Abstract
The pediatric brain may be particularly vulnerable to social deficits after traumatic brain injury (TBI) due to the protracted nature of psychosocial development through adolescence. However, the majority of pre-clinical studies fail to assess social outcomes in experimental pediatric TBI. The current study evaluated social behavior in mice subjected to TBI at post-natal day (p)21. Social behaviors were assessed by a partition test, resident-intruder, three-chamber, and tube dominance tasks during adolescence (p35-42) and again during early adulthood (p60-70), during encounters with unfamiliar, naïve stimulus mice. Despite normal olfactory function and normal social behaviors during adolescence, brain-injured mice showed impaired social investigation by adulthood, evidenced by reduced ano-genital sniffing and reduced following of stimulus mice in the resident-intruder task, as well as a loss of preference for sociability in the three-chamber task. TBI mice also lacked a preference for social novelty, suggestive of a deficit in social recognition or memory. By adulthood, brain-injured mice exerted more frequent dominance in the tube task compared to sham-operated controls, a finding suggestive of aggressive tendencies. Together these findings reveal reduced social interaction and a tendency towards increased aggression, which evolves across development to adulthood. This emergence of aberrant social behavior, which parallels the development of other cognitive deficits in this model and behaviors seen in brain-injured children, is consistent with the hypothesis that the full extent of deficits is not realized until the associated skills reach maturity. Thus, efficacy of therapeutics for pediatric TBI should take into account the time-dependent emergence of abnormal behavioral patterns.
Key words: behavioral assessments, cognitive function, outcome measures, pediatric brain injury, social behavior
Introduction
Impairment in social functioning is among the most disabling and persistent consequences of childhood traumatic brain injury (TBI).1 In addition to abundant anecdotal evidence, a systematic review of clinical studies and case reports recently confirmed that children and adolescent survivors of TBI have an elevated risk of social dysfunction, reporting low self-esteem, loneliness, social maladjustment, aggressive tendencies, depression, and social isolation.1–4 For both children and adult survivors of TBI, such social problems can significantly impact daily living, including the maintenance of relationships and participation within the community, thereby greatly reducing overall quality of life.4–6
TBI sustained during early childhood typically results in poorer outcomes and longer recovery times compared to children who sustain injury in later childhood or adolescence.7–9 The immature brain is particularly vulnerable to injury,10 likely due to the ongoing maturation of cognitive functions, which parallel the specialization and differentiation of higher brain regions.11 Social functioning, or the use of social skills to interact with others in a social context, emerges gradually during childhood and is consolidated during adolescence. The protracted nature of this maturation means that any disruption to this process by injury during childhood may impair future progress and the acquisition of new social skills.4,12 Supporting this hypothesis, Greenham and colleagues12 found that early brain insult before the age of 2 years was associated with a more significant long-term social impairment compared to older children. Two other studies comparing children who sustained TBI either before or after 12 years of age similarly found that a younger age of insult resulted in poorer social development and social problem-solving skills.13,14
In addition to a young age at insult, the degree of social impairment after childhood TBI has been attributed to a number of other complex, interacting risk factors, including injury severity, pathology to frontal or corpus callosal regions, and environmental factors such as pre-existing social disadvantage.4,9,15 The prefrontal cortex, amygdala, and hippocampus have all been implicated in social processing, leading to the current view that social skills are likely mediated by an integrated neural network, many components of which are susceptible to disruption by brain injury.16
Despite the clinical consequences of social dysfunction after childhood TBI, the majority of experimental TBI studies that assess animal behavior after injury primarily focus on physical and cognitive outcomes. Aberrant social behaviors seen in TBI patients, such as social isolation and poor social skills, can be evaluated in rodents by quantification of social investigation or by the use of social choice tasks, in which mice are presented with a choice between spending time in the proximity of other mice or remaining alone. Few TBI studies have incorporated social behavior as an outcome measure, primarily in rats, and assessed within 2 weeks of injury. Shultz and colleagues17,18 examined the distance between paired rats during free exploration, a measure which is reportedly not affected by a single or repeated mild fluid percussion injury in adult rats. Another group of investigators, who scored social and passive interactions between pairs of adult rats after diffuse impact acceleration injury, demonstrated a significant reduction in TBI animals,19 interpreted by the authors to reflect anxiety- and depression-like behaviors.20 However, social behavior paradigms are not yet standardized, such that test execution and quantification methods differ considerably between laboratories, potentially confounding interpretations.18,19,21 Optimization of such measures should be encouraged, as manipulations or therapeutics that may either directly or indirectly modify social behavior outcomes have the potential to significantly improve quality of life for TBI patients.
The current study thus aimed to evaluate social behaviors in mice after experimental TBI to the immature brain, using methods both existing and new to the experimental TBI field. We have previously characterized a model of frontoparietal controlled cortical impact to mice at post-natal day (p)21, an age approximating a 2- to 3-year-old child.22,23 These mice display a hyperactive, anxiolytic phenotype, with delayed-onset impairments in spatial memory and learning,24,25 consistent with common neurobehavioral sequelae seen in brain-injured children.26–29 These behavioral deficits coincide with progressive neuronal loss and atrophy in the cortex, ipsilateral hippocampus, and dorsal thalamus.24,25 Using a battery of tasks to evaluate social interactions, we here compared injured and sham-operated mice at adolescence and adulthood following TBI at p21, providing evidence that clinically relevant social behavior deficits emerge with increasing age after injury to the immature brain.
Methods
Animals
Male C57Bl/6J mouse pups at p17 with an accompanying lactating mother were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in the Laboratory Animal Resource Center at the University of California, San Francisco. Standard rodent chow and tap water were available ad libitum, and the room was maintained on a 12 h light/dark cycle at approximately 20°C. All surgical and behavioral procedures were conducted randomized and blinded, were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and approved by the UCSF Institutional Animal Care and Use Committee.
Controlled cortical impact model
At p21, pups were weaned and anesthetized with 1.25% 2,2,2-tribromoethanol in isotonic saline i.p. at 0.02 mL/g body weight. Mice from each litter were randomly allocated for either TBI or sham operation. After positioning the head in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), the skull was exposed by a midline skin incision and reflection of the soft tissues. Using a micro-drill, a 5.0-mm-diameter circular craniotomy was made midway between bregma and lambda, with the medial edge 0.5 mm lateral to the midline. TBI mice (n=9) were then positioned beneath the injury device (Custom Design and Fabrication, eCCI-6.3), still within the stereotaxic frame, and subjected to a controlled cortical impact injury.24,30 The injury was generated at 4.5 m/sec velocity, 1.7 mm depth of penetration, for a sustained depression of 150 msec, using a 3.0 mm convex impactor tip. Mice were maintained on a water-circulating heating pad throughout surgery and recovery. Following impact, the scalp was closed with sutures and each animal administered 1.0 mL of isotonic saline subcutaneously to prevent postoperative dehydration. Sham-operated mice (n=9) underwent identical surgical procedures, including craniotomy, without receiving the cortical impact. Following surgery, mice were group-housed until the beginning of behavioral testing (4–5/cage, a mix of TBI and sham mice) and provided with standard bedding supplemented with a cardboard dome house and nestlet square. Weights were monitored weekly post-surgery.
Behavioral tasks
A series of behavioral assessments were performed in succession from least stressful (involving indirect contact for a short duration) to most stressful (direct contact for a longer time period). The order was thus (1) partition; (2) tube dominance; (3) three-chamber; and (4) resident-intruder (Fig. 1). Testing was conducted within the Neurobehavioral Core for Rehabilitation Research at UCSF, and mice were housed within the core throughout testing. Mice were placed into individual clean cages in a designated procedure room 24 h prior to commencement of testing for overnight isolation and habituation. Testing was first conducted during adolescence (p35-42; ∼20 g weight). Mice were then returned to group-housing (the same social group as prior to testing) and isolated again 24 h prior to a second round of testing during early adulthood (p60-70; ∼30 g weight). “Stimulus” animals were naïve male C57Bl/6J mice, purchased at the same time from Jackson Laboratory and group-housed on the opposite side of the same room. Each “test” mouse (TBI or sham) encountered a novel stimulus mouse on one occasion only. Stimulus mice (n=5) were used repeatedly, but were rotated to ensure rest periods between encounters. Testing was conducted blinded to surgical treatment, between 9:00 am and 6:00 pm daily, in dimmed lighting with a background of constant white noise. The apparatus was cleaned between mice with 10% bleach followed by 3% acetic acid to eliminate odors.
