Abstract
It is well established that a relatively brief exposure to environmental enrichment (EE) enhances motor and cognitive performance after experimental traumatic brain injury (TBI), but it is not known whether the benefits can be sustained after EE is discontinued. To address this important rehabilitation-relevant concern, anesthetized rats received a controlled cortical impact (CCI) or sham injury, and for phase 1 of the experiment were randomly assigned to either 3 weeks of EE or standard (STD) housing. Neurobehavioral outcome was assessed by established motor and cognitive tests on postoperative days 1–5 and 14–18, respectively. Beam-balance and spatial learning were facilitated in the TBI + EE more than the TBI + STD group (p<0.0001). In phase 2 of the experiment, half of the rats in EE were transferred to STD conditions (TBI + EE + STD and sham + EE + STD), and neurobehavior was re-assessed once per month for 6 months. The TBI + EE and TBI + EE + STD groups performed markedly better in the water maze than the TBI + STD group (p<0.0001), and did not differ from one another (p=0.53). These data replicate those of several studies from our laboratory showing that EE enhances recovery after CCI injury, and extend those findings by demonstrating that the cognitive benefits are maintained for at least 6 months post-rehabilitation. The persistent benefits shown with this paradigm provide further support for EE as a pre-clinical model of rehabilitation that can be further explored, either alone or in combination with pharmacotherapies, for optimal neurorehabilitation after TBI.
Key words: controlled cortical impact, environmental enrichment, functional recovery, learning and memory, Morris water maze, traumatic brain injury
Introduction
Environmental enrichment (EE) consists of novel stimuli and increased social interaction in a spacious milieu.1 Several studies in experimental models of traumatic brain injury (TBI) have shown that a typical 3-week EE paradigm improves motor performance, facilitates spatial learning, and enhances memory retention after controlled cortical impact,1–6 and fluid percussion brain injury.7–9 Additionally, EE also provides histological protection as evidenced by decreased hippocampal CA1/3 cell loss,3,5 and smaller cortical lesions.8,10 Furthermore, EE attenuates the TBI-induced loss of acetyltransferase-positive (ChAT+) medial septal cells.4 Given the plethora of benefits mediated by EE after TBI, it constitutes a pre-clinical model of neurorehabilitation.
However, a potential limitation to the current model is that it is unknown if the improvement conferred by the relatively brief EE exposure paradigm is limited to the period when treatment is ongoing, or if the benefits are sustained after enrichment is discontinued. The answer to this question has significant clinical implications because the current time frame of EE exposure plus behavioral evaluation is akin to clinical rehabilitation, in which therapy is provided for a finite period after TBI.11,12,13 Hence the current study was designed to determine whether the EE-mediated motor and cognitive benefits persist after its withdrawal. The findings could help refine current clinical rehabilitation practice.
Methods
Forty-four male Harlan Sprague-Dawley rats, weighing 300−325 g on the day of surgery, were housed in standard steel-wire mesh cages in a temperature-controlled (21±1°C) and light-controlled (lights on at 7:00 am to 7:00 pm) environment, with continuing access to food and water ad libitum. After 1 week of acclimatization, the rats were prepared for surgery as previously described.3,4,5,14 Briefly, anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. After endotracheal intubation the rats were placed in a stereotaxic frame and ventilated mechanically. All rats were maintained at 37±0.5°C during surgery with a heating blanket. Utilizing aseptic techniques a craniectomy was made in the right hemisphere with a hand-held trephine, and the TBI was produced by impacting the exposed parietal cortex 2.8 mm at 4 m/sec. After the impact, anesthesia was discontinued, the incision was promptly sutured, and the rats were extubated and placed in a temporary acrylic glass holding cage until the effects of anesthesia abated, and then they were placed in their assigned housing conditions. Sham rats underwent similar surgical procedures, but were not subjected to the cortical impact.
Following surgery, the rats were randomly assigned to two TBI groups (EE or STD housing; n=22 and 8, respectively), and two sham groups (EE or STD housing; n=9 and 5, respectively). The EE groups were much larger initially (i.e., phase 1), as half of these were returned to STD housing (i.e., phase 2), thus producing additional comparison groups requiring statistical power.
