Abstract
Summary: Forced interspecific hybridization has been used in yeasts for many years to study speciation or to construct artificial strains with novel fermentative and metabolic properties. Recent genome analyses indicate that natural hybrids are also generated spontaneously between yeasts belonging to distinct species, creating lineages with novel phenotypes, varied genetic stability, or altered virulence in the case of pathogens. Large segmental introgressions from evolutionarily distant species are also visible in some yeast genomes, suggesting that interspecific genetic exchanges occur during evolution. The origin of this phenomenon remains unclear, but it is likely based on weak prezygotic barriers, limited Dobzhansky-Muller (DM) incompatibilities, and rapid clonal expansions. Newly formed interspecies hybrids suffer rapid changes in the genetic contribution of each parent, including chromosome loss or aneuploidy, translocations, and loss of heterozygosity, that, except in a few recently studied cases, remain to be characterized more precisely at the genomic level by use of modern technologies. We review here known cases of natural or artificially formed interspecies hybrids between yeasts and discuss their potential importance in terms of genome evolution. Problems of meiotic fertility, ploidy constraint, gene and gene product compatibility, and nucleomitochondrial interactions are discussed and placed in the context of other known mechanisms of yeast genome evolution as a model for eukaryotes.
INTRODUCTION
The possibility to form hybrids between living species was recognized long ago, along with its usefulness for agronomy and other biotechnological applications. At the same time, reproductive isolation between species has been a basic concept of biology ever since Darwin's time, and following the pioneering works of Bateson, Dobzhansky, and Muller (reviewed in references 127, 167, and 168), it is now interpreted in terms of genetic incompatibility. But much remains to be learned about the diversity of the molecular mechanisms involved, as well as the importance of interspecies genetic exchanges in evolution. In most metazoans or plants, the existence of efficient prezygotic and/or postzygotic barriers between species prevents large-scale interspecies genetic exchanges from occurring. Extant hybrids are often limited to crosses between closely related species, or even varieties, most often producing sterile diploid lines. Hybrid speciation, however, sometimes occurs, producing fertile lines of multispecies ancestry (128, 156). The second important aspect influencing the role of interspecies hybridization in natural evolution is the fitness of hybrids. Hybrid vigor can sometimes be observed by breeding different species, in a manner similar to heterosis, obtained by breeding distinct genetically pure lines within a species. But interspecies hybridization is intrinsically prone to eroding established gene pools in organisms with obligate sexual reproduction (7). With their ability for indefinite clonal propagation and generally weak prezygotic barriers, yeasts and also many fungi offer specific interests for studying the general problem of interspecies hybridization. The question can therefore be raised about the role played by interspecific genetic exchanges in the natural evolution of these organisms.
It was only a few decades ago that natural interspecies hybrids were first suspected in yeasts, in particular with the polyploid brewing strains that do not sporulate (154). Part of the difficulty in identifying yeast hybrids was due to the imprecise definition of yeast species themselves prior to the advent of molecular methods (101). With early gene cloning and sequencing methods, the simultaneous presence within a strain of two or more alleles with significantly different sequences was regarded as an indication of its hybrid nature. But this criterion was not sufficient to indicate the overall importance of the phenomenon at the genome scale. A new dimension has now been reached with the considerable progress in yeast genomics over the last decade (40). Today, the genomes of over 60 different yeast species have been sequenced and analyzed, and this figure is increasing rapidly owing to novel sequencing methods. Furthermore, polymorphic variations within species have now been examined at the genomewide level, revealing population structures and their mode of propagation (82, 114, 115, 126, 189). Genome data are also particularly powerful for reconstructing phylogenies, one of the criteria used to define species in addition to the classical morphological and biological criteria (214). These data, although still limited relative to the large number of extant yeast isolates (101), were already sufficient to reveal the presence of introgressions between distantly related species (95, 141, 149, 159), as well as the existence of natural hybrid genomes at various stages in their evolution (12, 43, 105, 164).
In this article, we review the variety of naturally occurring hybrids observed between defined yeast species, as well as those constructed experimentally by interspecific matings or protoplast fusions. We then review the stabilization of hybrid lines, paying particular attention to the few recent examples of fully sequenced genomes, and discuss the molecular mechanisms that may explain the observed natural introgressions. We finally discuss the important problems of the meiotic fertility of hybrids and the genetic compatibility between parental genomes. Such questions are obviously not specific to yeasts. But in contrast to metazoans or plants with obligate sexual reproduction, yeasts can also propagate clonally, and the impact of horizontal genetic exchanges during this phase can be evaluated. If conditions are appropriate, haploid or diploid yeast clones multiply mitotically for long periods without undergoing a sexual cycle. Some yeast species are even described as asexual. The nature and role of interspecies genetic exchanges in yeasts are therefore interesting to consider not only as model for the many other unicellular eukaryotes but also, by comparison, for multicellular organisms. (Species definitions and taxonomy used in this article follow reference 101. For species designations at time of original publications, refer to the tables in the supplemental material.)
NATURAL YEAST HYBRIDS AND THEIR SIGNIFICANCE
A Brief History
The diagnosis of interspecies hybrids in yeasts has long been problematic due to the lack of appropriate molecular tools, combined with the difficulty in precisely delineating species. Classically, prior to DNA-DNA reassociation studies, yeast species were defined by a combination of morphological and metabolic criteria (101). But the genetic basis of the distinct physiological properties of the different isolates from natural niches or industrial fermentations remained unclear for a long time. As for all fungi in general (214), three major operational criteria, i.e., morphological, biological, and phylogenetic, can now be used for species recognition in yeasts. Within the biological criterion, the meiotic fertility of diploids was used extensively to define “genetic species” in the Saccharomyces sensu stricto group in particular (143, 144). This criterion, however, cannot be applied in the absence of sporulation, as is the case in several other lineages. The development of genomics has now considerably increased the importance of the phylogenetic criterion.
