Abstract
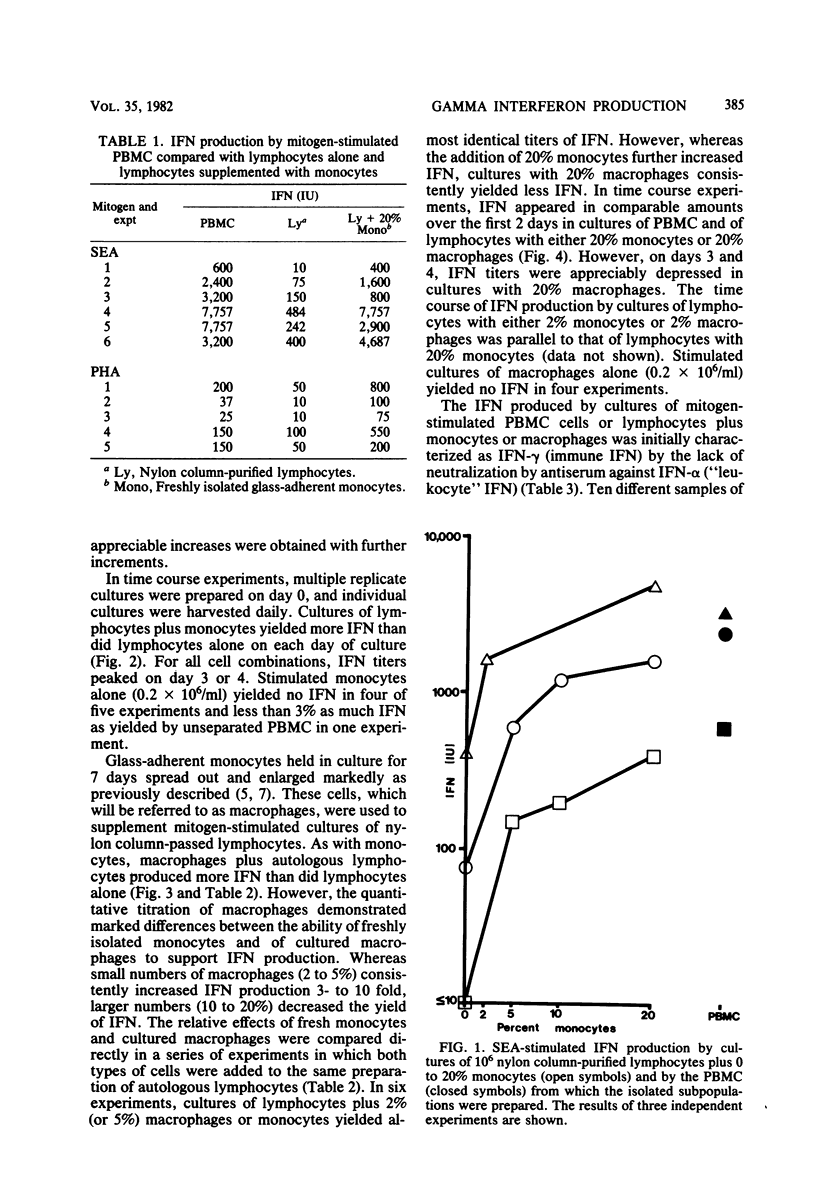
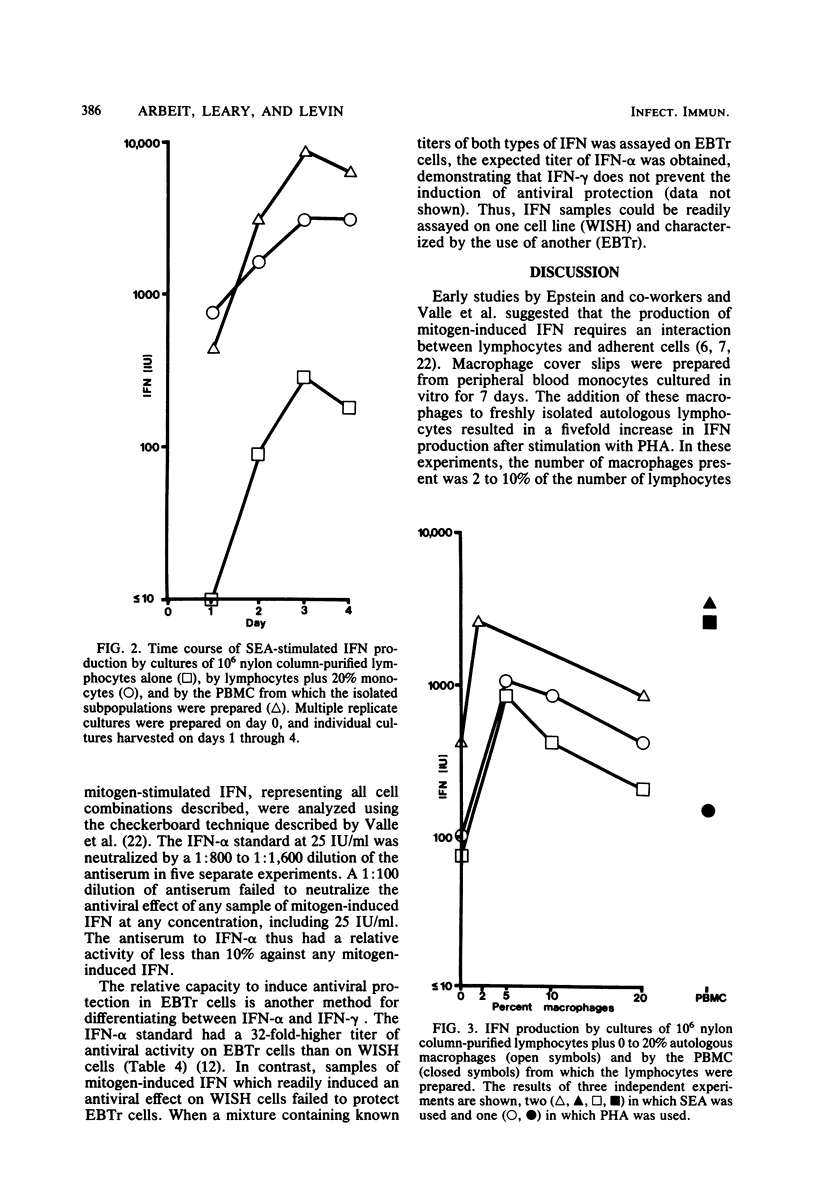
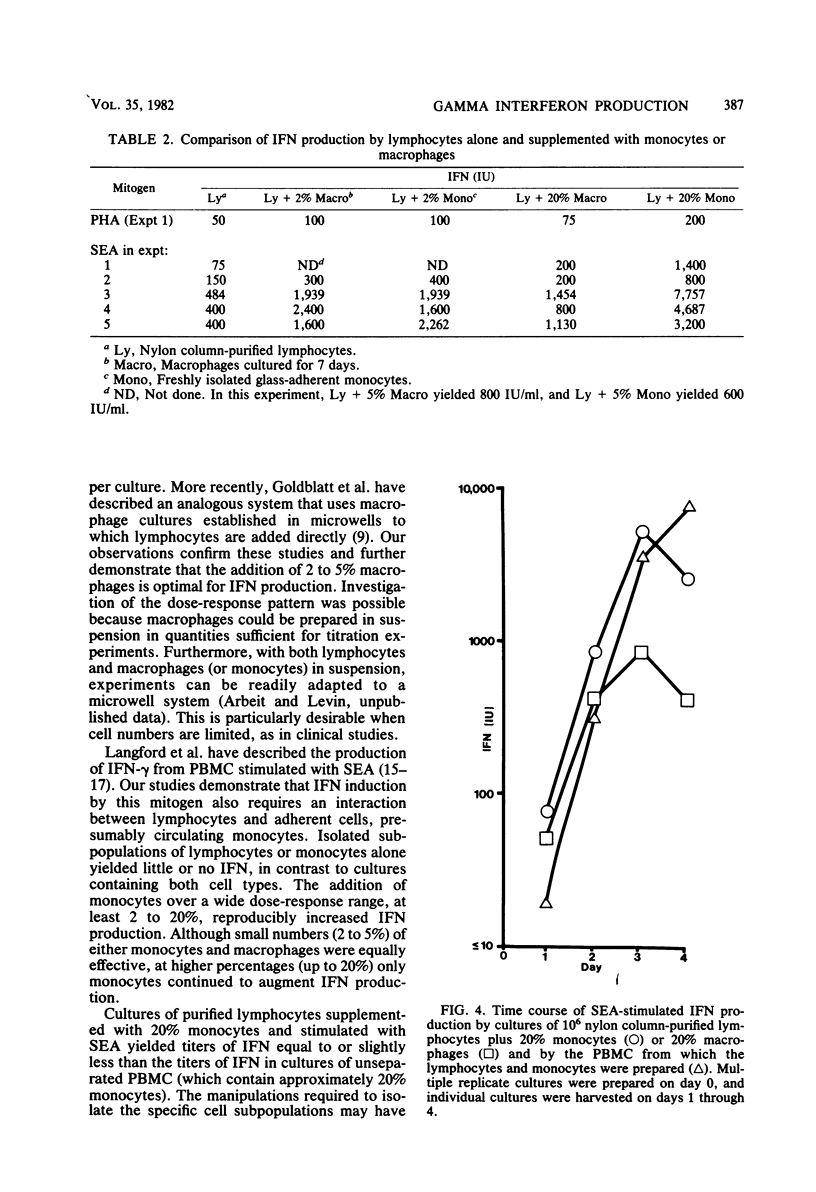
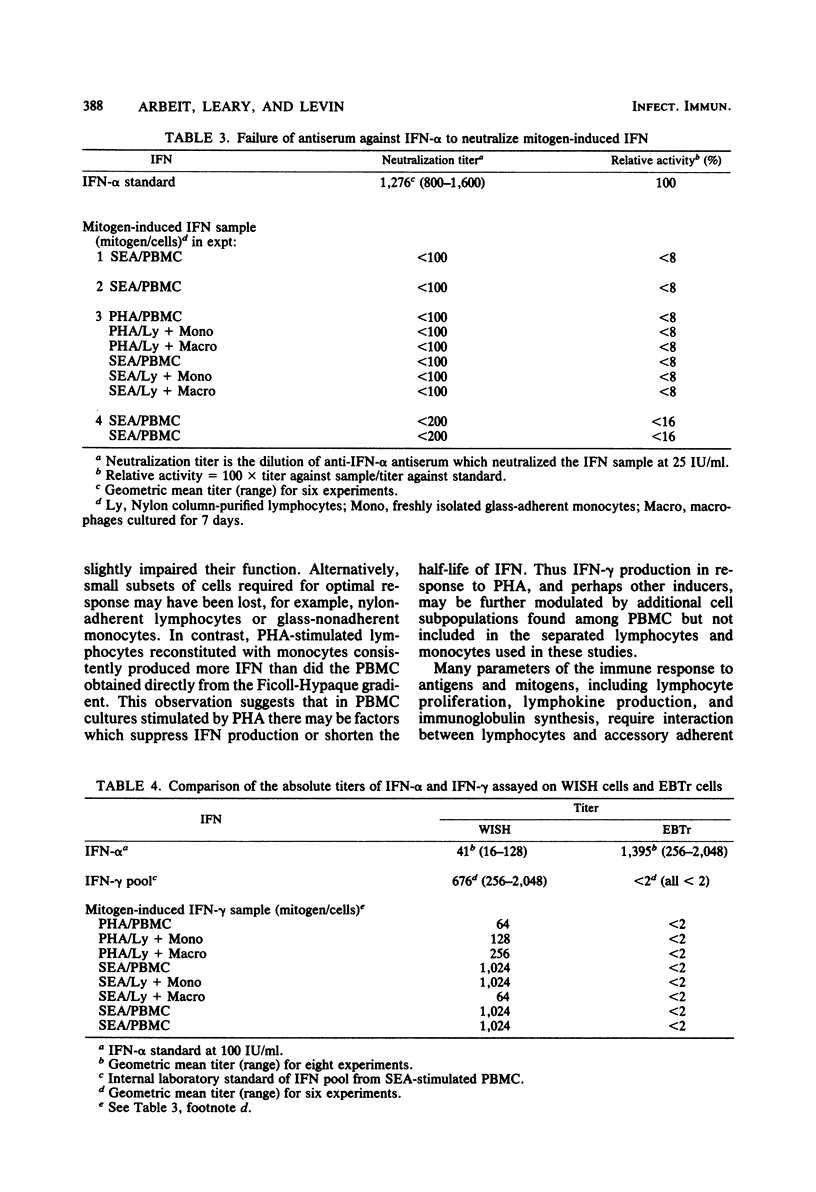
Mitogen-induced interferon (IFN) production was studied using human peripheral blood mononuclear cells and subpopulations of lymphocytes, monocytes, and cultured macrophages. Cell populations were prepared in suspension to permit quantitative analysis of the interactions among different cell types. After stimulation by staphylococcal enterotoxin A, nylon column-purified lymphocytes produced only 5% as much IFN as the peripheral blood mononuclear cells from which they were prepared. When lymphocytes were supplemented with as little as 2% monocytes, IFN production increased two- to eightfold; with the addition of up to 20% monocytes, IFN production increased further, to levels approximating those of peripheral blood mononuclear cells. Monocytes alone produced no or very little IFN. Macrophages were derived from monocytes by culturing in vitro for 7 days. The addition of 2 to 5% autologous macrophages augmented IFN production to the same extent as 2 to 5% monocytes. However, more macrophages consistently resulted in less, rather