FIG. 1.
Behavioral assays used to evaluate social function in mice following TBI or sham operation. The resident-intruder task (A) and partition test (B) were used to examine social investigation. The tube dominance task (C) involves the test and stimulus mouse being released simultaneously from opposite ends of a plexiglass tube. The three-chamber task is shown (E,F) from side and overhead views, respectively. Stage 2 is shown here, in which the test mouse can choose to interact with either a novel stimulus mouse or an empty metal cup. (D) Typical investigation of the stimulus mouse in this task.
The partition test
In this task, a perforated partition containing 1×1 cm square holes was placed in the center of a standard empty mouse cage (16×30 cm). The stimulus mouse was placed on one side of the partition and the test mouse on the other, and the cage topped with a clear plexiglass sheet to begin the recorded trial period (5 min).31,32 The amount of time that the test mouse spent at the partition (nose-touching, sniffing, climbing) was recorded across five consecutive 1-min time-bins as a measure of investigative behavior. This was recorded live using a stopwatch, as well as by subsequent independent quantification of video recordings.
The tube dominance task
In this task, test and stimulus mice were released simultaneously into opposite ends of a 30 cm long clear plexiglass tube raised 2.0 cm above the bench surface. The tube diameter (3.2 cm) aimed to ensure that mice could not turn around or move past each other. Typically, one mouse will exert dominance and force the other to back out of the tube, which is thought to reflect a tendency for aggression.33,34 The trial concluded when one mouse placed his forepaws out of the tube. Two blinded observers recorded the result live, with the mouse remaining in the tube designated the winner (score of 1) and the retreating, submissive mouse designated the loser (score of 0). Trials in which no resolution was made within 2 min were ceased and a score of 0.5 was recorded for the test mouse. Each test mouse underwent three trials (paired with the same stimulus mouse), with a 1 h inter-trial interval. Trials were video-recorded for later quantification of trial duration (latency to exit tube). While this assay was conducted in both adolescent and adult mice, we found that the tube diameter was too wide for adolescent mice. As such, test and stimulus mice could pass one another or turn around in the tube. As this occurred on approximately 75% of all trials during adolescence, insufficient data were obtained to form any conclusions at this age; thus presented results are for the adult cohort only.
The three-chamber task
The three-chamber paradigm was established by Crawley and colleagues35–37 and has been used to date primarily to examine mouse sociability in models of autism and psychiatric disorders. The design allows for the evaluation of two distinct aspects of social behavior—social affiliation (sociability) and social recognition memory. A plexiglass three-chambered box was custom-built (Tap Plastics, San Francisco, CA) as previously described.37–39 Doorways in the two dividing walls had sliding covers to control access to the outer-side chambers, if desired. The three consecutive stages of the test lasted for 10 min each, following overnight acclimatization in the testing room.
Stage 1 involved habituation of the test mouse in the apparatus, with free exploration allowed through all three chambers. This stage also allows for detection of any innate side preferences. Following the 10-min period, the test mouse was gently encouraged into the central chamber and confined there briefly by closing the side chamber doors. For stage 2, a custom-made, inverted stainless-steel barred cup (6.5×15 cm) was placed in one of the side chambers. A novel stimulus mouse, previously habituated to the chamber, was placed into an identical inverted cup in the other side chamber. The cup bars (8 mm apart) were designed to be sufficiently wide apart to allow nose-to-nose contact while minimizing the possibility of aggressive interactions and limiting the initiation of interactions to the test mouse. Filled water bottles were placed on top of each inverted cup to prevent the test mouse from climbing on top. The side containing the novel object (empty cup) and stimulus mouse alternated between the left and right chambers across subjects. After both stimuli were positioned according to marks on the chamber floor, the side doors were simultaneously opened and the test mouse was allowed access to all three chambers for the 10-min session. During this stage, a preference for sociability was defined as the test mouse spending more time in the side chamber with the novel mouse in it compared to the chamber with the novel object alone. Movement of the test mouse was tracked by an overhead mounted camera connected to Noldus EthoVision software, which allowed for determination of the time spent in each chamber, as well as the distance travelled. At completion of stage 2, the test mouse was again briefly confined to the central chamber, and a second novel stimulus mouse was placed into the empty, inverted cup for stage 3. Here, a preference for social novelty, an indicator of social memory and recognition, was defined as the test mouse spending more time in the chamber with the second stimulus mouse compared to the first (now familiar) stimulus mouse. Data were expressed as time spent in each chamber (percent of total time).
The resident-intruder task
This task examines the social response of the test mouse to an intruding stimulus mouse that has entered his home cage. To begin, the lid and food containers were removed and the test mouse's home cage gently placed under the video camera. The cage was topped with a thin sheet of clear plexiglass for a 10-min acclimatization period. A stimulus mouse (identified by a small piece of tape on its rump) was then presented into the cage, and behaviors were video-recorded for 5 min.40 Behaviors of the test mouse were scored blinded from videos using Stopwatch+© (Center for Behavioral Neuroscience, Georgia State University), and categorized based on published literature,41,42 as follows: head/torso sniff, ano-genital sniff, following (defined as test mouse following stimulus mouse nose-to-tail); mutual circling, biting/fighting, and grooming (test mouse grooming the stimulus, or vice-versa). Both the duration and number of occurrences of behaviors were recorded. In addition, the total investigative time was calculated (sum of total time for head/torso sniff, ano-genital sniff, following, mutual circling, and grooming behaviors).
The buried food task
Social behaviors in mice are predominantly mediated by olfactory cues, such that anosmic mice show abnormal socially interactive and aggressive behavior.43 The buried food task is simply designed to assess the ability of mice to detect a hidden volatile odor.44 This task was performed once only, following completion of the adult testing period. For 2 consecutive days prior to the test, a food stimulus (Honey Nut Cheerios, General Mills, MN) was placed in the test mouse's home cage to establish odor familiarization. All mice at least partly consumed the food stimulus during this time, indicating that it was palatable. All food sources were removed from the cages for overnight fasting in the procedure room the evening prior to the test day, with water available ad libitum. On the test day, each mouse was placed into a clean cage containing a 3.0 cm deep layer of clean bedding for a 5-min acclimatization period. The mouse was then transferred briefly to a temporary holding cage while the food stimulus was buried and hidden in a random corner of the test cage (the location alternated across subjects). The mouse was then reintroduced into the testing cage and a blinded observer recorded the latency to find the food source, defined as uncovering and beginning to eat or holding the food with its forepaws.
Statistical analysis
Statistics were performed using Prism GraphPad V5.0 (San Diego, CA), with a significance level of p<0.05. Repeated measures (RM) ANOVAs were used, with factors of injury (TBI or sham) and time, trial number or chamber, for the partition, tube-dominance, and three-chamber tasks, respectively. Two-way ANOVAs were used to analyze sniff duration in the resident-intruder test, with factors of injury and sniff location. Non-sniff behaviors in the resident-intruder task (i.e., following, circling, grooming) and latency in the buried food task were analyzed by unpaired t-tests between groups. Individual groups were compared using Bonferroni's post-hoc tests as appropriate following a significant ANOVA result. Post-hoc analyses are stated as p<0.05, p<0.01, or p<0.001 and annotated graphically.
