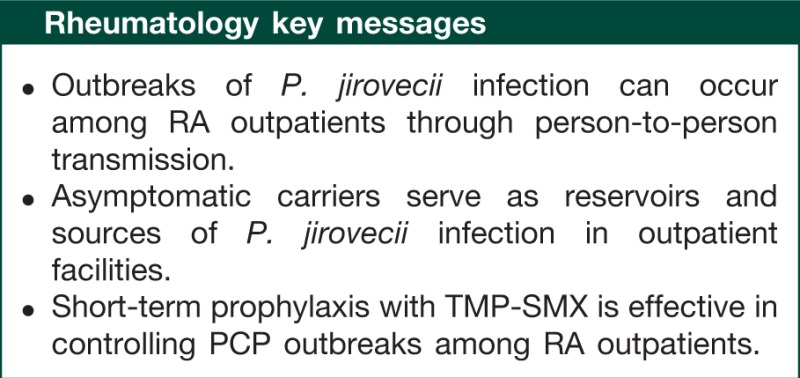
Abstract
Accompanying the increased use of biologic and non-biologic antirheumatic agents, patients with RA have been exposed to an increased risk of Pneumocystis jirovecii infection, which causes acute fulminant P. jirovecii pneumonia (PCP). Mortality in this population is higher than in HIV-infected individuals. Several guidelines and recommendations for HIV-infected individuals are available; however, such guidelines for RA patients remain less clear. Between 2006 and 2008 we encountered a clustering event of P. jirovecii infection among RA outpatients. Through our experience with this outbreak and a review of the recent medical literature regarding asymptomatic colonization and its clinical significance, transmission modes of infection and prophylaxis of PCP, we have learned the following lessons: PCP outbreaks among RA patients can occur through person-to-person transmission in outpatient facilities; asymptomatic carriers serve as reservoirs and sources of infection; and short-term prophylaxis for eradication of P. jirovecii is effective in controlling PCP outbreaks among RA outpatients.
Keywords: Pneumocystis jirovecii, rheumatoid arthritis, colonization, transmission, outbreaks, prophylaxis
Introduction
Over the past decade, the treatment of RA has dramatically changed. Early use of MTX as the first-line DMARD and the emergence of innovative biologic agents targeted at specific molecules and pathways in the immune system have altered the course of RA and improved patient and social outcomes [1, 2]. However, accompanying the increased use of biologic and non-biologic antirheumatic drugs, RA patients have been exposed to an increased risk of Pneumocystis jirovecii pneumonia (PCP) [3–6]. CSs, widely used in the treatment of RA, are also implicated as a major predisposing factor for PCP development [7]. RA itself also contributes to increased risk of infection because of its immunomodulatory effects [8].
Pneumocystis jirovecii (formerly known as Pneumocystis carinii f. sp. hominis) is an important opportunistic fungal pathogen in humans who have depressed immune function [9, 10]. The onset of PCP in RA patients often presents with abrupt severe oxygenation impairment and this complication is potentially life threatening [11]. Recently we reported a PCP outbreak among RA outpatients [12]. This is the first report indicating the possibility that clustering events of nosocomial P. jirovecii infection can occur among such patient groups, although sporadic cases of PCP have been reported. In the years ahead, more RA patients will be treated with immunosuppressive therapy, with increasing therapeutic durations. Therefore rheumatologists should be prepared for an increased risk of PCP outbreaks among RA patients. In this mini-review, we examine the recent literature with the goal of developing a preventative strategy against outbreaks of P. jirovecii infection among RA outpatients.
History
Pneumocystis jirovecii was first recognized as a pathogen in premature infants developing an epidemic form of interstitial plasma cell pneumonitis in European countries during the Second World War. In 1952 the Czech pathologists Vanek and Jirovec [13] described Pneumocystis as the causative agent of this endemic pneumonia [14]. Since then PCP has only been reported sporadically in premature infants and malnourished young children [15, 16]. In the 1960s PCP started to be recognized as an opportunistic pathogen in immunocompromised children with congenital T cell immunodeficiency and in patients with haematological neoplasm such as acute lymphoblastic leukaemia or lymphomas [17, 18]. After the introduction of trimethoprim–sulphamethoxazole (TMP-SMZ) as PCP chemoprophylaxis, however, its incidence markedly decreased [19]. With the emergence of the human immunodeficiency virus (HIV) pandemic, there was a dramatic increase in the incidence of PCP. In the 1980s PCP was the most common opportunistic infection in patients with AIDS, and among them >60% developed PCP during their disease course [20]. In the 1990s the routine use of PCP prophylaxis and the widespread use of highly active anti-retrovirus therapy brought a substantial decline in the incidence of PCP among HIV-infected persons [21, 22]. As a result of the increased number of patients receiving immunosuppressive or cytotoxic therapy for solid tumours and haematological malignancies, organ and bone marrow transplantation, and inflammatory and rheumatic diseases, the clinical significance of PCP has been recognized in HIV-negative immunocompromised individuals [23–28].
Immunosuppressive therapy and PCP
Roles of immunosuppressive therapy in PCP development
Host defence against P. jirovecii involves a complex series of interactions between CD4+ T lymphocytes, alveolar macrophages, polymorphonuclear cells and various pro-inflammatory mediators released by these cells [29, 30]. Immunosuppressive agents apparently influence such host immune systems. In particular, it is likely that CSs promote PCP development through depletion of CD4+ T cells [31]. This therapy has therefore been identified as a risk factor of PCP in HIV-negative patients who have a variety of underlying conditions [7, 25, 32–34]. Even low or moderate doses of CSs can increase the risk of PCP [24, 35–37]. In most cases CSs were being given as pulse therapy with sudden discontinuation or the dose was lowered on diagnosis of PCP [23, 28]. Withdrawal of CSs can lead to reconstitution of the immune system and result in immune-mediated damage to the lungs [38]. Inflammatory responses directed against P. jirovecii are essential for clearance of P. jirovecii from the body; however, excessive inflammation can cause severe lung injury and impairment of pulmonary function [39]. These findings may be related to the fact that PCP often occurs in HIV-negative rheumatic disease patients with CD4+ T cell counts >200/µl [11, 12, 34, 37, 40].
Incidence and mortality of PCP in RA patients and those with other rheumatic diseases during immunosuppressive therapy
Recent post-marketing surveillance (PMS) reports by the Japan College of Rheumatology (JCR) indicated a high incidence of PCP in RA patients receiving the anti-TNF-α agents infliximab (0.4% of 5000 patients), etanercept (0.2% of 7091 patients) and adalimumab (0.3% of 3000 patients) [41–43]. A review of US Food and Drug Administration data between 1998 and 2003 identified 84 cases of PCP following infliximab therapy [44]. Regarding a humanized monoclonal anti-IL-6 receptor antibody tocilizumab, a PMS conducted by the JCR reported that the incidence of PCP is 0.28/100 patient-years [45]. Low-dose MTX can also increase the risk of developing PCP in RA patients [3, 4, 37, 46]. As shown in Table 1, the most recent surveillance by individual pharmaceutical companies in Japan indicates a high number of PCP cases in RA patients during treatment with low-dose MTX (S. Mori, June 2012, personal communication).
Table 1.
