Abstract
Existing data suggest that antipyretic medications may have deleterious effects on immune function and may increase mortality in human infection. This study was designed to evaluate the impact of antipyretic therapy on 28-day in-hospital mortality when administered early in the course of gram-negative severe sepsis or septic shock. This study was a single-center retrospective cohort study at a 1,111-bed academic medical center of all febrile patients with gram-negative bacteremia hospitalized with severe sepsis or septic shock (n = 278) between Jan 2002 and Feb 2008. Although the raw mortality was lower in the group that received an early antipyretic medication (22 vs. 35 %, p = 0.01), patients in the early antipyretic group had higher mean arterial pressure (58.0 vs. 52.7, p = 0.01) and higher 24-h Tmax (39.3 vs. 39.0, p < 0.01). Early antipyretic therapy was not significantly associated with 28-day in-hospital mortality (adjusted OR 0.55, 0.29–1.03) in a multivariable logistic regression model controlling for APACHE-II score, hypotension, pneumonia, surgery during hospitalization, persistent fever, and in-hospital dialysis. In conclusion, early antipyretic therapy is not associated with increased mortality.
Keywords: Fever, Antipyretics, Septic shock, Sepsis, Bacteremia, Acetaminophen
Introduction
Infection is a leading cause of non-procedural admissions to intensive care units (ICUs) in the United States [1]. Ninety percent of patients with severe sepsis have a fever at the time of diagnosis [2]. Despite the commonality of fever in the critically ill patient, very little is understood about the role of fever in combating infection.
As early as the 1970s, animal trials suggested that subjects allowed to mount fever to severe infection had improved survival [3, 4]. More recently, Su et al. [5] demonstrated with a sheep model that fever in sepsis improved oxygen delivery, shock resolution, and survival compared with animals whose febrile response was attenuated.
Observational data suggest that fever is important both for immune function and for its bacteriostatic properties. Children pre-treated with acetaminophen seem to have less acquired immunity after vaccination [6], in vitro laboratory studies suggest that fever may augment antimicrobial function [7], and there is growing recognition that heatshock proteins may protect the host in severe infection [8, 9].
Despite these data, there is a paucity of human trials to guide the clinician in the decision to treat fever. Only two small prospective clinical trials have tried to address the question of fever control in sepsis. The first study randomized patients to either aggressive (T < 38 °C) or permissive (T < 40 °C) fever control. The trial was stopped early (n = 82) because the mortality in the aggressive fever control group was higher (15.9 vs. 2.6 %, p = 0.06) [10]. The second was a very small randomized study (n = 38) using external cooling versus no cooling, and no difference in mortality (11 vs. 15 % p = 0.55) was observed [11].
Based on these data, we hypothesize that administration of antipyretic medications within 6 h of the clinical recognition of severe sepsis or septic shock is associated with a higher mortality. We estimated effects of early antipyretic therapy and potential confounders on 28-day in-hospital mortality. We collected retrospective data on severe sepsis and septic shock patients in our institution to evaluate the strength of the association between early antipyretic medication and mortality.
Methods
Patients and setting
This study was a retrospective cohort analysis of febrile, gram-negative bacteremic adult patients (age ≥ 18 years) hospitalized at a 1,111-bed academic medical center between January 2002 and February 2008. All patients had fever (Tmax ≥ 38.3 °C) within 24 h of blood culture and met criteria for severe sepsis or septic shock by ICD-9 criteria as reported by Angus et al. and Martin et al. [12, 13]. We restricted the data set to gram-negative pathogens to focus the study population on a presumed homogenous cause of fever (i.e., lipopolysaccharide release, and because most clinicians recognize a gram negative organism in a blood culture to be pathogenic whereas many gram positive organisms may be treated as culture contaminants. Patients were excluded if gram-positive or fungal organisms grew in simultaneous blood cultures. Fever was required for inclusion because afebrile septic patients would not be expected to receive antipyretic therapy.
