Abstract
Summary: Type IV pili (T4P) are multifunctional protein fibers produced on the surfaces of a wide variety of bacteria and archaea. The major subunit of T4P is the type IV pilin, and structurally related proteins are found as components of the type II secretion (T2S) system, where they are called pseudopilins; of DNA uptake/competence systems in both Gram-negative and Gram-positive species; and of flagella, pili, and sugar-binding systems in the archaea. This broad distribution of a single protein family implies both a common evolutionary origin and a highly adaptable functional plan. The type IV pilin is a remarkably versatile architectural module that has been adopted widely for a variety of functions, including motility, attachment to chemically diverse surfaces, electrical conductance, acquisition of DNA, and secretion of a broad range of structurally distinct protein substrates. In this review, we consider recent advances in this research area, from structural revelations to insights into diversity, posttranslational modifications, regulation, and function.
INTRODUCTION
Type IV pilins are small (∼7 to 20 kDa) structural proteins with a conserved, hydrophobic α-helical N terminus that is both a transmembrane (TM) domain and a protein-protein interaction domain. In general, the proteins function through their reversible polymerization into helical fibers by dedicated assembly/disassembly systems. In current models, system-specific hexameric ATPases are predicted to undergo conformational changes upon ATP hydrolysis, converting chemical energy into mechanical energy (285, 348). In ways that are not yet clear, the force generated by resulting domain movements in the ATPases is thought to move subunits from the cytoplasmic membrane into the fiber during polymerization and, for type IV pili (T4P), from the fiber back into the membrane during depolymerization. Fibers are predicted to grow by the addition of subunits at the base, with an estimated 12 protomers forming the short type II secretion (T2S) system pseudopilus—a length sufficient to span the periplasm of Gram-negative bacteria—and 500 to 1,000 (or more) subunits forming the quaternary structure of a T4P (349). This dynamic assembly and disassembly of type IV pilin-like proteins is important for the functioning of T4P and T2S systems, as well as for DNA transfer or uptake by a number of naturally competent species (30, 112, 295, 309).
The Signature Type III Signal Sequence
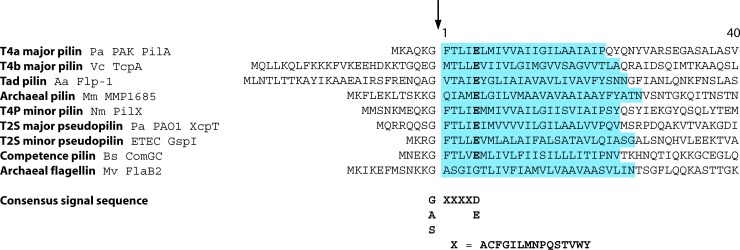
Although type IV pilin proteins are quite divergent in sequence, their defining characteristic is a distinctive N-terminal signal sequence (Fig. 1), denoted “type III” (379) to distinguish it from the type I (recognized by signal peptidase I) and type II (characteristic of lipoproteins) signal sequences. Canonical type I and type II signal sequences are cleaved at the exterior of the cytoplasmic membrane, C-terminal to a stretch of hydrophobic residues. In contrast, proteins with the unique type III signal sequence are called prepilins and are not competent for assembly until the signal is cleaved at the cytoplasmic face of the membrane (369). Prepilins are inserted into the cytoplasmic membrane by the Sec system (22, 144), and the signal sequence is removed by dedicated aspartyl proteases called prepilin peptidases (301). The positively charged signal sequence is likely important for the correct orientation of pilin proteins, with their C-terminal domains outside the cytoplasmic membrane. However, its polar nature may inhibit subsequent extraction of the subunits from the membrane and/or protein-protein interactions between subunits required for fiber assembly. The type III signal sequence motif (Fig. 1) has been used to develop an algorithm called PilFind (http://signalfind.org/pilfind.html) that can accurately identify putative pilins (195).
Fig 1.
Alignment of N-terminal sequences of type IV pilin proteins. The leader peptide and first 40 N-terminal residues of representative mature type IV pilin protein sequences were aligned based on the prepilin peptidase cleavage site (marked by an arrow). Pilin-like proteins share the type III signal sequence, which is cleaved before the hydrophobic stretch between the Gly (−1) and Phe (+1) residues, although the +1 residue can vary. The consensus signal sequence used by the PilFind algorithm (195) to identify putative type IV pilin proteins is shown below the alignment. The hydrophobic N terminus of mature pilin proteins is situated in the inner membrane and contains the highly conserved Glu5 (+5) residue (shown in bold); in GspK orthologs, there is a hydrophobic residue at that position. The transmembrane segments, as predicted by Geneious Pro v5.0.3 (Biomatters Ltd.) using TMHMM, are highlighted in blue. Pa, P. aeruginosa; Vc, V. cholerae; Aa, Aggregatibacter (Actinobacillus) actinomycetemcomitans; Mm, Methanococcus maripaludis; Nm, N. meningitidis; ETEC, enterotoxigenic E. coli; Bs, Bacillis subtilis; Mv, Methanococcus voltae.
Both T4P and T2S systems include multiple type III signal sequence-carrying proteins (183). The fibers are composed predominantly of a single subunit, referred to as the major pilin in T4P and the major pseudopilin in T2S. Additional pilin-like proteins are present at lower abundances, and for that reason, they are called the minor pilins/pseudopilins. Some are common to most systems (referred to here as “core” minor pilins/pseudopilins), and the genes encoding them are typically clustered, while other minor subunits are unique to particular species or genera and may or may not be encoded with the core minor subunits (Table 1). Although the minor subunits are present at low levels relative to the major subunits, they can have profound effects on function (see below).
Table 1.
Type IV pilin proteins in select model systems
| Component | Pilin protein(s) | References | ||
|---|---|---|---|---|
| Type IVa pili | Pseudomonas aeruginosa | Neisseria spp. | Vibrio cholerae (MSHAa) | |
| Major subunit | PilA | PilE | MshA | 270, 282, 307 |
| Core minor subunits | FimU, PilV, PilW, PilX | PilH, PilI, PilJ, PilK | MshB, MshC, MshD, MshO | 13–15, 270, 416 |
| Noncore minor subunits | PilE | PilX (PilL), PilV, ComP | 175, 340, 415 | |
| Prepilin peptidase | PilD | PilD | VcpD | 146, 271, 298 |
| Type IVb pili | EPECb (bundle-forming pili) | Vibrio cholerae (Tcpc) | R64 thin pili | |
| Major subunit | BfpA | TcpA | PilS | 123, 135, 221 |
| Core minor subunits | BfpI, BfpJ, BfpK | TcpB | PilV | 221, 265, 368 |
| Prepilin peptidase | BfpP | TcpJ | PilU | 221, 246, 368 |
| Tad pili | Aggregatibacter actinomycetemcomitans | Caulobacter crescentus | Pseudomonas aeruginosa | |
| Major subunit | Flp | PilA | Flp | 117, 210, 359 |
| Core minor subunits | TadE, TadF | NAd | TadF | 42, 393 |
| Prepilin peptidase | TadV | CpaA | FppA | 117, 359, 392 |
| Type II pseudopili | Klebsiella oxytoca | Vibrio cholerae | Pseudomonas aeruginosa | |
| Major subunit | PulG | EpsG | XcpT | 299, 331, 347 |
| Core minor subunits | PulH, PulI, PulJ, PulK | EpsH, EpsI, EpsJ, EpsK | XcpU, XcpV, XcpW, XcpX | 299, 331, 347 |
| Prepilin peptidase | PulO | VcpD | XcpA | 37, 271, 321 |
Mannose-sensitive hemagglutinin pili.
Enteropathogenic E. coli.
Toxin-coregulated pili.
NA, not applicable.
Proteins with a type III signal sequence have been identified as components of DNA uptake (Com) systems in a wide variety of Gram-negative genera, as well as Gram-positive Bacillus, Clostridium, and Streptococcus species (30); in plasmid-encoded DNA transfer systems (221); as subunits of archaeal flagella (for recent reviews, see references 9 and 151); and even as electrically conductive nanowires in Geobacter (327). This broad distribution of type IV pilin proteins among eubacteria and archaea suggests an ancient and versatile modular architecture that has been adapted for many functions.
Type IVa versus Type IVb Pilins
A number of distinctive features divide major pilins into two classes, called T4aP and T4bP. The T4a pilins are a relatively homogeneous class and are found in plant, animal, and human pathogens such as Pseudomonas, Neisseria, and Dichelobacter, as well as in environmental genera such as Thermus, Myxococcus, Deinococcus, Bdellovibrio, and Shewanella (311). The T4b class is more diverse and is best characterized for enteric bacteria such as enteropathogenic, enterohemorrhagic, and enterotoxigenic Escherichia coli, Salmonella enterica serovar Typhi, and Vibrio cholerae. T4b pilins are further divided into subtypes, including the tight adherence pili (Tad; also called Flp or Fap) that were first identified in Aggregatibacter (Actinobacillus) actinomycetemcomitans (196, 210, 313). Tad pili are distributed among a variety of Gram-positive and Gram-negative species, including well-studied environmental bacteria such as Caulobacter (195, 393). Tad pili have smaller subunits (∼7 to 8 kDa) than other T4P and T2S systems (∼15 to 20 kDa). Other T4b subtypes include the plasmid-encoded longus pili of enterotoxigenic E. coli, so called because they can reach lengths of 20 μm or more (157). Some species can express multiple kinds of T4P (83, 117, 155, 195), while a subset of archaea coexpress pili and archaeal flagella (200, 296), both of which are formed from subunits of the type IV pilin protein family.
T4a and T4b major pilins have traditionally been distinguished by differences in the lengths and sequences of their leader peptides (135). The leader peptides of T4a major pilins are usually short (6 or 7 residues), while those of T4bP are longer (15 to 30 residues). The T4a major pilins most often have a methylated Phe at the N terminus following removal of the signal sequence, while T4b pilins can have other, typically hydrophobic, residues at the same position.
Major pseudopilins generally have short leader peptides (6 or 7 residues), although there are exceptions, such as Erwinia OutG (22 residues). The archaeal pilins and flagellins have leader sequences ranging from 3 to 20 residues (315). The PilFind algorithm (195) will allow for a more complete bioinformatic analysis of type IV pilin proteins, which in turn will provide better statistics on what is considered a “typical” leader in terms of length and sequence characteristics.
The size of the mature (processed) pilin has also been used as a criterion to distinguish the two subclasses, with T4b pilins being larger, on average, than T4a pilins (∼180 to 200 residues versus 150 to 175 residues), except for the Flp pilins, which are significantly smaller (∼50 to 80 residues). As more type IV pilin proteins with a range of overlapping sizes are identified through high-throughput sequencing and metagenomic approaches (195), sequence length is likely to become a less useful feature for distinguishing between the two classes. A more informative way to determine whether the pilin-like proteins in a particular bacterial strain are T4a, T4b, or T2S subunits is to consider their corresponding assembly system(s), as each has characteristic components (31). For example, T4a but not T4b assembly systems often include homologs of the PilMNOP inner membrane complex proteins, while T2S systems typically have GspLMC components instead (31, 125, 311, 386). Among the exceptions to this rule of thumb are T4a pili encoded in single large clusters (likely part of former mobile elements), such as the mannose-sensitive hemagglutinin (MSHA) pili of V. cholerae and other environmental bacteria (57, 149, 270, 350, 408). T4b assembly systems are more diverse with respect to their specific components, but both Tad and Com systems have characteristic elements (195). For comprehensive comparisons of T4a, T4b, and T2S assembly systems and their components—which are beyond the scope of this article—the reader is directed to previously published references (31, 99, 112, 113, 125, 139, 195, 205, 275, 309, 311, 313, 393).
STRUCTURES OF TYPE IV PILIN PROTEINS
Outside their conserved N termini, type IV pilin proteins can have widely divergent sequences. Despite this limited sequence identity, X-ray crystallography and nuclear magnetic resonance (NMR) studies have revealed the remarkable underlying structural similarity in this protein family. For comprehensive reviews on the particulars of type IV pilin structure, the reader is directed to previous references (112, 113, 168, 311). Below, we highlight recent findings regarding the structures of type IV pilin proteins.
Although several structures have been solved, thus far they are limited to Gram-negative major pilins (both T4a and T4b subfamilies) (26, 36, 114, 297, 306, 326, 420), a Neisseria-specific minor pilin from Neisseria meningitidis (176), and T2S major and minor pseudopilins from a number of species (16, 233, 236, 421, 422). To date, there are no structures available for the core minor pilins (named FimU, PilV, PilW, and PilX in Pseudomonas aeruginosa and PilH, PilI, PilJ, and PilK in Neisseria) (Table 1) from any T4P system, for the Tad/Flp pilins, for competence-specific pilins, or for any of the archaeal type IV flagellin or pilin subunits. This lack of structural depth is unfortunate, as it precludes a more complete understanding at this time of the flexibility and adaptability of the predicted T4P fold in other members of this family. The poor sequence conservation of the C-terminal domains of type IV pilin proteins usually prevents the generation of high-confidence models based on existing structures and the use of molecular replacement methods in crystallography studies.
Ironically, even though the extended N-terminal helix of type IV pilin proteins is their most distinctive trait, the majority of structures solved lack most of this region. To date, only three full-length structures are available (114, 169, 306), all of which are for T4a pilins. As techniques for purification and manipulation of membrane proteins improve, it will be important to add additional full-length structures for comparison. The pilins are typically truncated by ∼28 residues or more to remove the hydrophobic first half of the N-terminal α-helix, improving their solubility. The minimal impact of this deletion on pilin structure was confirmed by comparison of the N-terminally truncated and full-length versions of the P. aeruginosa PAK pilin, which were essentially identical, with a root mean square deviation for all atoms of 0.69 Å (114). Interestingly, it is possible to induce the formation of hollow pilus-like nanotubes from N-terminally truncated pilins by using a hydrophobic small molecule to replace the missing hydrophobic domains (27).
Although the N-terminal segment appears dispensable for folding of the C terminus, it has important functional, and potentially regulatory, roles in T4P biology (see below), and thus minor sequence differences in this region can be quite significant. For example, Aas et al. (4) showed that point mutations at the highly conserved Glu5 residue (Fig. 1) of the Neisseria gonorrhoeae major pilin, PilE, precluded assembly unless the mutant protein was coexpressed with wild-type subunits. The mixed fibers had altered T4P phenotypes, including differences in retraction dynamics, which were dependent upon the nature of the substitution. Similarly, a recent study of Myxococcus xanthus (424) showed that mutation of the Ala residue at position 20 of the mature pilin (Ala32 in prepilin sequence numbering) to Val resulted in formation of adhesive pili that were deficient in retraction. Alteration to Gly or Ser gave wild-type phenotypes, while other substitutions resulted in unstable pilins or pilins that were unable to assemble. These studies show that very small variations at key residues can have major consequences for function.
General Architecture of Type IV Pilin Proteins
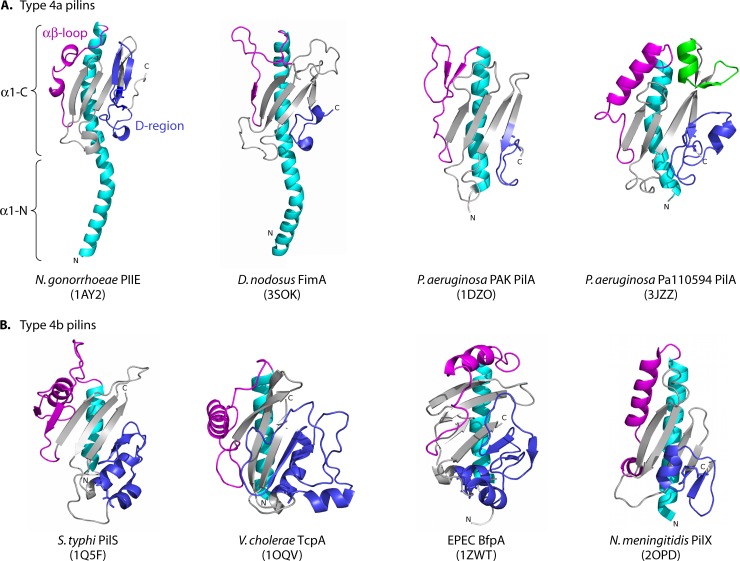
Full-length type IV pilin proteins resemble a lollipop or a ladle (Fig. 2). The extended N-terminal α-helix has two subdomains: α1-N (spanning amino acids ∼1 to 28) and α1-C (amino acids ∼29 to 52). The hydrophobic α1-N region protrudes from the globular C-terminal domain and forms the central core of the assembled pilus fiber (113). The α1-N domain is multifunctional, acting as a transmembrane segment to retain individual pilin subunits in the cytoplasmic membrane prior to assembly, as a protein interaction domain for subunit-subunit interactions in the fiber, and potentially as a regulatory domain (see below). In the case of T4P, where hundreds to thousands of subunits can form a single fiber, mature subunits disassembled from the pilus when it is retracted are thought to reenter the cytoplasmic membrane via the α1-N domain for use in subsequent rounds of assembly. In contrast, subunits of the short T2S pseudopilus may be degraded as a means of pseudopilus retraction (129). The α1-C region embedded in the C-terminal globular domain is amphipathic and packs against the head domain, which generally consists of a 4- to 7-stranded antiparallel β-sheet oriented 45° or more relative to the long axis of the α1 helix. Sequence differences in the loop regions and in the orientation of secondary structure elements within the C-terminal domain contribute to the structural diversity associated with these proteins.
Fig 2.
Structures of T4 pilins and minor pilins. Type IV pilins are characterized by conserved N-terminal α-helices (cyan) connected to a β-sheet (gray) by the αβ-loop (magenta). The N-terminal α-helix is divided into two subdomains, α1-N (amino acids ∼1 to 28) and α1-C (amino acids ∼29 to 52), as indicated on the N. gonorrhoeae PilE structure. In most pilins, two cysteine residues form a disulfide bond to form the variable D region (blue). Although the Dichelobacter nodosus FimA protein lacks these characteristic residues, its C terminus retains a similar architecture through alternative interactions (169). (A) T4a pilins, represented by Neisseria gonorrhoeae PilE (Protein Data Bank [PDB] accession no. 1AY2), Dichelobacter nodosus FimA (PDB accession no. 3SOK), Pseudomonas aeruginosa PAK PilA (PDB accession no. 1DZO), and P. aeruginosa Pa110594 PilA (PDB accession no. 3JZZ), share a shallow S-shaped N-terminal helix and a four-stranded continuous antiparallel β-sheet. The architectures of the αβ-loop and the D region of T4a pilins vary, with defined secondary structure in the GC pilin structure (top left) and the Pa110594 PilA structure (top right). Pa110594 PilA has an extended loop region, with an additional α-helix and β-strand between β3 and β4 (green) differentiating it from other T4a pilins. (B) T4b pilins, represented by Salmonella Typhi PilS (PDB accession no. 1Q5F), Vibrio cholerae TcpA (PDB accession no. 1OQV), and enteropathogenic E. coli BfpA (PBD accession no. 1ZWT), have a different protein fold, with discontinuous β-strands making up the β-sheet. The D region is embedded in the protein, with the C terminus forming the last strand of the β-sheet. The αβ-loop contains an α-helix oriented roughly 90° relative to α1. The structure of the noncore minor pilin PilX from N. meningitidis (PDB accession no. 2OPD) is similar to that of the major pilins but has two α-helices in the αβ-loop. The D region contains a short α-helix with a hook implicated in function (176). Figures were prepared using MacPymol (DeLano Scientific).
In the full-length structures of the T4a pilins P. aeruginosa PAK PilA and N. gonorrhoeae PilE, there is a shallow S-shaped kink in the N-terminal helix (114, 306), generated by Pro22 and Gly/Pro42 (26, 114, 126, 172, 219, 297, 306). The resulting curvature is thought to reduce intersubunit packing, thereby contributing to the flexibility of the fiber (67, 114, 251, 306), but may also contribute to fiber assembly-disassembly dynamics by making otherwise rigid transmembrane domains more flexible (78). Although a full-length structure of a T4b pilin has yet to be solved, N-terminal sequences of that subclass lack the characteristic Pro residues of T4a sequences. Instead, they have Gly residues that could impart flexibility. Li et al. (251) showed that solvent exposure of assembled V. cholerae TcpA subunits was similar to that of unassembled monomers, suggesting that limited interactions between T4b subunits in fibers exposed large areas of their surfaces. Portions of the N-terminal α-helices—previously assumed to be buried in the assembled fiber—were also solvent exposed. Those data suggested that despite the lack of N-terminal Pro residues, the packing of T4b pilins in the pilus is looser than that of T4a pilins, contributing to fiber flexibility. The looser packing means that they are less resistant to proteases than T4a pili, which are quite stable and, amazingly, remain intact even in 8 M urea (250).
The N. meningitidis noncore minor pilin PilX lacks a kink in the N-terminal helix, despite having a Gly residue at position 42; however, the full-length protein may have a curved N-terminal helix, as it has a Pro residue at position 22 (176). Although there are no other T4P minor pilin structures yet available, analysis of the representative P. aeruginosa minor pilins shows that only FimU and PilE have a conserved Pro22 residue, while the N. meningitidis minor pilin PilH, equivalent to FimU, also has a Pro residue at position 22. The major pseudopilin GspG—named XcpT and EpsG in Pseudomonas and Vibrio, respectively—has a conserved Pro22 residue. The minor pseudopilin GspH and its relatives have a Pro residue in the α1-C domain. Korotkov and Hol (236) solved the structure of a heterotrimeric complex of GspIJK, and based on its architecture, they hypothesized that it may form the tip of the T2S pseudopilus. It is possible that the Pro22 residue is important only for controlling the extent of intersubunit packing along the fiber to provide flexibility and is less important for those subunits located at the pilus or pseudopilus tip.
The 4- to 7-stranded β-sheet of type IV pilin proteins is a conserved structural motif that forms the majority of the C-terminal domain of the protein. In all of the T4a pilins, as well as the minor pilin and pseudopilin structures solved to date, this region is composed of a β-meander with 3 to 4 β-strands having nearest-neighbor connectivity (16, 112, 176, 233, 236, 244, 421, 422). The T4b pilins are somewhat different, in that the antiparallel β-sheet is composed of 5 to 7 β-strands in the case of V. cholerae TcpA and S. Typhi PilS or of a mix of parallel and antiparallel β-strands in the case of BfpA from enteropathogenic E. coli (EPEC) (Fig. 2) (36, 114, 326, 420), with non-nearest-neighbor connectivity.
What Makes Type IV Pilin Proteins So Diverse?
Although type IV pilins have generally similar architectures, their amazing functional diversity comes from the hypervariable loop regions connecting the core structural elements. Even with the limited number of structures currently available, differences in shape and surface properties arising from sequence diversity in loop regions are readily apparent. Evolution of loops is constrained, however, by the need to accommodate multiple protein-protein interactions with neighboring subunits during fiber assembly. Expression of even closely related pilins in a single background yields only fibers of homologous composition (308), providing strong evidence that interactions among or a lack of steric clashes between loop regions is important for polymerization. There are 3 important hypervariable regions: the loop connecting the conserved N-terminal α-helix to the remainder of the protein, the loops between β-strands of the β-sheet, and the C-terminal loop.
The loop connecting the main N-terminal α-helix to the C-terminal β-sheet is called the αβ-loop in pilins and the variable loop in pseudopilins. In the T4a pilins, the αβ-loop can contain a minor β-sheet like that in the pilin of P. aeruginosa strain PAK, α-helices such as those of N. gonorrhoeae PilE and P. aeruginosa Pa110594 PilAV (the latter of which has a loop region with helical character like that of the P. aeruginosa strain K122-4 pilin), or it can be largely unstructured, as in Dichelobacter nodosus FimA (Fig. 2) (26, 114, 115, 169, 172, 219, 306). The αβ-loop of N. gonorrhoeae PilE has a short, 1-turn α-helix that is O-glycosylated at Ser63, whereas PilAV of P. aeruginosa Pa110594 has a 3-turn unmodified α-helix (114, 143, 297, 306). This α-helix is reminiscent of the one in N. meningitidis PilX, which is a 4-turn α-helix in the corresponding region. In addition, there is a prominent α-helix in the αβ-loops of most T4b pilin structures solved to date (36, 114, 176, 326, 420), although its ∼90° angle relative to the extended α1-helix differs from that of the T4a proteins (Fig. 2). The exception is CofA of E. coli; its αβ-loop has a 10-residue insertion that forms an irregular loop containing a short 310 helix (234).