One of the first indications of the existence of natural interspecies hybrids in yeasts came from an early genetic characterization of a commonly used brewer's yeast strain, Saccharomyces carlsbergensis (154). It was noted that after transfer of chromosome III from this yeast into a laboratory strain of S. cerevisiae by use of a kar1 mutant, no meiotic recombination occurred along a large part of this chromosome (155). Furthermore, two distinct allelic sequences were found in S. carlsbergensis for each of the (few) genes studied. Together, these facts suggested that S. carlsbergensis was not merely a polyploid strain of S. cerevisiae, as previously suspected, but contained genomic portions of a different phylogenetic origin (19, 77, 154). Subsequently, DNA-DNA reassociation studies (a method that became familiar for the definition of yeast species in the mid-1980s) indicated that the genome of S. carlsbergensis was a partial allodiploid arisen by natural hybridization between S. cerevisiae and S. bayanus (96, 221). A similar conclusion was drawn for isolates of S. pastorianus (222), the name now used to design all S. cerevisiae-S. bayanus hybrids (101). Additional analyses of S. pastorianus strains based on amplified fragment length polymorphism (AFLP) and hybridization with subtelomeric sequences of S. cerevisiae further characterized them as hybrids (24, 50). More recently, CGH arrays (43) and complete sequencing (142) showed that the different strains of S. pastorianus were produced by independent hybridization events followed by distinct subsequent evolutionary trajectories (see Evolution of Hybrid Genomes).
Natural Hybrids between Saccharomyces Sensu Stricto Species
The importance of Saccharomyces yeasts for industrial fermentations, combined with the considerable attractiveness of S. cerevisiae for molecular genetic studies, stimulated numerous investigations on the Saccharomyces sensu stricto complex of species and their hybrids during the last decade. From early DNA-DNA reassociation studies and analyses of meiotic fertility, it is known that the Saccharomyces complex is formed of five genetically delineated species, i.e., S. cerevisiae, S. paradoxus, S. mikatae, S. kudriavzevii, and S. bayanus (113, 144, 145, 196), to which S. arboricolus was recently added (147, 225). The originally defined species S. cariocanus does not differ in sequence from S. paradoxus (113) and is, therefore, now considered a synonym despite the low spore viability of diploids issued from crosses between S. cariocanus and S. paradoxus haploid strains (125). Nucleotide sequence divergence between species of this complex is very high (188). S. paradoxus, the closest relative to S. cerevisiae, already exhibits ca. 15% nucleotide substitution, on average, over its entire genome, and ca. 30 to 35% nucleotide sequence divergence is observed between S. cerevisiae and either S. kudriavzevii or S. bayanus. Despite these long evolutionary distances, Saccharomyces sensu stricto species show no or only limited prezygotic barriers between them. In the laboratory, haploid strains of the different species mate with each other and form viable diploids capable of unlimited clonal propagation. Therefore, the reproductive isolation between these species in nature must essentially rely on the low frequency of ascospore viability in tetrads. Note that this low viability, which is generally less than 1%, was the original criterion used to genetically define the Saccharomyces species (143). However, traces of recent introgressions found in their genomes support the idea that, in nature, the boundary between these species is fuzzy.
In this context, it is not so much the existence of natural hybrids between Saccharomyces species that is surprising, but their number and diversity among strains used in fermentations (see Table S1 in the supplemental material). Molecular characterization of wine, beer, or cider yeasts revealed numerous independently formed hybrids between S. cerevisiae and S. kudriavzevii (8, 9, 22, 57, 63, 64, 120, 121, 164, 198), between S. cerevisiae and S. bayanus (60, 78, 106, 133, 134, 141, 146, 175, 198), and even (triple hybrids) between S. cerevisiae, S. kudriavzevii, and S. bayanus (18, 32, 62). Such hybrids often exhibit more robust characteristics than the parental strains, such as tolerance to various stresses induced by artificial fermentations (11, 174, 175, 216). Natural hybrids between S. cerevisiae and the closely related yeast S. bayanus have also been isolated and described (113, 238). The abundance of these hybrids may therefore reflect adaptation. But it is also possible that the stressful conditions themselves trigger the hybridization events (178). In any case, the resulting hybrids show traces of posthybridization genome stabilization events, affecting the two parental genomes at various degrees. These events include gross chromosomal rearrangements and modification of the relative genetic contributions of the parents by aneuploidy or partial chromosome loss. Uniparental inheritance of mitochondrial DNA (mtDNA) is also observed. In the cider yeast CID1, for example, nuclear DNA originates from two different species and mitochondrial DNA originates from a third parent (73).