Patients were excluded for factors that served as an indication for, or a contraindication to, antipyretic therapy, including cirrhosis, elevations in hepatic enzymes (aspartate aminotransferase or alanine transaminase >5 times normal, total bilirubin >3 times normal), acute brain injury (including traumatic brain injury, stroke, or intracerebral hemorrhage) diagnosed on admission or during hospitalization before culture, and allergy to acetaminophen. Patients were excluded for incomplete data only if the chart did not reflect a disposition or if insufficient information was available to apply exclusion criteria. Only the first episode of severe sepsis or septic shock was included for those patients with more than one infection during the study period. This study was approved by the Washington University Human Research Protection Office under waiver of informed consent.
Intervention
Doses of antipyretic medications (i.e., acetaminophen, ibuprofen) recorded on the medication administration record were collected. Combination medications containing an antipyretic as one component (e.g., hydrocodone/acetaminophen) were included. For analysis, patients were divided into an exposed cohort (i.e., receiving at least one dose of antipyretic medication within 6 h), and a control cohort.
Definitions
As recommended by a consensus of the American College of Critical Care Medicine and the Infectious Diseases Society of America [14], fever was defined as temperature greater than or equal to 38.3 °C. The index time was standardized to be the time the positive blood culture was drawn. Early antipyretic therapy was defined as a dose of medication containing either acetaminophen or ibuprofen between 4 h before and 6 h after the index time (diagnostic blood culture). This time window was selected to incorporate the anticipated duration of effect of the medication (most commonly acetaminophen), and to capture all doses that could be used to treat the earliest fevers observed and attributable to a diagnosis of severe sepsis or septic shock. APACHE-II [15] scores were calculated at the index time (no points were assigned for APACHE-II variables not measured within 24 h of index time.) The source of infection was assigned by one of the authors [STM] by comparing with culture data available from other body fluids (e.g., urine, bronchoalveolar lavage, operative culture), and the clinical circumstances. Home medications were listed on the hospital admission documentation, and hospital medications were recorded on the medication administration record. Inappropriate antibiotics were defined as either failure to administer antibiotics within 24 h of culture, or administration of antibiotics to which the pathogen was later found to be resistant. Surgery included any surgical procedure during hospitalization performed in an operating suite (excluding tracheostomy, gastrostomy, diagnostic bronchoscopy, endoscopy, or procedures performed by interventional radiology). New dialysis included any renal replacement therapy (e.g., intermittent hemodialysis, continuous venovenous hemodialysis) performed on a patient not previously known to have end-stage renal disease at the time of admission. Mechanical ventilation included only ventilation via an endotracheal tube or tracheostomy tube (excluding non-invasive ventilation), and liberation from mechanical ventilation was defined as 24 continuous hours with no ventilator support. Vasopressors were defined as norepinephrine, vasopressin, dopamine, or phenylephrine infusions, with the stop time being the time when the infusion was stopped if it was not restarted for 24 h. For those patients on mechanical ventilation or vasopressor infusions at the index time, the start time for both interventions was listed as the index time.
Data collection
Data were collected either by computerized query of the medical record, or by manually abstracting data from the paper or computerized medical record by one of the authors, and recording it on a paper case report form. The three data collectors [NMM, HPM, RLA] were all physicians who were at least PGY-2 trainees, who were trained in data collection prior to abstracting data. Charts were randomly selected to be reviewed by two data collectors independently and verified for agreement. Manually collected data were transferred to an electronic database for analysis.
Outcomes
The primary outcome was 28-day in-hospital mortality. Pre-defined secondary outcomes were subsequent requirement for mechanical ventilation (within 72 h after diagnostic culture), use of vasopressors, or new renal failure requiring dialysis.
Statistical analysis
The primary outcome analysis was performed as a multivariable logistic regression to estimate the effect of early antipyretic administration and to account for baseline differences in covariates between the two groups. Associations between baseline characteristics and outcomes were tested using the Student t test, Mann–Whitney U test, or Pearson Chi-squared test, as appropriate. No correction was made for multiple comparisons (i.e., Bonferroni) because omitting the correction is a more conservative approach for a comparison designed to show baseline similarities. Variables expected to be related to disease severity were evaluated in a univariable logistical regression for their relationship to mortality. Variables that were significantly related to antipyretic exposure and to mortality were included in the multivariable logistic regression model. Predictors were included in a main effects explanatory model. Continuous covariates were modeled as linear variables. Where appropriate, outcomes are presented with 95 % confidence intervals, and all tests are considered significant when p < 0.05 using two-tailed tests.