Incidence and mortality rate of P. jirovecii pneumonia in RA patients during immunosuppressive therapy in Japan
| Anti-RA agents | PCP incidence, n (%)a | Mortality, n (%)a |
|---|---|---|
| MTX | 236 | 28 (11.9) |
| Tacrolimus | 14 | 4 (28.6) |
| Infliximab | 188 (0.3) | 19 (10.1) |
| Etanercept | 81 (0.1) | 15 (18.5) |
| Adalimumab | 54 (0.3) | 10 (18.5) |
| Golimumab | 1 (0.03) | 0 |
| Tocilizumab | 14 (0.2) | 2 (14.3) |
| Abatacept | 9 (0.1) | 2 (22.2) |
aPCP incidence and mortality are expressed as numbers of patients who developed PCP during treatment with the respective immunosuppressive agents and who died due to this pneumonia, respectively. Exact numbers of patients who used MTX and tacrolimus were not available. Data were obtained from the most recent surveillance reports by individual pharmaceutical companies in Japan (S. Mori, June 2012, personal communication).
In typical cases of untreated or delayed-treatment PCP, progressive alveolar damage leads to death. Despite the availability of medications for treatment, the mortality rate of PCP still remains high. Most studies indicate better survival rates for HIV-positive PCP patients (86–92%) than HIV-negative PCP patients with various underlying conditions (51–80%) [47]. HIV-negative PCP patients appear to rapidly develop fulminate pneumonia with severe oxygenation impairment, diffuse alveolar damage and respiratory failure, whereas PCP in HIV-positive individuals presents as a subacute disease course [23, 25–28, 40, 48–51]. Differences in inflammatory responses of the lungs apparently contribute to such marked differences between the two types of PCP in clinical presentations, outcomes and mortality [29]. High mortality rates have been reported in RA patients and those with other rheumatic diseases who developed PCP during immunosuppressive therapy [11, 32–34, 37, 44, 52]. High numbers of fatal PCP cases during treatment with biologic or non-biologic agents for RA have been reported by pharmaceutical companies in Japan (Table 1).
Reactivation from lifelong latency or de novo infection?
It has long been debated whether PCP development is due to a reactivation of latent childhood infection or de novo acquisition. There is evidence that contact with P. jirovecii occurs early in life (primary infection). Pifer et al. [53] found that two-thirds of normal children have acquired antibody against this organism by 4 years of age, analogous to other opportunistic infections, indicating early exposure to this organism. Vargas et al. [54] also showed that seroconversion developed in 85% of healthy infants by 20 months and P. jirovecii DNA was frequently detected during episodes of mild respiratory infection in 32% of normal infants. These findings suggest that subclinical P. jirovecii infection is highly prevalent in healthy infants. PCP events occurring in adults were therefore considered to be mostly due to a reactivation of latent infection of P. jirovecii.
However, this theory has been challenged by recent findings that clustering of specific genotypes is associated with the place of diagnosis (residence) rather than the place of birth [55–57]. Other studies also demonstrated geographic clustering of PCP cases among HIV-infected individuals living in specific zip codes of San Francisco and Cincinnati [58, 59]. These findings have suggested that P. jirovecii infections are actively acquired from a common environmental source or person-to-person contact [60]. Several studies have indicated that recurrent episodes of PCP in HIV-infected individuals were caused by de novo infection rather than by reactivation of latent infection, because genetically distinct strains were isolated during each episode of PCP [61–64].
Serial examinations of pulmonary specimens indicated that persistent P. jirovecii cysts at the end of antimicrobial treatment for acute PCP are gradually cleared from the lungs of HIV-infected patients [65]. Wakefield et al. observed asymptomatic carriage of P. jirovecii for no longer than 9.5 months in HIV-positive patients after a PCP episode [66]. These findings provide support for the conclusion that, instead of lifelong latency, the relationship between P. jirovecii and its host appears to be transient colonization. PCP development seems to result from new infection rather than reactivation of latent childhood infection. Using mouse or rat models, several groups showed that immunocompromised animals naturally acquired P. carinii infection as well as developed pneumonia, but with the recovery of the immune system, the hosts completely cleared this organism within relatively short periods (3 weeks to 1 year) [67–69]. The carrier status of Pneumocystis organisms in immunocompetent hosts seems a time-limited phenomenon.
Establishing the major route for infection: a common environmental source or interhuman transmission?
Possible infectious sources of de novo infection of P. jirovecii have not yet been established, but they may include the environment, asymptomatic carriers and patients with active PCP. Utilizing data from genotyping and contact tracing, several studies of PCP clusters among HIV-infected individuals or immunosuppressive patients from other conditions have suggested that person-to-person transmission may occur but does not constitute the majority route of acquisition [70–72]. Another group reported a PCP episode due to genetically distinct P. jirovecii strain in each member of three HIV-infected couples, and therefore ruled out direct transmission within each couple [73]. Wakefield [74] detected P. jirovecii DNA in ambient air collected from a number of spore traps, suggesting that P. jirovecii is a common component of air spores in a rural area in England. Pneumocystis jirovecii DNA has also been identified in air samples obtained from hospital environments, which suggests an environmental risk to susceptible persons [75, 76]. At the same time, this finding may indicate the presence of nosocomial person-to-person transmission via the airborne route (aerosol spread). Choukri et al. [77] indicated that detection rates of P. jirovecii in air samples decrease with increasing distance from hospitalized patients with PCP and thereby proposed a possible risk of direct airborne transmission of P. jirovecii from close contact with PCP patients. In each PCP patient reported in that study, P. jirovecii genotypes in surrounding air samples closely matched those in pulmonary specimens, confirming that P. jirovecii organisms in the air of hospital rooms were exhaled by PCP patients [78]. Animal models also indicate a direct airborne transmission route of Pneumocystis species among immunosuppressed mice, from immunocompetent mice to highly susceptible mice, and among healthy mice [79–81].
Humans do not appear to contract Pneumocystis pneumonia from animals. Pneumocystis species are transmissible only to the same host species and cross-transmission among different mammalian species has not been documented [82, 83]. Molecular analysis also showed that rats and humans harbour distinct types of Pneumocystis species [84]. In addition, the challenging task of continuously culturing this species outside of the host lung has not as yet been successful; namely, Pneumocystis cannot propagate outside an infected host [85].
Asymptomatic carriers of P. jirovecii as the infectious reservoir
Recent studies have shown the presence of asymptomatic carriers of P. jirovecii and their participation in the transmission cycle as an infectious reservoir for susceptible individuals in the community. High rates of prevalence of P. jirovecii colonization are reported among HIV-positive individuals who were hospitalized with non-PCP pneumonia (68%) or who died from causes other than PCP (46%) [86, 87]. Of particular note is the fact that carriage of this organism has been described in HIV-negative individuals with immunosuppressive conditions, those with chronic pulmonary disease and even immunocompetent healthy persons.
