Abstract
The study objective was to evaluate the effect of occupational lead exposure on blood concentrations of zinc, iron, copper, selenium and proteins related to them, such as transferrin, caeruloplasmin and haptoglobin. The examined group consisted of 192 healthy male employees of zinc–lead works. By the degree of lead exposure, the exposed group was subdivided into three subgroups. The control group was composed of 73 healthy male administrative workers. The markers of lead exposure (blood levels of lead and zinc protoporphyrin) were significantly elevated in the exposed group compared with the control group. Additionally, concentrations of copper and caeruloplasmin were raised. The significant increase in haptoglobin level was observed only in the low exposure group. Selenium levels were significantly decreased, whereas iron, zinc and transferrin levels were unchanged in the exposed group compared with the control group. There were positive correlations between the lead toxicity parameters and the copper and caeruloplasmin levels. In conclusion, the effect of occupational exposure to lead on the metabolism of trace metals appears to be limited. However, significant associations between lead exposure and levels of copper and selenium were shown. Changed levels of positive acute-phase proteins, such as caeruloplasmin and haptoglobin, were also observed.
Keywords: Lead poisoning, Trace elements, Acute-phase proteins, Haptoglobin, Caeruloplasmin, Transferrin
Introduction
Although lead induces physiological, biochemical and behavioural disturbances in humans, exposure to this xenobiotic is unavoidable because of its accumulation in the environment and use in industrial applications [1, 2]. Being present in contaminated water, air, food and dust [3], lead is mostly absorbed by the lungs and gastrointestinal tract, whereas percutaneous absorption of inorganic lead is minimal. In adults, 40–50 % of inhaled and approximately 10 % of ingested lead are transferred to the bloodstream and then distributed by plasma throughout the soft tissues and bones [4].
One of the major targets for lead toxicity is the thiol group of enzymes. Consequently, lead has an inhibitory effect on delta-aminolevulinic acid dehydratase. Because lead also inhibits ferrochelatase, it impairs the chain reaction that leads to the formation of haem; this impairment results in anaemia and the accumulation of delta-aminolevulinic acid (ALA) and zinc protoporphyrin (ZPP) in erythrocytes [5, 6]. Concentrations of ALA and ZPP are used as biomarkers of human lead exposure [4].
Lead is a redox inactive metal [5]. However, lead has pro-oxidative activity and can generate reactive oxygen species (ROS) and reduce cell antioxidant defences, such as antioxidant enzymes and glutathione [2]. Moreover, ALA that accumulates in saturnism has pro-oxidant properties [7].
Furthermore, lead interacts with some essential metals [2]. One of them is selenium (Se), which plays an important role as an antioxidant [8]. Se is a cofactor of glutathione peroxidase, decreases the amount of lipid peroxidation and protects DNA, RNA and proteins from oxidative damage. Additionally, Se forms inactive selenium–lead complexes [9] and, consequently, reduces the availability of free lead ions in the body [10].
A lead–zinc interaction has been observed [11]. Zinc (Zn) is essential for cellular membrane integrity and metabolism [2] as a central part of over 300 enzymes and proteins [12]. Similar to Se, Zn has been shown to possess antioxidant properties caused by its requirement for superoxide dismutase (SOD) activity [13]. Therefore, Zn not only reduces lead-induced oxidative stress but also competes with lead for similar binding sites [11]. Competitive binding to metallothionein-like transport protein in the rat duodenum suggests the ability of Zn to reduce lead absorption [11, 14].
Additionally, copper (Cu) has been reported to bind to metallothionein-like transport proteins [14]. Cu is contained in caeruloplasmin, an α2-globulin having enzymatic properties, and is responsible for the oxidation of ferrous to ferric iron and catalyses the transport of iron to transferrin, which transfers bound ions to cells. Because lead binds to both caeruloplasmin and transferrin, iron (Fe) metabolism in exposed individuals could be impaired [15, 16]. Another antagonism between these metals may occur in the intestine because low dietary intake of Fe should increase the absorption of lead [17]. Moreover, limited Fe in the mitochondria may enhance lead-induced haem synthesis inhibition [11].
In short, the presence of metals, such as Se, Zn, Cu and Fe, modifies lead toxicity, but their interactions are unclear.
Therefore, the present study was undertaken to determine the effect of occupational exposure to lead on blood levels of the above-mentioned trace metals and proteins that are related to them.
Materials and Methods
Study Population
The experimental protocol has been approved by the Bioethics Committee of the Medical University of Silesia in Katowice no. NN-6501-36/I/06. The examined group included 192 male employees of zinc and lead works localised in the southern region of Poland with an age range of 22–58 years. The study subjects had been exposed to lead for 4 to 37 years. Workers suffering from chronic diseases were excluded.