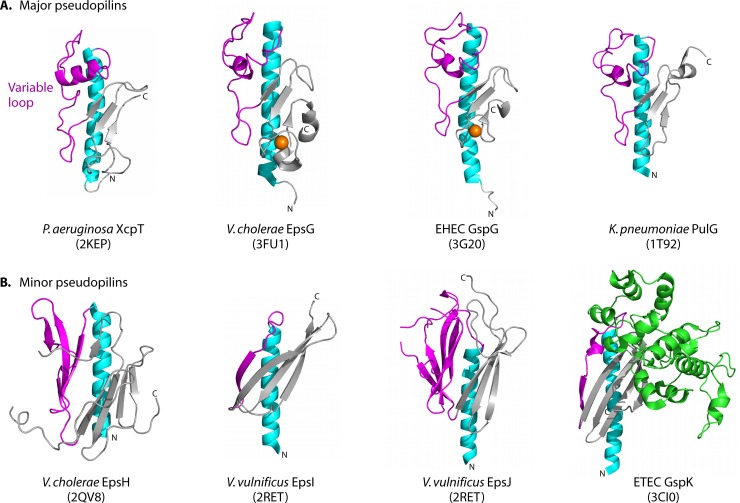
In contrast to the T4 pilins, all of the major pseudopilin structures solved thus far have a mostly unstructured loop with a single helix connecting the N-terminal α-helix and β-sheet domains (Fig. 3). Although referred to as a variable loop, this region has a conserved motif (LPXDPWGXXY) that is found only in major pseudopilins (16, 233, 236). Until recently, the major pseudopilins were thought to be a single protein class, since they were among the few functionally interchangeable components of T2S systems from different species. However, Durand et al. (127) showed that the HxcT major pseudopilin from P. aeruginosa could not complement a P. aeruginosa mutant lacking XcpT, the major pseudopilin from its predominant T2S system. Biochemical and mutagenesis studies confirmed that the Hxc (homolog of Xcp) system is the archetype of a similar but separate T2S subfamily. The lack of conservation in sequence and secondary structure of the variable loops in minor pseudopilin orthologues across species may reflect a role in selection of unique sets of secreted substrates, a hypothesis supported by the recent demonstration of direct interactions between minor pseudopilins and effector proteins in P. aeruginosa (124). The minor pseudopilin GspI (EpsI) from Vibrio vulnificus has a single β-strand in the variable loop, whereas GspI from enterotoxigenic E. coli has a short α-helix followed by a β-strand, similar to that found in the variable region of GspK (236, 421, 422). The variable regions of GspH and GspJ are substantially larger than those of other type IV pilin proteins and are composed mainly of β-strands (236, 422). In particular, the variable segment of GspH forms a five-stranded antiparallel β-sheet, although the strands are not continuous (Fig. 3) (422). The structures of the orthologous proteins GspJ (from enterotoxigenic E. coli), EpsJ (from V. vulnificus), and XcpW (from P. aeruginosa) vary in this region, but they are mostly β-strands (244).
Fig 3.
Structures of T2S pseudopilins and minor pseudopilins. T2S pseudopilins are structurally similar to the T4 pilins, with an N-terminal α-helix (cyan) connected to a β-sheet (gray) by a variable loop (magenta). (A) The major pseudopilins, represented by Pseudomonas aeruginosa XcpT (PDB accession no. 2KEP), Vibrio cholerae EpsG (PDB accession no. 3FU1), enterohemorrhagic E. coli GspG (PDB accession no. 3G20), and Klebsiella pneumoniae PulG (PDB accession no. 1T92), have variable loops with a helical character followed by a 3-stranded β-sheet. Near the C terminus is a calcium-binding motif (with a calcium ion shown in orange) in EpsG and GspG, although calcium binding is expected to occur in all major pseudopilins (235). (B) The minor pseudopilins, represented by GspH from V. cholerae (PDB accession no. 2QV8), GspI and GspJ from V. vulnificus (PBD accession no. 2RET), and GspK from enterotoxigenic E. coli (PDB accession no. 3CI0), vary in architecture, with a large α-domain insertion (green) in GspK. Figures were prepared using MacPymol (DeLano Scientific).
Loops connecting the β-strands that form the C-terminal β-sheet of type IV pilins and pseudopilins are mainly unstructured, though shorter loops can form β-turn motifs. Exceptions include PilAV from P. aeruginosa Pa110594 and the minor pseudopilin GspK, where defined secondary structure elements appear in specific loops. Pa110594 PilAV has an α-helix and a β-strand between β3 and β4, whereas the loop region between β2 and β3 of GspK has a massive α-domain insertion composed of 12 α-helices and 4 short β-strands (Fig. 2 and 3) (236, 297). The α-domain of GspK also contains a disulfide bond that likely stabilizes the domain, as well as a dinuclear metal binding site predicted to contain calcium.
The loop regions may participate in interactions with neighboring subunits, although the β3-β4 loop of Pa110594 PilAV—located at the “top” of the structure, adjacent to the αβ-loop—has also been hypothesized to interact with its dedicated accessory protein, TfpZ (112, 113, 115, 297). Interestingly, D. nodosus FimA has a large unstructured β1-β2 loop that occupies a position similar to that of the β3-β4 loop in PilAV (169) and is coexpressed with a TfpZ-like accessory protein, FimB (220). For T4b pili, limited interactions between subunits (251) suggest only minimal contact between loops of adjacent monomers in the assembled fiber. However, two recent studies (207, 255) showed that specific residues in the loop regions of T4b pilins play critical roles in interfiber bundling and aggregation, as mutations at those positions affect lateral interactions between filaments. These data suggest that surface topography created by variable loops can affect pilus function in ways that are independent of effects on interactions between adjacent subunits in the same fiber.
The fiber surface generated by loops of adjacent subunits can create contiguous cavities or grooves that provide binding sites for other molecules. For example, the cystic fibrosis transmembrane regulator protein, a host receptor for S. Typhi T4bP, is proposed to bind in a pocket of complementary charge formed by adjacent PilS subunits (35). Where T4P are involved in competence, binding of extracellular DNA can be facilitated by positively charged furrows on fiber surfaces (115, 396). In the case of the minor pseudopilins, the large, arrowhead-shaped α-domain insertion of GspK and the organization of the GspIJK heterotrimer with the α-domain at the top of the complex led to the suggestion that the insertion domain may be positioned at the tip of the pseudopilus, where it could induce secretin opening or interact with secreted proteins (142, 236). These hypotheses were recently strengthened by elegant studies showing that minor pseudopilins equivalent to GspH, GspI, and GspK from the Xcp system of P. aeruginosa interact directly with the T2S substrate, elastase (124), and with the periplasmic portion of the secretin (330).
The last important hypervariable region of type IV pilin proteins is located at the C terminus. In this region, most major pilins and some minor pilins have a conserved structural element known as the disulfide-bonded loop (DSL), or D region (Fig. 2, shown in blue). In the majority of major pilins characterized to date, two Cys residues near the C terminus form a disulfide bond that staples the C terminus of the protein to the β-sheet. An important disulfide is also present in the ComGC competence pseudopilin, involved in formation of the DNA uptake system in Bacillus subtilis and other Gram-positive species (94). Interestingly, the recently characterized structure of D. nodosus FimA from serotype A (169) revealed a D-region architecture similar to that of other major pilins, even though it lacks the typical C-terminal disulfide bond (Fig. 2). Instead, a noncovalent network of hydrogen bonds maintains the orientation of the loop. There are two Cys residues in FimA, but they form a disulfide bond linking the αβ-loop—which contains a large unstructured region—to the start of β2; a similar bond is present in the P. aeruginosa K122-4 pilin, which has the more typical C-terminal disulfide as well (26).
The length and secondary structure of the D region vary between pilins of different species, even among pilins from different strains of the same species (242). In the P. aeruginosa PAK and K122-4 pilin structures, the D region is short and has a type I β-turn followed by a type II β-turn (26, 172). Although the Pa110594 PilAV pilin has a larger D region, with an additional α4-helix, the structure of a type I β-turn followed by a type II β-turn is conserved (297). The D region of the N. gonorrhoeae pilin contains a prominent β-hairpin (306) whose sequence is among the most hypervariable among antigenic variants.
The D region of major pilins has a structural role, as mutations that lead to a loss of C-terminal disulfide bond formation—including mutations in both general and dedicated disulfide bond isomerases—prevent pilus assembly (94, 390, 403, 427, 429). For some species, a lack of disulfide bond formation was reported to result in pilin instability. This finding could be due to a loss of reactivity with pilin-specific antisera, as the D region is an important immunodominant epitope. In P. aeruginosa, mutation of either of the Cys residues or deletions within the loop region impact twitching motility by impairing assembly (170). Because the D region contacts adjacent subunits in the fiber, altering its conformation could impede subunit-subunit interactions. The D region continues to have a key role after polymerization of the subunits, because treatment of assembled pili with reducing agents leads to their rapid disintegration (250). In B. subtilis, mutation of the Cys residues in ComGC to Ser resulted in a loss of transformation capacity, a loss of higher-order ComGC complexes, and a marked decrease in detectable membrane levels of monomeric ComGC, suggesting instability of the mutant proteins (94). It will be interesting to determine if mutations that disrupt the hydrogen bond network that stabilizes the FimA C-terminal region (169) will similarly affect stability or pilus assembly in D. nodosus.
A number of studies suggested that the D region of the T4a major pilin can function as the adhesive component of the pilus, as specific antibodies or competitive peptide inhibitors were specifically able to block pilus-mediated binding to a variety of surfaces (156, 249, 374). To explain how D regions with diverse sequences can provide similar functions, their adhesive properties were attributed to main chain- rather than side chain-based interactions (172). Those data led to a focus on development of anti-P. aeruginosa vaccines containing peptides corresponding to the D region (71, 72, 75, 162, 216). Newer information showing that the minor pilins are present in sheared pilus fractions (154, 416) and that the orthologous PilC1 or PilC2 (Neisseria and Kingella) and PilY1 (Pseudomonas) proteins are potentially pilus associated and required for adherence to—and manipulation of—the host (174, 204, 217, 288, 289, 384) suggests that additional studies are needed to unequivocally identify all T4aP adhesins. Given the wide range of surfaces to which T4P bind, there may be multiple players that contribute to adherence under specific circumstances.
The T4b pilins have substantially larger D regions than those of the T4a pilins, containing more defined secondary structure elements (36, 114, 176, 326, 420). The connectivity between strands of the β-sheet varies, and the characteristic disulfide bond joins residues that are more distant from one another in the primary sequence than the case with the T4a pilins. In V. cholerae, point mutations in a portion of the TcpA D region that alter its conformation affect pilus morphology or stability, whereas mutations in the area involved in lateral interactions between fibers have effects on pilus-mediated aggregation and host cell colonization (207, 227, 255).
The D region in the noncore N. meningitidis minor pilin, PilX, is small but has defined secondary structure (176). Deletions within this region severely impair pilus-mediated aggregation and adhesion, supporting a key role for the region in PilX function (176). The PilX D region has a hook-like conformation, proposed to protrude from assembled fibers. Upon retraction of adjacent but antiparallel fibers, the hook-like domains of PilX were hypothesized to catch upon one another, antagonizing pilus retraction and thus contributing to cell-cell aggregation (176). Although sequence analyses of other core and noncore minor pilins revealed that many have two or more Cys residues near the C terminus, there are no structures yet available to confirm the formation of predicted disulfide bonds, nor have their roles in minor pilin stability or function yet been examined.
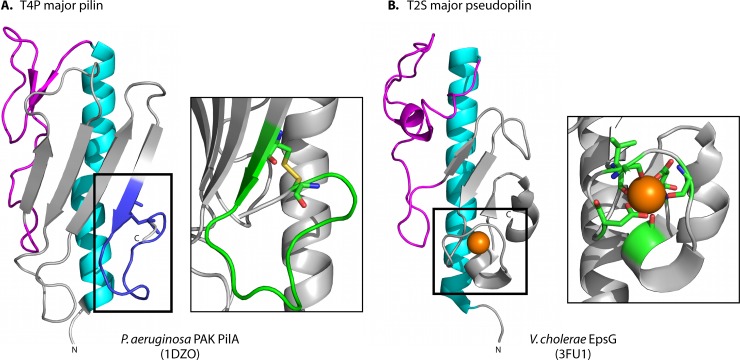
Unlike most major T4 pilins, major pseudopilins lack a stabilizing disulfide bond near the C terminus. Instead, crystal structures of the major pseudopilins from V. cholerae, V. vulnificus, and enterohemorrhagic E. coli (EHEC) revealed a calcium-binding site in this region (Fig. 4) (235). Calcium is coordinated in an octahedral manner by different residues in the V. cholerae and EHEC proteins, suggesting that they are the archetypes of at least two structural subclasses. Both contribute carboxylates from two conserved Asp residues, but in the V. cholerae pseudopilin, four main chain carbonyl oxygens complete the Ca2+ coordination site, while the same function is provided by two main chain carbonyl oxygens and a Thr/Ser side chain oxygen in the case of EHEC (235). Between the conserved Asp residues involved in calcium binding, there is an additional α-helix in the Vibrio-like pseudopilins that is not present in EHEC-like proteins. Although structures of the major pseudopilins from Klebsiella oxytoca and P. aeruginosa have been solved by X-ray crystallography and NMR spectroscopy, respectively, a calcium-binding site was not identified (16, 233). However, a β-strand swap between protomers in the dimeric structure of GspG from K. oxytoca may have disrupted the coordination site (233, 235). Loss of secretion upon mutation of the Asp residues involved in calcium binding suggested that they are integral to T2S system function (235). Based on the importance of the D-region conformation to pilus assembly, loss of secretion was likely related to the inability of mutant pseudopilins to form a pseudopilus (see below).
Fig 4.
Comparison of the T4 pilin D region and the T2S pseudopilin calcium-binding domain. One of the defining differences between T4 pilins (left) (P. aeruginosa PAK PilA [PBD accession no. 1DZO]) and T2S pseudopilins (right) (V. cholerae EspG [PBD accession no. 3FU1]) is found at the C terminus, where the majority of T4 pilins have a disulfide-bonded loop (D region) and the pseudopilins have a calcium-binding motif (with a calcium ion shown in orange) (235). The disulfide bond creates a loop with two β-turns (left inset), while calcium binding by both main chain and side chain residues stabilizes the corresponding region in the pseudopilins (green, right inset). Both structural motifs are implicated in function. Figures were prepared using MacPymol (DeLano Scientific).
FROM SUBUNIT TO FIBER
A defining functional characteristic of type IV pilin proteins is their ability to reversibly assemble into polymeric fibers, which in the case of pili can further interact to form higher-order bundles or tangled aggregates. In all cases examined to date, the subunits assemble in a helical manner. Several T4P fiber models have been proposed, including both right- and left-handed single-start (172, 219, 306) and multistart (114, 115, 251) helical models. Although under native conditions T2S pseudopili are predicted to be only long enough to span the periplasm of Gram-negative bacteria, overexpression of the major pseudopilin leads to the formation of long, surface-exposed pseudopili that are amenable to structural analyses (77, 128, 349, 400). In the case of archaeal flagella, only limited structural information on the fibers is available, but thus far, right-handed filament models predominate (378). In some archaeal species, the flagella are made of multiple subunit types, making model building more challenging.
Type IV Pilus Models
Crystallographic and cryo-electron microscopy (cryo-EM) evidence suggests that the pilin monomers of the best-characterized T4a pilus from N. gonorrhoeae are arranged in a 3-start left-handed helix, which can alternately be viewed as a 1-start right-handed or 4-start right-handed helix, with a predicted 3.6 subunits per turn (Fig. 5) (115). The fiber has an outer diameter of ∼60 Å (115), consistent with the ∼65-Å-diameter opening of the T4aP secretin through which the fiber passes to the cell's exterior (49, 103, 104). The fiber is stabilized by hydrophobic and electrostatic subunit-subunit interactions between the N-terminal α-helices, which form the central core of the pilus. The methylated, positively charged N-terminal residue of one subunit is thought to neutralize the charge of the Glu +5 residue of the adjacent subunit and to act as a means of registration to ensure the correct degree of vertical displacement between one subunit and the next (115, 306).
Fig 5.
Models of pilus and pseudopilus fibers. Using structural subunit information, low-resolution EM data, and biochemical data, fiber models of the T4aP (left), T4bP (center), and pseudopilus (right) were generated. The T4aP model can be viewed as a one-start or four-start right-handed helix or a three-start left-handed helix (one strand is pictured, with two subunits shown as a green cartoon) with a diameter of approximately 60 Å. Tight packing of the N-terminal helices holds the structure together, with additional polar interactions between the C-terminal head groups. The three-start left-handed helix T4bP model has a larger diameter, at approximately 90 Å, due to the larger subunit size along with the more loose packing of the subunits, exposing portions of the N-terminal helix and producing deep grooves and bulges along the fiber (model kindly provided by Lisa Craig). The right-handed one-start helix T2S pseudopilus model is slightly larger than the T4aP model, at 65 Å, with hydrophobic and electrostatic interactions stabilizing subunit interactions (model kindly provided by Olivera Francetic). Figures were prepared using MacPymol (DeLano Scientific).
The N-terminal α-helices form staggered helical bundles that spiral along the length of the pilus. A single α-helix participates in 3 different helical bundles, contacting neighboring subunits at residues 1 to 13, 4 to 19, and 24 to 39 (115). Packing of the α-helices is facilitated by the S-shaped curvature created by residues Pro22 and Gly42 (113). The C-terminal domains form subunit-subunit contacts, mainly in loop regions. The outer surface of the pilus, formed by the C-terminal domains of the subunits, is characterized by deep grooves separating the head groups (115). From the 3-start left-handed helix view, there are polar interactions between the αβ-loop of one subunit and the D region of the next subunit, while in the 4-start right-handed helix view, there are interactions between the loop regions connecting strands of the β-sheets (Fig. 5) (115). Li et al. (250) reported that stacking of conserved aromatic residues, particularly the mature N-terminal Phe with Tyr24 and Tyr27 of the α1-N domain of the adjacent subunit, likely contribute additional stabilizing interactions in the pilus core. Combined, these features allow for a highly flexible structure that can withstand the piconewton-scale forces required for twitching motility (263).
While a 70-Å-diameter opening was reported for the T4bP secretin, T4bP fiber models predict a wider pilus of approximately 90 Å (114, 115). Current models and experimental data support a 3-start left-handed helix (114, 250, 251). The C termini of T4b pilin monomers are bulkier in three dimensions than their T4a pilin counterparts, and they protrude outward. Like T4aP, T4bP is held together by tight interactions of the subunits' N-terminal helices in the core of the pilus; however, hydrogen-deuterium-exchange mass spectrometry (MS) data comparing monomeric with assembled subunits suggested that the C-terminal head groups are loosely packed (251). The only interactions of the head groups occur via polar and hydrophobic interactions between the αβ-loop and a portion of both α1-N and α3, not α4 of the D region as previously thought. This minimal level of interaction leaves the rest of the pilin subunit—including the majority of the D region—solvent exposed, and it results in the presence of bulges and deep grooves that expose an amphipathic portion of the N-terminal helix, previously thought to be buried completely in the fiber's core (251). The loose packing of the pilins' C termini is thought to underlie the reduced resistance of T4bP to heat, proteases, and chaotropic agents compared with that of T4aP (250).
Due to technical and computational constraints, pilus models are usually derived from X-ray diffraction or cryo-electron microscopy analyses of straight segments of assembled pili, into which crystal or NMR structures of individual subunits are fitted. There are a number of caveats to this approach. At the moment, only major subunit structures are considered during the model-building process, although it is becoming clear that minor subunits may also be part of the fiber (100, 154, 416). Pronounced flexibility is an inherent property of T4P, and the interactions between pilin subunits in the straightened segments of fibers used to collect diffraction or cryo-EM data may be different from those that occur in acutely bent segments. The resolutions that can be obtained are low, presumably because the filaments are not uniform (250).
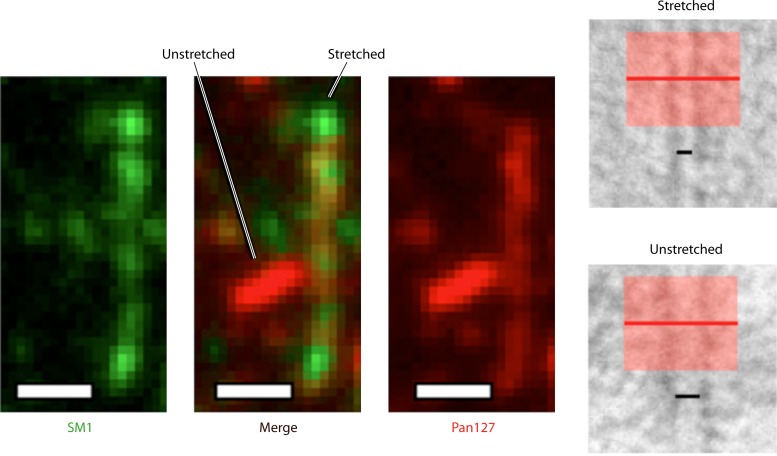
Interestingly, Biais and colleagues (46) demonstrated that the T4a pili of N. gonorrhoeae can undergo dramatic and reversible conformational changes upon application of threshold levels of force, stretching in their longer dimension to become ∼40% narrower than unstretched pili, with a concomitant reduction of ∼2/3 of the mass per unit length (Fig. 6). These changes alter the nature of interactions occurring between subunits, as they expose epitopes on the pilins that are normally occluded in unstretched fibers. In some cases, the deformations occur in localized regions, implying that there are multiple types of interactions that can occur between pilin subunits in a single intact fiber. The ability to stretch without breaking was suggested to provide a buffer against force fluctuations along the length of a fiber, preventing detachment during transient increases in shear forces (46). The ability to undergo deformation was recently linked to the presence of the Neisseria-specific minor pilin, PilX (63). Pili from PilX-expressing N. meningitidis underwent the structural transition that exposes the epitope for the monoclonal antibody (MAb) SM1 upon binding to host cells, while pilX mutants or strains expressing mutant forms of PilX did not show this change. In light of PilX's proposed function as a retraction antagonist (175, 176), it is possible that deformations are initiated when PilX-PilX interactions between adjacent but antiparallel fibers that are being retracted in opposite directions temporarily increase local forces. Affinity differences in the subunit-subunit interaction interfaces of PilE-PilE versus PilE-PilX may allow for initiation of deformation within a specific fiber.
Fig 6.
Force-dependent conformational changes in T4aP fibers. Biais et al. (46) showed that applying pulling forces just below those that cause Neisseria pili to break can lead to stretching of the pili in their long dimension, making them ∼40% narrower, with only 2/3 the mass per unit length of normal fibers (right). This conformational distortion was reversible and exposed epitopes (left) for the SM1 monoclonal antibody (green) that were hidden in normal fibers (red). (Left panel reprinted from reference 46 with permission of the publisher; right panel courtesy of Nicolas Biais.)
The propensity of T4P to form bundles that mediate bacterial aggregation has been well established (365, 411). However, the ability of such bundles to cooperatively generate retraction forces that exceed those produced by single filaments was not appreciated until recently. Biais et al. (47) developed a clever assay in which they used micropillars made from an elastic hydrogel whose stiffness could be varied from 100 to 500 pN/μm. Piliated N. gonorrhoeae was inoculated onto a single micropillar and incubated until the pili became attached to adjacent micropillars, whose lateral displacement upon pilus retraction was monitored by video microscopy. By manipulation of the growth medium, retraction events were biased toward those generated by single pili or by laterally associated bundles. Bundles of pili, containing up to 10 individual fibers, generated retraction forces 8 to 10 times higher than those generated by a single pilus, suggesting that T4P can retract in a cooperative manner and impose substantial forces. Further studies by Holz et al. (184) confirmed that cooperative retraction of multiple pili increases the persistence of Neisseria movement.
Type II Secretion Pseudopilus Models
The structure of the pseudopilus in the T2S system has been more challenging to elucidate, since native fibers are short and not exposed on the cell surface. However, some major pseudopilins are capable of forming long, surface-exposed fibers when overexpressed (127, 128, 349, 400). Such hyperpseudopili measure 6 to 9 nm in diameter. This dimension is consistent with initial electron microscopy analysis of the T2S secretin, which predicted a 95-Å-diameter opening (49). However, more recent cryo-EM data from the V. cholerae system suggest that the secretin channel varies in diameter, with a 75-Å opening that constricts to 55 Å followed by a chamber of 100 Å (330).
Initial analysis of the pseudopilus suggested a one-start left-handed helical arrangement of pseudopilins (233); however, recent computational models calculated from sparse data suggest otherwise (76, 77). Instead of docking the crystal structure of the pseudopilin subunit within high-resolution cryo-EM data (as was done to generate the T4aP fiber model), Campos et al. (76, 77) used low-resolution EM data, conformational restraints, and molecular modeling to generate a suite of models. On average, the pseudopilus fiber model is similar to the GC pilus model, with a diameter of 65 Å and a one-start right-handed helical arrangement (Fig. 5). Taking the restraint energy into account during structure calculations confirmed the right-handed helical character, as no models with a left-handed helix were generated. The smaller size of pseudopilins than of pilins is consistent with the observation of ∼4.25 subunits per turn for the pseudopilus, compared to ∼3.6 for the pilus. There are several subunit interactions mediated by hydrophobic and electrostatic contacts, where each subunit interacts to various extents with three upper and three lower subunits (77). Intermolecular salt bridges help to stabilize the structure, with interactions between Asp44-Arg88 and Asp48-Arg87 being key to pilus assembly and secretion. Asp44 and Asp48 are part of the α1-C domain, while Arg87 and Arg88 are in the variable loop region. A positive patch within the variable loop is involved in interactions with the crucial calcium-binding motif, characterized by two conserved Asp residues (77, 235). Interestingly, only 18% of the models generated included a potential salt bridge between Glu5 and the positively charged N-terminal residue, as was predicted for T4P subunits; instead, the majority of structures showed interaction between Glu5 and the side chains of Lys residues in the N-terminal helix. In support of this alternative model, mutation of Lys28 abolished piliation and reduced secretion (77).
How Do Individual Subunits Assemble into a Fiber?
Among the (many) unresolved mysteries in the field is how type IV pilins are extracted from the membrane and assembled into (and then subsequently disassembled from) a fiber. How is the process initiated, and by which components? Is the rate of assembly similar to the rate of disassembly, which is estimated to be between 1,000 and 1,500 subunits per second (281, 358)? How might disassembly rates be affected by bundling of pili or by binding of extraneous molecules such as DNA? Do pilins interact directly with the motor ATPases that power assembly/disassembly or with membrane-bound components that function to transduce the mechanical energy generated by ATP hydrolysis to the subunits (285)? How is the incorporation of core and nonconserved minor subunits (154, 175, 176, 416) into the fibers controlled? Although many of these questions remain unanswered, substantial progress is being made on some fronts.