Healthy individuals
Ponce et al. [88] reported a high prevalence (more than half) of mild infection of P. jirovecii in the autopsy lungs of the general adult population. The authors have proposed that immunocompetent individuals can develop frequent self-limiting reinfection throughout life after primary infection. Medrano et al. [89] also showed evidence that P. jirovecii DNA can be detected in the respiratory tract of 20% of healthy adults without underlying pulmonary disease or immunosuppression. This infection was short-lived: the DNA was not detected in >75% of colonized individuals after 6 months of follow-ups. Such colonization has also been demonstrated in older adults (21.5%) and healthy infants (32%) [54, 90]. Totet et al. [91] reported shared features of P. jirovecii genotypes between immunocompetent infants with a primary infection and immunocompromised adults with PCP. These findings strongly support the idea that the general population is a reservoir and source of P. jirovecii infection.
Transmission by contact of health-care workers to PCP patients
Molecular evidence has accumulated that immunocompetent health-care workers are at risk of P. jirovecii colonization by occupational close contact with patients who have developed PCP [92, 93]. In addition, several groups observed that P. jirovecii persists for limited periods of time in HIV-positive patients who have clinical recuperation after PCP [65, 66]. Thus transmission of this infection through hospital staff may continue for some time after patients’ recovery from PCP. Health-care workers may serve as vectors of this infection. In contrast, Lundgren et al. [94] have claimed, based on serological and molecular testing, that immunocompetent hospital staff treating PCP patients are not a potentially infectious source of P. jirovecii for immunocompromised patients.
Patients with pulmonary disease
Asymptomatic carriage of P. jirovecii has been recognized in immunocompetent patients who have primary pulmonary disorders such as chronic obstructive pulmonary disease (COPD), lung cancer, interstitial lung disease, tuberculosis and cystic fibrosis, suggesting that lung tissue injury may favour colonization by this organism [95–102]. CS therapy was an independent risk factor for colonization in patients undergoing diagnostic bronchoscopy and those suspected of bacterial pneumonia [103, 104]. Montes-Cano et al. [105] showed the presence of a continuous cycle of colonization and clearance in cystic fibrosis patients during a 1-year follow-up period. Considering that the patients with chronic pulmonary diseases are sputum producers, they may represent a reservoir for P. jirovecii with the potential ability of transmission to susceptible hosts. In fact, Rivero et al. [106] reported a case of P. jirovecii transmission from a grandfather with chronic bronchitis and sputum production to a grandmother and an infant via the airborne route. Morris et al. [107] reported a strong association between P. jirovecii colonization and severity of airway obstruction in smokers, suggesting that this organism may play a pathogenic role in COPD progression. Recently we have shown that bronchiolar abnormalities are commonly seen in RA patients, especially those with long-standing RA [108, 109]. In addition, bronchiectasis was the most frequent finding in both patients with early RA and those with long-standing RA [108]. Such modifications of bronchial and bronchiolar structures in the lungs of RA patients may provide a favourable environment for infection and colonization by P. jirovecii.
Patients with underlying diseases associated with immunosuppression
Pneumocystis jirovecii can colonize patients who have immunosuppressive conditions [46, 110–113]. Underlying diseases comprise haematological malignancies, solid tumours, inflammatory and rheumatic diseases, and organ transplant recipients. Many of these patients received immunosuppressive drugs and/or long-term CS therapy. Risk factors for colonization have remained controversial in this clinical setting. Nevez et al. [114] showed that an increased risk of P. jirovecii colonization is significantly associated with a CD4+ T cell count <400/µl in HIV-negative patients with underlying disease associated with immunosuppression. Mekinian et al. [113] found a high prevalence of P. jirovecii colonization (16%) in patients with systemic autoimmune diseases and identified high-dose CS therapy and low total lymphocyte counts as risk factors for colonization. Fritzsche et al. [115] showed that patients with autoimmune inflammatory diseases, especially those over the age of 60, have a high prevalence of P. jirovecii colonization. They also indicated that 28.5% of those patients are colonized with this organism, without significant influences of CS dose or immunosuppressive co-medication. We found that 10.9% of RA patients have asymptomatic carriage of this organism and the mean age of these carriers is significantly older than non-carrier RA patients. There were no significant differences in lymphocyte counts or prednisolone use [46]. A high rate of colonization (25.6%) was also reported among patients with rheumatic diseases receiving infliximab [116].
PCP outbreaks among renal transplant recipients
An increased number of PCP outbreaks among kidney transplant recipients have been reported worldwide. Through a systemic review of a total of 15 articles published from 1980 onwards, de Boer et al. [117] indicated that the settings of PCP outbreaks are all marked by the following three characteristics: no adequate prophylaxis with antibiotics was introduced, frequent person-to-person contact did exist and no measures were taken to isolate PCP patients during their hospitalization. Many studies have presented epidemiological evidence that there were infectious encounters and exposures between patients carrying P. jirovecii [118–125]. In addition, genotyping results have shown that each outbreak was caused by a single or a predominant strain of P. jirovecii [119–127]. The findings have suggested that interhuman transmission occurred in hospital environments, including outpatient facilities and inpatient wards. Sassi et al. [128] revealed that two geographically distinct clusters of PCP among renal transplant recipients in Europe were due to a single strain of P. jirovecii. The findings may be explained either by the existence of a common source of transmission or by differences in virulence of P. jirovecii strains.
Between May 2008 and April 2010, Le Gal et al. [129] encountered 12 cases of PCP and 6 cases of P. jirovecii colonization in organ recipients in their renal transplantation unit. Ten recipients were identified as potential infectious sources of a predominant strain of P. jirovecii: three were colonized by this fungus and seven developed PCP. The findings strongly suggest that asymptomatic carriers of P. jirovecii play a critical role in its circulation among renal transplant recipients.
An outbreak of P. jirovecii infection among RA outpatients
To determine the prevalence of asymptomatic carriage of P. jirovecii in RA patients and identify individuals with a high risk of PCP, between March 2005 and October 2009 we performed PCR tests for P. jirovecii on respiratory specimens from 132 outpatients with RA [12]. During the first 2 years only one case of PCP was observed and no asymptomatic carriers were found. However, between November 2006 and October 2008 we found nine cases of asymptomatic carriage of this organism. Among these carriers, three had not received any prophylactic antibiotics and developed PCP within 1 month. The other six obtained negative results for P. jirovecii DNA after 2–4 weeks of primary prophylaxis with TMP-SMX or pentamidine isethionate (PI) [130]. During this period we encountered an additional five cases of PCP in RA outpatients who had not yet undergone PCR testing.
Tracing of person-to-person transmission
All the members of this cluster, except two, had potentially infectious encounters at the outpatient facility within at least 4 months before the first detection of P. jirovecii. Of the two exceptions, one was in the same inpatient ward for joint surgery at the time when a PCP patient was hospitalized for treatment. The other was a PCP patient’s spouse. Person-to-person transmission in hospital environments seems to be a frequent event among RA outpatients. No geographic clustering by postal code was noted, suggesting that a regional environmental source outside the hospital was less likely. During the outbreak period, no PCP occurrence was noted among outpatients of other clinical sections who had shared the same waiting room with RA outpatients, suggesting that P. jirovecii circulation is limited to the RA patient group.
Clinical presentation
Patients’ respiratory symptoms were non-specific and non-severe at diagnosis of PCP, but all cases complained of slight general fatigue. Their chest radiographs were almost normal, but high-resolution CT scans revealed diffuse ground-glass opacities. In addition, oxygen saturation was in the normal range at rest, but it dropped to low levels after motion. As mentioned above, PCP in HIV-negative patients is likely to cause fulminant respiratory failure within the first several days and the mortality rate is higher. We therefore stress that those RA patients receiving immunosuppressive therapy should be followed up for signs and symptoms of PCP development, with a high index of suspicion. PCP should be included in the differential diagnosis of acute-onset diffuse interstitial pneumonia in RA patients receiving immunosuppressive therapy [131].