than more, IFN, so that lymphocytes with 20% monocytes produced three- to eightfold more IFN than did lymphocytes with 20% macrophages. Thus, whereas the addition of monocytes over a broad dose-response range (2 to 20%) progressively augmented IFN production, macrophages showed an optimal effect at 2 to 5%, with higher percentages being inhibitory. The IFN induced by stimulation with staphylococcal enterotoxin A was characterized as IFN-gamma by its resistance to neutralization by antibody to IFN- alpha and its inability to induce antiviral protection in embryonic bovine trachea cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carpentieri U., Thorpe L., Sordahl L. A. An improved method for purification of lymphocytes. Am J Clin Pathol. 1977 Dec;68(6):763–765. doi: 10.1093/ajcp/68.6.763. [DOI] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. The interaction of human macrophages and lymphocytes in the phytohemagglutinin-stimulated production of interferon. J Clin Invest. 1971 Apr;50(4):744–753. doi: 10.1172/JCI106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Goldblatt D., McManus N. H., Epstein L. B. A micromethod for preparation of human macrophage cultures for the study of lymphocyte-macrophage interaction in immune interferon production and blastogenesis. Immunopharmacology. 1978 Dec;1(1):13–20. doi: 10.1016/0162-3109(78)90004-8. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfo S., Marcucci F., Giovarelli M., Forni G. Does macrophage production of interferon after alloantigen stimulation depend on factors from activated T lymphocytes? Transplantation. 1980;29(1):87–89. [PubMed] [Google Scholar]
- Langford M. P., Georgiades J. A., Stanton G. J., Dianzani F., Johnson H. M. Large-scale production and physicochemical characterization of human immune interferon. Infect Immun. 1979 Oct;26(1):36–41. doi: 10.1128/iai.26.1.36-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Georgiades J. A., Johnson H. M., Stanton G. J. Antibody to staphylococcal enterotoxin A-induced human immune interferon (IFN gamma). J Immunol. 1981 Apr;126(4):1620–1623. [PubMed] [Google Scholar]
- Levin M. J., Parkman R., Oxman M. N., Rappeport J. M., Simpson M., Leary P. L. Proliferative and interferon responses by peripheral blood mononuclear cells after bone marrow transplantation in humans. Infect Immun. 1978 Jun;20(3):678–684. doi: 10.1128/iai.20.3.678-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C., Sorg C. Immune interferon. I. Production by lymphokine-activated murine macrophages. Eur J Immunol. 1977 Oct;7(10):719–725. doi: 10.1002/eji.1830071014. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Orser M., Kaplan M. E. Human monocyte and macrophage modulation of lymphocyte proliferation. Cell Immunol. 1979 Apr;44(1):131–143. doi: 10.1016/0008-8749(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. In vitro production and cellular origin of murine type II interferon. Immunology. 1979 Apr;36(4):883–890. [PMC free article] [PubMed] [Google Scholar]
- Valle M. J., Jordan G. W., Haahr S., Merigan T. C. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975 Jul;115(1):230–233. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Suppressor cells in the regulation of the immune response. Prog Clin Immunol. 1977;3:155–199. [PubMed] [Google Scholar]
- de Ley M., van Damme J., Claeys H., Weening H., Heine J. W., Billiau A., Vermylen C., de Somer P. Interferon induced in human leukocytes by mitogens: production, partial purification and characterization. Eur J Immunol. 1980 Nov;10(11):877–883. doi: 10.1002/eji.1830101113. [DOI] [PubMed] [Google Scholar]