While early studies suggested that pilin dimers were the building blocks of the pilus (409), more recent models suggest that single pilin monomers are added to the growing pilus fiber (115). The discovery that minor subunits are present outside the cell in assembled fibers (154, 416) also meant that the order and stoichiometry of subunit incorporation needed to be considered. The structure of a heterotrimeric T2S core minor pseudopilin complex, which revealed a bulky α-domain insertion in GspK, implied that the complex was likely to form the tip of the fiber; it was difficult to envision how additional subunits could fit above it without significant steric hindrance (142, 236). If the above hypothesis was true, the core minor subunit complex would form first, and subsequent polymerization of major subunits beneath the complex would lead to fiber elongation. Based on this idea, GspK, and possibly its core minor pilin equivalent, PilX (in Pseudomonas) or PilK (in Neisseria), would be the first component to be extracted from the membrane. In both T2S and T4P systems, this particular subunit is unique in that it lacks the highly conserved Glu5 residue present in other type IV pilin proteins, instead having a nonpolar residue at position +5. The absence of a charged side chain may improve the ability of GspK orthologues to leave the membrane during assembly initiation. Core minor pilin/pseudopilin gene clusters can readily be identified due to the presence of the gene encoding this atypical subunit.
Recent data from an exciting study by Cisneros and colleagues (100) supported the idea that the minor subunits form an initiation complex that primes subsequent fiber assembly. They showed that GspI, GspJ, and GspK orthologues (PulI, PulJ, and PulK) from Klebsiella were important for efficient expression of pseudopili in an E. coli overexpression system (349) and in a strain missing all of the minor pseudopilins, that GspI and GspJ alone were sufficient for assembly to proceed. Pilus assembly in spheroplasts was examined to rule out the possibility that the minor pseudopilins participate in assembly by opening the secretin; pseudopili were observed only when the minor pseudopilins were expressed, regardless of secretin expression. Bacterial two-hybrid and cysteine cross-linking studies showed that GspI and GspJ (as well as GspI and GspK) interact in the membrane and that due to the resulting conformational changes, their α1-N segments become displaced vertically by ∼1 nm relative to their initial side-by-side orientation. This displacement is equivalent to the rise between subunits in an assembled fiber, hinting at the formation of a priming complex. Molecular dynamics simulations further supported the formation of a staggered, pseudohelical GspI-GspJ-GspK complex, from which GspK protruded and deformed the membrane. This complex was proposed to provide a stable nucleus, templating the subsequent addition of major subunits beneath it.
The core minor pilins of the T4aP system could similarly form an initiation complex that primes pilus assembly, leading to their incorporation into the fiber (154, 416), although further studies are needed to test this idea. Data from overexpression studies suggested that the stoichiometry of the P. aeruginosa GspK equivalent, PilX (called PilK in Neisseria), and GspH equivalent, FimU (called PilH in Neisseria), relative to other minor pilins was important for the control of pilus length, since mutants with excess PilX or FimU had extremely short but functional pili (154). Interestingly, interactions of PilX with other components of the T4aP assembly system were implied by cross-complementation studies using two strains of P. aeruginosa (PAO1 and PA14) encoding heterologous sets of minor pilins. Replacement of the entire set of minor pilins of one strain with those of the other did not restore pilus assembly until the native PilX protein was also provided (155).
Tad/Flp systems differ from other T4aP, T4bP, and T2S systems in that they lack obvious homologs of the 4 core minor pilins (393). Typically, they have one major subunit and two minor subunits that have not been detected in the fibers. Do those proteins function to initiate Tad pilus assembly? The lack of a retraction ATPase in Tad systems may preclude the need for a core minor pilin complex, as studies of the T4aP system showed that all minor pilins may be dispensable for pilus assembly in retraction-deficient backgrounds (79, 154, 416). However, this possibility needs to be tested formally, as T2S systems, which also lack a retraction ATPase, need the minor pseudopilins to function. This is a rapidly moving area of research, and further studies are necessary to determine whether T4P assembly is primed or optimized by one or more minor pilins.
FUNCTIONS OF TYPE IV PILIN PROTEINS
The relationship between the diverse sequences and structures of pilin proteins and their broad range of functions is among the fascinating but poorly understood aspects of type IV pilin protein biology. This puzzle persists because although proteins with widely divergent sequences can play similar roles—suggesting that substantial sequence variation can be tolerated—in some cases even single residue changes in otherwise identical proteins can markedly affect function (4, 424). Well-characterized roles include adherence to living and nonliving surfaces, including other bacteria; twitching motility; modulation of biofilm architecture; DNA uptake (competence) and exchange (conjugation); secretion of exoproteins; and bacteriophage susceptibility. More exotic functions include swimming motility and binding of sugars (both archaeal traits), electron transfer (in Geobacter), and manipulation of host cell biology (20, 327). Some systems have multiple functions; for example, the T4P of Neisseria are adhesins and motility organelles and are required for competence, while the T4P system of V. cholerae is involved in both adherence and protein secretion. Some bacteria express multiple types of T4P or both T4P and T2S systems. A recent bioinformatic analysis of sequenced genomes by Imam and colleagues (195) showed that the distribution of genes with the potential to encode pilin-like proteins is far broader than previously appreciated, and thus the list of processes in which they are involved is ripe for expansion. This section gives brief examples of the ongoing research into the functions of type IV pilin proteins.
Adherence and Aggregation
The most commonly reported function of T4 pili is adherence to a diverse range of surfaces, from metal, glass, plastics, and rocks to plants and various host tissues (48, 51, 123, 156, 164, 209, 217, 313, 341, 391, 393, 419, 431). T4P have repeatedly been shown to contribute to the infectivity of pathogens—even intracellular pathogens such as Francisella tularensis (344)—firmly establishing them as important virulence factors.
Although T4P promote adherence, the biophysical aspects of their adhesive mechanisms are not well characterized. In other words, how exactly do T4P stick to diverse surfaces, and with what affinities? Does adherence occur only at the tips of the fibers, or are additional points of contact made along the fiber length? A series of studies aimed at measuring the forces generated upon pilus retraction was carried out (47, 101, 281), which required, of course, that the pili be stuck to a surface. However, it is not possible to calculate binding affinities from studies that measure the maximum pulling forces that pili can withstand, as release could happen for a number of reasons beyond loss of cohesion with a surface. Alternatives include breakage of the fiber (disruption of subunit-subunit interactions) or the forcible—or possibly deliberate—separation of the fiber from the cell at its base. The latter mechanism has been proposed to explain shedding of S. Typhi pili (385).
The major subunits of T4P can act as the adhesive component, as has been reported for P. aeruginosa pilins (249), Neisseria pilins (351), the bundlin subunit of EPEC bundle-forming pili (194), and the PilS subunit of S. Typhi T4bP (394). For P. aeruginosa, the ability of pilins of diverse sequence to bind to the proposed receptor, the glycosphingolipid asialo-GM1, on host cells was suggested to occur through main chain rather than side chain interactions (172). However, the identity of a specific host receptor for P. aeruginosa has been called into question by a study showing that asialo-GM1 does not colocalize with piliated bacteria bound to epithelial cells (132). The major pilin from Neisseria, PilE, was reported to hemagglutinate erythrocytes (351) and to mediate binding to host proteins on endothelial cells (110). The receptor for Neisseria pili was proposed to be CD46 (214, 413), although newer evidence suggests otherwise (224).
Characterization of the binding mode of E. coli bundlin showed that it is a lectin that recognizes N-acetyllactosamine (LacNAc) moieties on host cells (191, 194). Interaction of bundlin with N-acetyllactosamine induces pilus retraction and the upregulation of virulence gene expression (192). A similar phenomenon has been reported for the T4aP of M. xanthus, where binding of T4P to its self-produced exopolysaccharide (EPS) matrix induces pilus retraction and helps to coordinate social motility for fruiting body formation (253).
Not all pilins are lectin-like, however; PilS from S. Typhi was reported to bind to the first extracellular loop of the cystic fibrosis transmembrane conductance regulator (CFTR) protein on intestinal epithelial cells (or peptide mimetics thereof), while its equivalent from S. enterica serovar Typhimurium does not (394). This specific interaction was thought to explain why only S. Typhi is capable of causing human epidemics. However, these data were recently challenged by immunofluorescence studies that did not find colocalization of bacteria and CFTR, although the pili were required for epithelial cell invasion (62). In a final example, expression of the PilA2 subunit from the Gram-positive bacterium Clostridium perfringens in a nonpiliated N. gonorrhoeae mutant provided the recombinant strain with the novel ability to bind to myoblast cell lines, similar to C. perfringens itself (333).
Alternatively, T4P can display minor pilins or other adhesive proteins that mediate binding. The N. gonorrhoeae noncore minor pilin PilV is critical for host cell adherence (415), while PilV from N. meningitidis binds the β-adrenergic receptor of endothelial cells. In Neisseria, the large (nonpilin) PilC1 and PilC2 proteins have been reported to act as cell contact-dependent pilus-associated adhesins (384), although the evidence is indirect and therefore somewhat controversial. N. meningitidis isolates use PilC1 and PilC2 to adhere to the uropods of neutrophils in order to escape phagocytosis (362). P. aeruginosa expresses an integrin-binding PilC homolog, called PilY1, that requires the T4P system for surface localization, although its direct association with pili has not yet been demonstrated convincingly (13, 53, 174, 204). Mutation of PilY1 homologs in plant pathogens has also been associated with defects in motility and biofilm formation (252, 310). T4P that are involved in DNA transfer via conjugation (below) display lectin-like adhesive proteins whose sequences dictate the range of potential recipients (198).
The propensity of T4P to aggregate laterally into bundles of fibers promotes microcolony formation, an important virulence trait for pathogens such as Neisseria, V. cholerae, S. Typhi, and enteropathogenic E. coli (227, 255, 258, 266, 290), and it increases the retraction forces generated by piliated cells (47). In Neisseria, pilus-mediated aggregation was linked to the noncore minor pilin, PilX, which was proposed to inhibit retraction of adjacent, antiparallel pili through interaction of D-region protrusions of PilX molecules on opposing fibers (175, 176). However, pilus-mediated aggregation must occur in a controlled manner. Mutations in the major subunit that do not affect pilus assembly but preclude bundling diminish the pathogenicity of V. cholerae (255), as do mutations that lead to formation of pilus bundles and bacterial aggregates that cannot subsequently separate (243). In E. coli, remodeling of bundled pili into even thicker bundles via the action of the retraction ATPase BfpF was required for microcolony dispersal (232), suggesting that the particular mode of pilus-pilus interaction affects the subsequent behavior of bacteria. A recent study of V. cholerae binding to intestinal epithelia revealed that aggregates of T4P enveloped the bacteria, potentially protecting them during the infection process (238). In S. Typhi, bundling of pili is negatively controlled by expression of one of two variants of the minor pilin, PilV (note that this is a different component than the Neisseria protein of the same name). When PilV is not expressed due to increased supercoiling of the encoding DNA under low oxygen tension—such as would be found in the small intestine—the pili are able to self-associate and mediate microcolony formation, enhancing pathogenicity (290).
Motility
The ability to generate surface-associated twitching motility makes T4P unique among bacterial pili and fimbriae (305). Bradley (59–61) discovered that T4P retract by his studies of pilus-specific filamentous bacteriophages. He saw that pili became shorter after phage attachment, bringing the phage in contact with the cell surface, and from there, he inferred that pilus retraction was responsible for flagellum-independent motility. Twitching, also known as crawling motility (108), or social motility in Myxococcus (213), arises from the alternate polymerization (extension) and depolymerization (retraction) of pili that translocate the bacteria along surfaces. The ability to retract pilus fibers is essential for motility, as mutants lacking the retraction ATPase PilT are nonmotile. Twitching motility has so far been associated exclusively with T4aP, as the ability of T4b to retract does not appear to be a universal property; many T4b systems lack a PilT homolog. In fact, some of the differences in the two classes of assembly systems may be related in part to the evolution of T4aP into an efficient motility apparatus. There are a few reports providing evidence that the T4b bundle-forming pili of EPEC (which has a PilT homolog) can retract (21, 192, 428), but they are not involved in twitching motility. Other modes of T4aP-dependent movement, including “walking” and “slingshot” motilities, have recently been reported for P. aeruginosa; all modes depend on pilus retraction (108, 153).
Twitching has been characterized extensively for species that express T4aP, including M. xanthus, N. meningitidis, N. gonorrhoeae, P. aeruginosa, and D. nodosus. There are also reports of T4P-mediated “gliding” motility in the clostridia, notably C. perfringens and Clostridium beijerinckii (397). Motility can be random or directed; for example, the cyanobacterium Synechocystis uses its T4P to move toward light sources, while M. xanthus and P. aeruginosa twitch up phospholipid gradients (54, 66, 284). Motility, or pilus retraction, is an important trait for many pathogens, as piliated but retraction-deficient mutants are attenuated in virulence (48, 107, 166, 280, 428). In some cases, loss of virulence in the absence of pilus retraction was linked to an impaired ability to engage the needle-like type III secretion system for the injection of toxins due to loss of intimate host cell contact (107, 215, 428), and toxicity could be restored by expression of alternative adhesins (375).
Pilus retraction is also an important virulence attribute in parasites of bacteria, such as Bdellovibrio (277). This small bacterium preys on other Gram-negative bacteria by ramming into them at high speed and then using its T4P to enter the periplasm, where it digests them from the inside (134). Bdellovibrio pilT mutants have an attenuated predation capacity (277). For detailed discussions of twitching motility that are beyond the scope of this review, the reader is directed to previous references (68, 69, 112, 273).
Archaeal flagellins are more similar to T4 pilins than to eubacterial flagellins, including a requirement for processing of their type III secretion signal by dedicated peptidases prior to assembly (388). However, archaeal flagellar filaments are functionally similar to bacterial flagella, providing a propulsive force through rotation, rather than retraction, of the fibers. It was recently proposed that these unique structures be called “archaella” to distinguish them from eubacterial flagella (199). Although many components of the archaellar assembly system have been identified by knockout studies, the mechanism by which the system generates torque by use of a pilus-like fiber remains elusive (247). For a comprehensive overview of archaeal flagellin assembly and function, we suggest some recent reviews (151, 199).
Biofilm Formation and Remodeling
All classes of T4P, and even the T2S hyperpseudopili produced upon overexpression of the major pseudopilin, can participate in biofilm development in a wide variety of bacterial and archaeal species by promoting initial (and often highly tenacious) adherence to surfaces, as well as cell-cell interactions (122, 128, 208, 209, 328, 398, 400). O'Toole and Kolter (304) first reported that P. aeruginosa mutants lacking T4P are deficient in biofilm formation. Since then, similar findings have been reported for a number of bacteria and archaea, including (but not limited to) V. cholerae, A. actinomycetemcomitans, Aeromonas caviae, Shewanella oneidensis, Legionella pneumophila, Caulobacter crescentus, Deinococcus geothermalis, C. perfringens, and Sulfolobus acidocaldarius (40, 44, 102, 133, 178, 342, 389, 398, 408, 431).
In addition to promoting adherence and aggregation in biofilms, T4aP participate in remodeling of biofilm architecture through twitching motility (see above). Studies by Klausen et al. (228, 229) showed that twitching motility allows bacteria to move to specific zones in biofilms. Chiang and Burrows (95) showed that twitching motility mutants formed abnormal biofilm architectures. More recently, pilus mutants were shown to form aberrant biofilms due to loss of efficient detachment (108). Biofilm formation by Shewanella requires not only pilus expression but also pilus retraction (350). The role of twitching motility in biofilm biology is complex, however, as stimuli that increase twitching motility during early development can reduce the extent of irreversible attachment, which is necessary for further stages to proceed. Studies of P. aeruginosa showed that stimulation of twitching through increased rhamnolipid synthesis, caused by the chelation of iron or treatment with small molecules, peptides, or complex natural products such as ginseng, decreased biofilm formation (118, 158, 356, 412, 417).
Manipulation of Host Cells
Although attachment via T4P is the first step in infection for many pathogens, the participation of pili in virulence does not necessarily stop there. The ability of some bacteria to manipulate host cell biology via their T4P is a very interesting feature. Both N. meningitidis and N. gonorrhoeae can modulate host cell function to promote infection and persistence, but they have distinct tissue tropisms and cause different symptoms; for example, N. meningitidis is capable of crossing the blood-brain barrier to cause meningitis (293). N. meningitidis can bind to and traverse both epithelial and endothelial cell layers, in a T4P-dependent manner (376), and it recruits a variety of host proteins to do its bidding. The bacteria bind to cells and form microcolonies in a manner that requires retractable T4P and the noncore minor pilins PilV and PilX, as well as the pilus-associated PilC1 and PilC2 proteins (63, 111, 225, 283, 287, 288, 362, 415).
T4P-mediated adherence and retraction lead to the rapid formation beneath the microcolonies of cortical plaques (179, 280, 303), structures enriched in the cytoskeletal proteins actin and ezrin and in signaling proteins such as caveolin, epidermal growth factor receptor (EGFR), CD44, and ICAM-1 (52). Neisseria causes cytoskeletal rearrangements that block bacterial uptake, a phenotype also caused by E. coli expressing bundle-forming pili (52), and induces membrane ruffling, enveloping adherent microcolonies and protecting them from shear forces (283). Neisseria also alters cell signaling and cytokine production (97, 314, 383, 399) and impairs adherens and tight junction integrity, allowing bacteria to traverse polarized cell layers, including those forming the blood-brain barrier (110, 111, 248, 334). Pilus retraction is a key facet of the Neisseria-host interaction, as infection with retraction-deficient mutants impairs cortical plaque formation and cytoprotective signaling (119), leading to higher levels of host cell apoptosis (179). For further information on Neisseria-host cell dynamics and the role of T4P, we suggest previous reviews and the references therein (80, 110, 180, 206, 294, 402).
Competence and Conjugation
Studies of bacterial evolution have revealed the extensive contribution of horizontal gene transfer to the shaping of bacterial genomes. T4P have long been recognized as important systems for the acquisition of exogenous DNA in both Gram-negative and Gram-positive bacteria, both by acting as receptors for transducing bacteriophages and by their involvement in competence and conjugation (28, 93). Because of these key functions, T4P genes are often identified and/or annotated in genome sequences as com or competence genes. Well-studied examples include those of Haemophilus, Neisseria, Legionella, Bacillus, and Thermus (8, 33, 82, 150, 338, 367). DNA uptake occurs by a series of steps that require both inner and outer membrane components of the T4P assembly system, some of which have been shown to bind DNA directly (24, 245). The exact mechanism by which T4P participate in DNA uptake is not yet clear, but mutants lacking pili or that are incapable of pilus retraction lose competency (70). Some bacterial families, such as the Neisseriaceae and Pasteurellaceae, exhibit a preference for DNA with particular repetitive uptake sequences (34, 141), for reasons that are not entirely clear. Other bacteria, such as V. cholerae, become competent in response to specific environmental cues (279).
In addition to playing a role in DNA uptake, T4P can participate in the transfer of self-encoding DNA to recipient cells by conjugation. The best-characterized example of such a system is the IncI1 plasmid R64's thin pilus, involved in DNA transfer under liquid growth conditions. The plasmid encodes 12 proteins required for biogenesis of a T4bP that binds to the lipopolysaccharide (LPS) of recipient cells by using 1 of 7 variants of the PilV adhesin, generated by a shufflon mechanism that randomly swaps the C-terminal domains while maintaining the highly conserved N-terminal α-helix region (198, 343, 426). Similar genes are found on a number of conjugative plasmids and on self-transmissible genomic islands in human and animal pathogens, such as enterotoxigenic E. coli, S. enterica, S. Typhi, Yersinia pseudotuberculosis, and P. aeruginosa (83, 105, 364). The frequent appearance of transposon-carried drug and toxin resistance genes in such transmissible elements suggests that they contribute to the spread of antibiotic resistance. In some cases, they have been implicated in pathogenesis, as deletion of the entire locus reduces virulence in animal models (105). For further information on the role of T4P in DNA transfer and uptake, we recommend several comprehensive reviews (28–30, 70, 93).
Electron Transfer
T4P of the environmental bacteria Geobacter sulfurreducens and Geobacter metallireducens have the fascinating property of electroconductivity, giving them the nickname “nanowires” (264, 327). The bacteria use their pili to make contacts with terminal electron acceptors (both nonliving and living, in the case of biofilms) external to the cell, allowing them to thrive in anaerobic environments. Their ability to reduce insoluble iron and manganese oxides and to reductively precipitate other potentially toxic metals, such as uranium, makes them important players in biogeochemistry and bioremediation (261). There is considerable interest in developing Geobacter-based “bio-batteries,” inspiring research efforts to understand the physicochemical basis for pilus conductivity (260). Geobacter pili were reported to belong to the T4a class, and the bacteria of these genus have the characteristic pilMNOPQ genes of the T4aP system in their genome, but the subunits that have been characterized are unusually small (∼61 residues) and lack most of the characteristic C-terminal domain, similar to Flp pilins (332). This unique pilin structure is suggested to have arisen during its evolution into an efficient conductor, because the pilins lack other motifs associated with electron transfer in cytochromes or photosynthetic reaction centers. Feliciano et al. (137) suggested that the conserved N-terminal α-helix has the necessary geometry for electronic coupling, with alignment of peptide bond dipole moments and an electrostatic field along the helix, from the positively charged N terminus to the negatively charged C terminus. Truncation of the C terminus compared with those of other T4a pilins is thought to prevent the neutralization of moving electrons before they jump to the next subunit. Specific charged and aromatic residues with delocalized electrons are conserved throughout pilins of the Geobacter family and may form a relay, allowing electrons to move easily along the pilus (137). Two start sites in the same open reading frame (ORF) result in 9-kDa and 7-kDa products, both of which are processed at the same site by the prepilin peptidase. Although the mature products are identical after processing, mutation of the gene to eliminate one or the other start site affects pilin function. Klimes et al. (230) reported that some Geobacter strains continue to produce pilus-like filaments in the absence of PilA but that the fibers are lost when minor pilin genes are inactivated. It is possible that G. sulfurreducens produces multiple classes of T4P and that some assembly components are shared.
Protein Secretion
The T2S system is broadly distributed in eubacteria, where it exports fully folded proteins and protein complexes from the periplasm across the outer membrane, where they remain associated with the cell surface or are released (98, 99, 205, 237). This pathway allows for the secretion of proteins folded with cofactors or associated with chaperones or inhibitory peptides that are released upon secretion. It was originally called the main terminal branch of the general secretory pathway, because its substrates are first exported to the periplasm by the Sec or Tat pathway; this function led to the Gsp designation of this system (319). The nature of the secreted substrates varies between systems, and they are usually not interchangeable, suggesting that compatibility between substrate and assembly system components is necessary. Well-studied examples of T2S systems include the Klebsiella oxytoca pullulanase (Pul) system; the V. cholerae Eps system, responsible for secretion of cholera toxin; the P. aeruginosa Xcp system, which secretes elastase, exotoxin A, and lipases; the Out system of Erwinia; the Exe system of Aeromonas hydrophila; and the Xps system of Xanthomonas campestris (140, 320, 339, 346). Since many T2S substrates are virulence factors in diseases of plants and animals, there is significant interest in understanding T2S function from a therapeutic standpoint.
The relatedness of the T2S and T4P systems was recognized early (183) and has only been strengthened by a wealth of data, including structural studies revealing that even proteins with poor sequence identity have similar architectures (31, 205, 236, 275, 322, 345). Current models of T2S function that invoke a piston-like action pushing substrate proteins through the secretin were informed by models of the extension and retraction of T4P and by studies showing that T2S pseudopilins could form pilus-like fibers under specific conditions (128, 190, 349, 400). Furthermore, there are several examples of proteins that are secreted by T4P systems, including the TcpF colonization factor of V. cholerae (226), proteases of D. nodosus (167), and virulence factors of Francisella tularensis (19, 163), showing that the systems are functionally similar.
The T2S system is the only system for which structures of all the major and minor pseudopilins are available, alone and in subcomplexes. Those data have been invaluable for the refinement of theories about the mechanism of fiber assembly and function. Other key information came from protein-protein interaction studies that showed the specific pattern of interactions, both stable and transient, between various minor pseudopilins, between pseudopilins and components of the assembly system, and even with the T2S substrates (124, 125, 236, 237, 244, 329). Among the important questions that remain to be answered is how the substrates of T2S (and T4P, where they are involved in secretion) are recognized against the background of myriad periplasmic proteins. No linear secretion signal has been identified, and protein folding is required for secretion competency, suggesting that the signal may be a three-dimensional one (145, 407). However, comparison of substrates for which structures are available does not suggest an obvious candidate(s), and thus studies aimed at identifying the elusive secretion signal continue.
REGULATION OF PILIN AND PSEUDOPILIN EXPRESSION
Although the pseudopili of the T2S systems are typically shorter than a dozen subunits, T4P and archaeal flagella can be several micrometers long, corresponding to thousands of subunits. Making a large amount of protein that is exposed on the cell surface—subject to accidental loss or, in some cases, deliberate shedding (385)—is metabolically expensive. The need for control of pilus biogenesis in response to environmental signals has contributed to the evolution of complex regulatory mechanisms, ranging from transcriptional to posttranslational mechanisms. Pilin gene expression is responsive to environmental cues that are relevant to the lifestyle of the specific organism. In many species, surface contact—or, perhaps, a local increase in viscosity or mechanical strain on membranes—leads to upregulation of pilin expression (43). In Haemophilus influenzae, alkaline pH is required for optimal pilus expression (33), while some archaea produce T4P in response to UV irradiation (7, 147). For many pathogens, T4P are made only in response to growth under specific culture conditions or at specific temperatures and/or in response to host cell contact (102, 254, 274).
Regulation of Amino Acid Composition
Although pili are strong (250, 281), they can be lost under conditions of strong shear forces. Smith and Chapman (361) showed that bacterial proteins destined to be surface exposed or extracellular tend to contain a preponderance of “cheap” amino acids (in terms of the number of ATP molecules used in their production), such as Gly, Ala, Ser, Glu, Thr, Gln, and Asn, and fewer “expensive” amino acids, such as Trp, Phe, Tyr, His, and Met. Inspection of the composition of representative major pilins from P. aeruginosa supports this idea, as the content of Gly, Ala, Ser, and Thr (GAST) in mature pilins ranges from ∼38% to 47%. The GAST contents of P. aeruginosa minor pilins are slightly lower, ranging from ∼31% to 37%. In contrast, the GAST content of the major pseudopilin, XcpT, is ∼26%, consistent with those of other proteins predicted to remain intracellular, such as the cytoplasmic PilB, PilT, and PilU ATPases, all of which have GAST contents of ∼27%. Similarly, the XcpUVWX minor pseudopilins that remain periplasmic have GAST contents of ∼24% to 32%.