Results
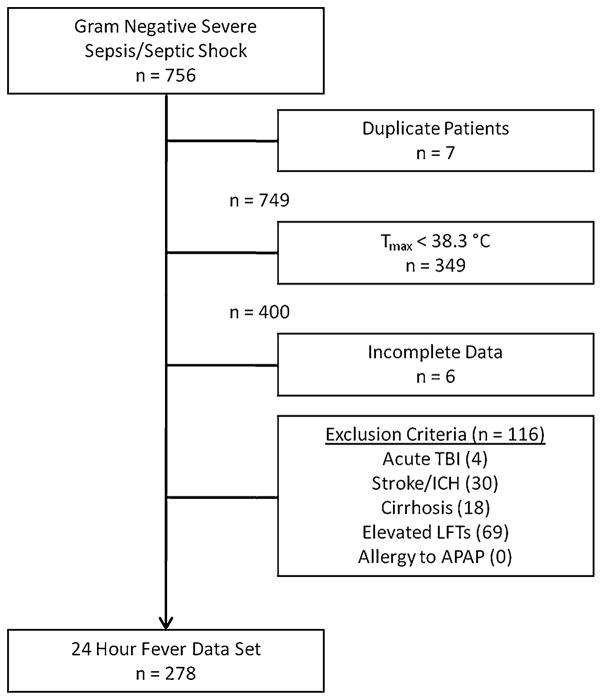
Of the 756 patients with gram-negative severe sepsis or septic shock over the six-year period, 278 met inclusion/exclusion criteria (Fig. 1). The most frequent reason for exclusion was the absence of fever at the time the culture was drawn. Patients were administered an early antipyretic in 130 (47 %) cases, and 129 of the total 133 doses administered (96.9 %) were acetaminophen. Of those doses of acetaminophen, 100 doses (77.5 %) were 650 mg.
Fig. 1.
Patient flow chart. The number of patients excluded is smaller than the sum of each exclusion criterion because some patients were excluded with multiple exclusion criteria. Tmax maximum temperature over period 24 h before and after index time, TBI traumatic brain injury, ICH intracerebral hemorrhage, LFT liver function tests, APAP acetaminophen
Patient characteristics
At baseline, patients treated with antipyretic therapy had higher temperature (p < 0.01), higher mean arterial pressure (p = 0.01), and were less likely to have been newly admitted to the hospital (p = 0.02) at study inclusion (Table 1). Although maximum temperature was statistically different, the difference between medians was small (0.3 °F). Otherwise, the groups were well matched on demographic factors and baseline condition.
Table 1.