To determine the amount of lead exposure (exposure to zinc was insignificant), the concentrations of lead and zinc protoporphyrin in the blood samples were determined, on average, every 3 months during the 2 years of observation. From the collected data, the mean blood concentrations of lead (PbBmean) and zinc protoporphyrin (ZPPmean) were calculated. In view of the obtained values, the examined population was divided into three subgroups: low exposure to lead (LE), medium exposure to lead (ME) and high exposure to lead (HE). The LE group consisted of 56 workers with PbBmean less than 40 μg/dl. The ME group included 67 workers with a PbBmean from 40 to 50 μg/dl and a ZPPmean from 5 to 7.5 μg/g Hgb, whereas 69 workers with a PbBmean greater than 45 μg/dl and a ZPPmean greater than 7.5 μg/g Hgb were classified as the HE group.
In the last collected blood samples, blood lead level (PbB), blood zinc protoporphyrin level (ZPP) and concentrations of iron, selenium, copper, zinc, caeruloplasmin, haptoglobin and transferrin were measured concomitantly.
The control group consisted of 73 healthy male administrative workers who were exposed to lead only environmentally and had no history of occupational exposure to lead. The age range of the control group was 21 to 60 years. No one from this group had PbB or ZPP levels greater than the normal levels, which were 10 μg/dl and 2.5 μg/g Hgb, respectively.
Sampling and Laboratory Procedures
By venipuncture, 10 ml of blood was collected into plain tubes to obtain serum, whereas 15 ml was placed in tubes containing an ethylenediaminetetraacetic disodium acid solution as an anticoagulant to obtain plasma and erythrocytes.
Whole blood was used for the analysis of PbB and ZPP. The determination of PbB was performed by graphite furnace atomic absorption spectrophotometry, using Unicam 929 and 939OZ Atomic Absorption Spectrometers with GF90 and GF90Z Graphite Furnaces. The data were reported in micrograms per deciliter. ZPP was measured using an Aviv Biomedical Hematofluorometer, Model 206. The results were expressed as micrograms per gram of haemoglobin.
After centrifugation of the remaining blood, plasma was separated for zinc, copper and selenium analysis. The sedimented red blood cells were washed three times with 0.9 % NaCl and then lysed with bidistilled water. In 10 % haemolysate, the concentration of haemoglobin was determined using the cyanmethaemoglobin method.
The concentrations of Zn, Cu and Se in plasma were determined by atomic absorption spectrophotometer using an acetylene-air flame. The results were reported in micrograms per deciliter. Serum Fe analysis was performed on A25 Clinical Analyzer (BioSystems, Spain) according to the manufacturer’s instructions. The obtained values were expressed in micromoles per liter.
The concentration of caeruloplasmin in serum was determined by Richterich [18] and expressed in micrograms per deciliter. Serum transferrin and haptoglobin levels were measured by immunoturbidimetric assays. Specific rabbit monoclonal antibodies (Dako-Cytomation, Denmark) were used according to the manufacturer’s instructions. The measurements were performed on Biochemical Analyzer EM 280 (Emapol, Poland).
Statistical Analysis
The statistical analysis was performed using Statistica 9.1 PL software. The statistical methods included the mean and standard deviation. Shapiro–Wilk’s test was used to verify normality, and Levene’s test was used to verify homogeneity of variances. Either an analysis of variance or Kruskal–Wallis ANOVA test was used for multiple comparisons of data. Additional statistical comparisons were made using either a t test, t test with separate variance estimates or a Mann–Whitney U test. A Spearman non-parametric correlation was calculated. A value of p < 0.05 was considered to be significant.
Results
There were no significant differences in age, body mass index and smoking habits between the examined and control groups (Table 1). Nevertheless, when comparing the control group with the subgroups, the mean age was significantly lower in the low exposure group (LE) by 10.5 %. The mean PbB and ZPP levels were significantly higher in the LE group by 492 and 242 %, respectively, in the medium exposure group (ME) by 650 and 346 %, respectively, and in the high exposure group (HE) by 680 and 420 %, respectively.
Table 1.