Transcriptional Regulation
The ability to reinsert subunits from existing fibers back into the cytoplasmic membrane upon depolymerization, where they can be available for another round of assembly, is an amazing bacterial example of recycling. This strategy conserves energy and provides a mechanism by which bacteria can monitor the amount of available subunits. In many T4P-expressing species, including M. xanthus, D. nodosus, G. sulfurreducens, Kingella kingae, Xanthomonas axonopodis, and P. aeruginosa, the major pilin subunit is expressed from a σ54-dependent promoter (Fig. 7) (182, 197, 218, 239, 418, 423). The complex formed by RpoN and RNA polymerase (RNAP) binds such promoters but remains stalled in a closed configuration. Conversion of the RNAP-σ54-DNA complex to a transcription-competent open form requires an enhancer binding protein, which in the case of pilin gene expression is PilR, the response regulator of the two-component PilR-PilS system.
Fig 7.
Proposed model of T4a pilin autoregulation by intramembrane interactions with the PilS sensor kinase. The PilR-PilS two-component system regulates major pilin biosynthesis in many T4aP-producing bacteria. Because PilS lacks a typical periplasmic sensor domain and controls the expression of pilins with diverse C-terminal sequences, it may instead detect the highly conserved N-terminal domain of PilA orthologs via intramembrane interactions (11). High intracellular levels of PilA, as in pilus assembly mutants, may inhibit pilA transcription in a manner that depends on sequences in the pilin's N terminus, possibly through modulation of PilS dimerization or stimulation of phosphatase activity of PilS on the response regulator, PilR (37, 338). When pilin levels are low—as in retraction-deficient mutants, where the pilins are trapped outside the cell—PilS kinase activity is stimulated, phosphorylating PilR and upregulating PilA transcription. The organization of the pilABCD genes in P. aeruginosa is shown. pilA is transcribed by σ54, RNAP, and phospho-PilR from a divergent promoter that also controls expression of PilB (the pilin polymerase), PilC (the platform protein), and PilD (the prepilin peptidase).
In P. aeruginosa, PilS is a membrane-bound histidine kinase that is autophosphorylated in response to an as yet unknown signal and, in turn, phosphorylates PilR, promoting pilin expression. PilS levels are critical to the control of PilA levels, as both lack of PilS and its overexpression had a detrimental effect on pilin gene transcription (58). Boyd and Lory (58) suggested that PilS overexpression titrated a crucial interaction partner, leading to decreased pilin gene transcription. In some species, including M. xanthus, PilR is required for pilin gene expression, but PilS is a negative rather than positive regulatory component (418). The pathway of major pilin regulation in M. xanthus has additional layers of complexity, since it can be upregulated in a pilRS-independent manner under nutrient-rich conditions. N. gonorrhoeae encodes a PilR-PilS hybrid, named Rsp, that binds to the major pilin promoter, but its inactivation has no effect on piliation, suggesting that its regulatory function was lost during evolution of that pathogen (81). In keeping with this regulatory rewiring, expression of the N. gonorrhoeae pilin is driven by σ70 (although a functional σ54 promoter is present), and the gene encoding σ54 is truncated in pathogenic Neisseria species.
Wu and Kaiser (418) showed that pilin mutants of M. xanthus had increased activity from a pilA transcriptional reporter, a finding that was corroborated by a recent study in the Engel lab (43), using P. aeruginosa. These data suggest that PilA negatively regulates its own expression, possibly through direct interactions with PilS (Fig. 7). In a P. aeruginosa mutant lacking the retraction ATPase PilT, the reporter was similarly activated, although an M. xanthus pilT mutant had only mildly elevated levels of pilA expression. In a pilT background, pilin levels in the cytoplasmic membrane would presumably be low due to the inability of the cells to retract their pili. In contrast, P. aeruginosa mutants lacking the assembly ATPase PilB had low levels of reporter activity (43). Such mutants accumulated pilins in the inner membrane due to the blockade in pilus assembly. The phenotypes of assembly and retraction mutants are consistent with the hypothesis that high levels of PilA in the inner membrane suppress pilA transcription. Similar findings have been reported for N. gonorrhoeae, where inactivation of pilT markedly increased the levels of transcription of the major subunit gene, pilE, while mutations that block assembly (in pilD, encoding the prepilin peptidase, or pilF, encoding the pilin polymerase) decreased its expression (120).
When pilA or rpoN was inactivated in P. aeruginosa, levels of specific pilus assembly system components were reduced (32). Interestingly, when pilR or pilS was inactivated, levels of the assembly system components were similar to those in the wild type, suggesting that even though there was no PilA expression in either of those mutants, the cells were unable to sense its absence and respond accordingly. In M. xanthus, pilB mutations that caused the accumulation of pilins in the inner membrane led to the downregulation of EPS synthesis, a phenotype that could be relieved partially by inactivating pilA in the pilB background (425). In contrast, mutations that led to the accumulation of PilA in the cytoplasm (signal sequence mutations) did not repress EPS synthesis. Both T4P and EPS are required for M. xanthus social motility, and these data suggest that they are coregulated in response to the levels of pilin present in membrane pools. In general, PilR-PilS may regulate other components in the cell in response to alterations in the amount of pilin detected. Bioinformatic studies of G. sulfurreducens promoters suggested that PilR might regulate the production of pili, flagella, secretion components, and proteins involved in cell envelope biogenesis (239).
The sensor kinase PilS, which has an atypical 6 transmembrane domains but no periplasmic sensor domain, could monitor levels of pilin through direct intramembrane interactions with the conserved hydrophobic N terminus of PilA (Fig. 7). High PilA levels could drive PilA-PilS interactions that, depending on the particular species, could either promote or prevent kinase activity. As pilin levels decrease, PilS might adopt an alternate conformation and act as a PilR phosphatase. Such a model for control of PilS activity is not unprecedented; regulation of sensor kinase activity by intramembrane interactions with hydrophobic peptides was recently reported for a number of systems (12). For example, Lippa and Goulian (256) showed that PhoPQ, a two-component system controlling important virulence pathways in many pathogens, is negatively regulated by the 47-residue hydrophobic peptide MgrB, in that direct interaction between PhoQ and MgrB inhibits the expression of PhoP-activated genes.
Szurmant et al. (381) showed that the YycH and YycI proteins of Bacillus subtilis—like PilA—each have a single TM domain, with the bulk of the proteins outside the cytoplasmic membrane. Short fragments from the N terminus of either protein regulated the activity of the YycG sensor kinase in a manner that could be disrupted by acidic point mutations within the hydrophobic TM domains (380). Wu and Kaiser (418) showed that mutation of the highly conserved Glu5 residue in the N-terminal helix of the major pilin to Lys recapitulated the phenotype of a pilA mutant; a reporter construct showed that pilin expression was upregulated, even though the E5K mutant protein was stable and present at wild-type levels in the cell. This particular residue is highly conserved among major pilins (and some minor pilins) in most species, suggesting that this proposed mechanism of regulation could accommodate diverse pilin alleles.
Regulation through Pilin Stability
Pilins are quite stable, and protein levels remain elevated even after cells are transferred to growth conditions (such as broth culture) that do not promote pilA expression (43). A reduced rate of pilin turnover is consistent with an energy conservation strategy in which pilins are retained in the inner membrane under conditions where twitching motility is not optimal but are ready for deployment if conditions become favorable. For T4P, there appears to be a minimum threshold of pilin expression that is necessary for motility to occur. In M. xanthus, Jelsbak and Kaiser (201) expressed the major pilin subunit from an inducible promoter and showed that motility was similar to that of uninduced strains until a level of ≥50% of the wild-type level was produced. The amount of motility was related to the number of surface pili rather than to total PilA levels. A similar phenomenon was seen in P. aeruginosa using an arabinose-inducible promoter; without inducer, the low level of PilA synthesized was insufficient for motility (170). When 0.1% arabinose or more was used, motility was restored to wild-type levels. Whether this phenomenon is related to pilus length is not clear, since even mutants with very short pili can be motile (154).
Minor Pilin Regulation
Regulation of minor pilin expression has not been well characterized. Because T4a (unlike T4b) genes are not clustered in the genome, it is possible to differentially regulate subsets of pilus genes. In P. aeruginosa, the core minor pilin operon is positively regulated by the AlgR-FimS two-component system (41, 257, 414), and algR mutants are consequently nonmotile. Although AlgR controls other operons as well, provision of the minor pilin genes in trans is sufficient to restore twitching motility. Inactivation of individual genes in the minor pilin operon of P. aeruginosa resulted in increased expression of others, implying a positive-feedback loop in the absence of specific components (53, 154). In Neisseria, the genes encoding the core minor pilins PilHIJK are clustered with the gene for the noncore minor pilin, PilX (called PilL in some N. gonorrhoeae strains [416]), while the loci encoding other noncore proteins (PilV and ComP) are unlinked. PilV and ComP are functionally connected to one another, however, since ComP-dependent DNA uptake is modulated in a reciprocal manner by PilV levels, because PilV is an antagonist of ComP trafficking (2, 64).
The minor pilin genes for T4b and T2S systems are generally clustered with those encoding the other components of the system and therefore are likely to be coregulated. For the T2S system, only a few major subunits are needed to produce a pseudopilus of sufficient length to cross the periplasm; therefore, coregulation of major and minor subunit expression to maintain their stoichiometry is likely straightforward. In the T4b system, the number and stoichiometry of minor components vary depending on the species and, in general, have not been well characterized.
PILIN DIVERSITY
Intra- and Interspecies Diversity of Pilin Proteins
Identifying functionally significant sequence differences among type IV pilin proteins is a challenge because of their immense diversity. As surface-exposed structures, they are likely under diversifying selection for variants that attach more or less persistently to various surfaces or receptors; for increased or decreased bacterial cell-cell aggregation, influencing dispersal and formation of biofilms; as receptors for bacteriophages; for uptake of DNA in competent species, which depends in part on pilin surface chemistry; or, in the case of pathogens, for avoidance of host immune recognition while retaining the ability to affect host biology. Although some bacteria can assemble heterologous pilins, a critical reading of the literature reveals that in most cases, (i) it is not possible to recapitulate all of the functions of the native protein and (ii) heterologous fibers are produced only in retraction-deficient backgrounds, suggesting that sequence differences reflect a balance between assembly dynamics and function.
The sequences of type IV pilins can undergo variation through horizontal gene transfer between species, by shufflon or other recombination events within species and strains, and by point mutation. Horizontal acquisition of new pilin-encoding DNA fragments whose 5′ sequences, encoding the highly conserved N-terminal helix, are very similar increases the probability of recombination with existing genes. In the case of pseudopilins, their diversity might be driven in part by their interactions with specific substrates secreted by the T2S system (124). The minor pseudopilins form functional complexes (100), suggesting that diversity may also reflect the coevolution of protein-protein interaction interfaces. In this section, we consider pilin diversity and its sources at the protein level; additional diversification can occur through decoration with various posttranslational modifications (PTMs) (see below). Type IV pilins are highly immunogenic and provoke protective serological responses, although typically only to homologous or closely related antigens (17). Therefore, a thorough understanding of pilin diversity and the extent to which major versus minor subunits are conserved is important for the design of broadly protective pilus-based vaccines (87). Detailed studies of type IV pilin protein diversity have so far been confined to a small number of well-characterized species, but the advent of inexpensive genome sequencing and identification algorithms will allow for comparisons among a larger pool of sequences.
Diversity in Neisseria Pilins
For obligate human pathogens such as Neisseria, which are continually exposed to immune surveillance, the ability to diversify their pilus composition is critical for survival. Because of their restricted host and niche range, they have limited opportunity to acquire new pilin genes by horizontal gene transfer. To circumvent this problem, Neisseria organisms have evolved an ingenious strategy involving intragenic recombination of a number of silent pilin gene cassettes (pilS) with a single expressed major pilin locus (pilE), in a process called gene conversion (181). Studies in male volunteers showed that strains recovered from infection with a single piliated strain had pilin sequences that were different from those of the parental strain (354, 377). Sequence analyses showed that during the course of infection, some or all of a pilS locus recombined into the active pilE locus (377). This recombination event can cause a hyperpiliated phenotype or, more commonly, a nonpiliated strain, where a frameshift in the pilE sequence creates a premature stop codon (116). A more recent study of isolates recovered from a scientist accidentally infected with a single N. meningitidis strain (302) showed that even with the limited number of in vivo cell divisions (∼25) estimated to have occurred before resolution of the infection by antibiotic therapy, at least 7 different pilin variants with different adhesive capacities arose, showing rapid diversification. Neisseria is naturally competent for DNA uptake, in a T4P-dependent manner, and can undergo intergenic recombination with DNA scavenged from its environment. Provision of exogenous DNA increased antigenic variation of the pilus in N. meningitidis (11), while addition of DNase during infection was shown to decrease antigenic diversity (152).
Most Neisseria have a single pilE locus, but in some cases, tandem alleles (pilE1 and pilE2) have been reported (6). The number of pilS alleles varies between strains, as 17 silent copies were identified in N. gonorrhoeae strain MS11, while 19 different pilS loci at 6 distinct chromosomal locations were identified in N. gonorrhoeae strain FA1090 (161, 165). Each pilS allele lacks a promoter, a ribosome binding site, and an approximately 150-bp region from the 5′ end of pilE (116, 161). These features prevent the expression of pilS alleles until they recombine with the functional pilE allele. Each pilS allele contains a semivariable region (5′ end) and a hypervariable region (3′ end). Within the variable regions are 1- to 34-bp sections that are conserved between pilS and pilE and predicted to allow for crossover between the two genes (116, 181). The combination of a series of repetitive elements between pilS genes, a putative recombinase site upstream of pilE, and a conserved Sma/Cla repeat downstream of pilE permits the nonreciprocal transfer of DNA from pilS to pilE though homologous recombination. Furthermore, recombination can occur between pilS alleles, adding to potential diversity.
The mechanism by which the initiation of high-frequency pilin gene conversion occurs in N. gonorrhoeae was recently discovered (74). Homologous recombination was previously shown to mediate pilin antigenic variation (231, 278, 353, 357), but no specific genetic element capable of triggering the recombination event had been identified. A cis-acting 16-base guanine-rich sequence—designated the G4-RIS or “recombination initiation sequence”—located upstream of pilE on the lagging strand, was shown to form a guanine quartet (G4) which facilitates nick formation within the G4 DNA (74). Mutation of any of the 12 G-C base pairs in the 16-bp region abolished pilin antigenic variation without affecting pilin expression, while mutation of the other 4 residues had no effect. Cahoon and Seifert further showed that the position, sequence, and orientation of the G4 structure were required for antigenic variation, as alterations in any of these factors prevented nicks from occurring on the G-rich strand (74). Although other predicted G4 structures were encoded in the gonococcal genome, only the specific sequence upstream of pilE was capable of promoting antigenic variation. Interestingly, this structure is conserved in pathogenic but not commensal species of Neisseria.
In addition to the pilins described above, which are referred to as class I pilins, specific clonal complexes of N. meningitidis express a second T4aP pilin type, designated class II (6, 88). Class II pilins are smaller (15 to 17 kDa) and do not bind the monoclonal antibody SM1, which recognizes both N. gonorrhoeae and N. meningitis class I pilins. Genetic and immunological studies suggest that some commensal species of Neisseria express class II pilins, suggesting the possibility of horizontal transfer between pathogenic and nonpathogenic species. Class II pilins are not subject to conversion and therefore are more antigenically stable than class I pilins (177). Class II pilins lack the C-terminal hypervariable region, although loss of this domain does not preclude class II pilin-expressing meningococci from causing invasive disease (88).
The impressive ability of Neisseria species to undergo rapid and frequent variation of their pilins has led to the abandonment of PilE (in particular, class I) as a vaccine candidate. However, Neisseria pili were also recently shown by a number of investigators to contain minor pilins, whose low abundance relative to that of the major subunit does not necessarily reflect their important contributions to pilus function and pathogenicity (63, 154, 175, 248, 283, 415, 416). To determine whether the level of protein diversity among minor pilins was lower than that among major pilins, and thus whether they would potentially make better vaccine candidates, Cehovin et al. (88) examined the prevalence and sequence similarity among specific minor pilin genes in a large set of pathogenic isolates of N. meningitidis. The pilX, pilV, and comP genes (encoding minor pilins involved in aggregation, adherence to host cells, and DNA uptake, respectively) were each present in all of the isolates tested, though sequence conservation among the predicted gene products varied from 64% identity for PilV orthologues to 97% for ComP orthologues, with that for PilX orthologues being intermediate, at 73%. The potential utility of the minor pilins as candidate antigens was supported by the identification of anti-minor-pilin antibodies in convalescent-phase patient sera (87). A more detailed characterization of their functions and proof-of-principle vaccination studies will be needed to show if they will be effective as protective antigens.
Diversity in Pseudomonas Pilins
Unlike Neisseria, Pseudomonas species occupy a broad range of environmental niches and therefore have access to a wider pool of DNA as a source of diversity. The best-characterized species, P. aeruginosa, can express multiple types of pili and fimbriae, including T4a, T4b, and Tad pili (42, 83, 395), but T4aP are the most commonly observed fibers on laboratory-grown cells. A recent examination of intraspecies diversity of the T4a pilin in P. aeruginosa and other Pseudomonas species provided evidence for a genomic pilin island—approximately 36 kb in the PAO1 reference strain—that encodes both the T4a major and minor pilins as well as the two-component PilRS system that controls major pilin expression (155). Remarkably, in at least one strain of P. aeruginosa (PA14), a set of genes expressing a separate, self-transmissible T4bP system as part of P. aeruginosa pathogenicity island 1 (PAPI-1) (83) is inserted within the T4aP pilin island, at three tandem tRNA genes (155). In other Pseudomonas species, the same locus is occupied by DNA insertions of up to ∼100 kb, suggesting that this position is an active hot spot for recombination.
Five different groups of T4a major pilins have been identified in P. aeruginosa, classified by sequence length, the size of the D region, and the identity of the pilin accessory protein encoded downstream of the pilin (242). The first identified groups were groups I and II (86). The common laboratory strains PAO1, PAK, and PA103 express group II pilins; such strains lack accessory genes between pilAII and the downstream tRNAThr gene. In contrast, strain 1244, the Liverpool epidemic strain (LES), and strain PA2192 have a slightly larger group I pilin and an additional ORF, between the pilAI and tRNAThr genes, involved in glycosylation of group I pilins at Ser148 (85, 106, 242). In 1995, the prototype of the group III allele was identified in a single P. aeruginosa isolate. Spangenberg et al. described the group III pilin of 173 amino acids—longer than the previously identified pilins—and an open reading frame of unknown function, now named tfpY, carried downstream of the pilA gene (242, 363). Additional isolates with group III alleles (including the well-characterized PA14 strain), as well as isolates with two new pilin alleles (groups IV and V), were subsequently identified in a survey of >300 P. aeruginosa strains (242). Since analysis of this relatively small sample size readily led to identification of novel pilins, examination of a greater number of isolates from the broad spectrum of environments colonized by this species will likely reveal additional diversity. Group V strains encode pilins and accessory proteins (TfpZ) most similar to, but distinct from, those of group III, as polyclonal antisera raised against pilins of the two groups do not cross-react. Group I alleles were most common, followed by groups II, III, and V (242), although a study by Römling et al. (335) found that clone C, the most common strain of P. aeruginosa distributed around the globe, belongs to group II. To date, group IV contains only two isolates, Pa5196 and PA7, which encode the TfpW and TfpX accessory proteins via genes downstream of pilAIV (242, 336). TfpW is a large membrane-bound glycosyltransferase that adds one or more d-arabinofuranose sugars to the pilin (241, 405). TfpX is a predicted pilus accessory protein most similar in sequence to TfpZ, the group V accessory protein. The specific function of the related accessory proteins TfpX, TfpY, and TfpZ is currently unknown, but mutagenesis studies show that they promote efficient assembly of their cognate pilin (23).
As in Neisseria, the minor pilins of P. aeruginosa were shown by Western blotting of sheared pili, as well as by immunogold labeling and transmission electron microscopy, to be associated with external pilus fractions. Using available P. aeruginosa genomes and additional sequencing of the minor pilin genes from selected strains, three unique sets of minor pilins were identified (23, 155). Similar to the case for Neisseria, there is less diversity among the minor pilins of P. aeruginosa than among the major pilins. However, the observed sequence diversity has functional implications, as the group II and III PilX proteins are not interchangeable (155). A thorough assessment of minor pilin diversity among other Pseudomonas species has not yet been done, in part because the reduced level of sequence identity between orthologous proteins has hampered their accurate annotation.
Moraxella and Dichelobacter Pilin Diversity
Moraxella bovis is the causative agent of infectious bovine keratoconjunctivitis (IBK), a highly contagious and economically significant disease that can cause permanent blindness in cattle (65), while Moraxella lacunata causes a similar disease in humans. Only piliated strains are able to bind to corneal tissue and cause IBK, highlighting the significance of T4P as a virulence factor. Two different pilin proteins have been identified in Moraxella, termed Q (quick) and I (intermediate) (148), and these form a total of 7 serogroups as determined by immunological cross-reactivity studies (286). The expression of these proteins is mutually exclusive, and switching between one or the other is dependent on the inversion of a 2.1-kb segment of DNA, as only one promoter is present. The inversion occurs in the N-terminal region, and the process is reversible, as Q strains can become I strains and vice versa (269). Further sequence analysis revealed that a 26-bp region including the sequence encoding the first 6 amino acids of PilQ was responsible for inversion of the pilin genes and subsequent expression of the leading gene (148). The Q pilin is associated with increased binding to corneal tissue compared with the I pilin (337). Recent studies of Moraxella catarrhalis, an emerging human pathogen responsible for a large proportion of otitis media and other upper respiratory tract infections, showed a low level of pilin diversity (262). All strains tested expressed either clade 1 (PilA1; 42%) or clade 2 (PilA2; 58%) pilins, with PilA1 having an 11-residue deletion at the C terminus compared with PilA2. PilA1 pilins from various strains showed 78 to 86% identity, while PilA2 pilins showed 88 to 99% identity, with 59 to 66% identity between the two types. Unlike the case for other Moraxella species, no phase variation was observed. The limited diversity of pilin types in Moraxella has allowed the pilins to be used successfully as vaccine antigens for IBK (17).
Dichelobacter (formerly Bacteroides) nodosus is the pathogen responsible for foot rot in sheep. The pili of this organism are important virulence factors and vaccine antigens, and the major pilin, FimA, is used to serotype isolates (220). Genome and microarray analyses (291) suggested that the fimA locus has a nucleotide composition that is atypical for the species and is divergent, suggesting hypervariability. Although D. nodosus pilins have been used successfully as vaccines (130), their ability to protect against homologous but not heterologous antigens and the presence of multiple strains on individual animals (430) mean that vaccines have to be multivalent, including the most prominent serotypes.
Tandem Chromosomal Pilin Loci
A number of groups have documented tandem major T4a pilin subunit genes on the chromosome. Initially, this arrangement suggested that the promoter-distal pilA allele might be used in antigenic variation, similar to the case for the Neisseria pilS loci. However, in species where the role of each allele has been investigated, the promoter-proximal pilA gene is required for surface piliation, while the second pilA gene appears to have little effect on surface piliation or motility.
Ruminococcus albus has two pilA loci, pilA1 and pilA2 (325). The pilA2 gene is located ∼115 bp downstream of the pilA1 stop codon, with a putative hairpin loop separating them. The two genes are 73% identical, but analysis of mRNA levels revealed that pilA2 was expressed at a level 73-fold greater than that of pilA2. While transcripts can be monocistronic (pilA1 only) or dicistronic, the former is predominant (325). Localization studies showed that while the pilus is composed of PilA1, PilA2 is confined to membrane fractions. Addition of anti-PilA2 sera did not block T4P-mediated adhesion, suggesting that PilA2 either was not incorporated into the pilus or was not the adhesin.
More than one potential major pilin gene is carried by the periodontal pathogen Eikenella corrodens, in a three-gene cluster of pilA1, pilA2, and pilB (homologous to the pilin accessory gene fimB in D. nodosus and the tfpY gene from group III strains of P. aeruginosa). Similar to the R. albus system, a hairpin loop is located between the pilA1 and pilA2 genes, and transcripts were found to be both monocistronic (pilA1 only) and polycistronic (pilA1–pilA2–pilB) (401). E. corrodens can modulate the expression of surface pili, which manifests as an observable change in colony morphology; piliated bacteria form small (or S-phase) colonies, while nonpiliated bacteria form large (or L-phase) colonies. This unique form of antigenic variation in E. corrodens is unidirectional, where an S-phase colony can become an L-phase colony but not the reverse (401). The reason for loss of surface pili in the L-phase variant is unknown, as both PilA1 and PilA2 are expressed. Although a small decrease in the pilA1–pilA2 transcript level in L-phase colonies was observed compared to the S-phase colonies, it was not considered significant enough to cause the nonpiliated phenotype (401). The authors attributed this transcript decrease to morphological differences between the variants; however, it is possible that a feedback loop resulting from higher PilA1 levels in the nonpiliated strain led to the overall decrease of pilA1 transcripts.