Patient characteristics (n = 278), univariable comparison
| No antipyretic (n = 148) | Early antipyretic (n = 130) | p | |
|---|---|---|---|
| Age [years (mean, SD)] | 58.1 (16.2) | 56.8 (16.2) | 0.494 |
| Male [no. (%)] | 84 (57) | 61 (47) | 0.101 |
| APACHE-II (mean, SD) | 24.3 (7.5) | 23.0 (6.0) | 0.092 |
| Tmax [°C (median, IQR)] | 39.0 (38.6–39.4) | 39.3 (38.7–39.7) | 0.004 |
| MAP [mmHg (mean, SD)] | 52.7 (19.0) | 58.0 (16.4) | 0.014 |
| History of transplant [no. (%)] | 14 (9) | 20 (15) | 0.132 |
| History of end-stage renal disease [no. (%)] | 18 (12) | 10 (8) | 0.217 |
| Home corticosteroid or immune suppressive use [no. (%)] | 33 (22) | 29 (22) | 0.998 |
| Neutropenia (ANC <1,000 neutrophils/μL) [no. (%)]a | 21 (15) | 19 (18) | 0.616 |
| Enrollment within 24 h of admission [no. (%)] | 64 (43) | 39 (30) | 0.023 |
| Antibiotics within 12 h of culture [no. (%)] | 94 (64) | 91 (70) | 0.253 |
| Inappropriate antibiotics [no. (%)] | 43 (29) | 29 (22) | 0.200 |
| Pneumonia [no. (%)] | 67 (45) | 59 (45) | 0.985 |
| Pseudomonas [no. (%)] | 31 (21) | 26 (20) | 0.845 |
SD standard deviation, no. number (count), APACHE-II Acute Physiology and Chronic Health Evaluation, 2nd revision (scores 1–63 with higher scores indicating higher level of illness), Tmax highest temperature achieved over 24-h period, °C degrees Celsius, IQR interquartile range, MAP mean arterial pressure, mmHg millimeters of mercury, ANC absolute neutrophil count
Since ANC was not measured on all patients, the percentage listed is the percentage of patients who had ANC value available who were neutropenic
Patients treated with antipyretic therapy were less likely to require ICU care (p < 0.01), mechanical ventilation (p < 0.01), and vasopressors (p < 0.01, Table 2). Eighty patients (28.8 %) died during the 28-day study follow-up, with fewer patients dying after receiving an antipyretic medication (22 vs. 35 %, p = 0.01). Most patients (63 %) were diagnosed with bacteremia greater than 24 h into their hospital stay, and many of these patients had nosocomial bacteremia.
Table 2.
Patient interventions and outcomes (n = 278), univariable comparison
| No antipyretic (n = 148) | Early antipyretic (n = 130) | p | |
|---|---|---|---|
| 28-day in-hospital mortality [no. (%)] | 52 (35) | 28 (22) | 0.012 |
| Mechanical ventilation within 72 h [no. (%)] | 80 (54) | 42 (32) | <0.001 |
| Vasopressor infusion within 72 h [no. (%)] | 74 (50) | 42 (32) | 0.003 |
| New hospital dialysis [no. (%)]a | 20 (15) | 16 (13) | 0.644 |
| Treatment with drotrecogin alfa [no. (%)] | 14 (9) | 5 (4) | 0.064 |
| Admitted to ICU during study period [no. (%)] | 115 (78) | 81 (62) | 0.005 |
| Surgery during hospitalization [no. (%)] | 48 (33) | 29 (19) | 0.060 |
| Treatment with new corticosteroid in hospital [no. (%)]a | 28 (24) | 28 (27) | 0.663 |
| Hospital length of stay [days (median, IQR)] | 9 (5–16) | 12.5 (6–23) | 0.025 |
| ICU length of stay [days (median, IQR)] | 5.5 (2.1–10.3) | 6.0 (2.0–13.5) | 0.299 |
no. number (count), IQR interquartile range, ICU intensive care unit
Since new treatment with dialysis or corticosteroids is relevant only for those patients not receiving interventions as outpatients, the total number of patients considered is restricted to this smaller population, and the percentage and p value is adjusted accordingly
Response to acetaminophen
Patients with temperature data both 4 h before culture and 4 h after culture (n = 179) were analyzed to evaluate for response to antipyretic treatment. Median temperatures over each period were calculated and did not significantly differ based on the administration of acetaminophen (temperature decreased 0.23 °C for no acetaminophen vs. 0.60 °C for acetaminophen, p = 0.06).
Primary outcome
Unadjusted 28-day in-hospital mortality was lower among patients who received antipyretic therapy (22 vs. 35 %, p = 0.012). Including measured covariates in our multivariable logistic regression model, early antipyretic exposure does not increase mortality in patients with gram-negative severe sepsis (adjusted OR = 0.55, p = 0.063, Table 3). Factors related to both exposure and outcome were included in a multivariable model, no significant collinearity existed, and no potential interactions were found to be significant. For the a priori subgroup analysis restricted only to patients cared for in the ICU (n = 196), the logistic regression model was very similar, with a nonsignificant contribution of antipyretic therapy to mortality.
Table 3.