Results
Brain-injured mice show reduced social investigation at adulthood but not during adolescence
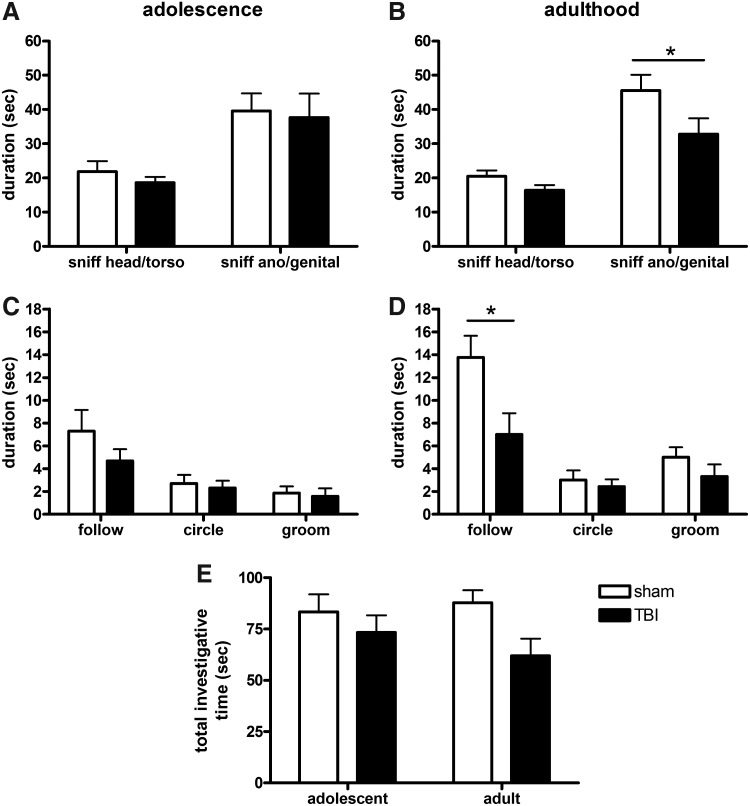
The resident-intruder task was used to examine social investigation and interactions by introducing an unfamiliar stimulus mouse into the home cage territory of the test mouse. Over a recorded 5-min period, we quantified the duration and number of occurrences of key interactive behavioral events performed by the test mouse. Of note, no overt signs of aggression (i.e., biting or fighting) were observed in any mice during this task. During adolescence, two-way ANOVA analysis revealed that test mice spent significantly more time sniffing the ano-genital region of the stimulus mouse compared to the head and torso (effect of region, F1,32=15.36, p=0.0004; Fig. 2A). This did not differ between sham and TBI mice (no effect of injury, F1,32=0.30, p=0.5860), indicating that TBI mice during adolescence show a normal level of interest in an unfamiliar mouse of the same strain.
FIG. 2.
TBI and sham mice exhibited normal social interest in the resident-intruder task during adolescence, with comparable time spent sniffing the head/torso and ano-genital region of the stimulus mouse (A). However, in adulthood (B), TBI mice spent less time sniffing the ano-genital region compared to sham mice (*p<0.05, post-hoc). Time involved in other interactive behaviors including following, circling, and grooming were also quantified, and were similar in both TBI and sham mice during adolescence (C). However, TBI mice spent less time following the stimulus mouse during adulthood (D; *p<0.05; t-test). When all observed interactive behaviors were combined to calculate the total investigative time (E), this measure was significantly lower in TBI mice compared to sham (two-way ANOVA, effect of injury, p=0.0311).
During adulthood, test mice again spent more time sniffing the ano-genital region of stimulus mice compared to the head and torso (effect of region, F1,32=36.11, p<0.0001; Fig. 2B). In contrast to adolescence, there was also a significant effect of injury (F1,32=5.95, p=0.0209), indicating that TBI mice showed reduced interactions with the stimulus compared to sham mice. This is most evident in the TBI group that spent significantly less time sniffing the ano-genital region compared to sham mice (post-hoc, p<0.05).
Other interactive behaviors between the test and stimulus mice were quantified separately (Fig. 2C). During adolescence, TBI and sham mice spent comparable time following (t1,16=1.24, p=0.2348), circling (t1,16=0.38, p=0.7055), and grooming (t1,16=0.32, p=0.7569) the stimulus mouse. At adulthood, the time spent circling and grooming was also equivalent between the TBI and sham groups (t1,16=0.54, p=0.5951 and t1,16=1.22, p=0.2428, respectively). However, following of the stimulus mouse was significantly lower in adult TBI mice compared to sham (t1,16=2.54, p=0.0224; Fig. 2D), indicating a reduction in social investigation.
We subsequently calculated the total cumulative time that test mice spent investigating stimulus mice, with a comparison between adolescence and adulthood (total investigative time=duration sniffing head/torso, ano-genital, following, mutual circling, and grooming, combined). Two-way ANOVA revealed that TBI mice engaged in significantly less investigation of the stimulus mouse compared to sham mice (effect of injury, F1.32=5.10, p=0.0311). However, the total investigation times during adolescence compared to adulthood was similar (no effect of age, F1,32=0.19, p=0.6651; Fig. 2E). Thus, while total investigation was reduced in response to TBI, there was no age-related effect of this metric, likely due to a more prominent TBI-dependent effect in the adult cohort, which is masked by the statistical comparison across the age groups.
As an alternative method to determine overall social investigation levels we conducted a partition task, which physically separated the test and stimulus mice by a perforated cage divider, quantified as the investigative time by the test mouse at the partition over a 5-min session. During adolescence, test mice typically spent 25–30 sec out of every 60 sec at the partition (Fig. 3A), with two-way RM ANOVA revealing similar investigative times in both TBI and sham mice (no effect of injury, F1,64=0.10, p=0.7562). Contrary to expectations that most mice would show increasing familiarity and thus a decrease in investigation over time, there was no significant change across the 5-min time course (no effect of time, F4,64=2.21, p=0.0780). Statistically, there was a significant interaction between injury and time (F4,64=3.21, p=0.0182); however, post-hoc analyses did not reveal any differences between sham and TBI during any of the 1 min time-bins.
FIG. 3.
Similar investigative times were shown by TBI and sham mice during the partition task at both adolescence (A) and adulthood (B), differing only in the final min 5 time-bin of testing at adulthood (*p<0.05, post-hoc). The cumulative (total) investigative time was also quantified for each group (C). Independent of injury, mice at adulthood spent significantly less time at the partition compared to mice at adolescence (two-way ANOVA effect of age, p=0.0018).
Similarly, at adulthood, there was no overall difference by two-way RM ANOVA in investigative time between sham and TBI mice (no effect of injury, F1,64=0.64, p=0.4347), nor was there an effect of time (F4,64=0.42, p=0.7919; Fig. 3B). However, there was again a significant interaction between time and injury (F4,64=3.12, p=0.0213), with TBI mice spending significantly less time at the partition compared to sham mice during minute 5 only (post-hoc, p<0.05). We next pooled the total time period (5 min) to examine the cumulative investigative time at both adolescence and adulthood by two-way ANOVA. Mice at adulthood spent significantly less time investigating at the partition compared to at adolescence (effect of age, F1,31=11.67, p=0.0018), which was independent of injury (no effect of injury, F1,31=0.32, p=0.5729; Fig. 3C).