Epidemiologic parameters: the blood lead level (PbB), zinc protoporphyrin concentration in blood (ZPP) and concentrations of iron (Fe), zinc (Zn), copper (Cu) and selenium (Se) in plasma as well as caeruloplasmin (CER), transferrin (TRF) and haptoglobin (HPG) serum levels in the study population (LE—the low exposure group, ME—the medium exposure group, HE—the high exposure group)
| Control group | LE group | ME group | HE group | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | Mean | SD | p value | Mean | SD | p value | p value | |
| Age | 41.5 | 9.23 | 37.2 | 8.80 | 0.007 | 43.0 | 10.2 | 0.382 | 43.9 | 7.53 | 0.102 | 0.100 |
| Years of work | 13.0 | 9.61 | 19.3 | 9.75 | 19.2 | 9.45 | 0.001 | |||||
| BMI (kg/m2) | 26.2 | 3.05 | 26.7 | 3.46 | 0.382 | 26.6 | 3.92 | 0.459 | 27.2 | 3.69 | 0.069 | 0.098 |
| Smokers (%) | 47 % | 45 % | 0.829 | 49 % | 0.753 | 62 % | 0.060 | 0.270 | ||||
| PbBmean (μg/dl) | 6.45 | 2.49 | 34.1 | 5.39 | <0.001 | 43.4 | 2.35 | <0.001 | 49.7 | 4.10 | <0.001 | <0.001 |
| PbB (μg/dl) | 6.39 | 2.47 | 37.8 | 10.0 | <0.001 | 47.9 | 6.75 | <0.001 | 49.8 | 5.98 | <0.001 | <0.001 |
| ZPPmean (μg/g Hb) | 1.93 | 0.47 | 6.03 | 3.07 | <0.001 | 8.15 | 4.13 | <0.001 | 10.2 | 2.41 | <0.001 | <0.001 |
| ZPP (μg/g Hb) | 1.96 | 0.51 | 6.68 | 3.73 | <0.001 | 8.72 | 5.06 | <0.001 | 10.2 | 3.10 | <0.001 | <0.001 |
| Fe (μmol/l) | 102 | 45.8 | 96.4 | 35.2 | 0.418 | 104 | 43.0 | 0.843 | 106 | 46.5 | 0.646 | 0.804 |
| Se (μg/dl) | 80.6 | 15.0 | 61.1 | 13.3 | <0.001 | 63.3 | 9.83 | <0.001 | 69.7 | 15.6 | 0.010 | <0.001 |
| Cu (μg/dl) | 69.1 | 8.48 | 77.3 | 13.9 | 0.003 | 78.5 | 16.4 | 0.002 | 77.4 | 14.2 | 0.004 | 0.028 |
| Zn (μg/dl) | 70.4 | 11.2 | 75.5 | 12.9 | 0.071 | 74.5 | 10.4 | 0.085 | 72.2 | 12.2 | 0.497 | 0.029 |
| CER (mg/dl) | 36.6 | 12.4 | 45.0 | 8.64 | <0.001 | 43.1 | 11.6 | 0.003 | 42.5 | 10.1 | 0.003 | <0.001 |
| HPG (mg/dl) | 117 | 51.8 | 153 | 63.2 | 0.002 | 132 | 58.2 | 0.212 | 130 | 48.4 | 0.211 | 0.062 |
| TRF (mg/dl) | 253 | 33.7 | 269 | 42.8 | 0.074 | 264 | 47.3 | 0.235 | 257 | 42.3 | 0.588 | 0.469 |
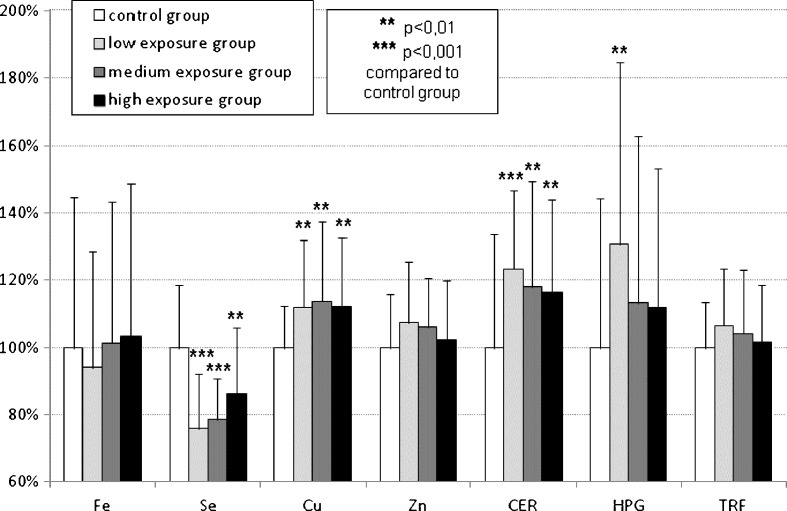
The concentrations of iron, selenium, copper, zinc, caeruloplasmin, haptoglobin and transferrin in the examined subgroups are shown in Fig. 1 as a percentage of the values obtained from the control group.
Fig. 1.
Plasma concentrations of iron (Fe), zinc (Zn), copper (Cu) and selenium (Se) as well as caeruloplasmin (CER), transferrin (TRF) and haptoglobin (HPG) serum levels in lead-exposed groups presented as 100 % of mean ± SD of control
The copper concentration significantly increased by 11.8 % in the LE group, by 13.6 % in the ME group and by 12.0 % in the HE group compared to the control group. The concentrations of caeruloplasmin increased by 23.2, 17.9 and 16.3 %, respectively, for the LE, ME and HE groups compared to the control group. However, the level of haptoglobin in the LE group was significantly raised by 30.6 %, whereas in the ME and HE groups, only an insignificant tendency to increase was observed. Only the selenium levels were significantly lower by 24.2 % in the LE group, by 21.4 % in the ME group and by 13.6 % in the HE group compared to the control group.