Multivariable logistic regression model for 28-day mortality
| Unadjusted OR | Adjusted OR | p | |
|---|---|---|---|
| Early antipyretic | 0.51 (0.30–0.87) | 0.55 (0.29–1.03) | 0.063 |
| APACHE-II (per 5 points) | 1.53 (1.15–2.04) | ||
| Pneumonia | 2.87 (1.53–5.36) | ||
| Hypotension (per 10 mmHg) | 1.41 (1.14–1.74) | ||
| Dialysis | 2.05 (1.00–4.22) | ||
| Surgery | 0.38 (0.18–0.82) |
Early antipyretic antipyretic medication given between 4 h before and 6 h after diagnostic blood culture with gram-negative bacteremia, APACHE-II Acute Physiology and Chronic Health Evaluation, 2nd revision (scores 1–63 with higher scores indicating higher level of illness), OR odds ratio
Secondary outcomes
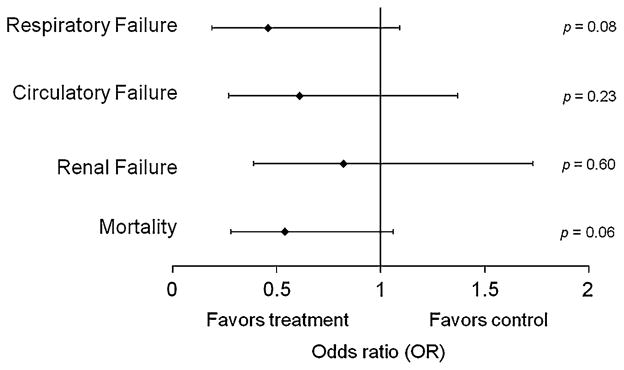
A similar multivariable logistic regression model that included patients not on mechanical ventilation at the index time (n = 194) controlled for covariates and showed that early antipyretic therapy was not associated with subsequent mechanical ventilation (adjusted OR 0.19–1.09, p = 0.08, Table 4). For patients not requiring vasopressors at the index time (n = 239), early antipyretic use was not associated with subsequent vasopressor use (adjusted OR 0.27–1.37, p = 0.23), and for those patients without end-stage renal disease (n = 250), early antipyretic use was not associated with acute renal failure requiring renal replacement therapy (adjusted OR 0.39–1.73, p = 0.60, Fig. 2).
Table 4.
Secondary outcomes adjusted for relevant covariates, multivariable logistic regression models
| Unadjusted OR | Adjusted OR | p | |
|---|---|---|---|
| Subsequent initiation of mechanical ventilation within 72 h (n = 194) | |||
| Early antipyretic | 0.40 (0.19–0.84) | 0.46 (0.19–1.09) | 0.076 |
| APACHE-II (per 5 points) | 1.69 (1.14–2.52) | ||
| Hypotension (per 10 mmHg) | 1.88 (1.32–2.65) | ||
| Inappropriate antibiotics | 2.88 (1.03–8.00) | ||
| Subsequent initiation of vasopressor therapy within 72 h (n = 239) | |||
| Early antipyretic | 0.57 (0.33–0.98) | 0.61 (0.27–1.37) | 0.229 |
| APACHE-II (per 5 points) | 1.81 (1.26–2.60) | ||
| Hypotension (per 10 mmHg) | 2.50 (1.69–3.57) | ||
| ICU residence | 23.07 (2.91–182.72) | ||
| Pneumonia | 0.42 (0.18–0.95) | ||
| Subsequent renal failure requiring renal replacement therapy during hospitalization (n = 250) | |||
| Early antipyretic | 0.85 (0.42–1.72) | 0.82 (0.39–1.73) | 0.595 |
| APACHE-II (per 5 points) | 1.22 (0.88–1.68) | ||
| Early culture (within 12 h) | 0.28 (0.11–0.73) | ||
| Vasopressor infusion | 1.99 (0.86–4.64) | ||
Patients not requiring each of these support modalities at the time of a diagnostic blood culture with isolated gram-negative bacteremia were included in the analysis to test the relationship between early antipyretic therapy and subsequent organ failure. Potential confounding variables were included to control for asymmetric distribution of illness severity between exposed and unexposed groups
Early antipyretic antipyretic medication given between 4 h before and 6 h after diagnostic blood culture with gram-negative bacteremia, APACHE-II Acute Physiology and Chronic Health Evaluation, 2nd revision (scores 1–63 with higher scores indicating higher level of illness), OR odds ratio
Fig. 2.