Brain-injured mice show deficits in sociability and social recognition in the three-chamber paradigm during adulthood but not adolescence
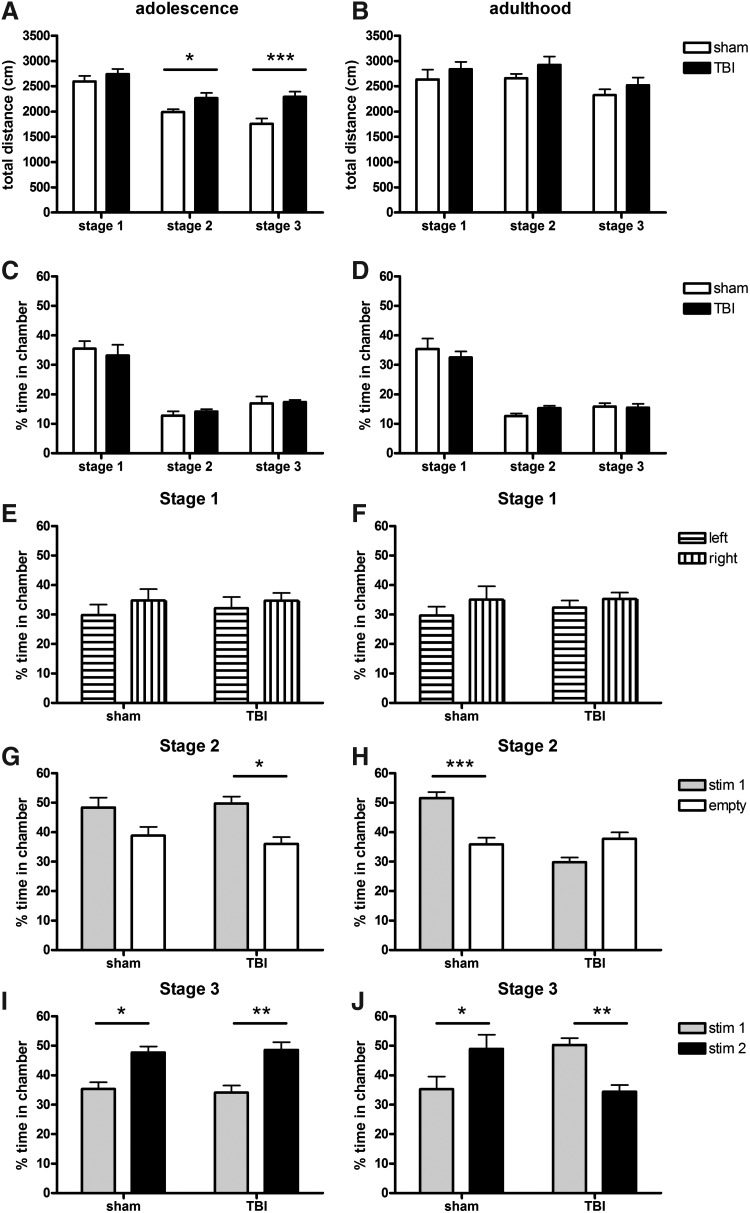
The three-stage, three-chamber paradigm allows for automated quantification of a test animals' preference for sociability and social novelty. Following habituation (stage 1), stage 2 allowed the test mouse to explore an empty cup in one outer chamber compared to a novel stimulus mouse restrained to another cup in the other outer chamber to determine any sociability preferences. Stage 3, in which a second novel stimulus mouse was introduced to the previously empty cup, evaluated a preference for social novelty. Firstly, we examined the total distance moved by test mice during each stage by two-way RM ANOVA, as a measure of general activity levels. Distance moved was strongly dependent upon the presence of stimulus mice, being significantly lower during stages 2 and 3 compared to stage 1 during both adolescence (effect of stage, F2,24=16.76, p<0.0001; Fig. 4A) and adulthood (F2,24=3.65, p=0.0340; Fig. 4B). Interestingly, we also found an increase in the total distance moved by adolescent TBI mice compared to sham (effect of injury, F1,24=29.47, p<0.0001), with a non-significant trend in the same direction during adulthood (F1,24=3.40, p=0.0719). This likely reflects the hyperactivity we have previously reported after brain injury in young mice25 and parallels what has been reported in brain-injured children.45,46
FIG. 4.
Adult mice showed deficits in sociability and social novelty preference on the three-chamber task. The left column (A,C,E,G,I) depicts adolescent data; the right column (B,D,F,H,J) depicts adult data. The total distance moved, an indication of activity level, tended to be elevated in TBI mice across the task stages (A,B; **p<0.01 and ***p<0.001, two-way RM ANOVA post-hoc). Time spent in the central chamber (C,D) was similar at adolescence and adulthood and comparable between TBI and sham mice. Time spent in each of the empty outer chambers during stage 1 (habituation) was also similar in TBI and sham mice at both ages (E,F). During stage 2, adolescent mice showed a preference for the chamber containing the stimulus mouse compared to the empty cup (G; *p<0.05). Sham mice at adulthood also displayed an interest in the stimulus mouse (H; ***p<0.001); however, this preference was absent in TBI mice. Stage 3 involved the addition of a second stimulus mouse into the previously empty cup. At adolescence (I), both TBI and sham mice show a strong preference for the newest stimulus mouse compared to the first mouse (*p<0.05, **p<0.01). At adulthood (J), while this preference persisted in sham mice (*p<0.05), TBI mice showed the opposite response, with a preference for the now-familiar first stimulus mouse (**p<0.01).
Time spent in the central chamber was separately quantified for all three test stages as a control measure. As expected, test mice spent approximately one third of their time in the central chamber during stage 1, and significantly less time here when stimulus mice were present in the other chambers during stages 2 and 3 (two-way RM ANOVA effect of stage, F2,24=60.45, p<0.0001 and F2,24=70.35, p<0.0001 for adolescence and adulthood, respectively). This was consistent between TBI and sham groups during both adolescence (no effect of injury, F1,24=0.01, p=0.9308; Fig. 4C) and adulthood (F1,24=0.01, p=0.9195; Fig. 4D). As expected, no differences were observed between time spent in each of the empty outer chambers during stage 1 (not significant for both adolescence and adulthood), indicating a lack of innate preference for either side (Fig. 4E,F).
In stage 2, adolescent sham and TBI mice showed a comparable preference for sociability, as evident by spending significantly more time in the chamber with stimulus mouse #1 compared to time spent in the chamber containing the empty cup (two-way RM ANOVA effect of chamber, F1,16=9.29, p=0.0077; no effect of injury, F1,16=0.75, p=0.3981; Fig. 4G). When tested during adulthood, there was no overall effect of chamber side (F1,16=2.19, p=0.1594), reflecting differential responses by sham and TBI mice (overall effect of injury F1,16=69.13, p<0.0001) and a significant interaction between chamber side and injury (F1,16=20.52, p=0.0004). Sham mice showed a strong preference for sociability consistent with that seen during adolescence (post-hoc, p<0.01; Fig. 4H). Adult TBI mice, however, showed the opposite response, typically spending more time in the chamber containing the empty cup compared to the chamber containing the stimulus mouse (post-hoc, p>0.05). This finding indicates that adult TBI mice not only lack a typical preference for sociability, but in fact appear to prefer avoiding social contact in this scenario.
During the subsequent stage 3, stimulus mouse #1 was left untouched in the same side chamber position, and a second novel mouse (stimulus mouse #2) was placed into the empty cup in the opposite side chamber. Adolescent mice spent significantly more time in the chamber containing the new stimulus mouse #2 compared to the now-familiar stimulus mouse #1 (overall effect of chamber, F1,16=19.24, p<0.0001), indicating a strong preference for social novelty (Fig. 4I). This effect was similar in both sham and TBI mice (no effect of injury, F1,16=0.02, p=0.7331). When tested during adulthood, sham mice also showed a similar preference for stimulus mouse #2 compared to #1 (no overall effect of chamber, F1,16=0.11, p=0.7417; no effect of injury, F1,16=0.00, p=0.9480; overall interaction F1,16=18.12, p=0.0002; post-hoc, p<0.05; Fig. 4J). In contrast, adult TBI mice spent significantly more time in the chamber containing stimulus mouse #1 compared to stimulus mouse #2 (post-hoc, p<0.01), indicating a strong preference for familiarity.
In summary, while showing normal social interest during adolescence, deficits in both sociability (stage 2) and social novelty preference (stage 3) are evident in TBI mice at adulthood.