The phenomenon of interspecies hybridization between Saccharomyces species is not limited to artificial fermentations. Hybrids have also been observed in natural environments (78, 164, 238). Indeed, it appears so universal between Saccharomyces yeasts that even some type strains, normally used to define the species, may eventually prove to be hybrids themselves after more extensive analysis. This is the case, for example, for the S. bayanus type strain, CBS380 (32, 151, 153). Recent molecular characterization of this genome revealed a predominant genetic background belonging to S. uvarum (a previously identified subspecies within the S. bayanus complex [173, 177]), a second uncharacterized species (temporarily designated S. lagerae), and several introgressions of S. cerevisiae fragments (152). A recent reconstitution of the ancestry of the S. bayanus type strain and of other lager-brewing strains, collectively designated S. pastorianus, is represented in Fig. 1. It was concluded that the current S. bayanus strains are segregants of tetrads issued from a cross of S. uvarum with NBRC1948 (or a similar strain). NBRC1948 is itself a hybrid of S. uvarum and the other contributor (S. lagerae), showing 2 to 6% sequence divergence with it. The draft genome sequencing of new cryotolerant yeast samples isolated from Nothofagus forests of Patagonia recently provided a clue to the origin of the wild genetic stock of the lager brewing yeast S. pastorianus (109). Some samples, designated S. eubayanus, showing 6% to 8% sequence divergence with S. uvarum, are nearly identical (99.5%) to the non-S. cerevisiae portion of the sequenced S. pastorianus genome (142).
Fig 1.
Example of multiple hybridizations at the origin of a type strain. Current S. bayanus strains, including CBS380T, are segregants of tetrads issued from a cross of S. uvarum with another strain (NBRC1948 or similar), itself a hybrid issued from a cross of S. uvarum with another genome, presently designated S. lagerae (see the text). This hybrid shows traces of introgression from an S. cerevisiae wine strain. (Reprinted from reference 152, which was published under a Creative Commons license.)
Natural Hybrids between Other Saccharomycotina Organisms
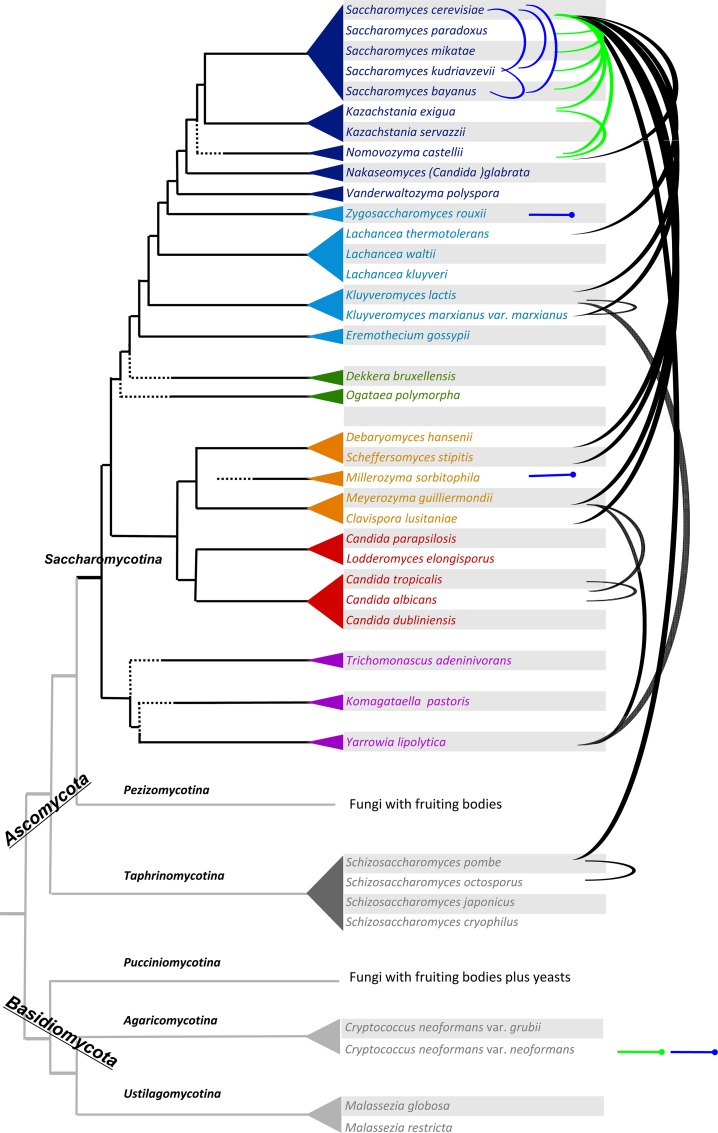
Natural hybrids are not limited to the Saccharomyces sensu stricto complex (Fig. 2). Examples have been reported among the food-associated strains of Zygosaccharomyces rouxii, used in the production of balsamic vinegar and soy sauce. While several isolates from this species are simply haploid, with a single allele for each gene (the type strain of Z. rouxii was recently sequenced [202]), others revealed two highly divergent alleles for each of the genes tested (ASE2, HIS3, and SOD2 [89]) or two distinct loci for the ZSOD gene (201). Nuclear and mitochondrial sequence data indicate that hybrid strains have acquired their nuclear genomes from two separate Zygosaccharomyces species, namely, Z. rouxii and a novel species with similarities to Zygosaccharomyces mellis. Independently, another strain of Z. rouxii was found by partial sequencing studies to be an allopolyploid formed between Z. rouxii and Z. pseudorouxii (67). Interestingly, in this case, and in contrast to other examples described later, the two parental ribosomal DNA (rDNA) sequences are retained simultaneously.
Fig 2.
Hybrids between some sequenced yeast species. The tree of sequenced yeast genomes was adapted from reference 40. Blue arcs join inferred parents of the natural hybrids observed. Note that actual parental strains are not identified in the case of natural hybrids but appear closely related to the sequenced strains indicated. Other arcs join parents of artificial hybrids constructed either by mating (green lines) or by protoplast fusion (black lines). Pins indicate that the other parents of the hybrids are not shown in the figure.