Primary and secondary outcomes for the effect of early antipyretic administration from multivariable analysis. The vertical line at unity shows the odds ratio of no effect, and each of the odds ratio confidence intervals cross unity
Discussion
In our cohort, early antipyretic therapy does not increase mortality in severe sepsis and septic shock. One of the conclusions of this analysis is one that has been reported previously: those who receive antipyretic therapy are different from those who do not. Clearly, patients with higher temperature seem more likely to receive antipyretic therapy, but they also may have a greater inflammatory burden of sepsis, and may have a different likelihood of survival (note that those without fever were excluded from this analysis). Furthermore, patients who are more ill tend to receive more interventions, so relating outcomes of organ failure to antipyretic exposure is difficult to do. This criticism is a recognized limitation of retrospective analyses, but by carefully limiting our study population, and applying statistical methods precisely, we have attempted to control for much of the asymmetric risk and potential bias in these two groups. We acknowledge that it is impossible to remove statistically the entire effect of potential confounding factors.
One of our hypotheses that antipyretic therapy might affect organ failure stemmed from animal data that suggested that fever augmented resolution from shock [5]. We determined a priori that looking at rates of new respiratory failure, circulatory failure, and renal failure (all measured by support modalities, including mechanical ventilation, vasopressor support, or renal replacement therapy) could aid in developing hypotheses to understand the benefits of fever. In our analysis, antipyretic therapy was not associated with subsequent organ failure, although a nonsignificant trend toward benefit was observed in subsequent respiratory failure. Although we have a temporal relationship from our data, we do not have a robust measure of respiratory compromise at study inclusion, so it is impossible to postulate that the impact of attenuating fever was causal in preventing intubation for these patients. It may be physiologically plausible that fever may contribute to tachypnea, high minute ventilation requirements, and clinical respiratory failure necessitating intubation.
Several prior studies have reported on animal effects [9, 16], fever in sepsis [17, 18], and prevention of infection with fever [10, 19, 20]. Human studies have had mixed results on the mortality effects of antipyretic therapy. A large prospective trial (n = 455) randomizing patients to ibuprofen or placebo did not control the use of other antipyretic drugs (i.e., acetaminophen), so patients in both groups received antipyretic medication [21]. Another human study was restricted to trauma patients, and study participants did not necessarily have evidence of infection at the time of inclusion. Although the findings did not reach statistical significance, there was a trend toward increased mortality (15.9 vs. 2.6 %, p = 0.06) prior to the study being stopped at an interim analysis [10]. Two observational studies correlating acetaminophen use to mortality in E. coli and Pseudomonas aeruginosa bacteremia were confounded by the increased use of acetaminophen later in their time analysis (when sepsis treatment likely had changed), and failure to account for specific severity of illness indices [22, 23]. Their findings may be difficult to compare with ours because acetaminophen use may have been collinear with some of our severity of illness variables. Our much larger analysis of patients with severe sepsis highlights that clinical equipoise remains, and that a prospective trial should be performed to either validate or refute prior findings.
Several reasons exist that might cause the direction of effect in our analysis to differ from prior studies. The most obvious two reasons are the observational nature of the analysis with statistically controlled covariates and the assignment to cohorts by a single early dose of medication only. Another possibility, however, is that most preclinical studies have examined the effect on clinical outcomes if fever is prevented. In actual practice, this is very difficult to do. In most cases, fever is not recognized until it exists, meaning that treatment cannot occur until fever has already occurred. Some preclinical data regarding fever in infection has examined the effect of heat shock proteins and their impact on survival. If the critical component of fever is magnitude rather than duration, it is conceivable that animal and human trials that prevent or delay an initial fever could lead to a very different outcome than one that considers treatment of fever after its onset in patients already diagnosed with bacteremia. Selecting patients with bacteremia and organ failure may have identified critically ill patients most likely to benefit from antipyretic therapy.