The enriched environment consists of specifically designed 36×30×20-inch steel-wire cages with three levels and ladders to ambulate from one level to another, where various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water are available (for depiction of an EE cage, see Kline and associates3 and Sozda and colleagues1). To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was twice per week. Groups of 10–12 rats, which included subsets of each of the conditions (i.e., TBI and sham controls), were housed together to minimize variability. During phase 1 of the experiment, all EE rats received our typical paradigm, which consisted of 3 weeks of exposure.1,3,15 During phase 2, half were returned to STD housing and the remaining animals received 6 additional months of EE. Rats in the STD conditions were placed in standard steel-wire mesh cages (2 rats per cage) with only food and water.
All experimental procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh, and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 2011). Every attempt was made to limit the number of subjects used and to minimize their discomfort.
Motor function was assessed with well-established beam tasks.3,5,16 Beam balance consisted of placing the rat on an elevated narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. The beam walk consisted of recording the elapsed time to traverse the beam (2.5 cm wide by 100 cm long). In the first phase of the experiment, testing was conducted immediately before surgery (to establish a baseline measure), as well as on postoperative days 1−5, and consisted of three trials (60 sec were allotted for each trial, with an inter-trial interval of 30 sec) per day on each task. The average daily scores for each subject were used in the statistical analyses. During the second phase of the experiment (i.e., determination of long-term effects), testing on the beam tasks again consisted of three trials on the testing day, but testing was done only once per month for 6 months.
Spatial learning was assessed in a Morris water maze (MWM),17 which is sensitive to cognitive function/dysfunction after TBI.4,5,10,14,15,18 Briefly, the maze consisted of a plastic pool (180 cm diameter, 60 cm high) filled with tap water (26±1°C) to a depth of 28 cm, and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear acrylic glass stand (10 cm diameter, 26 cm high), that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. For the first phase of the experiment, spatial learning acquisition began on postoperative day 14, and consisted of providing a block of four daily trials (4-min inter-trial interval) for 5 consecutive days (14−18) to locate the platform when it was submerged 2 cm below the water's