Other lineages of budding yeasts (the Saccharomycotina subphylum of Ascomycota) also contain natural hybrids. Among the large “CTG” group of yeasts sharing a specific alteration of the genetic code (185), the osmotolerant yeast Millerozyma (Pichia) sorbitophila, previously considered a regular species and isolated in 1980 from a highly concentrated sorbitol solution (182), proved, after complete genome sequencing, to be a partially homozygotized allodiploid between two highly divergent species of Millerozyma (105) (see Evolution of Hybrid Genomes).
Natural Hybrids between Pathogenic Basidiomycota Yeasts
Interspecific and intervarietal hybridizations also occur in pathogenic yeasts, with the potential to generate strains of altered virulence or susceptibility to antifungal drugs. Early sequence comparisons of four selected genes in different Cryptococcus neoformans strains isolated from patients from diverse geographical locations revealed incongruent individual gene phylogenies (232). The authors concluded that major lineages of this basidiomycetous yeast diverged tens of millions years ago and have undergone recent dispersion and hybridization between themselves. A subsequent phylogenetic analysis using several loci indicated that at least three independent hybridization events were at the origin of the 14 isolates analyzed (231). Hybrids were also reported, using AFLP analyses, between serotypes of C. neoformans and Cryptococcus bacillisporus differing by their geographical distributions (14). In a more recent study of patient-isolated Cryptococcus strains by AFLP analysis and sequencing of a few selected loci (20), naturally occurring hybrids were found between C. neoformans of serotype D (whose complete genome sequence was initially reported in reference 119) and Cryptococcus gattii, a subtropical species of serotype B affecting healthy individuals (the complete genome sequence and polymorphism of C. gattii were recently reported [39, 59]). The hybrid strains are serologically mixed (serotype BD) and show combinations of the two AFLP signatures. Note that a novel C. neoformans × C. gattii hybrid strain of serotype AB caused a lethal infection in a Canadian AIDS patient (21). Other hybrids of serotypes A and D were recently identified and studied at the molecular level (3, 31, 108, 116, 223). While most isolates of serotypes A and D are haploid, AD strains are diploid or aneuploid. They contain two sets of chromosomes and either two different mating-type alleles (107) or only one mating-type allele as a result of same-sex mating (112). The presence of the MATa allele in serotype A strains (a rare combination geographically restricted to Botswana) in many of these hybrids led the authors to conclude that a fusion occurred between sub-Saharan serotype A and D strains and subsequently propagated throughout the world, based on the increased fitness of the hybrids produced. Genomewide analysis by CGH arrays of three AD hybrids revealed unequal parental contributions for several chromosomes (86). A recent and more extensive analysis of C. neoformans AD strains, carried out on 64 isolates, suggested that multiple independent hybridization events took place between a relatively homogeneous population of serotype A strains and a much more diverse population of serotype D strains (108). Interestingly, the genomes of AD hybrids are highly dynamic, with continuous chromosome loss and extensive loss of heterozygosity, creating rapid genotypic and phenotypic variations that may facilitate the emergence of pathogens.
CONSTRUCTION OF YEAST HYBRIDS IN THE LABORATORY
Interspecific Mating
The simplest strategy for constructing hybrids in the laboratory is mass mating: haploid cells of opposite mating types from two different species can spontaneously form zygotes. This method has been applied successfully to produce a variety of interspecific hybrids between Saccharomyces sensu stricto species (see Table S2 in the supplemental material) in order to study speciation, epistasis, genetic compatibility, and stability of hybrid genomes (70, 192) or simply to produce new strains with novel physiological properties (5, 13, 99, 193). Mass mating was also applied successfully to produce hybrids between more distantly related yeasts (131). These crosses required complementation between distinct auxotrophic requirements to select hybrids because the efficiency of interspecific mating is usually low. Sometimes, putative hybrids could not be propagated, but viable hybrids were successfully obtained from a large variety of crosses. These include crosses between S. cerevisiae and Kazachstania exigua (previously designated Saccharomyces exiguus) or between S. cerevisiae and Naumovozyma castellii (previously S. castellii), as well as crosses between K. exigua and N. castellii. Similarly, hybrids were obtained successfully by mass mating between Yarrowia lipolytica and Saccharomycopsis fibuligera (150) or between novel Kazachstania species (88). Mass mating was also used to distinguish species of the large-spored Metchnikowia clade, an evolutionarily independent lineage relative to the above yeasts (130). Although interspecific mating efficiency is much lower than intraspecific mating efficiency, these experiments indicate the weakness of prezygotic barriers between yeast species and the limited degree of genetic incompatibility between highly divergent genomes with limited synteny conservation (15, 30, 41, 117). Finally, interspecies hybrids with normal karyogamy were also obtained by mating diploid strains of Candida albicans and Candida dubliniensis, homozygous for opposite mating types. In this case, the clones first underwent the white-opaque switching characteristic of this group of pathogenic yeasts (171).