An alternative possibility is that the findings of clinical studies are heterogeneous because the patient populations they study are different. The primary outcome in each study relates to a delicate balance between benefit and harm. Perhaps antipyretic therapy, while attenuating the immune response, also offers benefits in speeding volume resuscitation, enhancing lung function [24], decreasing metabolic load [25], preventing delirium, and reducing other metabolic derangements. If this balance is responsible for differing conclusions in the medical literature, then the populations studied may wildly exaggerate the benefits or harms of fever. Otherwise healthy young trauma patients, for example, may have a much different outcome related to antipyretic therapy than older, chronically ill patients.
One interesting finding is the minimal temperature response to acetaminophen in this cohort. This is a finding that has been reported previously [26, 27]. There are two possible explanations to this observation: (1) febrile critically ill patients may have wide fluctuations in their temperature patterns [28], so all patients tend to defervesce after a fever spike, or (2) acetaminophen has little efficacy in the critically ill. It is likely that patients who develop extreme fevers will regress toward a more normal temperature over time. It is also likely that treatment with antibiotic therapy, source control, and other sepsis therapies may decrease temperature as a reflection of clinical improvement. In this cohort the defervescence in the patients not treated with acetaminophen was very small (0.23 °C). The meager response to acetaminophen may be reflective of the minimal effect of antipyretic medications in such overwhelming systemic inflammation. Further, antipyretic medications may have effects in addition to their effect on temperature regulation that may impact clinical outcomes. Future studies may consider pharmacologic and direct thermal regulation of body temperature to elucidate the role of fever in sepsis. This limited temperature effect may partially explain the difficulty in showing a mortality difference in our study.
Several limitations must be considered for this study. First, the cohort is based on retrospective chart review, so some of the information gathered from the medical record may have been incomplete. We have strived to select measures of exposure (e.g., medication administration records), disease severity (e.g., laboratory values, vital signs), and outcomes (e.g., death, dialysis) that we expect are accurately recorded in the medical record, and most of the data were collected through computerized query of the medical record to reduce error. Although we have tried to limit bias by carefully limiting our inclusion cohorts and controlling for measured differences between our groups, there may be other confounders that are unmeasured, and are not controlled by our analysis. Further, we do not define why antipyretic medications were administered. In our population, 18 % of doses were given to patients in a time period without fever (meaning most doses were given in the presence of fever), so we cannot assure that each dose of medication was given for attenuation of fever. Despite not knowing the therapeutic intent, all included patients had severe sepsis, and fever, and were exposed to an early antipyretic medication. We also have assumed that the effect of antipyretics can be captured in the measurement of early doses, but it is possible that a cumulative dose or a projected fever dose may capture more appropriate data. We believe that the benefit or harm of antipyretic therapy is likely to be greatest early in disease when shock and inflammation is most severe, and the physiology of the heat shock protein response is likely captured in early doses of fever-attenuating medications.
Although the exclusion criteria were designed to better match the two cohorts in this observational study, they also likely limit the external validity of the study findings to only patient groups that were included in our analysis. Similarly, restricting our cohort to gram-negative bacteremia was intended to improve homogeneity of the study population, but it limits application of our findings to those with other ICU infections.
Conclusions
Early antipyretic exposure does not increase mortality in patients with gram-negative severe sepsis. Exposure to antipyretics was not associated with changes in respiratory failure, renal failure, or circulatory failure requiring vasopressors. Further prospective study will be required to elucidate the role of antipyretic therapy in survival from septic shock.
Acknowledgments
The authors would like to acknowledge Karen Steger-May, MA, Research Statistician, Division of Biostatistics, Washington University School of Medicine for her assistance with design of this study protocol. Support for this study was provided by the Washington University Department of Anesthesiology and by a grant from the Washington University Institute of Clinical and Translational Sciences (ICTS). Support from ICTS is made possible by grant numbers 1 UL1 RR024992-01, 1 TL1 RR024995-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Conflict of interest The authors have not disclosed any potential conflict of interest.