The cyanobacterium Synechocystis sp. PCC6803 has two morphologically different types of pili, termed thick and thin pili (45). Thin pili (2 to 3 nm in diameter and 500 to 1,000 nm long) are distributed around the entire surface of the cell, and their expression was unaffected in a pilA1 mutant. Thick pili (6 to 8 nm in diameter and 1,000 to 2,000 nm long) were found in asymmetrical clusters around the cell, and motility and surface expression of these pili were abolished in a pilA1 mutant (45). Mutation of the pilA2 gene did not affect surface piliation or motility. Analysis of the sequence directly upstream of pilA1 and pilA2 showed a Shine-Dalgarno (SD) sequence upstream of pilA1 but not pilA2 (45). The lack of an SD sequence for pilA2 suggests either that a polycistronic transcript is made, similar to the case for E. corrodens and R. albus, or that pilA2 is not expressed or expressed at levels below detection.
A tandem arrangement of pilin genes was also described for Pseudomonas stutzeri, with pilAI and pilAII being cotranscribed; however, pilAII expression was only 1/10 that of pilAI. Mutation of pilAI abolished piliation, while a pilAII mutant expressed surface pili and had increased transformability (159). Interestingly, the hypertransformation phenotype was maintained in a P. stutzeri pilAI pilAII double mutant expressing pilins from non-naturally transformable species such as P. aeruginosa (159). These data suggest that while pilAI and pilAII share 55% identity, their functional roles are distinct.
Diversity among T4b Pilins
The best-characterized T4b pilins with respect to diversity are the BfpA (bundlin) subunits of EPEC bundle-forming pili and the TcpA subunits of the toxin-coregulated pili of V. cholerae. In general, there is less diversity in T4b pilins than in T4a pilins. This finding may be related to the inability of the assembly systems to recognize heterologous subunits. For example, McNamara and Donnenberg (276) showed that TcpA could not be assembled into fibers by the E. coli Bfp system.
All EPEC strains carry one of two major subtypes, named α and β, which are further divided into numbered sequence variants (e.g., α1) (50, 138). The α proteins are 97% identical, while the β proteins are ∼80% identical to the α-bundlins. As is typical for pilins, sequence differences are concentrated near the C terminus. In terms of pilus formation, the two forms of bundlin are interchangeable and even form mixed fibers (138, 194), a phenomenon not observed when heterologous T4a pilins are coexpressed (308). However, there are functional differences between the two BfpA subtypes with respect to receptor interactions that mediate the important localized adherence phenotype required for EPEC colonization of intestinal epithelium. A number of studies (191–194) have reported the interaction of bundlin with the carbohydrate receptor N-acetyllactosamine (LacNAc). Mutagenesis studies which systematically targeted the surface-exposed sequence of α-bundlin (137-GENNI-141) and replaced it with the respective β-bundlin sequence (136-SPDST-140) and vice versa showed the LacNAc–α-bundlin binding to be specific. Conversion of the α-bundlin to the β-bundlin sequence caused loss of binding, while β-bundlin conversion to the 137-GENNT-141 sequence resulted in preferential binding to a fucosylated derivative of LacNAc, the Lewis X antigen (191). The specificity of the LacNAc–α-bundlin interaction could potentially explain its high level of sequence conservation compared with β-bundlin.
Most cases of epidemic cholera are caused by V. cholerae serogroups O1 and O139, with sporadic outbreaks caused by strains of other serogroups that express Tcp. The O1 serogroup contains the genetically similar classical and El Tor biotypes, which vary at only a few loci, including that for the major pilin subunit, TcpA (387). Comparison of TcpA sequences between those two serogroups showed that they were ∼80% identical, with the N terminus being the most highly conserved region. The primary mechanism responsible for generating TcpA diversity was proposed to be recombination rather than mutation (387). Even small sequence changes in TcpA have profound effects on pilus function, as single point mutations in the D region caused a loss of pilus bundling, a phenotype important to pathogenesis. Minor sequence differences between the El Tor and classical TcpA proteins generated different patterns of pilus bundling (255). TcpA proteins from classical strains formed large intertwined rope-like bundles, while those from El Tor strains formed a cross-hatched pattern. To understand the structural basis for these differences, Lim et al. (255) solved the structure of the El Tor subunit for comparison with the previously determined classical version (114) and used it to build a fiber model. Key residues that differed between the two forms were located on the protruding D regions and in the cavities between subunits, generating a combined “knob and hole” interaction surface. Mutagenesis of specific nonconserved residues to disrupt or switch the stereochemical complementarity of these interaction interfaces supported a role for them in pilus-pilus interactions leading to aggregation.
Boyd and Waldor (56) showed that despite sequence divergence of up to 33% from the classical biotype protein, TcpA variants from non-O1/non-O139 serogroup isolates supported infectivity and cholera toxin phage susceptibility, suggesting that they retained the functions of the well-characterized O1 and O139 TcpA proteins. Polymorphic sites were clustered in nonstructural regions of the proteins that were predicted to be surface exposed in the fiber. The diversity in tcpA was much higher than that in other loci, showing that it is a hypervariable locus subject to frequent horizontal gene transfer.
POSTTRANSLATIONAL MODIFICATIONS
All type IV pilin proteins identified to date undergo a unique and essential PTM, which is removal of the type III signal sequence by dedicated prepilin peptidases. Blocking of this step prevents the subunits from assembling into fibers (301, 312, 371, 373). The proteins are subject to additional modifications, including N-terminal methylation following removal of the signal sequence (373) and, depending on the species and strain, glycosylation or addition of other moieties to solvent-exposed regions. Neisseria is the current champion of pilin PTMs, with several different forms of modification identified, and sometimes multiple types of modification on a single subunit (1). With some exceptions that are detailed below, these decorations are not essential for fiber formation but can have important effects on filament function. Advances in mass spectrometry techniques that cause less damage to samples and increase sensitivity with respect to detection of nonstoichiometric modifications will likely lead to the identification of additional, potentially novel, PTMs.
N-Terminal Cleavage and Methylation
Although the mechanistic details of pilin N-terminal cleavage and methylation have been characterized most extensively using the V. cholerae (TcpJ and VcpD) and P. aeruginosa (PilD; also called XcpA) prepilin peptidases, homologs of these enzymes are found in all systems with type IV pilin proteins. Proteolysis and methylation were initially proposed to depend on four Cys residues in an N-terminal cytoplasmic loop, but it was later discovered that some PilD orthologues lacked the relevant Cys residues and had proteolytic activity even in the presence of sulfhydryl-reactive inhibitors. XcpO—a PilD homologue in Xanthomonas campestris—lacks all four Cys residues but complemented a P. aeruginosa pilD mutant (189), suggesting that the Cys residues are not part of the PilD active site. Further studies showed that two highly conserved Asp residues at the membrane boundary of a predicted C-terminal cytoplasmic loop are essential for protease activity; therefore, the enzymes were reclassified as aspartyl proteases (246). Prepilin peptidases are members of a larger family of aspartyl proteases, including mammalian presenilins, that catalyze the intramembrane proteolysis of their substrates. The first structure of a member of this protease family was solved in 2011 (188).
The discovery of similar peptidases associated with archaeal flagellar systems strengthened the hypothesis that these flagella are more similar to T4P than to eubacterial flagella (9, 10, 39). The enzymes FlaK (Methanococcus maripaludis and Methanococcus voltae) and PibD (Sulfolobus solfataricus) cleave archaeal type IV pilin-like proteins. FlaK and PibD have conserved C-terminal Asp residues, suggesting that the archaeal peptidases may likewise function as aspartyl proteases. FlaK peptidase activity was detected in M. voltae membrane fractions (109), and site-directed mutagenesis of the conserved C-terminal Asp residues abrogated peptidase activity (39). The recently solved FlaK crystal structure is proposed to show an inactive conformation, because the two Asp residues are relatively far apart (188).
P. aeruginosa PilD cleaves the leader peptide of PilA between Gly (−1) and Phe (+1) to form the mature pilin, and subsequently adds a single methyl group to the new N-terminal residue, using S-adenosylmethionine (AdoMet) as the methyl donor (37, 369). Other pilin-like proteins in the P. aeruginosa type IV pilus system (FimU, PilV, PilW, PilX, and PilE) and type II secretion system (XcpT, XcpU, XcpV, XcpW, and XcpX) are similarly cleaved by PilD, although subsequent methylation has been confirmed only for the minor pseudopilins (38, 154, 300, 372). The N-terminal processing of pilin subunits precedes and is independent of pilus assembly, as mutants lacking key components of the assembly machinery—such as the pilin polymerase, PilB—accumulate pools of processed subunits in the inner membrane. PilD has high specificity for particular residues within the leader peptide, as a complete loss of peptidase activity was observed when Gly (−1) was mutated, while mutation of Phe (+1) resulted in a reduced rate of proteolysis (369, 370). In vitro studies of the prepilin peptidase showed that peptidase and methylase activities are independent; methylation could be blocked without affecting peptidase activity, indicating that these processes occur at two distinct active sites (312, 372, 373). Site-directed mutagenesis of the two putative active sites demonstrated that while peptidase activity was essential, loss of N-methyltransferase activity did not affect surface piliation (312). Although methylation of type IV pilin proteins in T4P and T2S systems has been demonstrated in a number of species, this modification has not been reported for the Com or Flp pilins, archaeal flagellins, or pili. The FlaK and PibD peptidases are smaller by 54 to 60 residues than PilD, which may account for their lack of methylase activity.
In A. actinomycetemcomitans and P. aeruginosa, the fppA gene is located downstream of the flp-tad-rcp locus, whose gene products are responsible for assembly of Flp pili. At 160 amino acids, FppA is smaller than PilD (290 amino acids), with 4 predicted TM domains versus 6 for PilD (117). Sequence alignments of FppA and PilD showed that the active site Asp residues are conserved; however, FppA lacks the N-terminal domain containing the conserved Cys residues and the methyltransferase box found in PilD.
While cleavage of the type III signal is necessary for pilin function, it can also be used as a regulatory mechanism. V. cholerae can express 3 different kinds of type IV pili in different environmental niches: chitin-regulated pili, mannose-sensitive hemagglutinin (MSHA) T4a pili, and toxin-coregulated (Tcp) T4b pili. MSHA pili are important for persistence of V. cholerae in the environment as biofilms on zooplankton (96), while Tcp pili are involved in colonization of mammalian hosts. Hsiao et al. (187) found that the major subunit of MSHA, MshA, could be processed by the Tcp prepilin peptidase, TcpJ. However, this processing event led to degradation of MshA, preventing expression of MSHA and resulting clearance from the host, when the Tcp genes were activated by the regulator, ToxT, in response to host cues.
Because it is dispensable for fiber assembly, the specific role of pilin N-terminal methylation is so far unclear. It may enhance the stability of pilin subunits, extending their life span for recycling. Other possibilities include increased hydrophobicity of the N terminus, to allow subunits to be extracted more easily from the cytoplasmic membrane by the assembly machinery during fiber formation. Similarly, methylation may contribute to increased rates of depolymerization by increasing the propensity of the subunits to reinsert into the inner membrane. Interactions with the transmembrane domains of assembly proteins could be improved by methylation. Since the N termini of pilins are buried within the core of assembled fibers, methylation could contribute to the interactions that give assembled fibers their impressive capacity to resist disassembly under force.
In addition to methylation of the mature N terminus, C-terminal methylation of pilins was recently reported for the cyanobacterium Synechocystis (223). Using high-field Fourier transform ion cyclotron resonance (FT-ICR) MS—a form of MS that reveals small changes in mass in large proteins—a trimethylated Lys residue (versus a potentially acylated species of nearly identical mass) was identified at the C terminus of the major pilin subunit, PilA1. This unusual PTM was important for function and possibly stability of the subunits, as site-directed mutagenesis of the C-terminal Lys to Gln (K168Q) disrupted pilus-mediated gliding motility, reduced the intracellular levels of pilins, and resulted in the formation of fewer and significantly shorter pili on the cell surface than those of the wild type.
Production of Soluble Pilins
When pilus assembly is impaired, membrane-bound N. gonorrhoeae pilins can undergo an additional posttranslational cleavage event between residues 39 and 40 of the mature subunit to form shorter, soluble (S) pilins that are secreted (4, 160). Essentially, this cleavage event—carried out by an as yet unidentified protease—removes the hydrophobic N-terminal helix portion of the protein. Point mutations adjacent to the cleavage site prevented formation of S pilins and, depending on the nature of the mutation, blocked pilus assembly but did not affect competence for DNA transformation (4). A Neisseria-Pseudomonas chimera in which residues 37 to 43 from P. aeruginosa PilA replaced the corresponding residues in PilE was defective for assembly and competence, even though native P. aeruginosa pilins support competence in Neisseria (4, 5). The S pilins bind to human cells, but not animal cells, in a CD46-specific manner (341). Pilin glycosylation (below) was required for production of S pilins by N. meningitidis, but N. gonorrhoeae can produce nonglycosylated S pilins (although glycosylation increased the rate of S-pilin production) (268). The specific role that S pilins play in the pathogenesis of Neisseria disease is obscure at present, and truncated soluble forms of pilin have not been reported for other bacteria.
Glycosylation of Type IV Pilin Proteins
In addition to the variety in type IV pilin proteins arising from differences in their primary sequences, PTMs of the subunits with sugars or other moieties can increase antigenic and functional diversity. After processing of the N terminus, the most frequently identified PTM of type IV pilin proteins is O-glycosylation. Only T4a pilins have thus far been reported to have this modification (Fig. 8) (3, 73, 92, 136, 185, 186, 241, 318, 352, 405). Also, only major subunits are modified, although their relative abundance may simply make such modifications easier to detect. In addition to contributing to diversity, the presence or absence of glycosylation and the nature of the sugars that are attached to the subunit can modulate function. Kim and coworkers (222) inactivated a predicted acyl- or nucleotidyltransferase gene, sll0899, altering the Synechocystis O-linked pilin glycan. The sll0899 mutant produced pilins of increased mass compared with the parent strain but was significantly impaired for motility, even though the amount of surface-recoverable protein was similar. Mass spectrometry analysis revealed that the mutant pilins were modified with glycans containing the deoxyhexose rhamnose in addition to the xylose and fucose residues found on wild-type pilins. The mechanism by which this change alters function is unknown, but it may affect retraction dynamics, interactions with the surface necessary for motility, or even interactions with minor components involved in adherence.
Fig 8.
Type IV pilin glycosylation systems. Three different type IV pilin O-glycosylation systems have been characterized to date, one from Neisseria and two from Pseudomonas aeruginosa. The Pgl system (purple) from Neisseria generates undecaprenol-linked di- or trisaccharides via the action of cytoplasmic enzymes PglA, PglB, and PglE and translocates them via the activity of the flippase PglF (purple) to the periplasmic face of the membrane, where they are attached to Ser63 of PilE by one of two oligosaccharyltransferases, PglL or PglO (tan) (199, 256, 257). In P. aeruginosa, group I pilins are modified at the C-terminal Ser148 by TfpO (PilO) (tan) with an undecaprenol-linked O-antigen subunit generated by the Wbp enzymes of the lipopolysaccharide pathway (orange) and are flipped to the periplasmic face of the membrane by the O-unit flippase, Wzx (orange) (67, 87, 150). Group IV pilins are modified at multiple Ser and Thr residues by monomers, dimers, and longer polymers of d-arabinofuranose synthesized by cytoplasmic enzymes on a lipid carrier to form decaprenol-arabinofuranose (DPA) (blue), polymerized into α1,5-linked homopolymers, translocated to the periplasm by an unknown enzyme(s), and attached to the pilin by TfpW (tan) (241, 242, 405). TfpW is significantly larger than TfpO (694 versus 461 amino acids) and may be responsible for both the flippase and α1,5-arabinosyltransferase (AT) functions (yellow) in the group IV system.
Neisseria Pilin Glycosylation
Type IV pilin glycosylation was first identified in N. meningitidis, when mass spectrometry analysis of the major pilin PilE revealed a covalent Gal(β1-4)-Gal(α1-3)-2,4-diacetamido-2,4,6-trideoxyhexose (DATDH) trisaccharide modification (366). Soon after, the crystal structure of PilE from N. gonorrhoeae MS11 revealed covalent modification of Ser63 with Gal(α1-3) GlcNAc (306), with similar findings for N. meningitidis strain 8013SB (267). Pilins of N. gonorrhoeae strain N400 are modified with a hexose attached to 2,4-diacetamino-2,4,6-trideoxyhexose (HexDATDH) (173). Together, these studies suggested that more than one type of sugar could be attached to the pilins in Neisseria, but the glycan biosynthetic pathways, the mechanism by which glycans were added to PilE, and the extent of structural diversity among the sugars were unknown. Since those initial discoveries, T4P glycosylation has been identified in other bacterial species and in archaea.
Although S-pilin processing, glycosylation, and other posttranslational modifications have been studied extensively in Neisseria, the order in which these events happen is still being clarified. Long and colleagues put various forms of pilE (with and without a modifiable Ser63 residue) under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter and followed pilus-related phenotypes across a range of induction levels (259). S pilins released into the supernatant showed the same distribution of glycosylated and nonglycosylated forms as full-length pilins. Together, these data suggest that glycosylation likely occurs before proteolysis.
Studies of both N. gonorrhoeae and N. meningitidis led to the identification of the pgl (pilin glycan) locus, which encodes synthesis of the pilin glycan, its translocation to the periplasmic face of the inner membrane, and its covalent attachment to the pilins (173, 203, 212, 316, 317) (Fig. 8). The Pgl proteins work in tandem to sequentially add sugars to an undecaprenol lipid carrier to form the glycan, which is subsequently flipped across the inner membrane and transferred to the periplasmic C-terminal domain of an individual pilin monomer (318). Changes in composition of the pilin glycan occur through phase variation of the gene encoding the O-oligosaccharyltransferase (pglL or pglO) and through specific alleles of the sugar biosynthesis genes. Neisseria's glycosylation system is not specific to pilins, as it was recently shown to modify other surface proteins with the glycan derived from the Pgl pathway (240).
In addition to providing antigenic variation, the pilin glycan was recently shown to play a role in host cell invasion (202). In what has been called a “sticky and sweet” model (211), only glycosylated pili induce a high-affinity conformation of the I domain of the CR3 receptor on primary human cervical epithelial cells, which is normally present at low levels on uninfected cells.
Even though the minor pilins of Neisseria have not been reported to be glycosylated, they play an important role in control of other PTMs, as does the size of the glycan decorating the pilins (18). In particular, the abundance of the noncore minor pilin PilV controls the extent and type of alternate phospho-form modifications (see below) (1).
Pseudomonas Pilin Glycosylation
Two distinct pilin O-glycosylation systems have been identified in P. aeruginosa. In the first, glycosylation is mediated by TfpO (PilO), an O-oligosaccharyltransferase that (in another clever example of bacterial economy) adds an O-antigen unit synthesized by the LPS biosynthetic pathway (Fig. 8) to the C-terminal Ser of group I pilins (PilAI) (84, 106, 185). tfpO mutants still express surface pili and exhibit twitching motility, suggesting that glycosylation is not necessary for function. Instead, this PTM was suggested to provide a pathogenic advantage, since tfpO mutants were outcompeted by their wild-type parent in an acute mouse pneumonia coinfection model (360).
TfpO is a large protein with multiple membrane-spanning domains and is encoded immediately downstream of the pilAI gene. A stem-loop transcriptional terminator is present between the genes and likely functions to attenuate the level of tfpO expression from the strong pilA promoter (84). The very low abundance of TfpO required concentration of membranes before the protein could be detected by Western blotting (324). Together, these data suggest that the resulting stoichiometry would be such that a single TfpO enzyme could glycosylate multiple pilins. Transfer of the pilAI-tfpO gene cassette to strains of other LPS serotypes resulted in modification of the pilins with the corresponding O-antigen units of the new host, showing that TfpO has broad glycan specificity (121). Since only group I pilins that end in Ser are glycosylated by TfpO, the properties of PilAI that make it suitable for modification were investigated. Only Thr could substitute for the C-terminal Ser, and compatibility of the surface charge of the pilin's D region with TfpO was important for modification (185). In a pilin with an incompatible D-region charge, extension of the length of the C-terminal tail improved accessibility of the target residue and led to robust glycosylation. It is interesting that Cys-to-Ala mutations of the group I pilin from P. aeruginosa 1244 did not block glycosylation of the protein, suggesting that either the conformation of the D region was not crucial for substrate recognition by TfpO or other interactions maintained the architecture needed for TfpO activity (185). Pilins were modified in a pilD mutant, showing that processing of the N terminus was not required for glycosylation and that glycosylation can precede processing.
The second pilin glycosylation system in P. aeruginosa was identified in group IV strains Pa5196 and PA7, which lack tfpO (Fig. 8) (241, 242, 405). An O-antigen mutant of strain Pa5196 expressed glycosylated pilins, suggesting that the pilin glycan was derived from another pathway (405). Mass spectrometry and nuclear magnetic resonance studies revealed that the major pilin PilAIV was modified at multiple Thr and Ser residues by monomers, dimers, trimers, and potentially longer homo-oligosaccharides of α-1,5-linked arabinofuranose, a glycan typically found as part of the cell wall polymers of mycobacteria. Further studies showed that TfpW, the large protein encoded downstream of PilAIV, was required for pilin glycosylation (241). Mutants lacking TfpW produced unmodified pilins and few surface pili, suggesting that glycosylation of PilAIV is important for its assembly or stability. More recently, the d-Araf biosynthetic pathway in Pseudomonas was defined by reconstitution of the pilin glycosylation system in a laboratory strain of P. aeruginosa (171). Based on the genes required, the sugar precursor is synthesized as an unusual polyprenyl-phosphate intermediate, similar to that in mycobacteria. d-Araf biosynthetic mutants had the same phenotype as tfpW mutants, with unmodified pilins and few surface-exposed fibers even in retraction-deficient backgrounds, showing a profound assembly defect in the absence of glycosylation. Since the Pa5196 assembly system is not specific for glycosylated pilins, the lack of glycosylation probably affects PilAIV conformation, making it incompetent for assembly in the absence of PTM. The inability of TfpW to glycosylate other P. aeruginosa pilins—even those with Ser or Thr at similar positions to those modified in PilAIV—suggested that recognition of the atypical conformation of unmodified PilAIV could underlie TfpW's substrate specificity (171).
Glycosylation of Archaeal Flagellins and Pilins
Glycosylation of some archaeal flagellins and pilins has been reported. However, unlike the O-linked glycans identified on bacterial pilins, the glycans on archaeal subunits are N-linked. For M. voltae, glycosylation of 14 of 15 possible Asn residues on each of the four structural flagellin proteins (FlaA, FlaB1, B2, and B3) was reported (404). Mass spectrometry showed that a unique trisaccharide (β-ManpNAcA6Thr-(1-4)-β-Glc-pNAc3ACA-(1-3)-β-GlcpNAc) was linked to both flagellin and S-layer proteins, suggesting a common glycosylation system. Insertional inactivation of genes potentially involved in flagellin glycosylation showed two to be essential not only for modification but also for flagellin assembly (90). These genes, termed agl (for archaeal glycosylation) genes, encode a glycosyltransferase (AglA) with sequence similarity to the glycosyltransferases in P. aeruginosa and Thermus spp. and an oligosaccharyltransferase (AglB) similar to the STT3 homologue in Campylobacter spp. (PglB), responsible for transfer of glycans to a protein acceptor (90, 295, 382). Such similarities between the archaeal and bacterial systems suggest that archaea may have homologues of other members of the pgl locus. Some of the genes thought to be involved in glycan synthesis and translocation have been identified by bioinformatics and mutant analysis (89).
Other Forms of Pilin Posttranslational Modification
Besides glycosylation, modification of pilins with phosphate, phosphoethanolamine (PE), phosphocholine (PC), or phosphoglycerol (PG) has been reported, although thus far only for Neisseria species (143, 173). In the first example of such a modification, crystallographic analysis of the N. gonorrhoeae PilE protein revealed an electron density peak corresponding to a phosphate group at Ser68 (143).
In N. gonorrhoeae, the phase-variable PptA enzyme is responsible for transfer of both PC and PE to specific Ser residues of the pilin, PilE (1, 292). PptA is related to enzymes involved in PE modifications of LPS and is sufficient for PE but not PC transfer to PilE when expressed in a heterologous host, P. aeruginosa. It is possible that PE is converted to PC after attachment (292). As with the Neisseria pilin glycosylation system, the phospho-form modification system has additional periplasmic targets of unknown function. There is interplay between the glycosylation and phospho-form modification systems, as mutations that block glycosylation of substrate proteins or decrease the size of the glycan attached to the pilin at Ser63 result in PE/PC microheterogeneity (18). This change is separate from that mediated by altered PilV abundance, since pilV mutants exhibit no change in glycosylation.
Hegge et al. (173) suggested that N. gonorrhoeae modulates its pilin biochemistry through regulation of pilV levels. Wild-type strains have only PE modifications, while pilV null mutants have either PE or PC at Ser68 and Ser156 of PilE. Overexpression of PilV substantially reduced PE modification (173). Changing from a PE pilus to a PE/PC pilus could potentially modulate infection, as PC can function as a receptor for both C-reactive protein and platelet activating factor (355).
An elegant study by Chamot-Rooke et al. (91) recently provided an interesting biological rationale for modification of N. meningitidis pilins with PG. Exposure of N. meningitidis to host cells upregulated the enzyme PptB, responsible for the addition of PG at Ser69 and Ser93. Modification at Ser69 was similar in the presence or absence of host cell contact, while the increase in PptB expression associated with host cell contact led to Ser93 being the primary site of modification. The PG modification reduced the pI of the pilins, with the negative charge on Ser93 specifically reducing interfiber interactions (bundling) normally mediated by a patch of 5 Lys residues in the same region. Bundling of Neisseria pili is an important facet of their function, enhancing bacterial aggregation and substantially increasing force generation compared to that produced by single fibers (47). Both S93A and pptB mutations increased pilus-mediated aggregation, while overexpression of PptB blocked aggregation in a manner that could be reversed by the S93A substitution. Together, these data show that PG modification of Ser93 is a primary driver of pilus disaggregation. PG modification did not affect initial adherence or microcolony formation but was important for subsequent detachment and transmigration of cells across epithelial monolayers, thus favoring bacterial dissemination and invasion of host cells.