Outcomes
Within 2 weeks of hospitalization and treatment with TMP-SMX, P. jirovecii DNA disappeared in all PCP cases. Since P. jirovecii was eradicated from PCP cases and asymptomatic carriers, the DNA of this fungus was not detected during the follow-ups of these patients without any additional prophylactic intervention, even after resuming immunosuppressive therapy for RA. These findings suggest that RA outpatients with asymptomatic carriage can serve as an infectious reservoir for P. jirovecii. If PCP prophylaxis had not been introduced for such asymptomatic carriers, this outbreak may have escalated and resulted in wider transmission. Now we recommend PCR tests for all RA patients, especially aged individuals, on their first visit to our facility, because there is the possibility that they may carry a new infection into our patient cohort. Regular PCR testing during immunosuppressive therapy is not recommended because of its high cost.
Control of P. jirovecii infection and prevention of PCP outbreaks among RA outpatients
Primary PCP prophylaxis is recommended for HIV-infected individuals with CD4+ T cell counts of <200/µl [132, 133]. Renal transplant guidelines have recommended PCP prophylaxis with TMP-SMX for 3–12 months [134–136]. Considering the poor survival rates of PCP cases, prophylaxis of P. jirovecii infection should be discussed for RA outpatients who are scheduled to receive immunosuppressive therapy. However, guidelines for the administration of prophylactic antibiotics to such patients remain less clear. Universal routine prophylaxis is impractical because of the inherently long-term nature of anti-RA therapy. Prophylactic agents against PCP often induce severe adverse effects following MTX therapy for RA, such as allergic pancytopenia to TMP-SMX [137]. Development of Pneumocystis resistance to prophylaxis has also been reported [138, 139].
Eradication of P. jirovecii from asymptomatic carriers
Various reports have suggested that it is necessary to identify those HIV-negative patients whose risk of developing PCP is great enough to warrant prophylaxis in spite of the adverse effects [7, 24, 34–37, 140–145]. However, no quantitative markers clearly correlate with the risk of PCP in patients with rheumatic diseases, as CD4+ T cell count does in HIV-infected individuals. If reactivation of latent childhood infection is the predominant mode of PCP development in humans, prophylactic antibiotic usage for patients at high risk is the only method of preventing the disease. If new acquisition of P. jirovecii can occur in hospital environments, lifelong prophylaxis may theoretically be required for susceptible individuals. Alternatively, early identification of asymptomatic carriers as sources and reservoirs of infection leads to other strategies for prevention of PCP outbreaks in RA outpatients. Avoiding interhuman contacts between asymptomatic carriers and susceptible RA patients in a medical waiting room is difficult as standard practice. Rather, we should consider measures to eradicate P. jirovecii from asymptomatic carriers.
Duration of measures to eradicate P. jirovecii outbreaks
In reducing the potential for outbreaks of PCP among RA outpatients, it is important to consider when prophylactic antibiotics can safely be discontinued. In the outbreak observed among our RA outpatients, P. jirovecii was eliminated from carriers and PCP patients with a very short-term course (2–4 weeks) of treatment with TMP-SMX or PI [12, 130]. Through this procedure the outbreak was resolved and no new PCP outbreaks were observed in this patient group. Similarly, Saito et al. [33] observed that in almost all of their patients with PCP and rheumatic diseases, P. jiroveci disappeared within 7–10 days after commencement of TMP-SMX treatment and no recurrence of this pneumonia was observed. Godeau et al. [32] also reported that during follow-ups of 22 months on average, no relapse of PCP was seen among survivors who had been treated with TMP-SMX for a mean of 17 days, even though they continued to receive immunosuppressive drugs for rheumatic diseases without secondary prophylaxis. In contrast, Suryaprasad and Stone [146] reported three cases of new PCP development occurring after discontinuation of primary prophylaxis in patients with rheumatic diseases suffering from profound lymphopenia. These cases may result from new acquisition of P. jirovecii from unidentified reservoirs. There is always the possibility that patients with rheumatic diseases may be subject to reinfection as long as P. jirovecii reservoirs continue to exist in our patient group.
Conclusions
PCP development seems to result from a de novo infection rather than from reactivation of a latent childhood infection, and hospital-acquired, person-to-person transmission would appear to be the most likely mode of acquisition of new infection in RA outpatients. Identification of P. jirovecii carriers would lead to the prompt introduction of PCP prophylaxis when rheumatologists consider immunosuppressive therapy for RA. Once a new case of PCP occurs in an outpatient clinic, physicians should take prompt action not only to treat the patient but also to prevent other patients from becoming new reservoirs of P. jirovecii. In this case, short-term prophylaxis for all other RA patients visiting this clinic may be effective and even justified in preventing the increased risk of a PCP outbreak. Understanding the potential role of asymptomatic carriers in the circulation of P. jirovecii among RA outpatients will allow us to undertake effective action to control P. jirovecii infection and to prevent future outbreaks of PCP.
