, et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oguz FM, Oguz A, Uzunlulu M. The effect of infliximab treatment on insulin resistance in patients with rheumatoid arthritis. Acta Clin Belg. 2007;62:218–222. doi: 10.1179/acb.2007.035. [DOI] [PubMed] [Google Scholar]
- 60.Allanore Y, Kahan A, Sellam J, Ekindjian OG, Borderie D. Effects of repeated infliximab therapy on serum lipid profile in patients with refractory rheumatoid arthritis. Clin Chim Acta. 2006;365:143–148. doi: 10.1016/j.cca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Dahlqvist SR, Engstrand S, Berglin E, Johnson O. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35:107–111. doi: 10.1080/03009740500474578. [DOI] [PubMed] [Google Scholar]
- 62.Jacobsson LT, Turresson C, Gulfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–1218. [PubMed] [Google Scholar]
- 63.Reich K, Langley RG, Lebwohl M, et al. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analysis of data from phase II and III clinical studies. Br J Dermatol. 2011;164:862–872. doi: 10.1111/j.1365-2133.2011.10257.x. [DOI] [PubMed] [Google Scholar]
- 64.Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–640. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 65.Gordon K, Langley R, Gottlieb A, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 monoclonal antibody, briakinumab, in moderate-to-severe psoriasis. J Invest Dermatol. 2011. Epub ahead of print. [DOI] [PubMed]
- 66.Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 67.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 68.Fact Sheet: The American Geriatrics Society (AGS). http://www.americangeriatrics.org/about_us/who_we_are/faq_fact_sheet/. Accessed 17 Aug 2011.
- 69.Health, United States, 2008 with Special Feature on the Health of Young Adults. 2008. Hyattsville, MD; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. http://www.cdc.gov/nchs/data/hus/hus08.pdf. Accessed 17 Aug 2011.
- 70.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Flammiger A, Maibach H. Dermatological drug dosage in the elderly. Skin Therapy Lett. 2006;11:1–7. [PubMed] [Google Scholar]
- 72.Yosipovitch G, Tang MB. Practical management of psoriasis in the elderly: epidemiology, clinical aspects, quality of life, patient education and treatment options. Drugs Aging. 2002;19:847–863. doi: 10.2165/00002512-200219110-00003. [DOI] [PubMed] [Google Scholar]
- 73.Gottlieb AB, Boehncke WH, Darif M. Safety and efficacy of alefacept in elderly patients and other special populations. J Drugs Dermatol. 2005;4:718–724. [PubMed] [Google Scholar]
- 74.Bressolle F, Bologna C, Kinowski JM, Arcos B, Sany J, Combe B. Total and free methotrexate pharmacokinetics in elderly patients with rheumatoid arthritis. A comparison with young patients. J Rheumatol. 1997;24:1903–1909. [PubMed] [Google Scholar]
- 75.Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399–408. doi: 10.1111/j.1365-2230.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 76.Kovarik JM, Koelle EU. Cyclosporin pharmacokinetics in the elderly. Drugs Aging. 1999;15:197–205. doi: 10.2165/00002512-199915030-00003. [DOI] [PubMed] [Google Scholar]
- 77.Setoguchi S, Schneeweiss S, Avorn J, et al. Tumor necrosis factor-alpha antagonist use and heart failure in elderly patients with rheumatoid arthritis. Am Heart J. 2008;156:336–341. doi: 10.1016/j.ahj.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heydendael VM, deBorgie CA, Spuls PI, Bossuyt PM, Bos JD, deRie MA. The burden of psoriasis is not determined by disease severity only. J Investig Dermatol Symp Proc. 2004;9:131–135. doi: 10.1111/j.1087-0024.2004.09115.x. [DOI] [PubMed] [Google Scholar]
- 79.Amatya B, Wennersten G, Nordlind K. Patients’ perspective of pruritus in chronic plaque psoriasis: a questionnaire-based study. J Eur Acad Dermatol Venereol. 2008;22:822–826. doi: 10.1111/j.1468-3083.2008.02591.x. [DOI] [PubMed] [Google Scholar]
- 80.van de Kerkhof PC, de Hoop D, de Korte J, Kuipers MV. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology. 1998;197:326–334. doi: 10.1159/000018026. [DOI] [PubMed] [Google Scholar]
- 81.Moore A, Gordon KB, Kang S, et al. A randomized, open-label trial of continuous versus interrupted etanercept therapy in the treatment of psoriasis. J Am Acad Dermatol. 2007;56:598–603. doi: 10.1016/j.jaad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Amgen and Inc. A randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of etanercept in treating scalp involvement in subjects with moderate to severe plaque psoriasis. http://clinicaltrials.gov/ct2/show/NCT00791765. Accessed 17 Aug 2011.
- 83.Bagel J, Tyring S, Lynde C, Kricorian G, Shi Y, Klekotka P. Etanercept therapy for moderate to severe plaque psoriasis with involvement of the scalp. 2011 American Academy of Dermatology annual meeting. J Am Acad Dermatol. 2011;50:P3322.
- 84.Menter A, Reich K, Guzzo O. Consistency of infliximab response in different body regions for treatment of moderate to severe psoriasis: results from controlled clinical trials.J Am Acad Dermatol. 2008;58:AB120. Abstract 2607.
- 85.Krell J, Nelson C, Spencer L, Miller S. An open-label study evaluating the efficacy and tolerability of alefacept for the treatment of scalp psoriasis. J Am Acad Dermatol. 2008;58:609–616. doi: 10.1016/j.jaad.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 86.Passeron T, Ortonne JP. Use of the 308-nm excimer laser for psoriasis and vitiligo. Clin Dermatol. 2006;24:33–42. doi: 10.1016/j.clindermatol.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 87.Morison WL, Atkinson DF, Werthman L. Effective treatment of scalp psoriasis using the excimer (308 nm) laser. Photodermatol Photoimmunol Photomed. 2006;22:181–183. doi: 10.1111/j.1600-0781.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 88.Lindelof B, Johannesson A. Psoriasis of the scalp treated with Grenz rays or topical corticosteroid combined with Grenz rays. A comparative randomized trial. Br J Dermatol. 1988;119:241–244. doi: 10.1111/j.1365-2133.1988.tb03206.x. [DOI] [PubMed] [Google Scholar]
- 89.Johannesson A, Lindelof B. Additional effect of Grenz rays on psoriasis lesions of the scalp treated with topical corticosteroids. Dermatologica. 1987;175:290–292. doi: 10.1159/000248836. [DOI] [PubMed] [Google Scholar]
- 90.FDA Public Health Advisory, Updated Safety Information about Raptiva (efalizumab). 2008, Federal Drug Administration. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm110605.htm. Accessed 17 Aug 2011.
- 91.FDA Statement on the Voluntary Withdrawl of Raptiva from the U.S. Market. 2009, Federal Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149561.htm. Accessed 17 August 2011.