Brain-injured mice exhibit increased tendencies towards dominance at adulthood
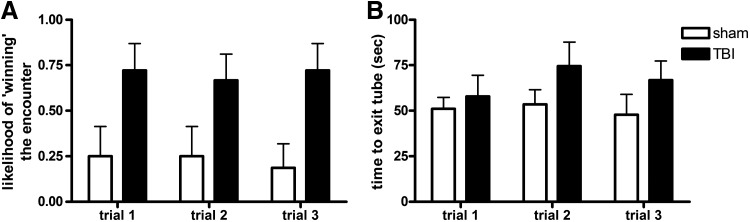
The tube dominance task involves an encounter between the test mouse and a novel stimulus mouse confined within a tube, ensuring a confrontation between animals that typically results in one mouse exerting dominance (score of 1) and forcing the other mouse to retreat from the tube (score of 0). During adulthood, no mice were seen to turn around or cross over each other during the task, so all trials were included in analysis by two-way RM ANOVA. The likelihood of the test mouse winning its encounter was found to be dependent on injury (effect of injury, F1,30=5.18, p=0.0380), indicating that TBI mice were more likely to exert dominance over the stimulus mouse, winning approximately 75% of their encounters, compared to sham mice who won only 25% of the time (Fig. 5A). Interestingly, this relationship between test and stimulus mouse appeared to generally be established during the first encounter, with 88% of encounters resulting in a consistent victor across all three trials (no overall effect of trial, F2,30=0.51, p=0.6073). The time until the first (submissive) mouse exited the tube was also quantified for all encounters, with the hypothesis that time would decrease across subsequent trials after the dominant-submissive relationship was established by the interacting pair. However, this parameter was not affected by either injury (F1,30=1.53, p=0.2345) or trial number (F2,30=0.97, p=0.3920; Fig. 5B).
FIG. 5.
Adult brain-injured mice displayed increased dominance in the tube dominance task. The chance of a test mouse winning its encounter with a stimulus mouse (A) was greater for TBI mice compared to sham mice (two-way RM ANOVA effect of injury, p=0.0380). The trial length, or time taken for the submissive mouse to exit the tube (B), was similar across all trials and independent of injury.
Brain-injured mice have normal olfactory function
Olfactory cues play a key role in mouse social behaviors.43 The buried food task was employed to quantify the latency to locate a hidden, odor-producing food source to determine whether this model of TBI induced any deficit in olfactory function, which may impact social behaviors. This was performed once only per mouse, upon completion of all other tests during adulthood, and after a period of familiarization with the food odor. We found no significant difference in the latency to find the food source in TBI compared to sham mice (t1,15=1.79, p=0.0926), indicating comparable olfactory function.
Discussion
Problems with social behavior are common after TBI in both adults and children, with widespread consequences on academic achievement, relationships with peers, and reintegration into society.4 The development of normal social behaviors is protracted through childhood and adolescence, suggesting that TBI at a young age is likely to adversely affect the acquisition and establishment of social skills. Here we have demonstrated that mice that receive TBI at a young age show deficits in social interactions, which emerge across development to adulthood. Interestingly, this trajectory parallels the development of other cognitive deficits previously characterized in this model25 and seen in brain-injured children,26,27 including spatial memory and learning problems, hyperactivity, and elevated anxiety. The observed changes in social behavior were not attributed to a simple deficit in olfaction, as TBI and sham mice shared a similar ability to rapidly detect the presence of a hidden food source.
To the best of our knowledge, this is the first application of the three-chamber task in the context of an acquired brain injury. Originally established and now extensively used for the characterization of autistic phenotypes in mouse models, our findings demonstrate this paradigm's applicability for quantifying social dysfunction after TBI. In fact, the time spent in each chamber by sham-operated mice is highly consistent with that of published literature for naïve C57Bl/6 mice,37,39 indicating a high degree of reproducibility between laboratories and control mice with this set-up. In comparison to open field or resident-intruder paradigms of social interactions, the three-chamber task is advantageous in that it limits initiation of social contact to the test mouse only by physically confining the stimulus mice. A potential limitation of this task is its time-consuming nature (3×10 min stages+transitions and post-test cleaning=∼40 min/mouse). However, it would be possible to establish parallel set-ups to enable simultaneous testing of several mice side-by-side. Using the three-chamber task, we have demonstrated that mice that received TBI at a young age show a deficit in general sociability during adulthood (test stage 2), while showing normal sociability when tested earlier during adolescence. Further, adult TBI mice lack a preference for proximity to an unfamiliar novel mouse over a now-familiar one (stage 3), which likely reflects a deficit in social recognition or social memory.35–37 This finding is consistent with work by Koliatsos and colleagues,21 who detected a social novelty deficit in adult mice after experimental blast injury. In their paradigm, TBI mice showed decreased interest in a novel stimulus mouse following several previous encounters with a familiar mouse.
In line with the three-chamber task findings, TBI mice also demonstrated reduced social interactions in the resident-intruder paradigm during adulthood but not adolescence. An overall difference in total investigative time in this task was primarily attributed to reduced ano-genital sniffing and following behavior in adult TBI mice compared to sham mice, when interacting with a novel intruding stimulus mouse. This is consistent with findings in adult rats after diffuse experimental TBI, in which unfamiliar pairs of brain-injured rats showed decreased social and passive interactions in a 5-min open-field encounter compared to pairs of sham-operated controls.19
Not all tests revealed age-related differences in behavior after TBI, as brain-injured and sham mice showed similar investigation times in the partition test across the 5-min session. This finding could be interpreted as a lack of difference between the groups; however, a clear deficit in social interactions was detected between TBI and sham mice on two other measures (resident-intruder and three-chamber). Therefore we argue that the partition task lacks sensitivity as a measure of social interaction. In addition, while we expected to see a gradual decrease in social interest over time due to increasing familiarity with the stimulus mouse, both TBI and sham mice instead showed a sustained high level of investigation at the partition at both ages. It is possible that a longer period of testing would thus yield an injury effect as detected by the three-chamber or resident-intruder paradigm. An additional confounding factor in this task was the partition itself, as interest in the partition could not be discriminated from interest in the stimulus mouse. This could be circumvented in future applications by increasing the habituation time to ensure familiarization of the test mouse with the partition prior to introduction of the stimulus mouse.
A key finding from this study was that adult brain-injured mice had an increased likelihood of exerting dominance in the tube dominance task compared to sham-operated controls, suggestive of a more aggressive phenotype.33,34 There was a high level of consistency between trials in which test mice encountered the same stimulus mouse, suggesting that the dominant-submissive relationship was established during the first interaction between the pair. This phenomenon of winning affecting future behavior has been reported previously in rodents and other animals, in which individuals that win aggressive confrontations are more likely to dominate in subsequent agonistic encounters.47,48 Contrary to expectation, however, encounters in the tube dominance task were not resolved more rapidly with increasing experience and familiarity, as a similar latency to exit the tube was found across all three trials, independent of injury or a win/loss for the test mouse.