Funding: This study was supported by research funds from the National Hospital Organization (NHO), Japan.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenger AA, Houtman PM, Bruyn GA, Eggink HF, Pasma HR. Pneumocystis carinii pneumonia associated with low dose methotrexate treatment for rheumatoid arthritis. Scand J Rheumatol. 1994;23:51–3. doi: 10.3109/03009749409102137. [DOI] [PubMed] [Google Scholar]
- 4.LeMense GP, Sahn SA. Opportunistic infection during treatment with low dose methotrexate. Am J Respir Crit Care Med. 1994;150:258–60. doi: 10.1164/ajrccm.150.1.8025760. [DOI] [PubMed] [Google Scholar]
- 5.Tai TL, O'Rourke KP, McWeeney M, Burke CM, Sheehan K, Barry M. Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology. 2002;41:951–2. doi: 10.1093/rheumatology/41.8.951. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Imamura F, Kiyofuji C, et al. Pneumocystis jiroveci pneumonia in a patient with rheumatoid arthritis as a complication of treatment with infliximab, anti-tumor necrosis factor alpha neutralizing antibody. Mod Rheumatol. 2006;16:58–62. doi: 10.1007/s10165-005-0454-2. [DOI] [PubMed] [Google Scholar]
- 7.Sowden E, Carmichael AJ. Autoimmune inflammatory disorders, systemic corticosteroids and pneumocystis pneumonia: a strategy for prevention. BMC Infect Dis. 2004;4:42. doi: 10.1186/1471-2334-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 9.Stringer JR, Beard CB, Miller RF. Spelling Pneumocystis jirovecii. Emerg Infect Dis. 2009;15:506. doi: 10.3201/eid1503.081060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis. 2002;8:891–6. doi: 10.3201/eid0809.020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokuda H, Sakai F, Yamada H, et al. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med. 2008;47:915–23. doi: 10.2169/internalmedicine.47.0702. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Cho I, Sugimoto M. A cluster of Pneumocystis jirovecii infection among outpatients with rheumatoid arthritis. J Rheumatol. 2010;37:1547–8. doi: 10.3899/jrheum.091294. [DOI] [PubMed] [Google Scholar]
- 13.Vanek J, Jirovec O. [Parasitic pneumonia. Interstitial plasma cell pneumonia of premature, caused by pneumocystis Carinii.] Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1952;158:120–7. [PubMed] [Google Scholar]
- 14.Gajdusek DC. Pneumocystis carinii: etiologic agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics. 1957;19:543–65. [PubMed] [Google Scholar]
- 15.Post C, Dutz W, Nasarian I. Endemic Pneumocystis carinii pneumonia in south Iran. Arch Dis Child. 1964;39:35–40. doi: 10.1136/adc.39.203.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutz W, Post C, Vessal K, Kohout E. Endemic infantile pneumocystis carinii infection: the Shiraz study. Natl Cancer Inst Monogr. 1976;43:31–40. [PubMed] [Google Scholar]
- 17.Hughes WT, Feldman S, Aur RJ, Verzosa MS, Hustu HO, Simone JV. Intensity of immunosuppressive therapy and the incidence of Pneumocystis carinii pneumonitis. Cancer. 1975;36:2004–9. doi: 10.1002/cncr.2820360912. [DOI] [PubMed] [Google Scholar]
- 18.Walzer PD, Schultz MG, Western KA, Robbins JF. Pneumocystis carinii pneumonia and primary immune deficiency diseases. Natl Cancer Inst Monogr. 1976;43:65–74. [PubMed] [Google Scholar]
- 19.Hughes WT, Kuhn S, Chaudhary S, et al. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1977;297:1419–26. doi: 10.1056/NEJM197712292972602. [DOI] [PubMed] [Google Scholar]
- 20.Selik RM, Starcher ET, Curran JW. Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS. 1987;1:175–82. [PubMed] [Google Scholar]
- 21.Chien SM, Rawji M, Mintz S, Rachlis A, Chan CK. Changes in hospital admissions pattern in patients with human immunodeficiency virus infection in the era of Pneumocystis carinii prophylaxis. Chest. 1992; 102:1035–9. doi: 10.1378/chest.102.4.1035. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl. 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 23.Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17(Suppl. 2):S416–22. doi: 10.1093/clinids/17.supplement_2.s416. [DOI] [PubMed] [Google Scholar]
- 24.Sepkowitz KA, Brown AE, Armstrong D. Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome. More patients, same risk. Arch Intern Med. 1995;155:1125–8. [PubMed] [Google Scholar]
- 25.Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993: an analysis of 78 cases. Arch Intern Med. 1995;155:2436–41. [PubMed] [Google Scholar]
- 26.Nuesch R, Bellini C, Zimmerli W. Pneumocystis carinii pneumonia in human immunodeficiency virus (HIV)-positive and HIV-negative immunocompromised patients. Clin Infect Dis. 1999;29:1519–23. doi: 10.1086/313534. [DOI] [PubMed] [Google Scholar]
- 27.Roblot F, Godet C, Le Moal G, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21:523–31. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard UM, Helweg-Larsen J. Pneumocystis jiroveci pneumonia (PCP) in HIV-1-negative patients: a retrospective study 2002–2004. Scand J Infect Dis. 2007;39:589–95. doi: 10.1080/00365540601150497. [DOI] [PubMed] [Google Scholar]
- 29.Hahn PY, Limper AH. The role of inflammation in respiratory impairment during Pneumocystis carinii pneumonia. Semin Respir Infect. 2003;18:40–7. doi: 10.1053/srin.2003.50004. [DOI] [PubMed] [Google Scholar]
- 30.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol. 2000;164:3755–63. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 31.Walzer PD, LaBine M, Redington TJ, Cushion MT. Lymphocyte changes during chronic administration of and withdrawal from corticosteroids: relation to Pneumocystis carinii pneumonia. J Immunol. 1984;133:2502–8. [PubMed] [Google Scholar]
- 32.Godeau B, Coutant-Perronne V, Le Thi Huong D, et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol. 1994;21:246–51. [PubMed] [Google Scholar]
- 33.Saito K, Nakayamada S, Nakano K, et al. Detection of Pneumocystis carinii by DNA amplification in patients with connective tissue diseases: re-evaluation of clinical features of P. carinii pneumonia in rheumatic diseases. Rheumatology. 2004;43:479–85. doi: 10.1093/rheumatology/keh071. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Inokuma S, Maezawa R, et al. Clinical characteristics of Pneumocystis carinii pneumonia in patients with connective tissue diseases. Mod Rheumatol. 2005;15:191–7. doi: 10.1007/s10165-005-0395-9. [DOI] [PubMed] [Google Scholar]
- 35.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa J, Harigai M, Nagasaka K, Nakamura T, Miyasaka N. Prediction of and prophylaxis against Pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod Rheumatol. 2005;15:91–6. doi: 10.1007/pl00021707. [DOI] [PubMed] [Google Scholar]
- 37.Iikuni N, Kitahama M, Ohta S, Okamoto H, Kamatani N, Nishinarita M. Evaluation of Pneumocystis pneumonia infection risk factors in patients with connective tissue disease. Mod Rheumatol. 2006;16:282–8. doi: 10.1007/s10165-006-0502-6. [DOI] [PubMed] [Google Scholar]
- 38.Wu AK, Cheng VC, Tang BS, et al. The unmasking of Pneumocystis jiroveci pneumonia during reversal of immunosuppression: case reports and literature review. BMC Infect Dis. 2004;4:57. doi: 10.1186/1471-2334-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104:1307–17. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enomoto T, Azuma A, Kohno A, et al. Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromized patients with and without HIV infection. Respirology. 2010;15:126–31. doi: 10.1111/j.1440-1843.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–94. doi: 10.1136/ard.2007.072967. [DOI] [PubMed] [Google Scholar]