There were no significant changes in iron, zinc and transferrin concentrations in the study population.
The Spearman correlation (Table 2) indicated that there is a positive correlation between the lead toxicity parameters (PbB, ZPP) and copper (R = 0.14–0.33) and caeruloplasmin (R = 0.14–0.28) levels. There were no correlations with other trace metals and proteins. However, caeruloplasmin correlated positively with copper (R = 0.43; p < 0.001).
Table 2.
Correlation between the study parameters (Spearman R values, p < 0.05, NS—non significant)
| Age | Years of work | BMI | PbB mean | PbB last | ZPP mean | ZPP last | Fe | Se | Cu | Zn | CER | HPG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years of work | 0.81 | ||||||||||||
| BMI | 0.23 | NS | |||||||||||
| PbBmean | 0.20 | 0.27 | 0.16 | ||||||||||
| PbB | 0.23 | 0.31 | NS | 0.82 | |||||||||
| ZPPmean | 0.18 | 0.24 | NS | 0.75 | 0.66 | ||||||||
| ZPP | 0.14 | 0.18 | NS | 0.71 | 0.71 | 0.94 | |||||||
| Fe | NS | NS | NS | NS | NS | NS | NS | ||||||
| Se | NS | NS | NS | NS | NS | NS | NS | NS | |||||
| Cu | 0.24 | 0.22 | NS | 0.14 | 0.16 | 0.28 | 0.33 | NS | NS | ||||
| Zn | NS | NS | NS | NS | NS | NS | NS | 0.25 | NS | 0.25 | |||
| CER | 0.23 | 0.26 | NS | NS | 0.14 | 0.28 | 0.27 | NS | NS | 0.43 | NS | ||
| HPG | 0.21 | 0.22 | 0.17 | NS | NS | NS | NS | NS | NS | 0.23 | NS | 0.27 | |
| TRF | NS | NS | 0.18 | NS | NS | NS | NS | NS | NS | 0.25 | NS | NS | 0.25 |
Discussion
The aim of the study was to evaluate the effect of occupational exposure to lead on blood levels of Zn, Fe, Cu, Se and related proteins, such as transferrin (TRF), caeruloplasmin (CER) and haptoglobin (HPG). The association between lead exposure and levels of Cu and Se was shown. Besides, altered levels of acute-phase proteins, such as CER and HPG, were observed.
The influence of trace metals on lead toxicity has been reported in many animal studies. Klauder and Petering [19] reported that adequate dietary Cu and Fe intake minimises the toxic effect of orally administered lead in rats. Inconsistent results were observed in a study by Cerklewski and Forbes [14], who suggested that high dietary Cu might increase lead toxicity. Conversely, other findings of Cerklewski and Forbes showed that there is a protective effect of dietary Zn and Se on lead toxicity in rats [20, 21]. Similar results have been reported by Batra et al. [22], who observed a significant reduction in lead content in the kidney, liver, spleen, testis, blood and bones because of Zn supplementation. The reports by Bandhu et al. [23] and Prasanthi et al. [24] are in agreement with this study. The protective effect from lead toxicity is attributed to dietary Se [8, 10, 25]; however, when Se was introduced through placental transfer by Sidhu and Nehru [26] or intramuscular injection by Othaman and El Missiry [9], consistent results were obtained.
Animal studies are concordant with those reports that included children populations. Zimmermann et al. [27] improved the Fe status in iron-deficient children exposed environmentally to lead thereby reducing their lead levels by 33 %. Because most environmental lead is absorbed in the intestine, the positive effects of Fe intake in this study might be a result of an iron–lead competitive binding to divalent metal transporter 1 (DMT1). Fe has a higher affinity to DMT1 and could inhibit lead uptake in the intestine [27]. Zn competes with lead analogically, which is in agreement with the results obtained by Ahamed et al. [11], who examined anaemic children environmentally exposed to lead and observed a significant negative correlation between both Fe and Zn blood levels and lead concentration. The Cu concentration in blood was not correlated with this parameter [11]. An association between increased blood lead levels and Fe deficiency was postulated by Muwakkit et al. [28] and Hegazy et al. [29]. Another investigation revealed that blood lead levels were negatively correlated with serum Zn and Se concentrations [30]. Additionally, Diouf et al. [5] demonstrated a negative significant correlation between Se levels and blood lead levels in children who were environmentally exposed to this xenobiotic.