CONCLUSIONS AND FUTURE DIRECTIONS
The incredibly broad distribution of type IV pilin proteins in eubacteria and archaea from every environment suggests that they are highly adaptable functional modules built from a common blueprint. Their signature domain, a highly conserved, multifunctional N-terminal alpha helix that contains the site for processing by dedicated bifunctional prepilin peptidases/N-methylases, makes them easy to identify, especially now that specific algorithms are available (195). The C-terminal domains of type IV pilin proteins exhibit wide sequence diversity, even in different strains of the same species, reflecting both their broad range of functions and evolutionary responses to environmental pressures.
These versatile molecular modules participate in a remarkable number of processes, including protein secretion, DNA uptake/competence, phage infection, adherence to both living and nonliving surfaces, twitching and swimming motility, and electron transport. It is highly likely that additional functions will be discovered in the coming years. Areas of research that need further study include the role of minor subunits in assembly and function of the fibers, how the subunits are recognized and polymerized/depolymerized by their respective assembly systems, how these proteins contribute to DNA uptake and thus horizontal gene transfer, how Flp pilins and their assembly systems differ in structure from the canonical T4a and T4b systems, and many more.
The expression of type IV pilin proteins by many pathogenic bacteria, as well as their potential utility as antivirulence drug targets and vaccine candidates, makes them interesting from a medical standpoint. Their small size and ability to mediate motility or transduce electrons highlight the potential for their exploitation for industrial purposes, including formation of nanotubes with emergent properties (25, 27), movement of nanoscale cargo (410), and powering of bacterial batteries (261). Their ability to tolerate bulky modifications such as sugars (55, 73, 131, 323, 405) or peptides (400) makes them amenable to synthetic biology approaches (272) for development of completely novel uses, once a comprehensive understanding of their functional logic is available. The use of filamentous phages that have T4P-like assembly systems, such as M13, has revolutionized many aspects of molecular biology (406), and the type IV pilins are equally suited to take us in new directions.
ACKNOWLEDGMENTS
We thank Lisa Craig, Olivera Francetic, and Nicolas Biais for sharing data and figures, members of the Howell laboratory for helpful discussions, and the reviewers for their useful corrections and suggestions. We are grateful to current and past members of the Burrows laboratory for their contributions to the study of type IV pilins, and we express our regrets to those in the field whose work was not covered due to space limitations.
Our work on type IV pilins is funded by the Canadian Institutes of Health Research (operating grant MOP 86639).
REFERENCES
- 1. Aas FE, et al. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281: 27712–27723 [DOI] [PubMed] [Google Scholar]
- 2. Aas FE, Lovold C, Koomey M. 2002. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to type IV pilus expression. Mol. Microbiol. 46: 1441–1450 [DOI] [PubMed] [Google Scholar]
- 3. Aas FE, Vik A, Vedde J, Koomey M, Egge-Jacobsen W. 2007. Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure. Mol. Microbiol. 65: 607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aas FE, et al. 2007. Substitutions in the N-terminal alpha helical spine of Neisseria gonorrhoeae pilin affect type IV pilus assembly, dynamics and associated functions. Mol. Microbiol. 63: 69–85 [DOI] [PubMed] [Google Scholar]
- 5. Aas FE, et al. 2002. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol. Microbiol. 46: 749–760 [DOI] [PubMed] [Google Scholar]
- 6. Aho EL, et al. 2005. Neisserial pilin genes display extensive interspecies diversity. FEMS Microbiol. Lett. 249: 327–334 [DOI] [PubMed] [Google Scholar]
- 7. Ajon M, et al. 2011. UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili. Mol. Microbiol. 82: 807–817 [DOI] [PubMed] [Google Scholar]
- 8. Albano M, Breitling R, Dubnau DA. 1989. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J. Bacteriol. 171: 5386–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albers SV, Pohlschroder M. 2009. Diversity of archaeal type IV pilin-like structures. Extremophiles 13: 403–410 [DOI] [PubMed] [Google Scholar]
- 10. Albers SV, Szabo Z, Driessen AJ. 2003. Archaeal homolog of bacterial type IV prepilin signal peptidases with broad substrate specificity. J. Bacteriol. 185: 3918–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexander HL, Richardson AR, Stojiljkovic I. 2004. Natural transformation and phase variation modulation in Neisseria meningitidis. Mol. Microbiol. 52: 771–783 [DOI] [PubMed] [Google Scholar]
- 12. Alix E, Blanc-Potard AB. 2009. Hydrophobic peptides: novel regulators within bacterial membrane. Mol. Microbiol. 72: 5–11 [DOI] [PubMed] [Google Scholar]
- 13. Alm RA, Hallinan JP, Watson AA, Mattick JS. 1996. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY1 encodes a gonococcal PilC homologue. Mol. Microbiol. 22: 161–173 [DOI] [PubMed] [Google Scholar]
- 14. Alm RA, Mattick JS. 1995. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol. Microbiol. 16: 485–496 [DOI] [PubMed] [Google Scholar]
- 15. Alm RA, Mattick JS. 1996. Identification of two genes with prepilin-like leader sequences involved in type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 178: 3809–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alphonse S, et al. 2010. Structure of the Pseudomonas aeruginosa XcpT pseudopilin, a major component of the type II secretion system. J. Struct. Biol. 169: 75–80 [DOI] [PubMed] [Google Scholar]
- 17. Angelos JA, Bonifacio RG, Ball LM, Hess JF. 2007. Prevention of naturally occurring infectious bovine keratoconjunctivitis with a recombinant Moraxella bovis pilin-Moraxella bovis cytotoxin-ISCOM matrix adjuvanted vaccine. Vet. Microbiol. 125: 274–283 [DOI] [PubMed] [Google Scholar]
- 18. Anonsen JH, et al. 2012. Novel protein substrates of the phospho-form modification system in Neisseria gonorrhoeae and their connection to O-linked protein glycosylation. Infect. Immun. 80: 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ark NM, Mann BJ. 2011. Impact of Francisella tularensis pilin homologs on pilus formation and virulence. Microb. Pathog. 51: 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arlehamn CS, Evans TJ. 2011. Pseudomonas aeruginosa pilin activates the inflammasome. Cell. Microbiol. 13: 388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aroeti B, Friedman G, Zlotkin-Rivkin E, Donnenberg M. 2012. Retraction of enteropathogenic E. coli type IV pili promotes efficient host cell colonization, effector translocation and tight junction disruption. Gut Microbes 3: 267–271 doi:10.4161/gmic.19814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arts J, van Boxtel R, Filloux A, Tommassen J, Koster M. 2007. Export of the pseudopilin XcpT of the Pseudomonas aeruginosa type II secretion system via the signal recognition particle-Sec pathway. J. Bacteriol. 189: 2069–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asikyan ML, Kus JV, Burrows LL. 2008. Novel proteins that modulate type IV pilus retraction dynamics in Pseudomonas aeruginosa. J. Bacteriol. 190: 7022–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Assalkhou R, et al. 2007. The outer membrane secretin PilQ from Neisseria meningitidis binds DNA. Microbiology 153: 1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Audette GF, Hazes B. 2007. Development of protein nanotubes from a multi-purpose biological structure. J. Nanosci. Nanotechnol. 7: 2222–2229 [DOI] [PubMed] [Google Scholar]
- 26. Audette GF, Irvin RT, Hazes B. 2004. Crystallographic analysis of the Pseudomonas aeruginosa strain K122-4 monomeric pilin reveals a conserved receptor-binding architecture. Biochemistry 43: 11427–11435 [DOI] [PubMed] [Google Scholar]
- 27. Audette GF, van Schaik EJ, Hazes B, Irvin RT. 2004. DNA-binding protein nanotubes: learning from nature's nanotech examples. Nano Lett. 4: 1897–1902 [Google Scholar]
- 28. Averhoff B. 2004. DNA transport and natural transformation in mesophilic and thermophilic bacteria. J. Bioenerg. Biomembr. 36: 25–33 [DOI] [PubMed] [Google Scholar]
- 29. Averhoff B. 2009. Shuffling genes around in hot environments: the unique DNA transporter of Thermus thermophilus. FEMS Microbiol. Rev. 33: 611–626 [DOI] [PubMed] [Google Scholar]
- 30. Averhoff B, Friedrich A. 2003. Type IV pili-related natural transformation systems: DNA transport in mesophilic and thermophilic bacteria. Arch. Microbiol. 180: 385–393 [DOI] [PubMed] [Google Scholar]
- 31. Ayers M, Howell PL, Burrows LL. 2010. Architecture of the type II secretion and type IV pilus machineries. Future Microbiol. 5: 1203–1218 [DOI] [PubMed] [Google Scholar]
- 32. Ayers M, et al. 2009. PilM/N/O/P proteins form an inner membrane complex that affects the stability of the Pseudomonas aeruginosa type IV pilus secretin. J. Mol. Biol. 394: 128–142 [DOI] [PubMed] [Google Scholar]
- 33. Bakaletz LO, et al. 2005. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect. Immun. 73: 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakkali M. 2007. Genome dynamics of short oligonucleotides: the example of bacterial DNA uptake enhancing sequences. PLoS One 2: e741 doi:10.1371/journal.pone.0000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balakrishna AM, Saxena AM, Mok HY, Swaminathan K. 2009. Structural basis of typhoid: Salmonella typhi type IVb pilin (PilS) and cystic fibrosis transmembrane conductance regulator interaction. Proteins 77: 253–261 [DOI] [PubMed] [Google Scholar]
- 36. Balakrishna AM, Tan YY, Mok HY, Saxena AM, Swaminathan K. 2006. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 62: 1024–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bally M, Ball G, Badere A, Lazdunski A. 1991. Protein secretion in Pseudomonas aeruginosa: the xcpA gene encodes an integral inner membrane protein homologous to Klebsiella pneumoniae secretion function protein PulO. J. Bacteriol. 173: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bally M, et al. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6: 1121–1131 [DOI] [PubMed] [Google Scholar]
- 39. Bardy SL, Jarrell KF. 2003. Cleavage of preflagellins by an aspartic acid signal peptidase is essential for flagellation in the archaeon Methanococcus voltae. Mol. Microbiol. 50: 1339–1347 [DOI] [PubMed] [Google Scholar]
- 40. Bechet M, Blondeau R. 2003. Factors associated with the adherence and biofilm formation by Aeromonas caviae on glass surfaces. J. Appl. Microbiol. 94: 1072–1078 [DOI] [PubMed] [Google Scholar]
- 41. Belete B, Lu H, Wozniak DJ. 2008. Pseudomonas aeruginosa AlgR regulates type IV pilus biosynthesis by activating transcription of the fimU-pilVWXY1Y2E operon. J. Bacteriol. 190: 2023–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bernard CS, Bordi C, Termine E, Filloux A, de Bentzmann S. 2009. Organization and PprB-dependent control of the Pseudomonas aeruginosa tad locus, involved in Flp pilus biology. J. Bacteriol. 191: 1961–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bertrand JJ, West JT, Engel JN. 2010. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. J. Bacteriol. 192: 994–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhattacharjee MK, Kachlany SC, Fine DH, Figurski DH. 2001. Nonspecific adherence and fibril biogenesis by Actinobacillus actinomycetemcomitans: TadA protein is an ATPase. J. Bacteriol. 183: 5927–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhaya D, Bianco NR, Bryant D, Grossman A. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37: 941–951 [DOI] [PubMed] [Google Scholar]
- 46. Biais N, Higashi DL, Brujic J, So M, Sheetz MP. 2010. Force-dependent polymorphism in type IV pili reveals hidden epitopes. Proc. Natl. Acad. Sci. U. S. A. 107: 11358–11363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Biais N, Ladoux B, Higashi D, So M, Sheetz M. 2008. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 6: e87 doi:10.1371/journal.pbio.0060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bieber D, et al. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280: 2114–2118 [DOI] [PubMed] [Google Scholar]
- 49. Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27: 209–219 [DOI] [PubMed] [Google Scholar]
- 50. Blank TE, Zhong H, Bell AL, Whittam TS, Donnenberg MS. 2000. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect. Immun. 68: 7028–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. 2002. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 45: 1255–1265 [DOI] [PubMed] [Google Scholar]
- 52. Boettcher JP, et al. 2010. Tyrosine-phosphorylated caveolin-1 blocks bacterial uptake by inducing Vav2-RhoA-mediated cytoskeletal rearrangements. PLoS Biol. 8: e1000457 doi:10.1371/journal.pbio.1000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bohn YS, et al. 2009. Multiple roles of Pseudomonas aeruginosa TBCF10839 PilY1 in motility, transport and infection. Mol. Microbiol. 71: 730–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonner PJ, Shimkets LJ. 2006. Phospholipid directed motility of surface-motile bacteria. Mol. Microbiol. 61: 1101–1109 [DOI] [PubMed] [Google Scholar]
- 55. Borud B, et al. 2010. Genetic, structural, and antigenic analyses of glycan diversity in the O-linked protein glycosylation systems of human Neisseria species. J. Bacteriol. 192: 2816–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boyd EF, Waldor MK. 2002. Evolutionary and functional analyses of variants of the toxin-coregulated pilus protein TcpA from toxigenic Vibrio cholerae non-O1/non-O139 serogroup isolates. Microbiology 148: 1655–1666 [DOI] [PubMed] [Google Scholar]
- 57. Boyd JM, et al. 2008. Contribution of type IV pili to the virulence of Aeromonas salmonicida subsp. salmonicida in Atlantic salmon (Salmo salar L.). Infect. Immun. 76: 1445–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boyd JM, Lory S. 1996. Dual function of PilS during transcriptional activation of the Pseudomonas aeruginosa pilin subunit gene. J. Bacteriol. 178: 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradley DE. 1972. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem. Biophys. Res. Commun. 47: 142–149 [DOI] [PubMed] [Google Scholar]
- 60. Bradley DE. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol. 26: 146–154 [DOI] [PubMed] [Google Scholar]
- 61. Bradley DE. 1972. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J. Gen. Microbiol. 72: 303–319 [DOI] [PubMed] [Google Scholar]
- 62. Bravo D, et al. 2011. Type IV(B) pili are required for invasion but not for adhesion of Salmonella enterica serovar Typhi into BHK epithelial cells in a cystic fibrosis transmembrane conductance regulator-independent manner. Microb. Pathog. 51: 373–377 [DOI] [PubMed] [Google Scholar]
- 63. Brissac T, Mikaty G, Dumenil G, Coureuil M, Nassif X. 2012. The meningococcal minor pilin PilX is responsible for type IV pilus conformational changes associated with signaling to endothelial cells. Infect. Immun. 80: 3297–3306 doi:10.1128/IAI.00369-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown DR, Helaine S, Carbonnelle E, Pelicic V. 2010. Systematic functional analysis reveals that a set of seven genes is involved in fine-tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis. Infect. Immun. 78: 3053–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brown MH, Brightman AH, Fenwick BW, Rider MA. 1998. Infectious bovine keratoconjunctivitis: a review. J. Vet. Intern. Med. 12: 259–266 [DOI] [PubMed] [Google Scholar]
- 66. Burriesci M, Bhaya D. 2008. Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC6803. J. Photochem. Photobiol. B 91: 77–86 [DOI] [PubMed] [Google Scholar]
- 67. Burrows LL. 2008. A nice return on the “stalk” exchange. Structure 16: 19–20 [DOI] [PubMed] [Google Scholar]
- 68. Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66: 493–520 [DOI] [PubMed] [Google Scholar]
- 69. Burrows LL. 2005. Weapons of mass retraction. Mol. Microbiol. 57: 878–888 [DOI] [PubMed] [Google Scholar]
- 70. Burton B, Dubnau D. 2010. Membrane-associated DNA transport machines. Cold Spring Harb. Perspect. Biol. 2: a000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cachia PJ, et al. 1998. The use of synthetic peptides in the design of a consensus sequence vaccine for Pseudomonas aeruginosa. J. Pept. Res. 52: 289–299 [DOI] [PubMed] [Google Scholar]
- 72. Cachia PJ, Hodges RS. 2003. Synthetic peptide vaccine and antibody therapeutic development: prevention and treatment of Pseudomonas aeruginosa. Biopolymers 71: 141–168 [DOI] [PubMed] [Google Scholar]
- 73. Cagatay TI, Hickford JG. 2008. Glycosylation of type-IV fimbriae of Dichelobacter nodosus. Vet. Microbiol. 126: 160–167 [DOI] [PubMed] [Google Scholar]
- 74. Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325: 764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Campbell AP, et al. 1997. Interaction of the receptor binding domains of Pseudomonas aeruginosa pili strains PAK, PAO, KB7 and P1 to a cross-reactive antibody and receptor analog: implications for synthetic vaccine design. J. Mol. Biol. 267: 382–402 [DOI] [PubMed] [Google Scholar]
- 76. Campos M, Francetic O, Nilges M. 2011. Modeling pilus structures from sparse data. J. Struct. Biol. 173: 436–444 [DOI] [PubMed] [Google Scholar]
- 77. Campos M, Nilges M, Cisneros DA, Francetic O. 2010. Detailed structural and assembly model of the type II secretion pilus from sparse data. Proc. Natl. Acad. Sci. U. S. A. 107: 13081–13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cao Z, Bowie JU. 2012. Shifting hydrogen bonds may produce flexible transmembrane helices. Proc. Natl. Acad. Sci. U. S. A. 109: 8121–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carbonnelle E, Helaine S, Nassif X, Pelicic V. 2006. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol. Microbiol. 61: 1510–1522 [DOI] [PubMed] [Google Scholar]
- 80. Carbonnelle E, et al. 2009. Meningococcal interactions with the host. Vaccine 27(Suppl 2): B78–B89 [DOI] [PubMed] [Google Scholar]
- 81. Carrick CS, Fyfe JA, Davies JK. 2000. The genome of Neisseria gonorrhoeae retains the remnants of a two-component regulatory system that once controlled piliation. FEMS Microbiol. Lett. 186: 197–201 [DOI] [PubMed] [Google Scholar]
- 82. Carruthers MD, et al. 2012. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J. Bacteriol. 194: 1927–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carter MQ, Chen J, Lory S. 2010. The Pseudomonas aeruginosa pathogenicity island PAPI-1 is transferred via a novel type IV pilus. J. Bacteriol. 192: 3249–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Castric P. 1995. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology 141: 1247–1254 [DOI] [PubMed] [Google Scholar]
- 85. Castric P, Cassels FJ, Carlson RW. 2001. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J. Biol. Chem. 276: 26479–26485 [DOI] [PubMed] [Google Scholar]
- 86. Castric PA, Deal CD. 1994. Differentiation of Pseudomonas aeruginosa pili based on sequence and B-cell epitope analyses. Infect. Immun. 62: 371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cehovin A, Kroll JS, Pelicic V. 2011. Testing the vaccine potential of PilV, PilX and ComP, minor subunits of Neisseria meningitidis type IV pili. Vaccine 29: 6858–6865 [DOI] [PubMed] [Google Scholar]
- 88. Cehovin A, et al. 2010. Sequence conservation of pilus subunits in Neisseria meningitidis. Vaccine 28: 4817–4826 [DOI] [PubMed] [Google Scholar]
- 89. Chaban B, Logan SM, Kelly JF, Jarrell KF. 2009. AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae. J. Bacteriol. 191: 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF. 2006. Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea. Mol. Microbiol. 61: 259–268 [DOI] [PubMed] [Google Scholar]
- 91. Chamot-Rooke J, et al. 2011. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science 331: 778–782 [DOI] [PubMed] [Google Scholar]
- 92. Chamot-Rooke J, et al. 2007. Alternative Neisseria spp. type IV pilin glycosylation with a glyceramido acetamido trideoxyhexose residue. Proc. Natl. Acad. Sci. U. S. A. 104: 14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen I, Dubnau D. 2003. DNA transport during transformation. Front. Biosci. 8: s544–s556 [DOI] [PubMed] [Google Scholar]
- 94. Chen I, Provvedi R, Dubnau D. 2006. A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J. Biol. Chem. 281: 21720–21727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chiang P, Burrows LL. 2003. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. J. Bacteriol. 185: 2374–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chiavelli DA, Marsh JW, Taylor RK. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67: 3220–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Christodoulides M, et al. 2002. Interaction of Neisseria meningitidis with human meningeal cells induces the secretion of a distinct group of chemotactic, proinflammatory, and growth-factor cytokines. Infect. Immun. 70: 4035–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cianciotto NP. 2009. Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol. 4: 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cianciotto NP. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13: 581–588 [DOI] [PubMed] [Google Scholar]
- 100. Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. 2011. Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation. EMBO 31: 1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Clausen M, Jakovljevic V, Sogaard-Andersen L, Maier B. 2009. High-force generation is a conserved property of type IV pilus systems. J. Bacteriol. 191: 4633–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Coil DA, Anne J. 2009. Twitching motility in Legionella pneumophila. FEMS Microbiol. Lett. 293: 271–277 [DOI] [PubMed] [Google Scholar]
- 103. Collins RF, Davidsen L, Derrick JP, Ford RC, Tonjum T. 2001. Analysis of the PilQ secretin from Neisseria meningitidis by transmission electron microscopy reveals a dodecameric quaternary structure. J. Bacteriol. 183: 3825–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Collins RF, et al. 2004. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J. Biol. Chem. 279: 39750–39756 [DOI] [PubMed] [Google Scholar]
- 105. Collyn F, et al. 2002. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect. Immun. 70: 6196–6205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Comer JE, Marshall MA, Blanch VJ, Deal CD, Castric P. 2002. Identification of the Pseudomonas aeruginosa 1244 pilin glycosylation site. Infect. Immun. 70: 2837–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Comolli JC, et al. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67: 3625–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Conrad JC, et al. 2011. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys. J. 100: 1608–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Correia JD, Jarrell KF. 2000. Posttranslational processing of Methanococcus voltae preflagellin by preflagellin peptidases of M. voltae and other methanogens. J. Bacteriol. 182: 855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Coureuil M, et al. 2012. Mechanism of meningeal invasion by Neisseria meningitidis. Virulence 3: 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Coureuil M, et al. 2009. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 325: 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Craig L, Li J. 2008. Type IV pili: paradoxes in form and function. Curr. Opin. Struct. Biol. 18: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Craig L, Pique ME, Tainer JA. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2: 363–378 [DOI] [PubMed] [Google Scholar]
- 114. Craig L, et al. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11: 1139–1150 [DOI] [PubMed] [Google Scholar]
- 115. Craig L, et al. 2006. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23: 651–662 [DOI] [PubMed] [Google Scholar]
- 116. Criss AK, Kline KA, Seifert HS. 2005. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 58: 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. de Bentzmann S, Aurouze M, Ball G, Filloux A. 2006. FppA, a novel Pseudomonas aeruginosa prepilin peptidase involved in assembly of type IVb pili. J. Bacteriol. 188: 4851–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de la Fuente-Nunez C, et al. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 56: 2696–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dietrich M, et al. 2011. Activation of NF-kappaB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell. Microbiol. 13: 1168–1182 [DOI] [PubMed] [Google Scholar]
- 120. Dietrich M, Mollenkopf H, So M, Friedrich A. 2009. Pilin regulation in the pilT mutant of Neisseria gonorrhoeae strain MS11. FEMS Microbiol. Lett. 296: 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. DiGiandomenico A, et al. 2002. Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol. Microbiol. 46: 519–530 [DOI] [PubMed] [Google Scholar]
- 122. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15: 167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Donnenberg MS, Giron JA, Nataro JP, Kaper JB. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6: 3427–3437 [DOI] [PubMed] [Google Scholar]
- 124. Douzi B, Ball G, Cambillau C, Tegoni M, Voulhoux R. 2011. Deciphering the Xcp Pseudomonas aeruginosa type II secretion machinery through multiple interactions with substrates. J. Biol. Chem. 286: 40792–40801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Douzi B, Filloux A, Voulhoux R. 2012. On the path to uncover the bacterial type II secretion system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dunlop KV, Irvin RT, Hazes B. 2005. Pros and cons of cryocrystallography: should we also collect a room-temperature data set? Acta Crystallogr. D 61: 80–87 [DOI] [PubMed] [Google Scholar]
- 127. Durand E, et al. 2011. The assembly mode of the pseudopilus: a hallmark to distinguish a novel secretion system subtype. J. Biol. Chem. 286: 24407–24416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Durand E, et al. 2003. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J. Bacteriol. 185: 2749–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Durand E, et al. 2005. XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus. J. Biol. Chem. 280: 31378–31389 [DOI] [PubMed] [Google Scholar]
- 130. Egerton JR, Burrell DH. 1970. Prophylactic and therapeutic vaccination against ovine foot-rot. Aust. Vet. J. 46: 517–522 [DOI] [PubMed] [Google Scholar]
- 131. Egge-Jacobsen W, et al. 2011. O-linked glycosylation of the PilA pilin protein of Francisella tularensis: identification of the endogenous protein-targeting oligosaccharyltransferase and characterization of the native oligosaccharide. J. Bacteriol. 193: 5487–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Emam A, et al. 2006. Laboratory and clinical Pseudomonas aeruginosa strains do not bind glycosphingolipids in vitro or during type IV pili-mediated initial host cell attachment. Microbiology 152: 2789–2799 [DOI] [PubMed] [Google Scholar]
- 133. Entcheva-Dimitrov P, Spormann AM. 2004. Dynamics and control of biofilms of the oligotrophic bacterium Caulobacter crescentus. J. Bacteriol. 186: 8254–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Evans KJ, Lambert C, Sockett RE. 2007. Predation by Bdellovibrio bacteriovorus HD100 requires type IV pili. J. Bacteriol. 189: 4850–4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Faast R, Ogierman MA, Stroeher UH, Manning PA. 1989. Nucleotide sequence of the structural gene, tcpA, for a major pilin subunit of Vibrio cholerae. Gene 85: 227–231 [DOI] [PubMed] [Google Scholar]