surface (i.e., invisible to the rat). For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west quadrants) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the four daily trials for each rat were averaged and used in the statistical analyses. During the second phase of the study, a similar daily block of trials was provided, but as indicated for the motor tasks, testing was conducted only once per month for 6 months. The data were obtained using a spontaneous motor activity recording and tracking (SMART) system (San Diego Instruments, San Diego, CA).
Statistical analysis
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). When the overall ANOVA revealed a significant effect, the data were further analyzed with Fisher's PLSD post-hoc test to determine specific group differences. The data are presented as the mean±standard error of the mean (SEM) and were considered significant when p values were ≤0.05.
Results
No differences were revealed in any behavioral measure between the EE and STD sham control groups in either phase 1 or phase 2 and thus the data were pooled.
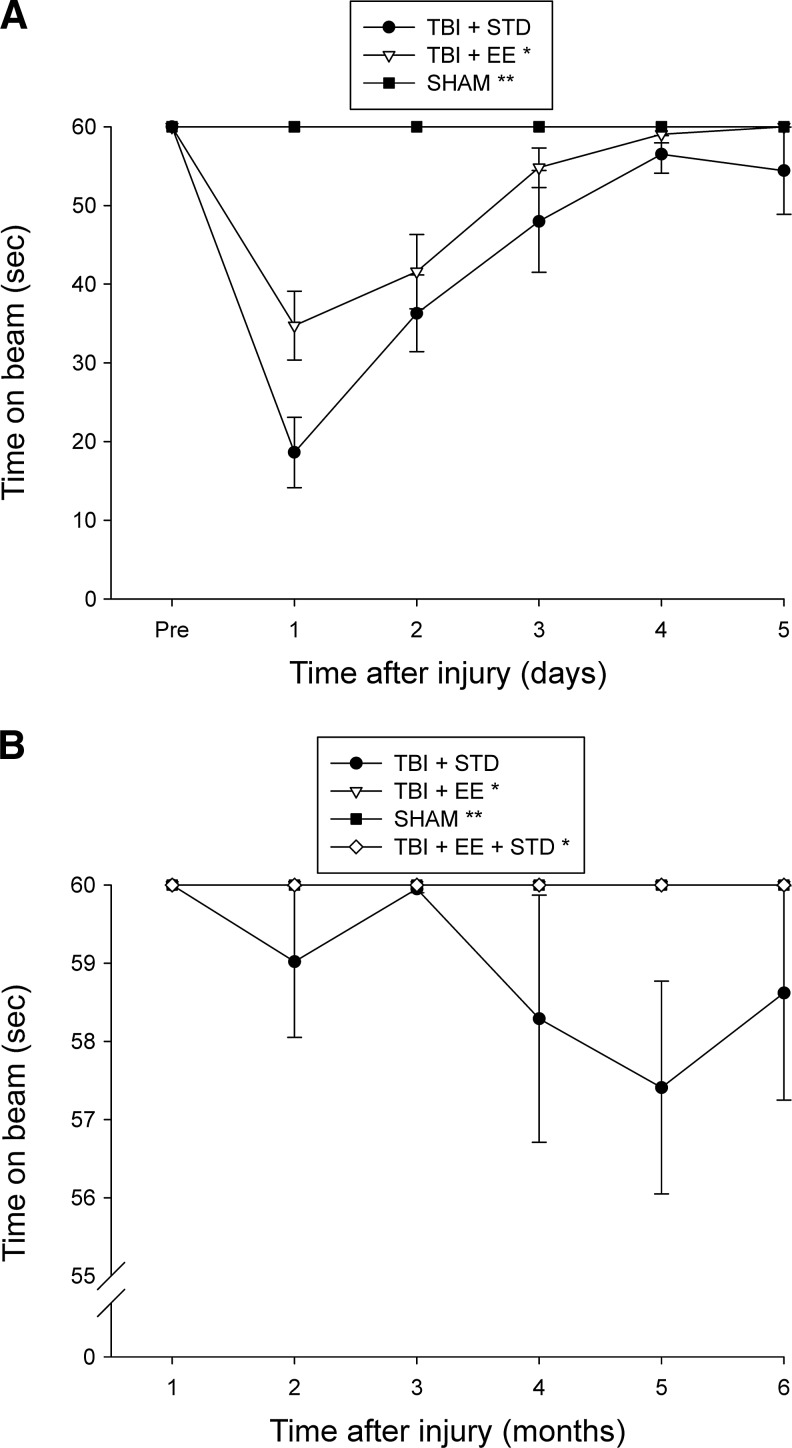
No pre-surgical differences were observed among groups in either the beam-balance or beam-walk tasks, as all rats were able to balance for the allotted 60 sec and traverse the beam within the 5-sec training criterion. However, following CCI injury significant impairments were detected in all TBI groups versus sham controls on both tests as expected (p<0.0001). Moreover, recovery of beam-balancing ability was facilitated in the EE group more than the STD controls (p=0.0117; Fig. 1A). While both TBI groups showed significant beam-walk impairments initially, there were no differences in the rate of recovery between the EE and STD groups, as they both reached baseline levels by the last day of testing (p=0.54). During phase 2 of the experiment, the TBI + STD group showed a statistically significant impairment on the beam-balance task versus the TBI + EE and TBI + EE + STD groups (p=0.0007 and 0.0021, respectively), which did not differ from one another (Fig. 1B). No overall differences were observed among the groups in beam-walk ability (p>0.05; data not shown due to a lack of significant differences).
FIG. 1.
Mean (±standard error of the mean) time (sec) balancing on an elevated narrow beam prior to and after traumatic brain injury (TBI) or sham injury. (A) Both TBI groups were significantly impaired relative to the sham group (**p<0.0001). Additionally, the TBI + EE group performed significantly better than the TBI + STD group (*p<0.0117). (B) *p<0.0021 versus the TBI + STD group (**p<0.0001 versus TBI + STD animals; EE, environmental enrichment; STD, standard housing; SHAM, sham injury).