- 42.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 43.Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol. 2011 doi: 10.1007/s10165-011-0541-5. Advance access published 13 Oct 2011, doi: 10.1007/s10165-011-0541-5. [DOI] [PubMed] [Google Scholar]
- 44.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481–4. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 45.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–51. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori S, Cho I, Ichiyasu H, Sugimoto M. Asymptomatic carriage of Pneumocystis jiroveci in elderly patients with rheumatoid arthritis in Japan: a possible association between colonization and development of Pneumocystis jiroveci pneumonia during low-dose MTX therapy. Mod Rheumatol. 2008;18:240–6. doi: 10.1007/s10165-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 47.Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii pneumonia. Infect Dis Clin North Am. 2010;24:107–38. doi: 10.1016/j.idc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–71. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 49.Limper AH, Offord KP, Smith TF, Martin WJ., 2nd Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–9. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 50.Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without AIDS. Lung. 2010;188:159–63. doi: 10.1007/s00408-009-9214-y. [DOI] [PubMed] [Google Scholar]
- 51.Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med. 2010;49:273–81. doi: 10.2169/internalmedicine.49.2871. [DOI] [PubMed] [Google Scholar]
- 52.Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum. 1999;42:780–9. doi: 10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Pifer LL, Hughes WT, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978;61:35–41. [PubMed] [Google Scholar]
- 54.Vargas SL, Hughes WT, Santolaya ME, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32:855–61. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 55.Beard CB, Carter JL, Keely SP, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis. 2000;6:265–72. doi: 10.3201/eid0603.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montes-Cano MA, de la Horra C, Martin-Juan J, et al. Pneumocystis jiroveci genotypes in the Spanish population. Clin Infect Dis. 2004;39:123–8. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 57.Miller RF, Lindley AR, Copas A, Ambrose HE, Davies RJ, Wakefield AE. Genotypic variation in Pneumocystis jirovecii isolates in Britain. Thorax. 2005;60:679–82. doi: 10.1136/thx.2004.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dohn MN, White ML, Vigdorth EM, et al. Geographic clustering of Pneumocystis carinii pneumonia in patients with HIV infection. Am J Respir Crit Care Med. 2000;162:1617–21. doi: 10.1164/ajrccm.162.5.9707101. [DOI] [PubMed] [Google Scholar]
- 59.Morris AM, Swanson M, Ha H, Huang L. Geographic distribution of human immunodeficiency virus-associated Pneumocystis carinii pneumonia in San Francisco. Am J Respir Crit Care Med. 2000;162:1622–6. doi: 10.1164/ajrccm.162.5.2002065. [DOI] [PubMed] [Google Scholar]
- 60.Beck JM. Pneumocystis carinii and geographic clustering: evidence for transmission of infection. Am J Respir Crit Care Med. 2000;162:1605–6. doi: 10.1164/ajrccm.162.5.ed11-00a. [DOI] [PubMed] [Google Scholar]
- 61.Keely SP, Stringer JR, Baughman RP, Linke MJ, Walzer PD, Smulian AG. Genetic variation among Pneumocystis carinii hominis isolates in recurrent pneumocystosis. J Infect Dis. 1995;172:595–8. doi: 10.1093/infdis/172.2.595. [DOI] [PubMed] [Google Scholar]
- 62.Keely SP, Baughman RP, Smulian AG, Dohn MN, Stringer JR. Source of Pneumocystis carinii in recurrent episodes of pneumonia in AIDS patients. AIDS. 1996;10:881–8. doi: 10.1097/00002030-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Latouche S, Poirot JL, Bertrand V, Roux P. Pneumocystis carinii hominis sequencing for reactivation or de novo contamination and for hypothetic transmission from person to person. APMIS Suppl. 1997;77:11–3. doi: 10.1111/j.1600-0463.1997.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 64.Keely SP, Stringer JR. Sequences of Pneumocystis carinii f. sp. hominis strains associated with recurrent pneumonia vary at multiple loci. J Clin Microbiol. 1997;35:2745–7. doi: 10.1128/jcm.35.11.2745-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Donnell WJ, Pieciak W, Chertow GM, Sanabria J, Lahive KC. Clearance of Pneumocystis carinii cysts in acute P. carinii pneumonia: assessment by serial sputum induction. Chest. 1998;114:1264–8. doi: 10.1378/chest.114.5.1264. [DOI] [PubMed] [Google Scholar]
- 66.Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients. J Infect Dis. 2003;187:901–8. doi: 10.1086/368165. [DOI] [PubMed] [Google Scholar]
- 67.Sepkowitz K, Schluger N, Godwin T, Armstrong D, Cerami A, Bucala R. DNA amplification in experimental pneumocystosis: characterization of serum Pneumocystis carinii DNA and potential P. carinii carrier states. J Infect Dis. 1993;168:421–6. doi: 10.1093/infdis/168.2.421. [DOI] [PubMed] [Google Scholar]
- 68.Chen W, Gigliotti F, Harmsen AG. Latency is not an inevitable outcome of infection with Pneumocystis carinii. Infect Immun. 1993;61:5406–9. doi: 10.1128/iai.61.12.5406-5409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vargas SL, Hughes WT, Wakefield AE, Oz HS. Limited persistence in and subsequent elimination of Pneumocystis carinii from the lungs after P. carinii pneumonia. J Infect Dis. 1995;172:506–10. doi: 10.1093/infdis/172.2.506. [DOI] [PubMed] [Google Scholar]
- 70.Helweg-Larsen J, Tsolaki AG, Miller RF, Lundgren B, Wakefield AE. Clusters of Pneumocystis carinii pneumonia: analysis of person-to-person transmission by genotyping. QJM. 1998;91:813–20. doi: 10.1093/qjmed/91.12.813. [DOI] [PubMed] [Google Scholar]
- 71.Manoloff ES, Francioli P, Taffe P, Van Melle G, Bille J, Hauser PM. Risk for Pneumocystis carinii transmission among patients with pneumonia: a molecular epidemiology study. Emerg Infect Dis. 2003;9:132–4. doi: 10.3201/eid0901.020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olsson M, Eriksson BM, Elvin K, Strandberg M, Wahlgren M. Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scand J Infect Dis. 2001;33:285–9. doi: 10.1080/003655401300077324. [DOI] [PubMed] [Google Scholar]
- 73.Latouche S, Poirot JL, Maury E, Bertrand V, Roux P. Pneumocystis carinii hominis sequencing to study hypothetical person-to-person transmission. AIDS. 1997;11:549. [PubMed] [Google Scholar]
- 74.Wakefield AE. DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J Clin Microbiol. 1996;34:1754–9. doi: 10.1128/jcm.34.7.1754-1759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsson M, Lidman C, Latouche S, et al. Identification of Pneumocystis carinii f. sp. hominis gene sequences in filtered air in hospital environments. J Clin Microbiol. 1998;36:1737–40. doi: 10.1128/jcm.36.6.1737-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartlett MS, Vermund SH, Jacobs R, et al. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J Clin Microbiol. 1997;35:2511–3. doi: 10.1128/jcm.35.10.2511-2513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choukri F, Menotti J, Sarfati C, et al. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin Infect Dis. 2010;51:259–65. doi: 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 78.Damiani C, Choukri F, Le Gal S, et al. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg Infect Dis. 2012;18:877–8. doi: 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Powles MA, McFadden DC, Pittarelli LA, Schmatz DM. Mouse model for Pneumocystis carinii pneumonia that uses natural transmission to initiate infection. Infect Immun. 1992;60:1397–400. doi: 10.1128/iai.60.4.1397-1400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dumoulin A, Mazars E, Seguy N, et al. Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur J Clin Microbiol Infect Dis. 2000;19:671–8. doi: 10.1007/s100960000354. [DOI] [PubMed] [Google Scholar]