Clinically, aggressive behaviors have been reported in both children and adult survivors of TBI. One study estimated the prevalence of aggression after first-time TBI in adults to be approximately 30%, which was associated with reports of poorer social functioning.49 Similarly, in a cohort of 23 children aged 7–13 years with severe TBI, 30% of parents reported aggressiveness as a prominent behavioral problem.50 Both anecdotal evidence and clinical studies indicate that aggression after a TBI is predominantly impulsive, verbal abuse rather than physical violence, and it is thought that the injury likely exacerbates or disinhibits pre-existing aggressive tendencies rather than eliciting an aggressive personality change per se.51,52 Such behaviors can pose a significant challenge during rehabilitation and negatively impact reintegration into the community and workforce.51
Our results indicate that injury to the mouse brain at an early age increases aggressive tendencies by adulthood, and are the first to show aggression in mice after TBI. The direct relevance of this finding to human TBI is likely to be controversial; while mice typically use aggression to exert dominance, it is unclear whether performance in the tube task is directly related to aggression.33,34 Interestingly, we did not see any overt signs of aggression (such as biting, clawing, or tail rattling) in either TBI or sham mice in any tests involving direct contact between mice. Consistent with previous findings, dominance in the tube task appeared to be enforced primarily by pushing the retreating mouse with head and torso.32 Based on this evidence, it has been suggested that dominance exerted in this task may in fact reflect a decrease in social anxiety.32 However, taking into account that TBI mice in the current study show reduced sociability in the resident-intruder and three-chamber tests, and we have previously demonstrated a hyper-anxiolytic phenotype with this injury model,25 we surmise that TBI mice in fact exhibit increased social anxiety. We therefore reason that dominance exerted by TBI mice in this task is unlikely to result from changes in social anxiety. Additional studies are needed to confirm and extend these findings. Future, more extensive testing for aggression and social dominance after TBI in rodents may include assays of agonistic behavior, urine marking, and ultrasonic vocalizations.34
Overall our study profiles age-related social deficits after pediatric TBI, a finding that parallels clinical evidence. We submit that carefully controlled metrics of social profiling should be considered when testing novel therapeutics aimed at improving functional and cognitive outcomes after TBI. Social behaviors are quite sensitive to numerous known and unknown factors, including housing and experimental conditions, handling, previous life experiences, and social isolation.38,41,53 As such, the evaluation of social parameters in experimental TBI studies requires meticulous control and evaluation of many subtle factors that may influence mouse behaviors and interfere with assessments.37 In addition, the choice of mouse strain is of critical importance. C57Bl/6J mice were used in the current study as they consistently show high sociability across multiple laboratories, as do FVB/NJ mice. In contrast, low sociability has been reported with BALB/cJ, C58/J, and 129S1/SvImJ strains, such that use of these mice may negatively impact the usefulness of social behavior outcome measures.37,54–56
While retesting the same group of animals during adolescence and at adulthood allows for evaluation across neurodevelopmental stages, this design could be a limitation of the current study. Retesting is generally discouraged in behavioral tasks, due to the risk that early life events could potentially shape behavioral phenotypes later in life, particularly for stress-inducing behavioral tasks that feature a learning component, such as the Morris water maze.57,58 However, it has been reported that social behavior tasks can be successfully repeated, provided that testing is separated by several weeks.37–39 The three-chamber task in particular, although longer in duration compared to other tasks, is thought to induce only minor stress in mice due to the large chamber size, which allows the test mouse to choose its proximity to the stimulus mice.59 Extensive characterization of different inbred and autism mouse models has also been conducted using a repeated study design, testing mice as juveniles and again as adults.60
It is also important to consider the limitations of modeling social behavior in mice, in terms of the direct relevance of this to the complex behaviors displayed by brain-injured children and adults. Negative social outcomes after TBI in humans are exacerbated by complex environmental factors, including socioeconomic status, rehabilitation access, and family psychopathology,5,61,62 factors that are difficult or impossible to model and explore in an experimental TBI setting.
The exact brain regions responsible for social behavior deficits after childhood TBI are still unclear and worthy of further investigation. The corpus callosum has been implicated in normal development of social function, as well as the disruption of normal behaviors by injury. Volumetric studies of patients after pediatric TBI have demonstrated atrophy, particularly in the posterior body, genu, and splenium of the corpus callosum.63–65 Further, individuals who sustained a severe TBI in childhood reportedly show reduced corpus callosum volumes when measured 10 years post-injury, with size significantly correlated with social skills, suggesting that callosal atrophy may be related to poorer social outcomes.6 Most recently, an evaluation of social skills by parental ratings in children who sustained TBI prior to 7 years of age similarly found an association between poorer social function and decreased corpus callosum surface area.9 In addition, the hippocampus proper and parahippocampal regions, including the fimbria, subiculum, and medial septum, have been implicated in the processing of social recognition memory, as evidenced by several focal lesion studies aiming to elucidate region-specific contributions.66–68 In agreement with these studies, we see considerable hippocampal and corpus callosal damage in this experimental TBI model. Although anatomical damage was not quantified in the current study, such structural changes may contribute to the observed deficits in sociability and social recognition in brain-injured mice.
Other regions commonly implicated in normal social functioning include the prefrontal cortex, temporo-parietal junction, cingulate cortex, insula, and amygdala, as well as their connecting pathways.16,69 In brain-injured adults, lesion location has been shown to contribute to social outcome, with injury to the frontal lobes and right hemisphere in particular being associated with behavioral deficits.12 Focal injury to medial temporal regions in neonatal monkeys70 and ventromedial frontal lesions in children71,72 have also been linked to an increased risk of long-term social impairment. In contrast, a recent study found that lesion location and laterality were not predictive of social outcome after brain insult in young children,12 suggesting that there is unlikely to be a direct relationship between lesion location and social behaviors in the pediatric brain. Prefrontal brain regions undergo a period of rapid development in infancy and preschool years, but do not reach full maturity until mid-to-late adolescence.73 Normal maturation of the prefrontal cortex is thought to be dependent on the development of extra-frontal brain regions, an idea supported by animal studies demonstrating that medial temporal damage early in life can permanently alter the structure and function of prefrontal regions.74 It is therefore probable that the integrity of the entire brain may be necessary for the development of social processes in childhood. As such, an early brain injury, regardless of the site of pathology, may be detrimental to the development of normal social function. Our findings are consistent with this hypothesis, as we see social behavior deficits despite a lack of pathology in the frontal cortex in this model.
Conclusion
Persistent problems with social functioning are common after TBI in both adults and children, with widespread consequences on academic achievement, relationships with peers, and reintegration into society. The development of normal social behaviors is protracted through childhood and adolescence, suggesting that TBI at a young age is likely to adversely affect the acquisition and establishment of social skills. Here we have demonstrated that mice that receive TBI at a young age show reduced social interaction and increased aggressive tendencies that emerge across development to adulthood. This trajectory parallels the development of other cognitive deficits previously characterized in this model and seen in brain-injured children, including spatial memory and learning problems, hyperactivity, and elevated anxiety. This is consistent with the hypothesis that the full extent of deficits may not be apparent until the associated skills reach maturity,75,76 and that poor long-term outcomes may result from a failure to acquire new cognitive and psychosocial skills in order to meet new developmental demands.25 In line with this reasoning, monkeys who received neonatal medial temporal lesions show social and emotional disturbances and aberrant behaviors when tested at 2 and 6 months of age77; however, this social dysfunction was even more pronounced when subjects were reassessed at adulthood (6–8 years), and was more severe compared to monkeys who received the same lesions during adulthood.70 Future studies are required to elucidate the extent to which the emergence and severity of social dysfunction are dependent on the age at the time of insult.
Despite potential limitations in extrapolating animal social behavior to the clinical scenario, the identification of social deficits in animal models of TBI paves the way for the elucidation of underlying cellular and genetic mechanisms that may be targeted to improve long-term psychosocial outcomes. Future studies examining social behaviors relative to injury severity and advanced neuroimaging correlates after TBI are warranted to determine the neuroanatomical basis underlying the development of social deficits following childhood TBI.
Acknowledgments
This study was supported by an NIH/NINDS R01 grant (NS050159) and a Core Exploratory Award from the UCSF Resource Allocation Program. The research was conducted in the Neurobehavioral Core for Rehabilitation Research at UCSF. B.D. Semple was also supported by a Sir Keith Murdoch Fellowship from the American Australian Association.
Author Disclosure Statement
No completing financial interests exist.