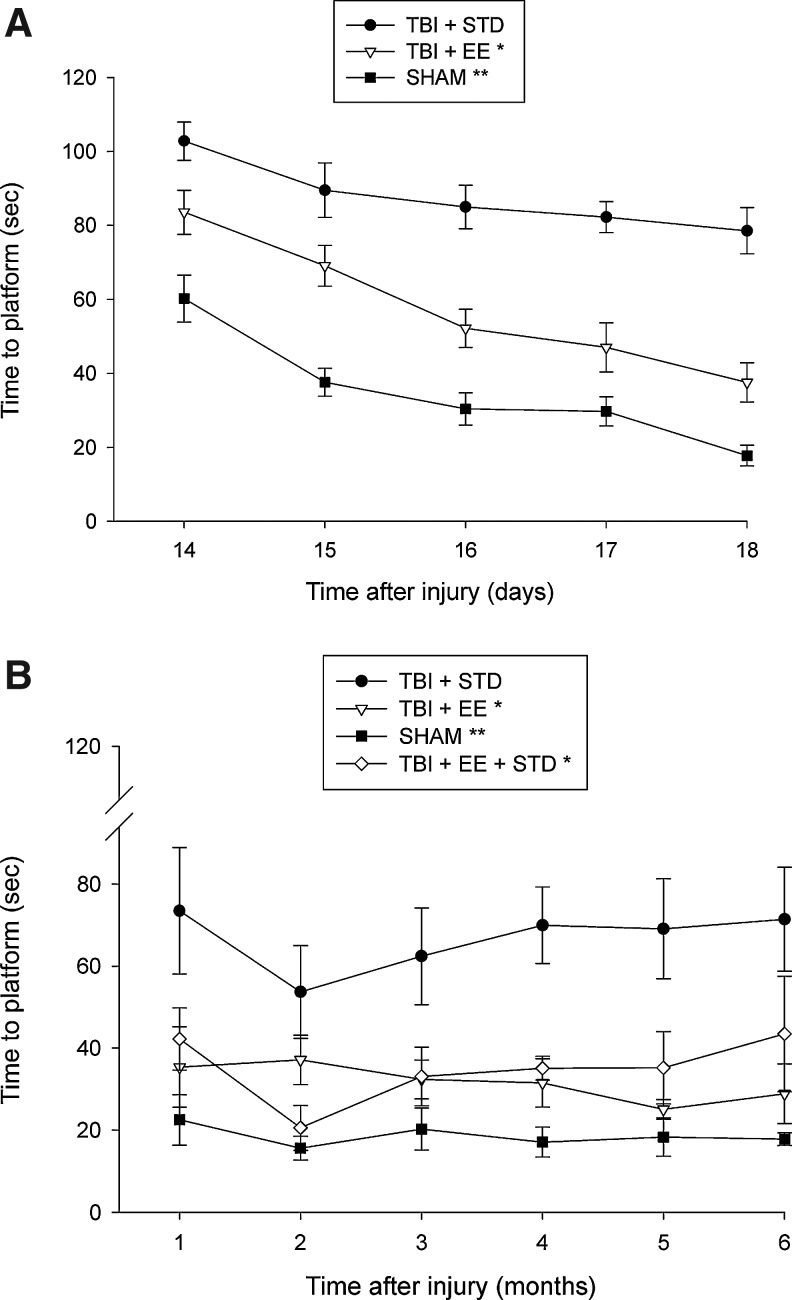
Analysis of the spatial acquisition data for phase 1 of the experiment revealed that the sham group was significantly better than all TBI groups (p<0.0001). Additionally, the TBI + EE group located the escape platform significantly quicker over time than the TBI + STD group (p<0.0001; Fig. 2A). Analysis of the phase 2 data showed that the sham control group was significantly better than all TBI groups (p<0.0022). Furthermore, the TBI + EE and TBI + EE + STD groups were significantly better at finding the escape platform than the TBI + STD group over the subsequent 6 months (p<0.0001), but did not differ from one another (p=0.53; Fig. 2B). No differences were observed among the TBI groups in visible platform performance (p=0.478; data not shown).
FIG. 2.