Protoplast Fusion
Natural mating, however, becomes impossible when more distantly related yeast species are considered, and protoplast fusion or electrofusion must be used instead (reviewed in reference 161). Polyethylene glycol-based methods of protoplast fusion (reviewed in reference 94) were developed long ago for intraspecific experiments. The method was used, for example, to fuse haploid cells of identical mating type for S. cerevisiae (65, 83, 220, 234), Kluyveromyces lactis (139), Schizosaccharomyces pombe (199), or Wickerhamomyces canadensis (previously Hansenula wingei) (209). Complementation between auxotrophic requirements of the parental strains was often used to select for rarely occurring fusions, whose frequencies ranged from 10−5 to 10−2. Other techniques were also applied to select fusions, such as the use of killer factors (75, 224), exchange of mitochondria (49, 129), or even the use of N-ethylmaleimide-inactivated protoplasts (98). Subsequently, a number of authors used protoplast fusion to perform interspecific yeast crosses. Hybrid clones were selected from their parents by their prototrophy, carbon source utilization, or respiratory proficiency (205, 235) and then characterized by cytological or ultrastructural observations, metabolic traits, electrophoretic karyotype (80, 229, 240), and even two-dimensional analysis of cellular proteins (74).
Artificial Hybrids Made by Protoplast Fusion of Saccharomycetaceae Yeasts
By use of the above methods, viable hybrids have been obtained between species belonging to a broad variety of evolutionary lineages with distinct genomic architectures (40), and even with distinct genetic codes (Fig. 2; see Table S3 in the supplemental material). In an early experiment (197), hybrids of low viability and altered morphology were obtained between Schizosaccharomyces pombe and Schizosaccharomyces octosporus, two distant members of the Taphrinomycotina subphylum of Ascomycota with sequence divergence between each other comparable to that of humans and fishes (179). These hybrids showed a tendency to segregate cells resembling S. octosporus.
Within the Saccharomycetaceae family, hybrids were obtained between yeasts that inherited from the ancestral whole-genome duplication, such as S. cerevisiae (230), and others that did not (protoploids), such as the osmotolerant yeasts Zygosaccharomyces mellis (104), Z. rouxii (204), and Torulaspora delbrueckii (124), the thermotolerant yeasts Lachancea fermentati (166) and Kluyveromyces marxianus (35, 205, 229, 240), or the lactose-utilizing yeast K. lactis (213). Similarly, viable hybrids were obtained between the duplicated yeast Nakaseomyces delphensis and the protoploid yeast K. lactis (91), as well as between the protoploid yeasts K. lactis and K. marxianus (91, 228). All of these species share a significant protein repertoire attributed to their common ancestry (202) but show extensive sequence divergence between them, with little synteny conservation.
But more surprisingly, viable interspecies hybrids were also obtained between members of the CTG clade and other yeasts that use the universal genetic code. For example, hybrids were reported between Candida tropicalis (CTG yeast) and Saccharomycopsis fibuligera (170) or between Schwanniomyces occidentalis (CTG yeast) and Kluyveromyces marxianus (103). Similarly, viable hybrids were obtained between S. cerevisiae and the CTG yeasts Schwanniomyces occidentalis (212), Debaryomyces hansenii (122), Clavispora lusitaniae (132), Meyerozyma guilliermondii (226), Scheffersomyces (Pichia) stipitis, Candida shehatae (44, 76, 160), and Spathaspora passalidarum (85). How translation operates in such hybrids remains unknown.
Artificial Hybrids Made by Protoplast Fusion of Other Yeasts
Very large evolutionary distances between yeasts do not seem to prevent the formation of viable hybrids. For example, S. cerevisiae is able to form hybrids with Lindnera jadinii (34, 163), Kuraishia capsulata (205, 206), Saccharomycopsis fibuligera (58, 97), and Pachysolen tannophilus (44, 80), four species that belong to yet other families of the Saccharomycotina (101). Even broader evolutionary distances exist between Yarrowia lipolytica and K. lactis, Lindnera jadinii, Meyerozyma guilliermondii, and Saccharomycopsis fibuligera, respectively, with which it forms hybrids (74, 84, 150, 203). Note, however, that the Y. lipolytica-K. lactis hybrids of intermediate morphology between the two parental strains were mitotically unstable, giving rise to segregants with the Y. lipolytica phenotype. Still broader evolutionary distances exist between yeasts and fungi belonging to distinct subphyla of the Ascomycota or, even more, to the Basidiomycota phylum. For example, the fission yeast S. pombe (a member of the Taphrinomycotina subphylum of Ascomycota) can form viable hybrids with the budding yeast S. cerevisiae (205, 210), as well as with the filamentous fungus Monoascus anka (a member of the Pezizomycotina subphylum) or the shiitake mushroom, Lentinula edodes (a member of the Basidiomycota) (26, 111, 211). Similarly, hybrids have been reported between S. cerevisiae and the basidiomycetous yeast Rhodotorula rubra (48).
EVOLUTION OF HYBRID GENOMES
Many naturally occurring and experimentally constructed yeast hybrids cited above were studied prior to the rise of genomics. The classical genetic and molecular methods applied, such as sporulation and crosses, karyotyping, AFLP analysis, DNA-DNA hybridization, and the partial sequencing of a few selected genes, were not sufficient to fully characterize the hybrid genomes. These methods were sufficient, however, to indicate that hybrid lines generally undergo progressive genome stabilization, during which large genomic rearrangements occur, including aneuploidization, chromosomal translocation, and partial or total chromosome loss (6, 42, 90, 99, 123, 174, 175, 198). Gene amplification also occurs, especially in subtelomeric regions of chromosomes. These alterations may be accelerated by stress (90). They depend on the initial hybrid genome structure (allodiploid, allotriploid, or other), the extent of synteny conservation between the parents, and, probably, a complex network of epistatic functional incompatibilities that are not yet precisely characterized. Against this background, recent data on the genomes of a few hybrid yeasts have considerably clarified these issues and have revealed at least some of their underlying mechanisms. These hybrid yeasts include several strains of the lager beer yeast S. pastorianus, wine S. cerevisiae-S. kudriavzevii hybrid strains, and the osmotolerant organism Millerozyma (Pichia) sorbitophila.