Investigations in adult male workers occupationally exposed to lead are inconsistent. The present study revealed that there is no association between Fe and blood lead levels, which is concordant with previous data [31–34]. However, Kim et al. [35] reported a decrease in the serum Fe level in lead-exposed workers, but a significantly lower dietary Fe intake was observed concurrently. Therefore, to expect that increased lead levels inhibit the uptake of Fe as is postulated by some authors would be unreasonable [28] because workers are exposed to lead primarily through the respiratory tract and competitive binding of lead and Fe or Zn to divalent metal transporters in the intestine should have marginal significance in occupational exposure.
In vitro studies indicate that lead not only impairs Fe binding to TRF [16] but also suppresses its synthesis thus, decreasing mRNA and protein levels [36]. There is no corroboration of this observation in our study because a decrease in TRF level was not observed.
In the present study, there was no significant difference in the Zn plasma levels between the examined and the control groups. Similar results have been reported by Mehdi et al. [34] and Chiba et al. [32], whereas Dioka et al. [37] observed that the Zn blood level decreased by 34 % in artisans who were occupationally exposed to lead. When examining zinc–lead miners, Malekirad et al. [2] observed a positive correlation amongst Zn and lead blood levels and significant elevation of these parameters in the examined workers compared with the control group. Because elevated total antioxidant status and lower DNA damage were also indicated in the examined workers, it is possible to expect that simultaneous exposure to Zn may improve antioxidant defence and, therefore, alleviate lead toxicity.
Se should act analogically. In the present study, the Se plasma level was significantly lower in the workers than in the control group. In addition, lead-exposed smelter workers observed by Gustafson et al. [38] had significantly lower plasma Se levels than the control group. Additionally, there was a significant negative correlation between blood lead level and plasma Se level [38]. The findings of Chiba et al. [32] support our study with the observation that plasma Se levels had a tendency to decrease, whereas the Se concentration in erythrocytes increased significantly with an increasing blood lead level. A possible explanation for this association may be that blood lead, which is predominantly present in erythrocytes [11], forms a complex with Se and reduces the lead level in plasma.
The findings concerning Cu levels are more difficult to interpret than those for Se. Studies by Mehdi et al. [34], Wasowicz et al. [39] and Chiba et al. [32] revealed no association between Cu and lead levels in workers, whereas Cu plasma levels in the present study were significantly higher compared with the control group and correlated positively with lead concentrations. Our earlier studies [40] showed that lead exposure is associated with an elevated activity of superoxide dismutase isoenzyme that contains Cu and Zn (CuZn-SOD) in both serum and erythrocytes. Therefore, an increase in the Cu level, which was observed in the present study, may be caused by increased CuZn-SOD activity. This enzyme is part of the antioxidant defence system and its activity may be elevated because of lead-induced oxidative stress [40, 41]. The increase in plasma Cu levels may also be caused by competitive displacement of the metal from tissues by lead ions. Moreover, lead and Cu compete for binding sites on proteins, such as the ATPase complex [42]. Increased bioavailability of displaced Cu may induce ROS generation via the Fenton reaction and contribute to oxidative stress enhancement.
The concentration of CER, which is an acute-phase protein, increased significantly. Therefore, in part, the increase in plasma Cu level may be secondary to the increase in the CER level. Mongiat et al. [43] obtained similar results in a study of welders and suggested that the increase in CER levels is related to the severity of the oxidative stress and plays an adaptative role. A slight increase in the CER level was observed in rats that were orally administered with lead acetate in a dose of 1,000 mg/l for 4 weeks [44]. Nevertheless, Mehdi et al. [34] and Wasowicz et al. [39] showed that there is no effect of lead exposure on CER levels. In contrast, Leelakunakorn et al. [15] postulated that inhibition of CER oxidase activity was lead mediated. This hypothesis is not in conflict with our study because a decrease in the enzymatic activity of CER does not necessarily mean a reduction in its concentration as a protein.
HPG is the second positive acute-phase protein indicated in our study. The mean HPG serum levels increased significantly in the LE group and insignificantly in the ME and HE groups. The increase in the serum levels of both proteins may be caused by the pro-inflammatory properties of lead [45]. Chang et al. [46] reported that 1 μM lead upregulates the transcription of genes encoding cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 in vascular smooth muscle cells. This causes an elevated PGE2 secretion. Consistently, Chou et al. [47] showed that low lead ion concentrations induce inflammation by increasing COX-2 gene expression via the EGFR/NF-κB signal transduction pathway in A431 carcinoma cells. Lead exposure may also be associated with altered levels of interleukin-6, interleukin-10 or tumour necrosis factor-alpha [48, 49]. Additionally, Khan et al. [50] observed increased levels of C-reactive protein (CRP) in lead-exposed workers (PbB = 29.1 μg/dl). There was a strong positive correlation between blood lead and CRP levels in this study (R = 0.75).