- 136. Faridmoayer A, Fentabil MA, Mills DC, Klassen JS, Feldman MF. 2007. Functional characterization of bacterial oligosaccharyltransferases involved in O-linked protein glycosylation. J. Bacteriol. 189: 8088–8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Feliciano GT, da Silva AJ, Reguera G, Artacho E. 2012. Molecular and electronic structure of the peptide subunit of Geobacter sulfurreducens conductive pili from first principles. J. Phys. Chem. A doi:10.1021/jp302232p [DOI] [PubMed] [Google Scholar]
- 138. Fernandes PJ, Guo Q, Donnenberg MS. 2007. Functional consequences of sequence variation in bundlin, the enteropathogenic Escherichia coli type IV pilin protein. Infect. Immun. 75: 4687–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Filloux A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694: 163–179 [DOI] [PubMed] [Google Scholar]
- 140. Filloux A, Michel G, Bally M. 1998. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol. Rev. 22: 177–198 [DOI] [PubMed] [Google Scholar]
- 141. Findlay WA, Redfield RJ. 2009. Coevolution of DNA uptake sequences and bacterial proteomes. Genome Biol. Evol. 1: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Forest KT. 2008. The type II secretion arrowhead: the structure of GspI-GspJ-GspK. Nat. Struct. Mol. Biol. 15: 428–430 [DOI] [PubMed] [Google Scholar]
- 143. Forest KT, Dunham SA, Koomey M, Tainer JA. 1999. Crystallographic structure reveals phosphorylated pilin from Neisseria: phosphoserine sites modify type IV pilus surface chemistry and fibre morphology. Mol. Microbiol. 31: 743–752 [DOI] [PubMed] [Google Scholar]
- 144. Francetic O, Buddelmeijer N, Lewenza S, Kumamoto CA, Pugsley AP. 2007. Signal recognition particle-dependent inner membrane targeting of the PulG pseudopilin component of a type II secretion system. J. Bacteriol. 189: 1783–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Francetic O, Pugsley AP. 2005. Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J. Bacteriol. 187: 7045–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Freitag NE, Seifert HS, Koomey M. 1995. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16: 575–586 [DOI] [PubMed] [Google Scholar]
- 147. Frols S, et al. 2008. UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation. Mol. Microbiol. 70: 938–952 [DOI] [PubMed] [Google Scholar]
- 148. Fulks KA, Marrs CF, Stevens SP, Green MR. 1990. Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. J. Bacteriol. 172: 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fullner KJ, Mekalanos JJ. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fussenegger M, Rudel T, Barten R, Ryll R, Meyer TF. 1997. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—a review. Gene 192: 125–134 [DOI] [PubMed] [Google Scholar]
- 151. Ghosh A, Albers SV. 2011. Assembly and function of the archaeal flagellum. Biochem. Soc. Trans. 39: 64–69 [DOI] [PubMed] [Google Scholar]
- 152. Gibbs CP, et al. 1989. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature 338: 651–652 [DOI] [PubMed] [Google Scholar]
- 153. Gibiansky ML, et al. 2010. Bacteria use type IV pili to walk upright and detach from surfaces. Science 330: 197. [DOI] [PubMed] [Google Scholar]
- 154. Giltner CL, Habash M, Burrows LL. 2010. Pseudomonas aeruginosa minor pilins are incorporated into the type IV pilus. J. Mol. Biol. 398: 444–461 [DOI] [PubMed] [Google Scholar]
- 155. Giltner CL, Rana N, Lunardo MN, Hussain AQ, Burrows LL. 2011. Evolutionary and functional diversity of the Pseudomonas type IVa pilin island. Environ. Microbiol. 13: 250–264 [DOI] [PubMed] [Google Scholar]
- 156. Giltner CL, et al. 2006. The Pseudomonas aeruginosa type IV pilin receptor binding domain functions as an adhesin for both biotic and abiotic surfaces. Mol. Microbiol. 59: 1083–1096 [DOI] [PubMed] [Google Scholar]
- 157. Giron JA, Gomez-Duarte OG, Jarvis KG, Kaper JB. 1997. Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili—a minireview. Gene 192: 39–43 [DOI] [PubMed] [Google Scholar]
- 158. Glick R, et al. 2010. Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 192: 2973–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Graupner S, Wackernagel W. 2001. Pseudomonas stutzeri has two closely related pilA genes (type IV pilus structural protein) with opposite influences on natural genetic transformation. J. Bacteriol. 183: 2359–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Haas R, Schwarz H, Meyer TF. 1987. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 84: 9079–9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Haas R, Veit S, Meyer TF. 1992. Silent pilin genes of Neisseria gonorrhoeae MS11 and the occurrence of related hypervariant sequences among other gonococcal isolates. Mol. Microbiol. 6: 197–208 [DOI] [PubMed] [Google Scholar]
- 162. Hackbarth C, Hodges RS. 2010. Synthetic peptide vaccine development: designing dual epitopes into a single pilin peptide immunogen generates antibody cross-reactivity between two strains of Pseudomonas aeruginosa. Chem. Biol. Drug Des. 76: 293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hager AJ, et al. 2006. Type IV pili-mediated secretion modulates Francisella virulence. Mol. Microbiol. 62: 227–237 [DOI] [PubMed] [Google Scholar]
- 164. Hahn HP. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192: 99–108 [DOI] [PubMed] [Google Scholar]
- 165. Hamrick TS, Dempsey JA, Cohen MS, Cannon JG. 2001. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology 147: 839–849 [DOI] [PubMed] [Google Scholar]
- 166. Han X, et al. 2008. Twitching motility is essential for virulence in Dichelobacter nodosus. J. Bacteriol. 190: 3323–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Han X, Kennan RM, Parker D, Davies JK, Rood JI. 2007. Type IV fimbrial biogenesis is required for protease secretion and natural transformation in Dichelobacter nodosus. J. Bacteriol. 189: 5022–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hansen JK, Forest KT. 2006. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J. Mol. Microbiol. Biotechnol. 11: 192–207 [DOI] [PubMed] [Google Scholar]
- 169. Hartung S, et al. 2011. Ultrahigh resolution and full-length pilin structures with insights for filament assembly, pathogenic functions, and vaccine potential. J. Biol. Chem. 286: 44254–44265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Harvey H, Habash M, Aidoo F, Burrows LL. 2009. Single residue changes in the C-terminal disulfide-bonded loop of the Pseudomonas aeruginosa type IV pilin influence pilus assembly and twitching motility. J. Bacteriol. 191: 6513–6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Harvey H, Kus JV, Tessier L, Kelly J, Burrows LL. 2011. Pseudomonas aeruginosa d-arabinofuranose biosynthetic pathway and its role in type IV pilus assembly. J. Biol. Chem. 286: 28128–28137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hazes B, Sastry PA, Hayakawa K, Read RJ, Irvin RT. 2000. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J. Mol. Biol. 299: 1005–1017 [DOI] [PubMed] [Google Scholar]
- 173. Hegge FT, et al. 2004. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc. Natl. Acad. Sci. U. S. A. 101: 10798–10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Heiniger RW, Winther-Larsen HC, Pickles RJ, Koomey M, Wolfgang MC. 2010. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell. Microbiol. 12: 1158–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Helaine S, et al. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55: 65–77 [DOI] [PubMed] [Google Scholar]
- 176. Helaine S, Dyer DH, Nassif X, Pelicic V, Forest KT. 2007. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 104: 15888–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Helm RA, Seifert HS. 2010. Frequency and rate of pilin antigenic variation of Neisseria meningitidis. J. Bacteriol. 192: 3822–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Henche AL, Koerdt A, Ghosh A, Albers SV. 2012. Influence of cell surface structures on crenarchaeal biofilm formation using a thermostable green fluorescent protein. Environ. Microbiol. 14: 779–793 [DOI] [PubMed] [Google Scholar]
- 179. Higashi DL, et al. 2007. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect. Immun. 75: 4743–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hill DJ, Virji M. 2012. Meningococcal ligands and molecular targets of the host. Methods Mol. Biol. 799: 143–152 [DOI] [PubMed] [Google Scholar]
- 181. Hill SA, Davies JK. 2009. Pilin gene variation in Neisseria gonorrhoeae: reassessing the old paradigms. FEMS Microbiol. Rev. 33: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hobbs M, Collie ES, Free PD, Livingston SP, Mattick JS. 1993. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 7: 669–682 [DOI] [PubMed] [Google Scholar]
- 183. Hobbs M, Mattick JS. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10: 233–243 [DOI] [PubMed] [Google Scholar]
- 184. Holz C, et al. 2010. Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys. Rev. Lett. 104: 178104 doi:10.1103/PhysRevLett.104.178104 [DOI] [PubMed] [Google Scholar]
- 185. Horzempa J, Comer JE, Davis SA, Castric P. 2006. Glycosylation substrate specificity of Pseudomonas aeruginosa 1244 pilin. J. Biol. Chem. 281: 1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Horzempa J, Dean CR, Goldberg JB, Castric P. 2006. Pseudomonas aeruginosa 1244 pilin glycosylation: glycan substrate recognition. J. Bacteriol. 188: 4244–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hsiao A, Toscano K, Zhu J. 2008. Post-transcriptional cross-talk between pro- and anti-colonization pili biosynthesis systems in Vibrio cholerae. Mol. Microbiol. 67: 849–860 [DOI] [PubMed] [Google Scholar]
- 188. Hu J, Xue Y, Lee S, Ha Y. 2011. The crystal structure of GXGD membrane protease FlaK. Nature 475: 528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hu NT, Lee PF, Chen C. 1995. The type IV pre-pilin leader peptidase of Xanthomonas campestris pv. campestris is functional without conserved cysteine residues. Mol. Microbiol. 18: 769–777 [DOI] [PubMed] [Google Scholar]
- 190. Hu NT, et al. 2002. XpsG, the major pseudopilin in Xanthomonas campestris pv. campestris, forms a pilus-like structure between cytoplasmic and outer membranes. Biochem. J. 365: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Humphries RM, et al. 2009. From alpha to beta: identification of amino acids required for the N-acetyllactosamine-specific lectin-like activity of bundlin. Mol. Microbiol. 72: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Humphries RM, et al. 2010. N-acetyllactosamine-induced retraction of bundle-forming pili regulates virulence-associated gene expression in enteropathogenic Escherichia coli. Mol. Microbiol. 76: 1111–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Humphries RM, Waterhouse CC, Mulvey G, Beck P, Armstrong GD. 2009. Interactions of enteropathogenic Escherichia coli with pediatric and adult intestinal biopsy specimens during early adherence. Infect. Immun. 77: 4463–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hyland RM, et al. 2008. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell. Microbiol. 10: 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Imam S, Chen Z, Roos DS, Pohlschroder M. 2011. Identification of surprisingly diverse type IV pili, across a broad range of Gram-positive bacteria. PLoS One 6: e28919 doi:10.1371/journal.pone.0028919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ishihara K, Honma K, Miura T, Kato T, Okuda K. 1997. Cloning and sequence analysis of the fimbriae associated protein (fap) gene from Actinobacillus actinomycetemcomitans. Microb. Pathog. 23: 63–69 [DOI] [PubMed] [Google Scholar]
- 197. Ishimoto KS, Lory S. 1992. Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J. Bacteriol. 174: 3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ishiwa A, Komano T. 2004. PilV adhesins of plasmid R64 thin pili specifically bind to the lipopolysaccharides of recipient cells. J. Mol. Biol. 343: 615–625 [DOI] [PubMed] [Google Scholar]
- 199. Jarrell KF, Albers SV. 2012. The archaellum: an old motility structure with a new name. Trends Microbiol. 20: 307–312 [DOI] [PubMed] [Google Scholar]
- 200. Jarrell KF, Stark M, Nair DB, Chong JP. 2011. Flagella and pili are both necessary for efficient attachment of Methanococcus maripaludis to surfaces. FEMS Microbiol. Lett. 319: 44–50 [DOI] [PubMed] [Google Scholar]
- 201. Jelsbak L, Kaiser D. 2005. Regulating pilin expression reveals a threshold for S motility in Myxococcus xanthus. J. Bacteriol. 187: 2105–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Jennings MP, Jen FE, Roddam LF, Apicella MA, Edwards JL. 2011. Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells. Cell. Microbiol. 13: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jennings MP, et al. 1998. Identification of a novel gene involved in pilin glycosylation in Neisseria meningitidis. Mol. Microbiol. 29: 975–984 [DOI] [PubMed] [Google Scholar]
- 204. Johnson MD, et al. 2011. Pseudomonas aeruginosa PilY1 binds integrin in an RGD- and calcium-dependent manner. PLoS One 6: e29629 doi:10.1371/journal.pone.0029629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Johnson TL, Abendroth J, Hol WG, Sandkvist M. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255: 175–186 [DOI] [PubMed] [Google Scholar]
- 206. Join-Lambert O, et al. 2010. Mechanisms of meningeal invasion by a bacterial extracellular pathogen, the example of Neisseria meningitidis. Prog. Neurobiol. 91: 130–139 [DOI] [PubMed] [Google Scholar]
- 207. Jude BA, Taylor RK. 2011. The physical basis of type 4 pilus-mediated microcolony formation by Vibrio cholerae O1. J. Struct. Biol. 175: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jurcisek JA, et al. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol. Microbiol. 65: 1288–1299 [DOI] [PubMed] [Google Scholar]
- 209. Kachlany SC, et al. 2000. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J. Bacteriol. 182: 6169–6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Kachlany SC, et al. 2001. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 40: 542–554 [DOI] [PubMed] [Google Scholar]
- 211. Kahler CM. 2011. Sticky and sweet: the role of post-translational modifications on neisserial pili. Front. Microbiol. 2: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kahler CM, et al. 2001. Polymorphisms in pilin glycosylation locus of Neisseria meningitidis expressing class II pili. Infect. Immun. 69: 3597–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kaiser D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. U. S. A. 76: 5952–5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kallstrom H, Liszewski MK, Atkinson JP, Jonsson AB. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25: 639–647 [DOI] [PubMed] [Google Scholar]
- 215. Kang PJ, et al. 1997. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24: 1249–1262 [DOI] [PubMed] [Google Scholar]
- 216. Kao DJ, Churchill ME, Irvin RT, Hodges RS. 2007. Animal protection and structural studies of a consensus sequence vaccine targeting the receptor binding domain of the type IV pilus of Pseudomonas aeruginosa. J. Mol. Biol. 374: 426–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kehl-Fie TE, Miller SE, St Geme JW, 3rd 2008. Kingella kingae expresses type IV pili that mediate adherence to respiratory epithelial and synovial cells. J. Bacteriol. 190: 7157–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kehl-Fie TE, Porsch EA, Miller SE, St Geme JW, 3rd 2009. Expression of Kingella kingae type IV pili is regulated by sigma54, PilS, and PilR. J. Bacteriol. 191: 4976–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Keizer DW, et al. 2001. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276: 24186–24193 [DOI] [PubMed] [Google Scholar]
- 220. Kennan RM, Dhungyel OP, Whittington RJ, Egerton JR, Rood JI. 2001. The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J. Bacteriol. 183: 4451–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kim SR, Komano T. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179: 3594–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kim YH, et al. 2009. Alteration in the glycan pattern of pilin in a nonmotile mutant of Synechocystis sp. PCC 6803. Proteomics 9: 1075–1086 [DOI] [PubMed] [Google Scholar]
- 223. Kim YH, et al. 2011. Identification of trimethylation at C-terminal lysine of pilin in the cyanobacterium Synechocystis PCC 6803. Biochem. Biophys. Res. Commun. 404: 587–592 [DOI] [PubMed] [Google Scholar]
- 224. Kirchner M, Heuer D, Meyer TF. 2005. CD46-independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect. Immun. 73: 3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kirchner M, Meyer TF. 2005. The PilC adhesin of the Neisseria type IV pilus-binding specificities and new insights into the nature of the host cell receptor. Mol. Microbiol. 56: 945–957 [DOI] [PubMed] [Google Scholar]
- 226. Kirn TJ, Bose N, Taylor RK. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49: 81–92 [DOI] [PubMed] [Google Scholar]
- 227. Kirn TJ, Lafferty MJ, Sandoe CM, Taylor RK. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35: 896–910 [DOI] [PubMed] [Google Scholar]
- 228. Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50: 61–68 [DOI] [PubMed] [Google Scholar]
- 229. Klausen M, et al. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48: 1511–1524 [DOI] [PubMed] [Google Scholar]
- 230. Klimes A, et al. 2010. Production of pilus-like filaments in Geobacter sulfurreducens in the absence of the type IV pilin protein PilA. FEMS Microbiol. Lett. 310: 62–68 [DOI] [PubMed] [Google Scholar]
- 231. Kline KA, Seifert HS. 2005. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J. Bacteriol. 187: 5347–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Knutton S, Shaw RK, Anantha RP, Donnenberg MS, Zorgani AA. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33: 499–509 [DOI] [PubMed] [Google Scholar]
- 233. Kohler R, et al. 2004. Structure and assembly of the pseudopilin PulG. Mol. Microbiol. 54: 647–664 [DOI] [PubMed] [Google Scholar]
- 234. Kolappan S, Roos J, Yuen A, Pierce OM, Craig L. 2012. Structural characterization of CFA/III and longus type IVB pili from enterotoxigenic Escherichia coli. J. Bacteriol. 194: 2725–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Korotkov KV, et al. 2009. Calcium is essential for the major pseudopilin in the type 2 secretion system. J. Biol. Chem. 284: 25466–25470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Korotkov KV, Hol WG. 2008. Structure of the GspK-GspI-GspJ complex from the enterotoxigenic Escherichia coli type 2 secretion system. Nat. Struct. Mol. Biol. 15: 462–468 [DOI] [PubMed] [Google Scholar]
- 237. Korotkov KV, Sandkvist M, Hol WG. 2012. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 10: 336–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Krebs SJ, Taylor RK. 2011. Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model. J. Bacteriol. 193: 5260–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Krushkal J, et al. 2010. Genome-wide survey for PilR recognition sites of the metal-reducing prokaryote Geobacter sulfurreducens. Gene 469: 31–44 [DOI] [PubMed] [Google Scholar]
- 240. Ku SC, Schulz BL, Power PM, Jennings MP. 2009. The pilin O-glycosylation pathway of pathogenic Neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem. Biophys. Res. Commun. 378: 84–89 [DOI] [PubMed] [Google Scholar]
- 241. Kus JV, et al. 2008. Modification of Pseudomonas aeruginosa Pa5196 type IV pilins at multiple sites with d-Araf by a novel GT-C family arabinosyltransferase, TfpW. J. Bacteriol. 190: 7464–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Kus JV, Tullis E, Cvitkovitch DG, Burrows LL. 2004. Significant differences in type IV pilin allele distribution among Pseudomonas aeruginosa isolates from cystic fibrosis (CF) versus non-CF patients. Microbiology 150: 1315–1326 [DOI] [PubMed] [Google Scholar]
- 243. Kuwae A, et al. 2011. NafA negatively controls Neisseria meningitidis piliation. PLoS One 6: e21749 doi:10.1371/journal.pone.0021749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lam AY, Pardon E, Korotkov KV, Hol WG, Steyaert J. 2009. Nanobody-aided structure determination of the EpsI:EpsJ pseudopilin heterodimer from Vibrio vulnificus. J. Struct. Biol. 166: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Lang E, et al. 2009. Identification of neisserial DNA binding components. Microbiology 155: 852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. LaPointe CF, Taylor RK. 2000. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J. Biol. Chem. 275: 1502–1510 [DOI] [PubMed] [Google Scholar]
- 247. Lassak K, et al. 2012. Molecular analysis of the crenarchaeal flagellum. Mol. Microbiol. 83: 110–124 [DOI] [PubMed] [Google Scholar]
- 248. Lecuyer H, Nassif X, Coureuil M. 2012. Two strikingly different signaling pathways are induced by meningococcal type IV pili on endothelial and epithelial cells. Infect. Immun. 80: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Lee KK, et al. 1994. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11: 705–713 [DOI] [PubMed] [Google Scholar]
- 250. Li J, Egelman EH, Craig L. 2012. Structure of the Vibrio cholerae type IVb pilus and stability comparison with the Neisseria gonorrhoeae type IVa pilus. J. Mol. Biol. 418: 47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Li J, et al. 2008. Vibrio cholerae toxin-coregulated pilus structure analyzed by hydrogen/deuterium exchange mass spectrometry. Structure 16: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Li Y, et al. 2007. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 153: 719–726 [DOI] [PubMed] [Google Scholar]
- 253. Li Y, et al. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. U. S. A. 100: 5443–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Liles MR, Viswanathan VK, Cianciotto NP. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect. Immun. 66: 1776–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Lim MS, et al. 2010. Vibrio cholerae El Tor TcpA crystal structure and mechanism for pilus-mediated microcolony formation. Mol. Microbiol. 77: 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lippa AM, Goulian M. 2009. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 5: e1000788 doi:10.1371/journal.pgen.1000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lizewski SE, et al. 2004. Identification of AlgR-regulated genes in Pseudomonas aeruginosa by use of microarray analysis. J. Bacteriol. 186: 5672–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Long CD, et al. 2001. Modulation of gonococcal piliation by regulatable transcription of pilE. J. Bacteriol. 183: 1600–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. 2003. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 71: 6279–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Lovley DR. 2012. Electromicrobiology. Annu. Rev. Microbiol. 66: 391–409 doi:10.1146/annurev-micro-092611-150104 [DOI] [PubMed] [Google Scholar]
- 261. Lovley DR, et al. 2011. Geobacter: the microbe electric's physiology, ecology, and practical applications. Adv. Microb. Physiol. 59: 1–100 [DOI] [PubMed] [Google Scholar]
- 262. Luke-Marshall NR, Sauberan SL, Campagnari AA. 2011. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene 477: 19–23 [DOI] [PubMed] [Google Scholar]
- 263. Maier B, Potter L, So M, Seifert HS, Sheetz MP. 2002. Single pilus motor forces exceed 100 pN. Proc. Natl. Acad. Sci. U. S. A. 99: 16012–16017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Malvankar NS, Lovley DR. 2012. Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem 5: 1039–1046 [DOI] [PubMed] [Google Scholar]
- 265. Manning PA. 1997. The tcp gene cluster of Vibrio cholerae. Gene 192: 63–70 [DOI] [PubMed] [Google Scholar]
- 266. Marceau M, Beretti JL, Nassif X. 1995. High adhesiveness of encapsulated Neisseria meningitidis to epithelial cells is associated with the formation of bundles of pili. Mol. Microbiol. 17: 855–863 [DOI] [PubMed] [Google Scholar]
- 267. Marceau M, Forest K, Beretti JL, Tainer J, Nassif X. 1998. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol. Microbiol. 27: 705–715 [DOI] [PubMed] [Google Scholar]
- 268. Marceau M, Nassif X. 1999. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J. Bacteriol. 181: 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Marrs CF, Ruehl WW, Schoolnik GK, Falkow S. 1988. Pilin-gene phase variation of Moraxella bovis is caused by an inversion of the pilin genes. J. Bacteriol. 170: 3032–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Marsh JW, Taylor RK. 1999. Genetic and transcriptional analyses of the Vibrio cholerae mannose-sensitive hemagglutinin type 4 pilus gene locus. J. Bacteriol. 181: 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Marsh JW, Taylor RK. 1998. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol. Microbiol. 29: 1481–1492 [DOI] [PubMed] [Google Scholar]
- 272. Martin CH, Nielsen DR, Solomon KV, Prather KL. 2009. Synthetic metabolism: engineering biology at the protein and pathway scales. Chem. Biol. 16: 277–286 [DOI] [PubMed] [Google Scholar]
- 273. Mattick JS. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56: 289–314 [DOI] [PubMed] [Google Scholar]
- 274. Mazariego-Espinosa K, Cruz A, Ledesma MA, Ochoa SA, Xicohtencatl-Cortes J. 2010. Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J. Bacteriol. 192: 2791–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. McLaughlin LS, Haft RJ, Forest KT. 2012. Structural insights into the type II secretion nanomachine. Curr. Opin. Struct. Biol. 22: 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. McNamara BP, Donnenberg MS. 2000. Evidence for specificity in type 4 pilus biogenesis by enteropathogenic Escherichia coli. Microbiology 146: 719–729 [DOI] [PubMed] [Google Scholar]
- 277. Medina AA, Shanks RM, Kadouri DE. 2008. Development of a novel system for isolating genes involved in predator-prey interactions using host independent derivatives of Bdellovibrio bacteriovorus 109J. BMC Microbiol. 8: 33 doi:10.1186/1471-2180-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Mehr IJ, Seifert HS. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Mol. Microbiol. 30: 697–710 [DOI] [PubMed] [Google Scholar]
- 279. Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310: 1824–1827 [DOI] [PubMed] [Google Scholar]
- 280. Merz AJ, Enns CA, So M. 1999. Type IV pili of pathogenic neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32: 1316–1332 [DOI] [PubMed] [Google Scholar]
- 281. Merz AJ, So M, Sheetz MP. 2000. Pilus retraction powers bacterial twitching motility. Nature 407: 98–102 [DOI] [PubMed] [Google Scholar]
- 282. Meyer TF, Billyard E, Haas R, Storzbach S, So M. 1984. Pilus genes of Neisseria gonorrhoeae: chromosomal organization and DNA sequence. Proc. Natl. Acad. Sci. U. S. A. 81: 6110–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Mikaty G, et al. 2009. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 5: e1000314 doi:10.1371/journal.ppat.1000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Miller RM, et al. 2008. Pseudomonas aeruginosa twitching motility-mediated chemotaxis towards phospholipids and fatty acids: specificity and metabolic requirements. J. Bacteriol. 190: 4038–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Misic AM, Satyshur KA, Forest KT. 2010. P. aeruginosa PilT structures with and without nucleotide reveal a dynamic type IV pilus retraction motor. J. Mol. Biol. 400: 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Moore LJ, Lepper AW. 1991. A unified serotyping scheme for Moraxella bovis. Vet. Microbiol. 29: 75–83 [DOI] [PubMed] [Google Scholar]