Molecular Analysis of Saccharomyces pastorianus Genomes
The genomes of several S. pastorianus strains were recently characterized in detail by genomic array hybridization (16, 43) and complete sequencing (142). In the former case, DNAs from 17 different S. pastorianus strains were compared with S. cerevisiae and S. bayanus probes. This analysis revealed two groups of hybrids correlated with their geographic locations and suggesting two independent hybridization events at their origin. In the first group, almost all of the S. bayanus genome is retained, but significant portions of the S. cerevisiae genome are lost. Loss of the S. cerevisiae genome is greater in the Saaz subgroup than among other strains. In the second group, hybrid strains retain almost the entire genomes of both parents. The loss of parental genome fragments concerns either entire chromosomes or large segments of chromosomes and may be compensated by amplification of other fragments. This amplification is often nonrandom. For example, subtelomeric regions of S. bayanus chromosomes are systematically replaced by S. cerevisiae counterparts in many S. pastorianus strains, and all show amplification of the subtelomeric MAL3 gene cluster of S. cerevisiae. Amplification of the left ends of chromosomes VII and XVI of S. cerevisiae, compensated by the loss of homologous regions from S. bayanus, is also regularly observed. Complete loss of chromosomes I and III of S. bayanus is observed in only two hybrid strains. The S. cerevisiae chromosome III, bearing the MAT locus, appears to be the most polymorphic. In some S. pastorianus strains, this chromosome is missing, in others it is duplicated, and in still others, the right arm fragment beyond the MAT locus is either missing or amplified. The S. cerevisiae genes FLO1, FLO5, FLO9, and PHO11 are often lost, leaving only the allele of non-S. cerevisiae origin (designated Lg-FLO1). Altogether, chromosomes III, V, VII, X, and XVI of S. cerevisiae show a higher density of breakpoint rearrangements than the rest of the genome. Such clusters of breakpoints tend to coincide with tRNA genes, Ty elements, solo long terminal repeats (LTRs), or replication origins, but intragenic recombination was also reported between alleles of the two homoeologous chromosomes (218). According to CGH array data, the 17 hybrids inherited the mitochondrial DNA of only the S. bayanus parent (43). However, a contribution of the S. cerevisiae parent was previously reported for 10 of them (176). The complete sequence of one S. pastorianus strain showed that genetic recombination occurred between the two parental mitochondrial genomes (142). More complete pictures of the inheritance of mtDNA molecules in Saccharomyces hybrids have been described previously (172, 200). Finally, the S. pastorianus strain Weihenstephan 34/70 shows a strong reduction of the S. bayanus rDNA cluster, with only 18 kb left, compared to that of S. cerevisiae, extending over more than 350 kb.
Reconstruction of the ancestral events at the origin of the two groups of S. pastorianus strains (43) suggests that the first hybridization event was probably a fusion between a haploid ascospore from an S. bayanus parent and another from an S. cerevisiae ale strain. This fusion was followed by a rapid loss of at least two chromosomes of S. cerevisiae to generate the first group of S. pastorianus strains, with the greatest imbalance of the two parental genomes. The second event, leading to the group of strains with the more balanced parental contribution, was probably a fusion between a diploid S. cerevisiae cell and a haploid S. bayanus cell, generating an allotriploid hybrid. This fusion was followed by the loss of the right end of chromosome XVI of S. cerevisiae in the last common ancestor of the studied strains. From analysis of the S. cerevisiae components in these hybrids, it was concluded that the diploid parent itself was probably a hybrid between two distinct S. cerevisiae genomes (Fig. 1).
Molecular Analysis of Wine Yeast Genomes
The genomes of four wine strains, recently identified as S. cerevisiae-S. kudriavzevii hybrids (62–64), were also analyzed in detail by genomic array hybridization, flow cytometry, AFLP analysis, and quantitative PCR (12). The two parental species share nearly complete synteny conservation but diverge extensively in sequence (ca. 30% nucleotide substitution). Genomes of hybrids display a ploidy of ca. 2.2 times the haploid content, corresponding to a limited aneuploidy: three copies are found for chromosomes V and XIV, one from each parent plus one chimera, compared to two copies for all other chromosomes. For 11 of these last pairs of chromosomes, the two homologous parental copies are preserved, with the exception of limited rearrangements in some subtelomeric regions. For each of the three remaining chromosomal pairs (IV, IX, and XV), however, one copy corresponds solely to S. cerevisiae, while the other is a chimeric chromosome between S. cerevisiae and S. kudriavzevii. In these pairs, a homozygotization process has taken place in favor of S. cerevisiae, affecting large segments from the chromosomal arms to the telomeres and overlapping (IV) or not overlapping (IX and XV) the centromeres. The process eliminates the S. kudriavzevii genes and replaces them with corresponding copies from S. cerevisiae. The high similarity of genome structures for the four hybrids studied (only two additional chimeric chromosomes are found when the four strains are compared) indicates that they originated from a single hybridization event. Junctions between the two parental subgenomes in the chimeric chromosomes occur either within large clusters of Ty elements and tRNA genes (chromosomes XIV and XV) or between conserved protein-encoding genes (chromosomes IV, V, and IX). Junctions within the rDNA locus on chromosome XII also exist in some hybrid strains.