In the present study, the elevations of CER and HPG levels were higher in the LE group than in the ME or HE groups. The LE group was composed of workers who had been exposed to significantly lower doses of lead for a significantly shorter period. Therefore, our study indirectly supports the hypothesis that reduced lead exposure exerts an immunostimulatory effect, whereas higher exposure may cause immunosuppression [51].
Conclusions
The effect of occupational exposure to lead on the metabolism of trace metals appears to be limited and concerns mainly their tissue distribution. However, results of the present study indicate that exposure to lead significantly influences blood levels of Cu and Se. Besides, altered levels of CER and HPG were shown. This changes in levels of the acute-phase proteins may be associated with lead-induced modifications of the immune system.
The accumulated data indicate that Fe, Zn, Se and Cu may reduce lead toxicity; thus, an adequate dietary intake of the above-mentioned trace metals is necessary. However, there is no evidence that additional supplementation would be beneficial.
Acknowledgments
Conflict of Interest
The authors report no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Abdel Moneim AE, Dkhil MA, Al-Quraishy S. Effects of flaxseed oil on lead acetate-induced neurotoxicity in rats. Biol Trace Elem Res. 2011;144:904–913. doi: 10.1007/s12011-011-9055-4. [DOI] [PubMed] [Google Scholar]
- 2.Malekirad AA, Oryan S, Fani A, Babapor V, Hashemi M, Baeeri M, Bayrami Z, Abdollahi M. Study on clinical and biochemical toxicity biomarkers in a zinc–lead mine workers. Toxicol Ind Health. 2010;26:331–337. doi: 10.1177/0748233710365697. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa F, Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669–1674. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai T. Biomarkers of lead exposure. Ind Health. 2000;38:127–142. doi: 10.2486/indhealth.38.127. [DOI] [PubMed] [Google Scholar]
- 5.Diouf A, Garçon G, Diop Y, Ndiaye B, Thiaw C, Fall M, Kane-Barry O, Ba D, Haguenoer JM, Shirali P. Environmental lead exposure and its relationship to traffic density among Senegalese children: a cross-sectional study. Hum Exp Toxicol. 2006;25:637–644. doi: 10.1177/0960327106074591. [DOI] [PubMed] [Google Scholar]
- 6.Olewińska E, Kasperczyk A, Kapka L, Kozłowska A, Pawlas N, Dobrakowski M, Birkner E, Kasperczyk S. Level of DNA damage in lead-exposed workers. Ann Agric Environ Med. 2010;17:231–236. [PubMed] [Google Scholar]
- 7.Hermes-Lima M, Pereira B, Bechara EJ. Are free radicals involved in lead poisoning? Xenobiotica. 1991;21:1085–1090. doi: 10.3109/00498259109039548. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Tang C. The accumulation effect of lead on DNA damage in mice blood cells of three generations and the protection of selenium. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng. 2001;36:501–508. doi: 10.1081/ESE-100103479. [DOI] [PubMed] [Google Scholar]
- 9.Othman AI, El Missiry MA. Role of selenium against lead toxicity in male rats. J Biochem Mol Toxicol. 1998;12:345–349. doi: 10.1002/(SICI)1099-0461(1998)12:6<345::AID-JBT4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Nehru B, Dua R, Iyer A. Effect of selenium on lead-induced alterations in rat brain. Biol Trace Elem Res. 1997;57:251–258. doi: 10.1007/BF02785293. [DOI] [PubMed] [Google Scholar]
- 11.Ahamed M, Singh S, Behari JR, Kumar A, Siddiqui MK. Interaction of lead with some essential trace metals in the blood of anemic children from Lucknow, India. Clin Chim Acta. 2007;377:92–97. doi: 10.1016/j.cca.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Cengiz B, Söylemez F, Oztürk E, Cavdar AO. Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects: a preliminary study. Biol Trace Elem Res. 2004;97:225–235. doi: 10.1385/BTER:97:3:225. [DOI] [PubMed] [Google Scholar]
- 13.Afridi HI, Kazi TG, Brabazon D, Naher S. Association between essential trace and toxic elements in scalp hair samples of smokers rheumatoid arthritis subjects. Sci Total Environ. 2011;412–413:93–100. doi: 10.1016/j.scitotenv.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Cerklewski FL, Forbes RM. Influence of dietary copper on lead toxicity in the young male rat. J Nutr. 1977;107:143–146. doi: 10.1093/jn/107.1.143. [DOI] [PubMed] [Google Scholar]