References
- 1.Yu W, Ash AS, Levinsky NG, Moskowitz MA. Intensive care unit use and mortality in the elderly. J Gen Intern Med. 2000;15(2):97–102. doi: 10.1046/j.1525-1497.2000.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med. 1999;27(4):699–707. doi: 10.1097/00003246-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188(4184):166–168. [PubMed] [Google Scholar]
- 4.Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. 1976;193(4249):237–239. doi: 10.1126/science.935867. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Nguyen ND, Wang Z, Cai Y, Rogiers P, Vincent JL. Fever control in septic shock: beneficial or harmful? Shock. 2005;23(6):516–520. [PubMed] [Google Scholar]
- 6.Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374(9698):1339–1350. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 7.Mackowiak PA, Marling-Cason M, Cohen RL. Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis. 1982;145(4):550–553. doi: 10.1093/infdis/145.4.550. [DOI] [PubMed] [Google Scholar]
- 8.Wong HR. Potential protective role of the heat shock response in sepsis. New Horiz. 1998;6(2):194–200. [PubMed] [Google Scholar]
- 9.Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol Regul Integr Comp Physiol. 1993;265(6):R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- 10.Schulman CI, Namias N, Doherty J, Manning RJ, Li P, Alhaddad A, Lasko D, Amortegui J, Dy CJ, Dlugasch L, Baracco G, Cohn SM. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt) 2005;6(4):369–375. doi: 10.1089/sur.2005.6.369. [DOI] [PubMed] [Google Scholar]
- 11.Gozzoli V, Schottker P, Suter PM, Ricou B. Is it worth treating fever in intensive care unit patients? Preliminary results from a randomized trial of the effect of external cooling. Arch Intern Med. 2001;161(1):121–123. doi: 10.1001/archinte.161.1.121. [DOI] [PubMed] [Google Scholar]
- 12.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through. N Engl J Med. 2000;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 16.Bernheim H, Kluger M. Fever and antipyresis in the lizard Dipsosaurus dorsalis. Am J Physiol. 1976;231(1):198–203. doi: 10.1152/ajplegacy.1976.231.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 18.Clemmer TP, Fisher CJ, Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. 1992;20(10):1395–1401. doi: 10.1097/00003246-199210000-00006. 1401. [DOI] [PubMed] [Google Scholar]
- 19.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 20.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134. [PubMed] [Google Scholar]
- 21.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, Higgins SB, Swindell BB. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336(13):912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 22.Kuikka A, Sivonen A, Emelianova A, Valtonen VV. Prognostic factors associated with improved outcome of Escherichia coli bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. 1997;16(2):125–134. doi: 10.1007/BF01709471. [DOI] [PubMed] [Google Scholar]
- 23.Kuikka A, Valtonen VV. Factors associated with improved outcome of Pseudomonas aeruginosa bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. 1998;17(10):701–708. doi: 10.1007/s100960050164. [DOI] [PubMed] [Google Scholar]
- 24.Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He J-R, Rice P, Frank M, Goldblum SE, Viscardi RM. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162(6):2005–2017. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poblete B, Romand JA, Pichard C, Konig P, Suter PM. Metabolic effects of i.v. propacetamol, metamizol or external cooling in critically ill febrile sedated patients. Br J Anaesth. 1997;78(2):123–127. doi: 10.1093/bja/78.2.123. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg RS, Chen H, Hasday JD. Acetaminophen has limited antipyretic activity in critically ill patients. J Crit Care. 2010;25(2):363.e361–363.e367. doi: 10.1016/j.jcrc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie I, Forrest K, Thompson F, Marsh R. Effects of acetaminophen administration to patients in intensive care. Intensive Care Med. 2000;26(9):1408. doi: 10.1007/s001340000614. [DOI] [PubMed] [Google Scholar]
- 28.Mohr NM, Hotchkiss RS, Micek ST, Durrani S, Fuller BM. Change in temperature profile may precede fever and be an early indicator of sepsis: a case report. Shock. 2011;36(3):318–320. doi: 10.1097/SHK.0b013e318224f5ee. discussion 320–311. [DOI] [PubMed] [Google Scholar]