- 81.Chabe M, Dei-Cas E, Creusy C, et al. Immunocompetent hosts as a reservoir of pneumocystis organisms: histological and RT-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis. 2004;23:89–97. doi: 10.1007/s10096-003-1092-2. [DOI] [PubMed] [Google Scholar]
- 82.Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun. 1993;61:2886–90. doi: 10.1128/iai.61.7.2886-2890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Durand-Joly I, Aliouat el M, Recourt C, et al. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol. 2002;40:1862–5. doi: 10.1128/JCM.40.5.1862-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stringer JR, Stringer SL, Zhang J, Baughman R, Smulian AG, Cushion MT. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993;40:733–41. doi: 10.1111/j.1550-7408.1993.tb04468.x. [DOI] [PubMed] [Google Scholar]
- 85.Sloand E, Laughon B, Armstrong M, et al. The challenge of Pneumocystis carinii culture. J Eukaryot Microbiol. 1993;40:188–95. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 86.Morris A, Kingsley LA, Groner G, Lebedeva IP, Beard CB, Norris KA. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS. 2004;18:793–8. doi: 10.1097/00002030-200403260-00011. [DOI] [PubMed] [Google Scholar]
- 87.Davis JL, Welsh DA, Beard CB, et al. Pneumocystis colonisation is common among hospitalised HIV infected patients with non-Pneumocystis pneumonia. Thorax. 2008;63:329–34. doi: 10.1136/thx.2007.088104. [DOI] [PubMed] [Google Scholar]
- 88.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–53. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 89.Medrano FJ, Montes-Cano M, Conde M, et al. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005;11:245–50. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vargas SL, Pizarro P, Lopez-Vieyra M, Neira-Aviles P, Bustamante R, Ponce CA. Pneumocystis colonization in older adults and diagnostic yield of single versus paired noninvasive respiratory sampling. Clin Infect Dis. 2010;50:e19–21. doi: 10.1086/649869. [DOI] [PubMed] [Google Scholar]
- 91.Totet A, Respaldiza N, Pautard JC, Raccurt C, Nevez G. Pneumocystis jiroveci genotypes and primary infection. Clin Infect Dis. 2003;36:1340–2. doi: 10.1086/374844. [DOI] [PubMed] [Google Scholar]
- 92.Vargas SL, Ponce CA, Gigliotti F, et al. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J Clin Microbiol. 2000;38:1536–8. doi: 10.1128/jcm.38.4.1536-1538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller RF, Ambrose HE, Wakefield AE. Pneumocystis carinii f. sp. hominis DNA in immunocompetent health care workers in contact with patients with P. carinii pneumonia. J Clin Microbiol. 2001;39:3877–82. doi: 10.1128/JCM.39.11.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lundgren B, Elvin K, Rothman LP, Ljungstrom I, Lidman C, Lundgren JD. Transmission of Pneumocystis carinii from patients to hospital staff. Thorax. 1997; 52:422–4. doi: 10.1136/thx.52.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calderon EJ, Regordan C, Medrano FJ, Ollero M, Varela JM. Pneumocystis carinii infection in patients with chronic bronchial disease. Lancet. 1996;347:977. doi: 10.1016/s0140-6736(96)91468-3. [DOI] [PubMed] [Google Scholar]
- 96.Armbruster C, Hassl A, Kriwanek S. Pneumocystis carinii colonization in the absence of immunosuppression. Scand J Infect Dis. 1997;29:591–3. doi: 10.3109/00365549709035900. [DOI] [PubMed] [Google Scholar]
- 97.Sing A, Roggenkamp A, Autenrieth IB, Heesemann J. Pneumocystis carinii carriage in immunocompetent patients with primary pulmonary disorders as detected by single or nested PCR. J Clin Microbiol. 1999;37:3409–10. doi: 10.1128/jcm.37.10.3409-3410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Probst M, Ries H, Schmidt-Wieland T, Serr A. Detection of Pneumocystis carinii DNA in patients with chronic lung diseases. Eur J Clin Microbiol Infect Dis. 2000;19:644–5. doi: 10.1007/s100960000329. [DOI] [PubMed] [Google Scholar]
- 99.Sing A, Geiger AM, Hogardt M, Heesemann J. Pneumocystis carinii carriage among cystic fibrosis patients, as detected by nested PCR. J Clin Microbiol. 2001;39:2717–8. doi: 10.1128/JCM.39.7.2717-2718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calderon E, de la Horra C, Medrano FJ, et al. Pneumocystis jiroveci isolates with dihydropteroate synthase mutations in patients with chronic bronchitis. Eur J Clin Microbiol Infect Dis. 2004;23:545–9. doi: 10.1007/s10096-004-1151-3. [DOI] [PubMed] [Google Scholar]
- 101.Respaldiza N, Montes-Cano MA, Dapena FJ, et al. Prevalence of colonisation and genotypic characterisation of Pneumocystis jirovecii among cystic fibrosis patients in Spain. Clin Microbiol Infect. 2005;11:1012–5. doi: 10.1111/j.1469-0691.2005.01276.x. [DOI] [PubMed] [Google Scholar]
- 102.Vidal S, de la Horra C, Martin J, et al. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin Microbiol Infect. 2006;12:231–5. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 103.Helweg-Larsen J, Jensen JS, Dohn B, Benfield TL, Lundgren B. Detection of Pneumocystis DNA in samples from patients suspected of bacterial pneumonia—a case-control study. BMC Infect Dis. 2002;2:28. doi: 10.1186/1471-2334-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maskell NA, Waine DJ, Lindley A, et al. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax. 2003;58:594–7. doi: 10.1136/thorax.58.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montes-Cano MA, de la Horra C, Dapena FJ, et al. Dynamic colonisation by different Pneumocystis jirovecii genotypes in cystic fibrosis patients. Clin Microbiol Infect. 2007;13:1008–11. doi: 10.1111/j.1469-0691.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 106.Rivero L, de la Horra C, Montes-Cano MA, et al. Pneumocystis jirovecii transmission from immunocompetent carriers to infant. Emerg Infect Dis. 2008;14:1116–8. doi: 10.3201/eid1407.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morris A, Sciurba FC, Lebedeva IP, et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–13. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 108.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35:1513–21. [PubMed] [Google Scholar]
- 109.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Mod Rheumatol. 2011;21:164–73. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nevez G, Raccurt C, Jounieaux V, Dei-Cas E, Mazars E. Pneumocystosis versus pulmonary Pneumocystis carinii colonization in HIV-negative and HIV-positive patients. AIDS. 1999;13:535–6. doi: 10.1097/00002030-199903110-00020. [DOI] [PubMed] [Google Scholar]
- 111.Hauser PM, Blanc DS, Bille J, Nahimana A, Francioli P. Carriage of Pneumocystis carinii by immunosuppressed patients and molecular typing of the organisms. AIDS. 2000;14:461–3. doi: 10.1097/00002030-200003100-00022. [DOI] [PubMed] [Google Scholar]
- 112.Nevez G, Pruna A, Jounieaux V, Makdassi R, Totet A, Raccurt C. A search for Pneumocystis carinii DNA by polymerase chain reaction on bronchoalveolar lavage fluids from patients with Wegener's granulomatosis. Rheumatology. 1999;38:1025–7. doi: 10.1093/rheumatology/38.10.1025. [DOI] [PubMed] [Google Scholar]
- 113.Mekinian A, Durand-Joly I, Hatron PY, et al. Pneumocystis jirovecii colonization in patients with systemic autoimmune diseases: prevalence, risk factors of colonization and outcome. Rheumatology. 2010;50:569–77. doi: 10.1093/rheumatology/keq314. [DOI] [PubMed] [Google Scholar]