- 287. Morand PC, et al. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 23: 2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Morand PC, Drab M, Rajalingam K, Nassif X, Meyer TF. 2009. Neisseria meningitidis differentially controls host cell motility through PilC1 and PilC2 components of type IV pili. PLoS One 4: e6834 doi:10.1371/journal.pone.0006834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Morand PC, Tattevin P, Eugene E, Beretti JL, Nassif X. 2001. The adhesive property of the type IV pilus-associated component PilC1 of pathogenic Neisseria is supported by the conformational structure of the N-terminal part of the molecule. Mol. Microbiol. 40: 846–856 [DOI] [PubMed] [Google Scholar]
- 290. Morris C, Yip CM, Tsui IS, Wong DK, Hackett J. 2003. The shufflon of Salmonella enterica serovar Typhi regulates type IVB pilus-mediated bacterial self-association. Infect. Immun. 71: 1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Myers GS, et al. 2007. Genome sequence and identification of candidate vaccine antigens from the animal pathogen Dichelobacter nodosus. Nat. Biotechnol. 25: 569–575 [DOI] [PubMed] [Google Scholar]
- 292. Naessan CL, et al. 2008. Genetic and functional analyses of PptA, a phospho-form transferase targeting type IV pili in Neisseria gonorrhoeae. J. Bacteriol. 190: 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Nassif X. 1999. Interaction mechanisms of encapsulated meningococci with eucaryotic cells: what does this tell us about the crossing of the blood-brain barrier by Neisseria meningitidis? Curr. Opin. Microbiol. 2: 71–77 [DOI] [PubMed] [Google Scholar]
- 294. Naumann M, Rudel T, Meyer TF. 1999. Host cell interactions and signalling with Neisseria gonorrhoeae. Curr. Opin. Microbiol. 2: 62–70 [DOI] [PubMed] [Google Scholar]
- 295. Ng SY, Chaban B, Jarrell KF. 2006. Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications. J. Mol. Microbiol. Biotechnol. 11: 167–191 [DOI] [PubMed] [Google Scholar]
- 296. Ng SY, et al. 2011. Genetic and mass spectrometry analyses of the unusual type IV-like pili of the archaeon Methanococcus maripaludis. J. Bacteriol. 193: 804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Nguyen Y, Jackson SG, Aidoo F, Junop M, Burrows LL. 2010. Structural characterization of novel Pseudomonas aeruginosa type IV pilins. J. Mol. Biol. 395: 491–503 [DOI] [PubMed] [Google Scholar]
- 298. Nunn D, Bergman S, Lory S. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 172: 2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Nunn DN, Lory S. 1993. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J. Bacteriol. 175: 4375–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Nunn DN, Lory S. 1992. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc. Natl. Acad. Sci. U. S. A. 89: 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Nunn DN, Lory S. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl. Acad. Sci. U. S. A. 88: 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Omer H, et al. 2011. Genotypic and phenotypic modifications of Neisseria meningitidis after an accidental human passage. PLoS One 6: e17145 doi:10.1371/journal.pone.0017145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Opitz D, Maier B. 2011. Rapid cytoskeletal response of epithelial cells to force generation by type IV pili. PLoS One 6: e17088 doi:10.1371/journal.pone.0017088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30: 295–304 [DOI] [PubMed] [Google Scholar]
- 305. Ottow JC. 1975. Ecology, physiology, and genetics of fimbriae and pili. Annu. Rev. Microbiol. 29: 79–108 [DOI] [PubMed] [Google Scholar]
- 306. Parge HE, et al. 1995. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378: 32–38 [DOI] [PubMed] [Google Scholar]
- 307. Pasloske BL, Finlay BB, Paranchych W. 1985. Cloning and sequencing of the Pseudomonas aeruginosa PAK pilin gene. FEBS Lett. 183: 408–412 [DOI] [PubMed] [Google Scholar]
- 308. Pasloske BL, Scraba DG, Paranchych W. 1989. Assembly of mutant pilins in Pseudomonas aeruginosa: formation of pili composed of heterologous subunits. J. Bacteriol. 171: 2142–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Peabody CR, et al. 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149: 3051–3072 [DOI] [PubMed] [Google Scholar]
- 310. Pehl MJ, et al. 2012. Genes that influence swarming motility and biofilm formation in Variovorax paradoxus EPS. PLoS One 7: e31832 doi:10.1371/journal.pone.0031832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Pelicic V. 2008. Type IV pili: e pluribus unum? Mol. Microbiol. 68: 827–837 [DOI] [PubMed] [Google Scholar]
- 312. Pepe JC, Lory S. 1998. Amino acid substitutions in PilD, a bifunctional enzyme of Pseudomonas aeruginosa. Effect on leader peptidase and N-methyltransferase activities in vitro and in vivo. J. Biol. Chem. 273: 19120–19129 [DOI] [PubMed] [Google Scholar]
- 313. Planet PJ, Kachlany SC, Fine DH, DeSalle R, Figurski DH. 2003. The widespread colonization island of Actinobacillus actinomycetemcomitans. Nat. Genet. 34: 193–198 [DOI] [PubMed] [Google Scholar]
- 314. Plant LJ, Jonsson AB. 2006. Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect. Immun. 74: 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Pohlschroder M, Ghosh A, Tripepi M, Albers SV. 2011. Archaeal type IV pilus-like structures—evolutionarily conserved prokaryotic surface organelles. Curr. Opin. Microbiol. 14: 357–363 [DOI] [PubMed] [Google Scholar]
- 316. Power PM, et al. 2000. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 146: 967–979 [DOI] [PubMed] [Google Scholar]
- 317. Power PM, et al. 2003. Genetic characterization of pilin glycosylation and phase variation in Neisseria meningitidis. Mol. Microbiol. 49: 833–847 [DOI] [PubMed] [Google Scholar]
- 318. Power PM, Seib KL, Jennings MP. 2006. Pilin glycosylation in Neisseria meningitidis occurs by a similar pathway to wzy-dependent O-antigen biosynthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 347: 904–908 [DOI] [PubMed] [Google Scholar]
- 319. Pugsley AP. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57: 50–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Pugsley AP, et al. 1997. Pullulanase: model protein substrate for the general secretory pathway of gram-negative bacteria. Folia Microbiol. 42: 184–192 [DOI] [PubMed] [Google Scholar]
- 321. Pugsley AP, Reyss I. 1990. Five genes at the 3′ end of the Klebsiella pneumoniae pulC operon are required for pullulanase secretion. Mol. Microbiol. 4: 365–379 [DOI] [PubMed] [Google Scholar]
- 322. Py B, Loiseau L, Barras F. 2001. An inner membrane platform in the type II secretion machinery of Gram-negative bacteria. EMBO Rep. 2: 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Qutyan M, Henkel M, Horzempa J, Quinn M, Castric P. 2010. Glycosylation of pilin and nonpilin protein constructs by Pseudomonas aeruginosa 1244. J. Bacteriol. 192: 5972–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Qutyan M, Paliotti M, Castric P. 2007. PilO of Pseudomonas aeruginosa 1244: subcellular location and domain assignment. Mol. Microbiol. 66: 1444–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Rakotoarivonina H, et al. 2005. The Ruminococcus albus pilA1-pilA2 locus: expression and putative role of two adjacent pil genes in pilus formation and bacterial adhesion to cellulose. Microbiology 151: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 326. Ramboarina S, et al. 2005. Structure of the bundle-forming pilus from enteropathogenic Escherichia coli. J. Biol. Chem. 280: 40252–40260 [DOI] [PubMed] [Google Scholar]
- 327. Reguera G, et al. 2005. Extracellular electron transfer via microbial nanowires. Nature 435: 1098–1101 [DOI] [PubMed] [Google Scholar]
- 328. Reguera G, Pollina RB, Nicoll JS, Lovley DR. 2007. Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation. J. Bacteriol. 189: 2125–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Reichow SL, et al. 2011. The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel. Channels 5: 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Reichow SL, Korotkov KV, Hol WG, Gonen T. 2010. Structure of the cholera toxin secretion channel in its closed state. Nat. Struct. Mol. Biol. 17: 1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Reyss I, Pugsley AP. 1990. Five additional genes in the pulC-O operon of the gram-negative bacterium Klebsiella oxytoca UNF5023 which are required for pullulanase secretion. Mol. Gen. Genet. 222: 176–184 [DOI] [PubMed] [Google Scholar]
- 332. Richter LV, Sandler SJ, Weis RM. 2012. Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation. J. Bacteriol. 194: 2551–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Rodgers K, Arvidson CG, Melville S. 2011. Expression of a Clostridium perfringens type IV pilin by Neisseria gonorrhoeae mediates adherence to muscle cells. Infect. Immun. 79: 3096–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Rodriguez-Tirado C, et al. 2012. Neisseria gonorrhoeae induced disruption of cell junction complexes in epithelial cells of the human genital tract. Microbes Infect. 14: 290–300 [DOI] [PubMed] [Google Scholar]
- 335. Römling U, Kader A, Sriramulu DD, Simm R, Kronvall G. 2005. Worldwide distribution of Pseudomonas aeruginosa clone C strains in the aquatic environment and cystic fibrosis patients. Environ. Microbiol. 7: 1029–1038 [DOI] [PubMed] [Google Scholar]
- 336. Roy PH, et al. 2010. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One 5: e8842 doi:10.1371/journal.pone.0008842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Ruehl WW, et al. 1993. Q pili enhance the attachment of Moraxella bovis to bovine corneas in vitro. Mol. Microbiol. 7: 285–288 [DOI] [PubMed] [Google Scholar]
- 338. Rumszauer J, Schwarzenlander C, Averhoff B. 2006. Identification, subcellular localization and functional interactions of PilMNOWQ and PilA4 involved in transformation competency and pilus biogenesis in the thermophilic bacterium Thermus thermophilus HB27. FEBS J. 273: 3261–3272 [DOI] [PubMed] [Google Scholar]
- 339. Russel M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279: 485–499 [DOI] [PubMed] [Google Scholar]
- 340. Russell MA, Darzins A. 1994. The pilE gene product of Pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol. Microbiol. 13: 973–985 [DOI] [PubMed] [Google Scholar]
- 341. Rytkonen A, Johansson L, Asp V, Albiger B, Jonsson AB. 2001. Soluble pilin of Neisseria gonorrhoeae interacts with human target cells and tissue. Infect. Immun. 69: 6419–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Saarimaa C, et al. 2006. Characterization of adhesion threads of Deinococcus geothermalis as type IV pili. J. Bacteriol. 188: 7016–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Sakai D, Komano T. 2002. Genes required for plasmid R64 thin-pilus biogenesis: identification and localization of products of the pilK, pilM, pilO, pilP, pilR, and pilT genes. J. Bacteriol. 184: 444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Salomonsson EN, Forslund AL, Forsberg A. 2011. Type IV pili in Francisella—a virulence trait in an intracellular pathogen. Front. Microbiol. 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Sampaleanu LM, et al. 2009. Periplasmic domains of Pseudomonas aeruginosa PilN and PilO form a stable heterodimeric complex. J. Mol. Biol. 394: 143–159 [DOI] [PubMed] [Google Scholar]
- 346. Sandkvist M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69: 3523–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Sandkvist M, Bagdasarian M, Howard SP. 2000. Characterization of the multimeric Eps complex required for cholera toxin secretion. Int. J. Med. Microbiol. 290: 345–350 [DOI] [PubMed] [Google Scholar]
- 348. Satyshur KA, et al. 2007. Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure 15: 363–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Sauvonnet N, Vignon G, Pugsley AP, Gounon P. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 19: 2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Saville RM, Dieckmann N, Spormann AM. 2010. Spatiotemporal activity of the mshA gene system in Shewanella oneidensis MR-1 biofilms. FEMS Microbiol. Lett. 308: 76–83 [DOI] [PubMed] [Google Scholar]
- 351. Scheuerpflug I, Rudel T, Ryll R, Pandit J, Meyer TF. 1999. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect. Immun. 67: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Schirm M, Schoenhofen IC, Logan SM, Waldron KC, Thibault P. 2005. Identification of unusual bacterial glycosylation by tandem mass spectrometry analyses of intact proteins. Anal. Chem. 77: 7774–7782 [DOI] [PubMed] [Google Scholar]
- 353. Sechman EV, Kline KA, Seifert HS. 2006. Loss of both Holliday junction processing pathways is synthetically lethal in the presence of gonococcal pilin antigenic variation. Mol. Microbiol. 61: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 93: 2744–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Serino L, Virji M. 2002. Genetic and functional analysis of the phosphorylcholine moiety of commensal Neisseria lipopolysaccharide. Mol. Microbiol. 43: 437–448 [DOI] [PubMed] [Google Scholar]
- 356. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417: 552–555 [DOI] [PubMed] [Google Scholar]
- 357. Skaar EP, Lazio MP, Seifert HS. 2002. Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J. Bacteriol. 184: 919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Skerker JM, Berg HC. 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 98: 6901–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Skerker JM, Shapiro L. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 19: 3223–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Smedley JG, 3rd, et al. 2005. Influence of pilin glycosylation on Pseudomonas aeruginosa 1244 pilus function. Infect. Immun. 73: 7922–7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Smith DR, Chapman MR. 2010. Economical evolution: microbes reduce the synthetic cost of extracellular proteins. mBio 1: e00131–10 doi:10.1128/mBio.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Soderholm N, Vielfort K, Hultenby K, Aro H. 2011. Pathogenic Neisseria hitchhike on the uropod of human neutrophils. PLoS One 6: e24353 doi:10.1371/journal.pone.0024353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Spangenberg C, Fislage R, Sierralta W, Tummler B, Romling U. 1995. Comparison of type IV-pilin genes of Pseudomonas aeruginosa of various habitats has uncovered a novel unusual sequence. FEMS Microbiol. Lett. 125: 265–273 [DOI] [PubMed] [Google Scholar]
- 364. Srimanote P, Paton AW, Paton JC. 2002. Characterization of a novel type IV pilus locus encoded on the large plasmid of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 70: 3094–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Stephens DS, Whitney AM, Rothbard J, Schoolnik GK. 1985. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J. Exp. Med. 161: 1539–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Stimson E, et al. 1995. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 17: 1201–1214 [DOI] [PubMed] [Google Scholar]
- 367. Stone BJ, Kwaik YA. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181: 1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Stone KD, Zhang HZ, Carlson LK, Donnenberg MS. 1996. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol. Microbiol. 20: 325–337 [DOI] [PubMed] [Google Scholar]
- 369. Strom MS, Lory S. 1991. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J. Biol. Chem. 266: 1656–1664 [PubMed] [Google Scholar]
- 370. Strom MS, Lory S. 1992. Kinetics and sequence specificity of processing of prepilin by PilD, the type IV leader peptidase of Pseudomonas aeruginosa. J. Bacteriol. 174: 7345–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Strom MS, Nunn D, Lory S. 1991. Multiple roles of the pilus biogenesis protein PilD: involvement of pilD in excretion of enzymes from Pseudomonas aeruginosa. J. Bacteriol. 173: 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Strom MS, Nunn DN, Lory S. 1994. Posttranslational processing of type IV prepilin and homologs by PilD of Pseudomonas aeruginosa. Methods Enzymol. 235: 527–540 [DOI] [PubMed] [Google Scholar]
- 373. Strom MS, Nunn DN, Lory S. 1993. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl. Acad. Sci. U. S. A. 90: 2404–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Sun D, Lafferty MJ, Peek JA, Taylor RK. 1997. Domains within the Vibrio cholerae toxin coregulated pilin subunit that mediate bacterial colonization. Gene 192: 79–85 [DOI] [PubMed] [Google Scholar]
- 375. Sundin C, Wolfgang MC, Lory S, Forsberg A, Frithz-Lindsten E. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33: 265–277 [DOI] [PubMed] [Google Scholar]
- 376. Sutherland TC, Quattroni P, Exley RM, Tang CM. 2010. Transcellular passage of Neisseria meningitidis across a polarized respiratory epithelium. Infect. Immun. 78: 3832–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Swanson J, et al. 1987. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 165: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Szabo Z, et al. 2007. Flagellar motility and structure in the hyperthermoacidophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 189: 4305–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Szabo Z, et al. 2007. Identification of diverse archaeal proteins with class III signal peptides cleaved by distinct archaeal prepilin peptidases. J. Bacteriol. 189: 772–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Szurmant H, Bu L, Brooks CL, 3rd, Hoch JA. 2008. An essential sensor histidine kinase controlled by transmembrane helix interactions with its auxiliary proteins. Proc. Natl. Acad. Sci. U. S. A. 105: 5891–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Szurmant H, Mohan MA, Imus PM, Hoch JA. 2007. YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J. Bacteriol. 189: 3280–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Szymanski CM, Logan SM, Linton D, Wren BW. 2003. Campylobacter—a tale of two protein glycosylation systems. Trends Microbiol. 11: 233–238 [DOI] [PubMed] [Google Scholar]
- 383. Taha MK. 2000. Neisseria meningitidis induces the expression of the TNF-alpha gene in endothelial cells. Cytokine 12: 21–25 [DOI] [PubMed] [Google Scholar]
- 384. Taha MK, et al. 1998. Pilus-mediated adhesion of Neisseria meningitidis: the essential role of cell contact-dependent transcriptional upregulation of the PilC1 protein. Mol. Microbiol. 28: 1153–1163 [DOI] [PubMed] [Google Scholar]
- 385. Tam CK, Morris C, Hackett J. 2006. The Salmonella enterica serovar Typhi type IVB self-association pili are detached from the bacterial cell by the PilV minor pilus proteins. Infect. Immun. 74: 5414–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Tammam S, et al. 2011. Characterization of the PilN, PilO and PilP type IVa pilus subcomplex. Mol. Microbiol. 82: 1496–1514 [DOI] [PubMed] [Google Scholar]
- 387. Tay CY, Reeves PR, Lan R. 2008. Importation of the major pilin TcpA gene and frequent recombination drive the divergence of the Vibrio pathogenicity island in Vibrio cholerae. FEMS Microbiol. Lett. 289: 210–218 [DOI] [PubMed] [Google Scholar]
- 388. Thomas NA, Chao ED, Jarrell KF. 2001. Identification of amino acids in the leader peptide of Methanococcus voltae preflagellin that are important in posttranslational processing. Arch. Microbiol. 175: 263–269 [DOI] [PubMed] [Google Scholar]
- 389. Thormann KM, Saville RM, Shukla S, Pelletier DA, Spormann AM. 2004. Initial phases of biofilm formation in Shewanella oneidensis MR-1. J. Bacteriol. 186: 8096–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Tinsley CR, Voulhoux R, Beretti JL, Tommassen J, Nassif X. 2004. Three homologues, including two membrane-bound proteins, of the disulfide oxidoreductase DsbA in Neisseria meningitidis: effects on bacterial growth and biogenesis of functional type IV pili. J. Biol. Chem. 279: 27078–27087 [DOI] [PubMed] [Google Scholar]
- 391. Tobe T, Sasakawa C. 2001. Role of bundle-forming pilus of enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell. Microbiol. 3: 579–585 [DOI] [PubMed] [Google Scholar]
- 392. Tomich M, Fine DH, Figurski DH. 2006. The TadV protein of Actinobacillus actinomycetemcomitans is a novel aspartic acid prepilin peptidase required for maturation of the Flp1 pilin and TadE and TadF pseudopilins. J. Bacteriol. 188: 6899–6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Tomich M, Planet PJ, Figurski DH. 2007. The tad locus: postcards from the widespread colonization island. Nat. Rev. Microbiol. 5: 363–375 [DOI] [PubMed] [Google Scholar]
- 394. Tsui IS, Yip CM, Hackett J, Morris C. 2003. The type IVB pili of Salmonella enterica serovar Typhi bind to the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 71: 6049–6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A. 2001. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 98: 6911–6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. van Schaik EJ, et al. 2005. DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol. 187: 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Varga JJ, et al. 2006. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other clostridia. Mol. Microbiol. 62: 680–694 [DOI] [PubMed] [Google Scholar]
- 398. Varga JJ, Therit B, Melville SB. 2008. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 76: 4944–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Velasquez L, et al. 2012. Neisseria gonorrhoeae pilus attenuates cytokine response of human fallopian tube explants. J. Biomed. Biotechnol. 2012: 491298 doi:10.1155/2012/491298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Vignon G, et al. 2003. Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides. J. Bacteriol. 185: 3416–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Villar MT, Hirschberg RL, Schaefer MR. 2001. Role of the Eikenella corrodens pilA locus in pilus function and phase variation. J. Bacteriol. 183: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Virji M. 2009. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7: 274–286 [DOI] [PubMed] [Google Scholar]
- 403. Vogt SL, et al. 2010. The Cpx envelope stress response both facilitates and inhibits elaboration of the enteropathogenic Escherichia coli bundle-forming pilus. Mol. Microbiol. 76: 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Voisin S, et al. 2005. Identification and characterization of the unique N-linked glycan common to the flagellins and S-layer glycoprotein of Methanococcus voltae. J. Biol. Chem. 280: 16586–16593 [DOI] [PubMed] [Google Scholar]
- 405. Voisin S, et al. 2007. Glycosylation of Pseudomonas aeruginosa strain Pa5196 type IV pilins with mycobacterium-like alpha-1,5-linked d-Araf oligosaccharides. J. Bacteriol. 189: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Vos WL, Nazarov PV, Koehorst RB, Spruijt RB, Hemminga MA. 2009. From ‘I’ to ‘L’ and back again: the odyssey of membrane-bound M13 protein. Trends Biochem. Sci. 34: 249–255 [DOI] [PubMed] [Google Scholar]
- 407. Voulhoux R, Taupiac MP, Czjzek M, Beaumelle B, Filloux A. 2000. Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa. J. Bacteriol. 182: 4051–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Watnick PI, Fullner KJ, Kolter R. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181: 3606–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Watts TH, Worobec EA, Paranchych W. 1982. Identification of pilin pools in the membranes of Pseudomonas aeruginosa. J. Bacteriol. 152: 687–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Weibel DB, et al. 2005. Microoxen: microorganisms to move microscale loads. Proc. Natl. Acad. Sci. U. S. A. 102: 11963–11967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Weiss RL. 1971. The structure and occurrence of pili (fimbriae) on Pseudomonas aeruginosa. J. Gen. Microbiol. 67: 135–143 [DOI] [PubMed] [Google Scholar]
- 412. Wenderska IB, Chong M, McNulty J, Wright GD, Burrows LL. 2011. Palmitoyl-dl-carnitine is a multitarget inhibitor of Pseudomonas aeruginosa biofilm development. Chembiochem 12: 2759–2766 [DOI] [PubMed] [Google Scholar]
- 413. Weyand NJ, et al. 2006. Monoclonal antibody detection of CD46 clustering beneath Neisseria gonorrhoeae microcolonies. Infect. Immun. 74: 2428–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Whitchurch CB, Alm RA, Mattick JS. 1996. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 93: 9839–9843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Winther-Larsen HC, et al. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. U. S. A. 98: 15276–15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Winther-Larsen HC, et al. 2005. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol. Microbiol. 56: 903–917 [DOI] [PubMed] [Google Scholar]
- 417. Wu H, et al. 2011. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol. 62: 49–56 [DOI] [PubMed] [Google Scholar]
- 418. Wu SS, Kaiser D. 1997. Regulation of expression of the pilA gene in Myxococcus xanthus. J. Bacteriol. 179: 7748–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Xicohtencatl-Cortes J, et al. 2007. Intestinal adherence associated with type IV pili of enterohemorrhagic Escherichia coli O157:H7. J. Clin. Invest. 117: 3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Xu XF, et al. 2004. NMR structure of a type IVb pilin from Salmonella typhi and its assembly into pilus. J. Biol. Chem. 279: 31599–31605 [DOI] [PubMed] [Google Scholar]
- 421. Yanez ME, Korotkov KV, Abendroth J, Hol WG. 2008. The crystal structure of a binary complex of two pseudopilins: EpsI and EpsJ from the type 2 secretion system of Vibrio vulnificus. J. Mol. Biol. 375: 471–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Yanez ME, Korotkov KV, Abendroth J, Hol WG. 2008. Structure of the minor pseudopilin EpsH from the type 2 secretion system of Vibrio cholerae. J. Mol. Biol. 377: 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Yang YC, Chou CP, Kuo TT, Lin SH, Yang MK. 2004. PilR enhances the sensitivity of Xanthomonas axonopodis pv. citri to the infection of filamentous bacteriophage Cf. Curr. Microbiol. 48: 251–261 [DOI] [PubMed] [Google Scholar]
- 424. Yang Z, et al. 2011. Alanine 32 in PilA is important for PilA stability and type IV pili function in Myxococcus xanthus. Microbiology 157: 1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Yang Z, Lux R, Hu W, Hu C, Shi W. 2010. PilA localization affects extracellular polysaccharide production and fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 76: 1500–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Yoshida T, Kim SR, Komano T. 1999. Twelve pil genes are required for biogenesis of the R64 thin pilus. J. Bacteriol. 181: 2038–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Yu J, Kroll JS. 1999. DsbA: a protein-folding catalyst contributing to bacterial virulence. Microbes Infect. 1: 1221–1228 [DOI] [PubMed] [Google Scholar]
- 428. Zahavi EE, et al. 2011. Bundle-forming pilus retraction enhances enteropathogenic Escherichia coli infectivity. Mol. Biol. Cell 22: 2436–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Zhang HZ, Donnenberg MS. 1996. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol. Microbiol. 21: 787–797 [DOI] [PubMed] [Google Scholar]
- 430. Zhou H, Hickford JG. 2000. Novel fimbrial subunit genes of Dichelobacter nodosus: recombination in vivo or in vitro? Vet. Microbiol. 76: 163–174 [DOI] [PubMed] [Google Scholar]
- 431. Zolghadr B, et al. 2010. Appendage-mediated surface adherence of Sulfolobus solfataricus. J. Bacteriol. 192: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]