- 15.Leelakunakorn W, Sriworawit R, Soontaros S. Ceruloplasmin oxidase activity as a biomarker of lead exposure. J Occup Health. 2005;47:56–60. doi: 10.1539/joh.47.56. [DOI] [PubMed] [Google Scholar]
- 16.Qian ZM, Xiao DS, Wang Q, Tang PL, Pu YM. Inhibitory mechanism of lead on transferrin-bound iron uptake by rabbit reticulocytes: a fractal analysis. Mol Cell Biochem. 1997;173:89–94. doi: 10.1023/A:1006884619972. [DOI] [PubMed] [Google Scholar]
- 17.Barton JC, Conrad ME, Nuby S, Harrison L. Effects of iron on the absorption and retention of lead. J Lab Clin Med. 1978;92:536–547. [PubMed] [Google Scholar]
- 18.Richterich R. Chemia kliniczna. Warszawa: PZWL; 1971. [Google Scholar]
- 19.Klauder DS, Petering HG. Protective value of dietary copper and iron against some toxic effects of lead in rats. Environ Health Perspect. 1975;12:77–80. doi: 10.1289/ehp.751277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerklewski FL, Forbes RM. Influence of dietary zinc on lead toxicity in the rat. J Nutr. 1976;106:689–696. doi: 10.1093/jn/106.5.689. [DOI] [PubMed] [Google Scholar]
- 21.Cerklewski FL, Forbes RM. Influence of dietary selenium on lead toxicity in the rat. J Nutr. 1976;106:778–783. doi: 10.1093/jn/106.6.778. [DOI] [PubMed] [Google Scholar]
- 22.Batra N, Nehru B, Bansal MP. The effect of zinc supplementation on the effects of lead on the rat testis. Reprod Toxicol. 1998;12:535–540. doi: 10.1016/S0890-6238(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 23.Bandhu HK, Dani V, Garg ML, Dhawan DK. Hepatoprotective role of zinc in lead-treated, protein-deficient rats. Drug Chem Toxicol. 2006;29:11–24. doi: 10.1080/01480540500408507. [DOI] [PubMed] [Google Scholar]
- 24.Prasanthi RP, Devi CB, Basha DC, Reddy NS, Reddy GR. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci. 2010;28:161–167. doi: 10.1016/j.ijdevneu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Abdollahi M, Rahmat-Jirdeh N, Soltaninejad K. Protection by selenium of lead-acetate-induced alterations on rat submandibular gland function. Hum Exp Toxicol. 2001;20:28–33. doi: 10.1191/096032701667736070. [DOI] [PubMed] [Google Scholar]
- 26.Sidhu P, Nehru B. Protective effects of selenium to placental lead neurotoxicity in rat pups. Toxicol Mech Methods. 2005;15:419–423. doi: 10.1080/15376520500194775. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF. Iron fortification reduces blood lead levels in children in Bangalore, India. Pediatrics. 2006;117:2014–2021. doi: 10.1542/peds.2005-2440. [DOI] [PubMed] [Google Scholar]
- 28.Muwakkit S, Nuwayhid I, Nabulsi M, al Hajj R, Khoury R, Mikati M, Abboud MR. Iron deficiency in young Lebanese children: association with elevated blood lead levels. J Pediatr Hematol Oncol. 2008;30:382–386. doi: 10.1097/MPH.0b013e318165b283. [DOI] [PubMed] [Google Scholar]
- 29.Hegazy AA, Zaher MM, Abd El-Hafez MA, Morsy AA, Saleh RA. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res Notes. 2010;3:133. doi: 10.1186/1756-0500-3-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osman K, Schütz A, Akesson B, Maciag A, Vahter M. Interactions between essential and toxic elements in lead exposed children in Katowice, Poland. Clin Biochem. 1998;31:657–665. doi: 10.1016/S0009-9120(98)00071-X. [DOI] [PubMed] [Google Scholar]
- 31.Alabdullah H, Bareford D, Braithwaite R, Chipman K. Blood lead levels in iron-deficient and noniron-deficient adults. Clin Lab Haematol. 2005;27:105–109. doi: 10.1111/j.1365-2257.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 32.Chiba M, Shinohara A, Matsushita K, Watanabe H, Inaba Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. Tohoku J Exp Med. 1996;178:49–62. doi: 10.1620/tjem.178.49. [DOI] [PubMed] [Google Scholar]
- 33.Lilis R, Eisinger J, Blumberg W, Fischbein A, Selikoff IJ. Hemoglobin, serum iron, and zinc protoporphyrin in lead-exposed workers. Environ Health Perspect. 1978;25:97–102. doi: 10.1289/ehp.782597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehdi JK, al-Imarah FJ, al-Suhail AA. Levels of some trace metals and related enzymes in workers at storage-battery factories in Iraq. East Mediterr Health J. 2000;6:76–82. [PubMed] [Google Scholar]