References
- 1.Anderson V. Godfrey C. Rosenfeld J.V. Catroppa C. 10 years outcome from childhood traumatic brain injury. Intl. J. Dev. Neurosci. 2012;30:217–224. doi: 10.1016/j.ijdevneu.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Andrews T.K. Rose R.F. Johnson D.A. Social and behavioral effects of traumatic brain injury in children. Brain Inj. 1998;12:133–138. doi: 10.1080/026990598122755. [DOI] [PubMed] [Google Scholar]
- 3.Levin H.S. Zhang L. Dennis M. Ewing-Cobbs L. Schachar R. Max J. Landis J.A. Roberson G. Scheibel R.S. Miller D.L. Hunter J.V. Psychosocial outcome of TBI in children with unilateral frontal lesions. J. Intl. Neuropsychol. Soc. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- 4.Rosema S. Crowe L. Anderson V. Social function in children and adolescents after traumatic brain injury: a systematic review 1989–2011. J. Neurotrauma. 2012 doi: 10.1089/neu.2011.2144. [DOI] [PubMed] [Google Scholar]
- 5.Yeates K.O. Swift E. Taylor H.G. Wade S.L. Drotar D. Stancin T. Minich N. Short- and long-term social outcomes following pediatric traumatic brain injury. J. Intl. Neuropsychol. Soc. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 6.Beauchamp M.H. Anderson V.A. Catroppa C. Maller J.J. Godfrey C. Rosenfeld J.V. Kean M. Implications of reduced callosal area for social skills after severe traumatic brain injury in children. J. Neurotrauma. 2009;26:1645–1654. doi: 10.1089/neu.2009.0916. [DOI] [PubMed] [Google Scholar]
- 7.Catroppa C. Anderson V. Morse S. Haritou F. Rosenfeld J. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) J. Ped. Psychol. 2008;33:707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- 8.Duval J. Braun C.M.J. Montour-Proulx I. Daigneault S. Rouleau I. Bgin J. Brain lesions and IQ: recovery versus decline depends on age of onset. J. Child Neurol. 2008;23:663–668. doi: 10.1177/0883073808314161. [DOI] [PubMed] [Google Scholar]
- 9.Ewing-Cobbs L. Prasad M.R. Swank P. Kramer L. Mendez D. Treble A. Payne C. Bachevalier J. Social communication in young children with traumatic brain injury: relations with corpus callosum morphometry. Intl. J. Dev. Neurosci. 2012;30:247–254. doi: 10.1016/j.ijdevneu.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferriero D.M. Miller S.P. Imaging selective vulnerability in the developing nervous system. J. Anat. 2010;217:429–435. doi: 10.1111/j.1469-7580.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson V. Catroppa C. Morse S. Haritou F. Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- 12.Greenham M. Spencer-Smith M.M. Anderson P.J. Coleman L. Anderson V.A. Social functioning in children with brain insult. Front. Hum. Neurosci. 2010;4:1–10. doi: 10.3389/fnhum.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donders J. Warschausky S. Neurobehavioral outcomes after early versus late childhood traumatic brain injury. J. Head Trauma Rehabil. 2007;22:296–302. doi: 10.1097/01.HTR.0000290974.01872.82. [DOI] [PubMed] [Google Scholar]
- 14.Hanten G. Wilde E.A. Menefee D.S. Li X. Lane S. Vasquez C. Chu Z. Ramos M.A. Yallampalli R. Swank P. Chapman S.B. Gamino J. Hunter J.V. Levin H.S. Correlates of social problem solving during the first year after traumatic brain injury in children. Neuropsychology. 2008;22:357–370. doi: 10.1037/0894-4105.22.3.357. [DOI] [PubMed] [Google Scholar]
- 15.Giza C.C. Kolb B. Harris N.G. Asarnow R.F. Prins M.L. Hitting a moving target: basic mechanisms of recovery from acquired developmental brain injury. Dev. Neurorehabil. 2009;12:255–268. doi: 10.3109/17518420903087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spikeman J.M. Timmerman M.E. Milders M.V. Veenstra W.S. van der Naalt J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J. Neurotrauma. 2012;29:101–111. doi: 10.1089/neu.2011.2084. [DOI] [PubMed] [Google Scholar]
- 17.Shultz S.R. Bao F. Omana V. Chiu C. Brown A. Cain D.P. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal of repeated concussion. J. Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- 18.Shultz S.R. MacFabe D.F. Foley K.A. Taylor R. Cain D.P. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav. Brain Res. 2011;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Pandey D.K. Yadav S.K. Mahesh R. Rajkumar R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 2009;205:436–442. doi: 10.1016/j.bbr.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 20.File S.E. Hyde J.R. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koliatsos V.E. Cernak I. Xu L. Song Y. Savonenko A. Crain B.J. Eberhart C.G. Frangakis C.E. Melnikova T. Kim H. Lee D. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- 22.Yager J.Y. Thornhill J.A. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci. Biobehav. Rev. 1997;21:167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- 23.Rice D. Barone S.J. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong W. Igarashi T. Ferriero D.M. Noble L.J. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp. Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- 25.Pullela R. Raber J. Pfankuch T. Ferriero D.M. Claus C.P. Koh S.-E. Yamauchi T. Rola R. Fike J.R. Noble-Haeusslein L.J. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev. Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- 26.Yeates K.O. Blumenstein E. Patterson C.M. Delis D.C. Verbal learning and memory following pediatric closed-head injury. Intl. J. Neuropsychol. Soc. 1995;1:78–87. doi: 10.1017/s1355617700000138. [DOI] [PubMed] [Google Scholar]
- 27.Levin H.S. Cognitive function outcomes after traumatic brain injury. Curr. Opin. Neurol. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Max J.E. Koele S.L. Castillo C.S. Lindgren S.D. Arndt S. Bokura H. Robin D.A. Smith W.L.J. Sato Y. Personality change disorder in children and adolescents following traumatic brain injury. J. Intl. Neuropsychol. 2000;6:279–289. doi: 10.1017/s1355617700633039. [DOI] [PubMed] [Google Scholar]
- 29.Konrad K. Gauggel S. Schurek J. Catecholamine functioning in children with traumatic brain injuries and children with attention-deficit/hyperactivity disorder. Brain Res. Cogn. Brain Res. 2003;16:425–433. doi: 10.1016/s0926-6410(03)00057-0. [DOI] [PubMed] [Google Scholar]
- 30.Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 199;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 31.Kudryavtseva N.N. Use of the “partition” test in behavioral and pharmacological experiments. Neurosci. Behav. Physiol. 2003;35:461–471. doi: 10.1023/a:1023411217051. [DOI] [PubMed] [Google Scholar]
- 32.Spencer C.M. Alekseyenko O. Serysheva E. Yuva-Paylor L.A. Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 33.Molina J. Carmona-Mora P. Chrast J. Krall P.M. Canales C.P. Lupski J.R. Reymond A. Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum. Mol. Genetics. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- 34.Wang F. Zhu J. Zhu H. Zhang Q. Lin Z. Hu H. Bidirectional control of social hierachy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 35.Nadler J.J. Moy S.S. Dold G. Trang D. Simmons N. Perez A. Young N.B. Barbaro R.P. Piven J. Magnuson T.R. Crawley J.N. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 36.Silverman J.L. Yang M. Lord C. Crawley J.N. Behavioral phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M. Silverman J.L. Crawley J.N. Automated three-chambered social approach task for mice. Curr. Prot. Neurosci. 2011;(Unit 8.26) doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawley J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M. Scattoni M.L. Zhodzishsky V. Chen T. Caldwell H. Young W.S. McFarlane H.G. Crawley J.N. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front. Behav. Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvoisin R.M. Villasana L. Davis M.J. Winder D.G. Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav. Brain Res. 2011;221:50–54. doi: 10.1016/j.bbr.