A recent investigation based on a set of 35 nuclear genes plus 1 mitochondrial gene revealed the presence of many new S. cerevisiae × S. kudriavzevii hybrids among isolates from clinical or dietary supplement origins (164). Interestingly, a high level of genomic diversity was found among these hybrids, despite a general reduction of the S. kudriavzevii genome fraction in most of them. A subsequent analysis of other S. cerevisiae-S. kudriavzevii hybrids from wine and beer confirmed the multiple patterns of chromosomal composition, despite the conservation of a common set of S. kudriavzevii genes possibly associated with cold adaptation (165). From the chromosomal rearrangements observed in hybrid genomes, it was concluded that multiple hybridizations took place, involving haploid and diploid parental strains. Further characterization of 24 other S. cerevisiae-S. kudriavzevii hybrids from Northern European wine-making environments revealed multiple ploidy levels (from 2n to 4n) and various amounts of S. kudriavzevii genetic content (46).
Molecular Analysis of the Millerozyma sorbitophila Genome
The third interesting example illustrating early steps of hybrid genome evolution is provided by the recent complete sequencing of the genome of Millerozyma (Pichia) sorbitophila (105), an osmotolerant yeast isolated in 1980 from a 70% sorbitol solution (182). This genome reveals seven chromosome pairs, as if the strain were diploid. But careful examination of their sequences showed that this genome is indeed a hybrid between two haploid parental yeasts sharing nearly complete synteny conservation but diverging in nucleotide sequence by an average of 15%. M. sorbitophila represents a remarkable example of a hybrid genome in the process of resolution by homozygotization of large chromosome fragments, or even entire chromosomes, with only a limited degree of single gene loss. The first evidence supporting this conclusion was the 1:2 ratio of sequencing coverage along assembled contigs. During assembly of the whole-genome shotgun sequence, heterozygous regions were represented as two distinct contigs of half coverage compared to homozygous regions. Distinction between the two parental genomes, arbitrarily designated Pγ and Pε, is nonambiguous, based on (i) a slight but significant difference in nucleotide composition (42% GC for Pγ and 41% GC for Pε), (ii) a considerable difference in the number of nuclear-mitochondrial DNA sequences (NUMTS) they contain (10 times more in Pε than in Pγ), and (iii) the near identity of allelic sequences attributed to the Pγ parent with a strain of Millerozyma farinosa (CBS2001), in contrast to the lower sequence similarity of the Pε alleles. Therefore, the Pγ parent of M. sorbitophila is a close relative of the CBS2001 strain. The Pε parent remains of unknown origin, but its nearly complete synteny with Pγ suggests that it belongs to the same “species complex.”
In the present state of its evolution, 59.7% of the M. sorbitophila genome has conserved heterozygous regions from its two parents. The rest was inherited from either the Pγ parent (35.5%) or the Pε parent (4.8%) after homozygotization of entire chromosomes or large fragments thereof (105). Interestingly, some chromosome pairs are mosaics of homozygous and heterozygous regions, suggesting that the homozygotization process is in progress, as discussed below. All protein-encoding genes appear to be functional, in both the homozygous and heterozygous regions of the genome. No trace of pseudogenization is visible at this stage of evolution. A total of 220 protein-encoding genes are present only in single copies, originating from either Pγ (104 genes) or Pε (116 genes). In the absence of genomic data on the actual parents of M. sorbitophila, it is impossible to conclude whether these genes were absent in one of the parental lineages prior to the formation of the hybrid or, more interestingly, if we are observing an early stage of a single gene deletion process in the hybrid. Among single-copy genes are those involved in maltose utilization, allantoate transport, nicotinic acid transport, and glutathione metabolism. As described for S. pastorianus above, the rDNA locus, which lies in a subtelomeric position in M. sorbitophila, is inherited from only one parent (Pγ). Its allele from the other parent shows only short remains after extensive deletion. Note that a single rDNA allele is also found in Dekkera bruxellensis strains, regarded as emerging from hybridization events because several distinct alleles exist for other genes (79). In M. sorbitophila, the mitochondrial DNA is inherited from the other parent (Pε). Note that if the rDNA sequence often used to define yeast species (101) had been used to define P. sorbitophila, it would have been classified as a strain of Pγ instead of a hybrid. It is therefore possible that many yeast hybrids have been overlooked so far.