Mean (±standard error of the mean) time (sec) to locate a submerged platform in a water maze. (A) *p<0.0001 versus traumatic brain injury (TBI) + STD; **p<0.0001 versus both TBI groups). (B) All TBI groups were significantly impaired relative to the sham group (**p<0.0022). Additionally, the TBI + EE and TBI + EE + STD groups were significantly better than the TBI + STD group (*p<0.0001), but did not differ from one another (p=0.53; EE, environmental enrichment; STD, standard housing; SHAM, sham injury).
Discussion
The rationale for this descriptive behavioral study was to determine whether the demonstrated benefits conferred by a relatively brief EE rehabilitation paradigm would be maintained after discontinuation. The relevance is that EE may be viewed as a preclinical model of rehabilitation for TBI. However, a requisite for a valid therapeutic intervention is that benefits are produced while treatment is ongoing and endure after its withdrawal. This later point is perhaps more relevant in the context of rehabilitation, as patients rarely have the opportunity to continue treatment due to cost and insurance limitations. Thus if EE is to move forward as a significant preclinical model of rehabilitation, then determination of its therapeutic longevity needs to be established.
The data showed that the typical 3-week EE paradigm enhanced motor and cognitive function after TBI, which replicates the results of several studies from our laboratory,1,3,4,6 and those of others.7–9 Additionally, the data showed that the benefits produced by EE are long-lasting, as the EE-induced motor and cognitive recovery was maintained for up to 6 months after discontinuation of EE. It is worth noting that while the beam-balance data showed a statistically significant difference between the continuously STD housed group (TBI + STD) and the group that received EE continuously (TBI + EE), or that received it initially and then returned to STD (TBI + EE + STD), the outcome may not be functionally relevant, as the effect is based on a difference of only 2–3 sec. In marked contrast, the ability of the TBI + EE + STD group to maintain its benefit in the spatial learning task is both statistically and functionally relevant, because the difference in time to locate the escape platform was consistently 30 sec or ∼50% faster than the STD-housed group, and did not differ from the continuous EE group.
This level of maintained performance is impressive, and suggests that the reparative and/or plasticity-associated effects of early and relatively brief EE are long-lasting. While we did not evaluate plasticity markers in this study, which was designed for behavioral manipulations, it is well known that EE induces a plethora of neuroplastic changes such as enhancing neurogenesis and increasing dendritic branching, spine density, and nerve growth factor mRNA.19–24 Any of these changes could have contributed to the enhanced and enduring benefits. Future studies will evaluate various markers of plasticity in continuous EE groups, as well as those with limited exposure, in order to determine a correlation between anatomical changes and functional outcome.
The data further showed that the TBI-induced cognitive deficits in untreated rats is long-term, which is in accord with previous studies following CCI injury,25,26 and lateral fluid percussion brain injury.27 Specifically, the time to locate the consistently-situated escape platform in the STD-housed rats did not change dramatically from postoperative day 18 (78.5±6.2 sec) to the last training session at 6 months (71.4±12.6 sec). This long-term TBI-induced cognitive deficit confirms the clinical literature, where it has been reported that memory is the most severely affected and the most frequently reported symptom by both TBI patients and relatives.28 The finding also validates the MWM as a sensitive indicator of cognitive function and dysfunction after experimental TBI.
An interesting new observation is that EE may have a limitation or threshold for producing benefits, as the rats in the continuous EE group did not perform significantly better than those that were returned to standard living conditions. Furthermore, even though the continuous EE rats did not differ statistically from the sham controls at a couple of time points, they did not quite reach their overall level of performance, suggesting that some TBI-induced impairment remains. EE has been shown to have a threshold for the minimal amount of daily exposure necessary to confer benefits,2 and here we show that there may also be a threshold at which EE has reached its maximum potential for spatial learning. Specifically, that threshold appears to be at 3 weeks, as indicated by the lack of statistically significant differences between the continuous EE and EE withdrawal groups. Based on our previous and current data, it seems that EE can provide benefits when provided for as little as 6 h per day after TBI,2 to as much as 3 weeks when provided continuously after TBI.1,3,4,6,15 Future studies will continue to establish the EE paradigm as a clinically-relevant model by assessing if limited EE each day (i.e., 6 h) provides long-term functional benefits when EE is withdrawn after 3 weeks.