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terranova M.L. Laviola G. Scoring of social interactions and play in mice during adolescence. Curr. Prot. Toxicol. 2005:13.10.1–13.10.11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- 42.McFarlane H.G. Kusek G.K. Yang M. Phoenic J.L. Bolivar V.J. Crawley J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 43.Ryan B.C. Young N.B. Moy S.S. Crawley J.N. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57Bl/6J mice. Behav. Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M. Crawley J.N. Simple behavioral assessment of mouse olfaction. Curr. Prot. Neurosci. 2009:8.21.1–8.24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konrad K. Gauggel S. Manz A. Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Inj. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- 46.Bloom D.R. Levin H.S. Ewing-Cobbs L. Saunders A.E. Song J. Fletcher J.M. Kowatch R.A. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:572–579. doi: 10.1097/00004583-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 47.van de Poll N.E. Smeets J. van Oyen H.G. van der Zwan S.M. Behavioral consequences of agonistic experience in rats: sex differences and the effects of testosterone. J. Compar. Physiol. Psychol. 1982;96:893–903. [PubMed] [Google Scholar]
- 48.Hsu Y. Earley R.L. Wolf L.L. Modulation of aggressive behavior by fighting experience: mechanisms and contest outcomes. Biol. Rev. Cambridge Philos. Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 49.Rao V. Rosenberg P. Bertrand M. Salehinia S. Spiro J. Vaishnavi S. Rastogi P. Noll K. Schretlen D.J. Brandt J. Cornwell E. Makley M. Miles Q.S. Aggression after traumatic brain injury: prevalence and correlates. J. Neuropsychiatry Clin. Neurosci. 2009;21:420–429. doi: 10.1176/appi.neuropsych.21.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braga L. Souza L. Najjar Y. Dellatolas G. Magnetic resonance imaging (MRI). findings and neuropsychological sequelae in children after severe traumatic brain injury: the role of cerebellar lesion. J. Child Neurol. 2007;22:1084–1089. doi: 10.1177/0883073807306246. [DOI] [PubMed] [Google Scholar]
- 51.Greve K.W. Sherwin E. Stanford M.S. Mathias C. Love J. Ramzinski P. Personality and neurocognitive correlates of impulsive aggression in long-term survivors of severe traumatic brain injury. Brain Inj. 2001;15:255–262. doi: 10.1080/026990501300005695. [DOI] [PubMed] [Google Scholar]
- 52.Dyer K.F.W. Bell R. McCann J. Rauch R. Aggression after traumatic brain injury: Analysing socially desirable responses and the nature of aggressive traits. Brain Inj. 2006;20:1163–1173. doi: 10.1080/02699050601049312. [DOI] [PubMed] [Google Scholar]
- 53.Pietropaoloa S. Branchia I. Cirullia F. Chiarottia F. Aloeb L. Alleva E. Long-term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: social versus physical enrichment. Physiol. Behav. 2004;81:443–453. doi: 10.1016/j.physbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Moy S.S. Nadler J.J. Young N.B. Perez A. Holloway L.P. Barbaro R.P. Barbaro J.R. Wilson L.M. Threadgill D.W. Lauder J.M. Magnuson T.R. Crawley J.N. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moy S.S. Nadler J.J. Young N.B. Nonneman R.J. Segall S.K. Andrade G.M. Crawley J.N. Magnuson T.R. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An X.L. Zou J.X. Wu R.Y. Yang Y. Tai F.D. Zeng S.Y. Jia R. Zhang X. Liu E.Q. Broders H. Strain and sex differences in anxiety-like and social behaviors in C47BL/6J and BALB/cJ mice. Exp. Anim. 2011;60:111–123. doi: 10.1538/expanim.60.111. [DOI] [PubMed] [Google Scholar]
- 57.D'Hooge R. De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 58.Engelmann M. Ebner K. Landgraf R. Wotjak C.T. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm. Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Kaidanovich-Beilin O. Lipina T. Vukobradovic I. Roder J. Woodgett J.R. Assessment of social interaction behaviors. J. Vis. Exp. 2011;48 doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moy S.S. Nadler J.J. Perez A. Barbaro R.P. Johns J.M. Magnuson T.R. Piven J. Crawley J.N. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 61.Cicerone K.D. Dahlberg C. Kalmar K. Langenbaln D.M. Malec J.F. Bergguist T.F. Felicetti T. Giacino J.T. Harley J.P. Harrington D.E. Herzog J. Kneipp S. Laatsch L. Morse P.A. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000;81:1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 62.Laatsch L. Harrington D. Hotz G. Marcantuono J. Mozzoni M.P. Walsh V. Hersey K.P. An evidence-based review of cognitive and behavioral rehabilitation treatment strategies in children with acquired brain injury. J. Head Trauma Rehabil. 2007;22:248–256. doi: 10.1097/01.HTR.0000281841.92720.0a. [DOI] [PubMed] [Google Scholar]
- 63.Benavidez D.A. Fletcher J.M. Hannay H.J. Bland S.T. Caudle S.E. Mendelsohn D.B. Yeakley J. Brunder D.G. Harward H. Song J. Perachio N.A. Bruce D. Scheibel R.S. Lilly M.A. Verger-Maestre K. Levin H.S. Corpus callosum damage and interhemispheric transfer of information following closed head injury in children. Cortex. 1999;35:315–336. doi: 10.1016/s0010-9452(08)70803-7. [DOI] [PubMed] [Google Scholar]
- 64.Johnson S.C. Pinkston J.B. Bigler E.D. Blatter D.D. Corpus callosum morphology in normal controls and traumatic brain injury: sex differences, mechanisms of injury, and neuropsychological correlates. Neuropsychol. 1996;10:408–415. [Google Scholar]
- 65.Verger K. Junqué C. Levin H.S. Jurado M.A. Pérez-Gómez M. Bartrés-Faz D. Barrios M. Alvarez A. Bartumeus F. Mereader J.M. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Injury. 2001;15:211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- 66.Maaswinkel H. Baars A.M. Gispen W.H. Spruijt B.M. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol. Behav. 1996;60:55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 67.Bannerman D.M. Lemaire M. Yee B.K. Iversen S.D. Oswald C.J.P. Good M.A. Rawlins J.N.P. Selective cytotoxic lesions of the retrohippocampal region produce a mild deficit in social recognition memory. Exp. Brain Res. 2002;142:395–401. doi: 10.1007/s00221-001-0938-z. [DOI] [PubMed] [Google Scholar]
- 68.Broadbent N.J. Squire L.R. Clark R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 70.Malkova L. Mishkin M. Suomi S.J. Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behav. Neurosci. 2010;124:742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eslinger P.J. Biddle K.R. Adolescent neuropsychological development after early right prefrontal cortex damage. Dev. Neuropsychol. 2000;18:297–329. doi: 10.1207/S1532694203Eslinger. [DOI] [PubMed] [Google Scholar]
- 72.Anderson V. Catroppa C. Advances in postacute rehabilitation after childhood-acquired brain injury: a focus on cognitive, behavioral, and social domains. Am. J. Phys. Med. Rehabil. 2006;85:767–778. doi: 10.1097/01.phm.0000233176.08480.22. [DOI] [PubMed] [Google Scholar]
- 73.Huttenlocher P.R. Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Compar. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs R. Harvey A.S. Anderson V. Are executive skills primarily mediated by the prefrontal cortex in childhood? Examination of focal brain lesions in childhood. Cortex. 2011;47:808–824. doi: 10.1016/j.cortex.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Eslinger P.J. Grattan L.M. Damasio H. Damasio A.R. Developmental consequences of childhood frontal lobe damage. Arch. Neurol. 1992;49:764–769. doi: 10.1001/archneur.1992.00530310112021. [DOI] [PubMed] [Google Scholar]
- 76.Barker L.A. Andrade J. Morton N. Romanowski C.A. Bowles D.P. Investigating the “latent” deficit hypothesis: age at time of head injury, implicit and executive functions and behavioral insight. Neuropsychologia. 2010;48:2550–2563. doi: 10.1016/j.neuropsychologia.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Bachevalier J. Málková L. Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2001;115:545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]