- 35.Kim HS, Lee SS, Hwangbo Y, Ahn KD, Lee BK. Cross-sectional study of blood lead effects on iron status in Korean lead workers. Nutrition. 2003;19:571–576. doi: 10.1016/S0899-9007(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 36.Barnum-Huckins KM, Martinez AO, Rivera EV, Adrian EK, Jr, Herbert DC, Weaker FJ, Walter CA, Adrian GS. A comparison of the suppression of human transferrin synthesis by lead and lipopolysaccharide. Toxicology. 1997;118:11–22. doi: 10.1016/S0300-483X(96)03586-X. [DOI] [PubMed] [Google Scholar]
- 37.Dioka CE, Orisakwe OE, Adeniyi FA, Meludu SC. Liver and renal function tests in artisans occupationally exposed to lead in mechanic village in Nnewi, Nigeria. Int J Environ Res Public Health. 2004;1:21–25. doi: 10.3390/ijerph2004010021. [DOI] [PubMed] [Google Scholar]
- 38.Gustafson A, Schütz A, Andersson P, Skerfving S. Small effect on plasma selenium level by occupational lead exposure. Sci Total Environ. 1987;66:39–43. doi: 10.1016/0048-9697(87)90075-1. [DOI] [PubMed] [Google Scholar]
- 39.Wasowicz W, Gromadzińska J, Rydzyński K. Blood concentration of essential trace elements and heavy metals in workers exposed to lead and cadmium. Int J Occup Med Environ Health. 2001;14:223–229. [PubMed] [Google Scholar]
- 40.Kasperczyk S, Birkner E, Kasperczyk A, Zalejska-Fiolka J. Activity of superoxide dismutase and catalase in people protractedly exposed to lead compounds. Ann Agric Environ Med. 2004;11:291–296. [PubMed] [Google Scholar]
- 41.Kasperczyk S, Birkner E, Kasperczyk A, Kasperczyk J. Lipids, lipid peroxidation and 7-ketocholesterol in workers exposed to lead. Hum Exp Toxicol. 2005;24:287–295. doi: 10.1191/0960327105ht528oa. [DOI] [PubMed] [Google Scholar]
- 42.Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The involvement of copper transporter in lead-induced oxidative stress in astroglia. Neurochem Res. 2005;30:429–438. doi: 10.1007/s11064-005-2677-1. [DOI] [PubMed] [Google Scholar]
- 43.Mongiat R, Gerli GC, Locatelli GF, Fortuna R, Petazzi A. Erythrocyte antioxidant system and serum ceruloplasmin levels in welders. Int Arch Occup Environ Health. 1992;64:339–342. doi: 10.1007/BF00379543. [DOI] [PubMed] [Google Scholar]
- 44.Flora SJ, Jeevaratnam K, Kumar D. Preventive effects of sodium molybdate in lead intoxication in rats. Ecotoxicol Environ Saf. 1993;26:133–137. doi: 10.1006/eesa.1993.1045. [DOI] [PubMed] [Google Scholar]
- 45.Khan MI, Islam N, Sahasrabuddhe AA, Mahdi AA, Siddiqui H, Ashquin M, Ahmad I. Ubiquitous hazardous metal lead induces TNF-α in human phagocytic THP-1 cells: primary role of ERK 1/2. J Hazard Mater. 2011;189:255–264. doi: 10.1016/j.jhazmat.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Chang WC, Chang CC, Wang YS, Wang YS, Weng WT, Yoshioka T, Juo SH. Involvement of the epidermal growth factor receptor in Pb²+-induced activation of cPLA2/COX-2 genes and PGE2 production in vascular smooth muscle cells. Toxicology. 2011;279:45–53. doi: 10.1016/j.tox.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Chou YH, Woon PY, Huang WC, Shiurba R, Tsai YT, Wang YS, Hsieh TJ, Chang WC, Chuang HY, Chang WC. Divalent lead cations induce cyclooxygenase-2 gene expression by epidermal growth factor receptor/nuclear factor-kappa B signaling in A431carcinoma cells. Toxicol Lett. 2011;203:147–153. doi: 10.1016/j.toxlet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Songdej N, Winters PC, McCabe MJ, Jr, van Wijngaarden E. A population-based assessment of blood lead levels in relation to inflammation. Environ Res. 2010;110:272–277. doi: 10.1016/j.envres.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Valentino M, Rapisarda V, Santarelli L, Bracci M, Scorcelletti M, Di Lorenzo L, Cassano F, Soleo L. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol. 2007;26:551–556. doi: 10.1177/0960327107073817. [DOI] [PubMed] [Google Scholar]
- 50.Khan DA, Qayyum S, Saleem S, Khan FA. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol Ind Health. 2008;24:611–618. doi: 10.1177/0748233708098127. [DOI] [PubMed] [Google Scholar]
- 51.Mishra KP, Singh VK, Rani R, Yadav VS, Chandran V, Srivastava SP, Seth PK. Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology. 2003;188:251–259. doi: 10.1016/S0300-483X(03)00091-X. [DOI] [PubMed] [Google Scholar]