It is also interesting to speculate whether the single day of training, which consisted of four trials in the cognitive task each month, served as a mini-rehabilitative session that allowed the TBI + EE + STD group to maintain their motor and cognitive benefits even without daily EE. If this is true, then this would suggest that clinical rehabilitative strategies could be far superior if patients were provided routine follow-up rehabilitation (i.e., “refresher rehab”), such that benefits could be maintained, if not strengthened over time, rather than weakened or lost altogether. Studies addressing these questions are ongoing in our laboratory.
Acknowledgment
Supported in part by National Institutes of Health grants HD043851, HD046700, and NS060005 (to A.E.K.)
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sozda C.N. Hoffman A.N. Olsen A.S. Cheng J.P. Zafonte R.D. Kline A.E. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Witt B.W. Ehrenberg K.M. McAloon R.L. Panos A.H. Shaw K.E. Raghavan P.V. Skidmore E.R. Kline A.E. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline A.E. Wagner A.K. Westergom B.P. Malena R.R. Zafonte R.D. Olsen A.S. Sozda C.N. Luthra P. Panda M. Cheng J.P. Aslam H.A. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kline A.E. McAloon R.L. Henderson K.A. Bansal U.K. Ganti B.M. Ahmed R.H. Gibbs R.B. Sozda C.N. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline A.E. Olsen A.S. Sozda C.N. Hoffman A.N. Cheng J.P. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman A.N. Malena R.R. Westergom B.P. Luthra P. Cheng J.P. Aslam H.A. Zafonte R.D. Kline A.E. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamm R.J. Temple M.D. O'Dell D.M. Pike B.R. Lyeth B.G. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- 8.Passineau M.J. Green E.J. Dietrich W.D. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- 9.Hicks R.R. Zhang L. Atkinson A. Stevenon M. Veneracion M. Seroogy K.B. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–637. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 10.Olsen A.S. Sozda C.N. Cheng J.P. Hoffman A.N. Kline A.E. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J. Neurotrauma. 2012;29:1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackerby W.F. Intensity of rehabilitation and length of stay. Brain Inj. 1990;4:167–173. doi: 10.3109/02699059009026162. [DOI] [PubMed] [Google Scholar]
- 12.Shiel A. Burn J.P. Henry D. Clark J. Wilson B.A. Burnett M.E. McLellan D.L. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin. Rehabil. 2001;15:501–514. doi: 10.1191/026921501680425225. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X.L. Poon W.S. Chan C.C.H. Chan S.S.H. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj. 2007;21:681–690. doi: 10.1080/02699050701468941. [DOI] [PubMed] [Google Scholar]
- 14.Kline A.E. Massucci J.L. Marion D.W. Dixon C.E. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 15.Matter A.M. Folweiler K.A. Curatolo L.M. Kline A.E. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J.P. Hoffman A.N. Zafonte R.D. Kline A.E. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 18.Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 19.Kempermann G. Kuhn H.G. Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 20.Torasdotter M. Metsis M. Henriksson B.G. Winblad B. Mohammed A.H. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 21.van Praag K. Kempermann G. Gage F.H. Neural consequences of environmental enrichment. Nat. Rev. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 22.Ip E.Y. Giza C.C. Griesbach G.S. Hovda D.A. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J. Neurotrauma. 2002;19:573–585. doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- 23.Leggio M.G. Mandolesi L. Federico F. Spirito F. Ricci B. Gelfo F. Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Olson A.K. Eadie B.D. Ernst C. Christie B.R. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 25.Lindner M.D. Plone M.A. Cain C.K. Frydel B. Francis J.M. Emerich D.F. Sutton R.L. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J. Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- 26.Dixon C.E. Kochanek P.M. Yan H.Q. Schiding J.K. Griffith R.G. Baum E. Marion D.W. DeKosky S.T. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- 27.Pierce J.E. Smith D.H. Trojanowski J.Q. McIntosh T.K. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 28.Binder L. Persistent symptoms after mild head injury: a review of the postconcussive syndrome. J. Clin. Exp. Neuropsychol. 1986;8:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]