- 114.Nevez G, Raccurt C, Vincent P, Jounieaux V, Dei-Cas E. Pulmonary colonization with Pneumocystis carinii in human immunodeficiency virus-negative patients: assessing risk with blood CD4+ T cell counts. Clin Infect Dis. 1999;29:1331–2. doi: 10.1086/313478. [DOI] [PubMed] [Google Scholar]
- 115.Fritzsche C, Riebold D, Munk-Hartig A, Klammt S, Neeck G, Reisinger E. High prevalence of Pneumocystis jirovecii colonization among patients with autoimmune inflammatory diseases and corticosteroid therapy. Scand J Rheumatol. 2012 doi: 10.3109/03009742.2011.630328. Advance access published 9 March 2012, doi: 10.3109/03009742.2011.630328. [DOI] [PubMed] [Google Scholar]
- 116.Wissmann G, Morilla R, Martin-Garrido I, et al. Pneumocystis jirovecii colonization in patients treated with infliximab. Eur J Clin Invest. 2011;41:343–8. doi: 10.1111/j.1365-2362.2010.02415.x. [DOI] [PubMed] [Google Scholar]
- 117.de Boer MG, de Fijter JW, Kroon FP. Outbreaks and clustering of Pneumocystis pneumonia in kidney transplant recipients: a systematic review. Med Mycol. 2010;49:673–80. doi: 10.3109/13693786.2011.571294. [DOI] [PubMed] [Google Scholar]
- 118.Chave JP, David S, Wauters JP, Van Melle G, Francioli P. Transmission of Pneumocystis carinii from AIDS patients to other immunosuppressed patients: a cluster of Pneumocystis carinii pneumonia in renal transplant recipients. AIDS. 1991;5:927–32. doi: 10.1097/00002030-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Rabodonirina M, Vanhems P, Couray-Targe S, et al. Molecular evidence of interhuman transmission of Pneumocystis pneumonia among renal transplant recipients hospitalized with HIV-infected patients. Emerg Infect Dis. 2004;10:1766–73. doi: 10.3201/eid1010.040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hocker B, Wendt C, Nahimana A, Tonshoff B, Hauser PM. Molecular evidence of Pneumocystis transmission in pediatric transplant unit. Emerg Infect Dis. 2005;11:330–2. doi: 10.3201/eid1102.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Boer MG, Bruijnesteijn van Coppenraet LE, Gaasbeek A, et al. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: interhuman transmission or a common environmental source? Clin Infect Dis. 2007;44:1143–9. doi: 10.1086/513198. [DOI] [PubMed] [Google Scholar]
- 122.Schmoldt S, Schuhegger R, Wendler T, et al. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J Clin Microbiol. 2008;46:966–71. doi: 10.1128/JCM.02016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation. 2009;88:380–5. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 124.Gianella S, Haeberli L, Joos B, et al. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis. 2010;12:1–10. doi: 10.1111/j.1399-3062.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 125.Phipps LM, Chen SC, Kable K, et al. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation. 2011;92:1327–34. doi: 10.1097/TP.0b013e3182384b57. [DOI] [PubMed] [Google Scholar]
- 126.Ripamonti C, Orenstein A, Kutty G, et al. Restriction fragment length polymorphism typing demonstrates substantial diversity among Pneumocystis jirovecii isolates. J Infect Dis. 2009;200:1616–22. doi: 10.1086/644643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomas S, Vivancos R, Corless C, Wood G, Beeching NJ, Beadsworth MB. Increasing frequency of Pneumocystis jirovecii pneumonia in renal transplant recipients in the United Kingdom: clonal variability, clusters, and geographic location. Clin Infect Dis. 2011;53:307–8. doi: 10.1093/cid/cir329. [DOI] [PubMed] [Google Scholar]
- 128.Sassi M, Ripamonti C, Mueller NJ, et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: implications for transmission and virulence. Clin Infect Dis. 2012;54:1437–44. doi: 10.1093/cid/cis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le Gal S, Damiani C, Rouille A, et al. A cluster of Pneumocystis infections among renal transplant recipients: molecular evidence of colonized patients as potential infectious sources of Pneumocystis jirovecii. Clin Infect Dis. 2012;54:e62–71. doi: 10.1093/cid/cir996. [DOI] [PubMed] [Google Scholar]
- 130.Mori S, Cho I, Sugimoto M. A followup study of asymptomatic carriers of Pneumocystis jiroveci during immunosuppressive therapy for rheumatoid arthritis. J Rheumatol. 2009;36:1600–5. doi: 10.3899/jrheum.081270. [DOI] [PubMed] [Google Scholar]
- 131.Kameda H, Tokuda H, Sakai F, et al. Clinical and radiological features of acute-onset diffuse interstitial lung diseases in patients with rheumatoid arthritis receiving treatment with biological agents: importance of Pneumocystis pneumonia in Japan revealed by a multicenter study. Intern Med. 2011;50:305–13. doi: 10.2169/internalmedicine.50.4508. [DOI] [PubMed] [Google Scholar]
- 132.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–29. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 133.U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA) 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. MMWR Recomm Rep (1999);48:1–59, 61–6. [PubMed] [Google Scholar]
- 134.Martin SI, Fishman JA. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl. 4):S227–33. doi: 10.1111/j.1600-6143.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 135.EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.7.1 Late infections. Pneumocystis carinii pneumonia. Nephrol Dial Transplant. 2002;17(Suppl. 4):36–9. [PubMed] [Google Scholar]
- 136.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 137.Chevrel G, Brantus JF, Sainte-Laudy J, Miossec P. Allergic pancytopenia to trimethoprim-sulphamethoxazole for Pneumocystis carinii pneumonia following methotrexate treatment for rheumatoid arthritis. Rheumatology. 1999;38:475–6. doi: 10.1093/rheumatology/38.5.475. [DOI] [PubMed] [Google Scholar]
- 138.Nahimana A, Rabodonirina M, Zanetti G, et al. Association between a specific Pneumocystis jiroveci dihydropteroate synthase mutation and failure of pyrimethamine/sulfadoxine prophylaxis in human immunodeficiency virus-positive and -negative patients. J Infect Dis. 2003;188:1017–23. doi: 10.1086/378239. [DOI] [PubMed] [Google Scholar]
- 139.Huang L, Crothers K, Atzori C, et al. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis. 2004;10:1721–8. doi: 10.3201/eid1010.030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mansharamani NG, Balachandran D, Vernovsky I, Garland R, Koziel H. Peripheral blood CD4+ T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712–20. doi: 10.1378/chest.118.3.712. [DOI] [PubMed] [Google Scholar]
- 141.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–9. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 142.Harigai M, Koike R, Miyasaka N. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med. 2007;357:1874–6. doi: 10.1056/NEJMc070728. [DOI] [PubMed] [Google Scholar]
- 143.Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum. 2009;61:305–12. doi: 10.1002/art.24283. [DOI] [PubMed] [Google Scholar]
- 144.Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum. 2011;41:497–502. doi: 10.1016/j.semarthrit.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 145.de Boer MG, Kroon FP, le Cessie S, de Fijter JW, van Dissel JT. Risk factors for Pneumocystis jirovecii pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis. 2011; 13:559–69. doi: 10.1111/j.1399-3062.2011.00645.x. [DOI] [PubMed] [Google Scholar]
- 146.Suryaprasad A, Stone JH. When is it safe to stop Pneumocystis jiroveci pneumonia prophylaxis? Insights from three cases complicating autoimmune diseases. Arthritis Rheum. 2008;59:1034–9. doi: 10.1002/art.23822. [DOI] [PubMed] [Google Scholar]