Processes of Hybrid Genome Stabilization
All genomes of S. pastorianus strains, S. cerevisiae-S. kudriavzevii hybrids, and M. sorbitophila show homozygotization of large chromosomal fragments. The chromosomal structure observed within the genome, with homozygous regions from one or the other parent alternating with heterozygous regions, is reminiscent of patterns recently reported for natural diploid strains of S. cerevisiae. In this case, a deep sequencing study of 11 diploid strains from wild, viticultural, or clinical origins (126) revealed the presence of multiple large homozygous regions within the otherwise heterozygous genomes. The lengths of these regions argue against classical gene conversion mechanisms (159). Instead, they imply a loss of heterozygosity (LOH) based upon aberrant chromosomal segregation events and/or break-induced replication (BIR) events. Similarly, in interspecies hybrids, the homozygous regions concern either entire chromosomes or large chromosomal regions, most often extending to telomeres, although a few cases of internal LOH were also observed (Fig. 3A and B). The molecular mechanism of such LOH is not entirely understood. A systematic experimental screen of S. cerevisiae using genetically marked strains indicated that no fewer than 61 genes alter the process when mutated, with or without an effect on entire chromosome loss (4). By analogy to the recombination-dependent chromosome loss mechanism (RDCL) proposed to explain infertile meioses of diploid Saccharomyces hybrids (see Meiotic Fertility of Hybrid Genomes and Hybrid Speciation), it has been suggested that the formation of chimeric chromosomes in S. cerevisiae-S. kudriavzevii hybrid lines results from a similar recombination-based mechanism operating mitotically (12). The fact that the borders of the homozygous segments correspond to well-conserved sequences between the two parents (clusters of Ty elements, rRNA genes, or conserved protein-encoding genes) supports this view. However, observed patterns of natural LOH are also consistent with the mechanism of BIR, which was characterized experimentally for S. cerevisiae (118). In this mechanism, the distal part of a broken chromosome arm is repaired by resynthesis using its homolog as the template, hence sequentially erasing the original allelic divergence between the two homologous chromosomes in a hybrid. The successive occurrence of BIR during clonal growth predicts the formation of chimeras of homozygous regions from one or the other of the two parental genomes, as shown in Fig. 3C. Long-range LOH was previously observed in other diploid yeast genomes as well. For example, the genome of C. albicans appears as a mosaic of heterozygous and homozygous regions (23, 27, 92, 219). Interestingly, in this yeast, the number of long-range LOH events was found to increase by passages through a mammalian host compared to laboratory cultivation (36, 52), suggesting that this process may be induced by stressful conditions. Perhaps related to this observation is the fact that in S. cerevisiae, the occurrence of long-range LOH increases with the age of the cells (137).
Fig 3.
Long-range loss of heterozygosity in hybrids between syntenic parental genomes, with a possible mechanism. Figures were constructed for the three best-characterized natural hybrids: S. pastorianus Weihenstephan (142), S. cerevisiae-S. kudriavzevii W27 (12), and M. sorbitophila (105). Homologous pairs (or triplets) are shown as colored bars and designated by letters, following chromosome numbering. Ovals symbolize centromeres. (A) Examples of LOH extending to chromosome ends. (B) Examples of chimeric chromosomes. (C) Hypothetical mechanism to explain the formation of both LOH and chimeric chromosomes during the successive mitotic divisions of hybrids. A first BIR event produces a long-range LOH segment extending to the telomere, as shown in panel A. A second, identical BIR event during a subsequent mitosis creates chimeric chromosomes, as shown in panel B. (Top row adapted from reference 142 by permission of Oxford University Press.)
Gene Deletion after Allo- or Autopolyploidization
Besides the reduction of each parental contribution by loss of large chromosomal fragments or entire chromosomes, the question emerges of the possible role of individual gene loss in the evolution of hybrid genomes. This question, however, can hardly be solved with natural hybrids because of the lack of data on their actual parents, and it was not addressed directly in the studies of artificially constructed hybrids reported above. In the case of the sequenced S. pastorianus strain Weihenstephan 34/70, a total of 61 coding DNA sequences (CDS) were found truncated by frameshifts or nonsense mutations, suggesting the beginning of a gene inactivation process within this hybrid (142). But so far the best evidence for the importance of single gene deletions during evolution of yeast genomes remains the massive gene loss subsequent to whole-genome duplication in the ancestry of Saccharomyces and related yeasts (30, 66, 187). In this case, however, it is assumed that the ancestral genome was an autopolyploid, i.e., that the two allelic copies were originally identical in sequence, such that besides a gene dosage imbalance, the loss of one or the other copy should be phenotypically equivalent. This is not the case for hybrids arising from divergent parental genomes, where functional differences between the products of allelic copies are expected. Deletion of one parental copy may be phenotypically very different from deletion of the other. This may explain the massive uniparental decrease of rDNA observed in both S. pastorianus (142) and M. sorbitophila (105).
INTROGRESSIONS AND OTHER NONCHROMOSOMAL EXCHANGES
Traces of Natural Introgressions in Yeast Genomes
Besides the variety of established hybrid lines cited above, the importance of interspecific genetic exchanges during the natural evolution of yeasts is further illustrated by the numerous traces of introgression observed in their genomes, i.e., genes or genome fragments of one species are observed in the genome of another. This phenomenon was mentioned a few years ago for Saccharomyces, based on classical molecular genetic methods (32, 148), and was recently extended using microsatellite typing (149). With complete genome sequences, introgression of delineated chromosomal fragments from one yeast species to another appears more frequent than initially anticipated. Sequences of S. cerevisiae strains isolated from patients (227) or a vineyard (38) revealed segments of chromosomes originating from S. paradoxus and not originally described in the reference sequence of S288c (61). The two species share, on average, ca. 85% sequence identity at the nucleotide level. In the S. cerevisiae genome, S. paradoxus segments that underwent introgression are found either at central locations within chromosomes or at subtelomeric locations. For example, a ca. 16-kb introgression internal to chromosome IV was found in all examined S. cerevisiae strains except for one isolated from a North American oak tree (47). Similarly, a 12-kb introgression exists on chromosome I in the clinically derived strain YJM789, as well as in the wild-type strain EM93, the progenitor of many laboratory strains. Another introgression, of ca. 15 kb, lies on chromosome XIV in both the vineyard and reference strains. Conversely, a 23-kb subtelomeric fragment of chromosome XIV that is nearly identical in sequence (99%) to that of S. cerevisiae is found in all European S. paradoxus strains, but not in Far Eastern or American isolates (113). Additional introgressions of S. paradoxus genes at five other internal chromosomal locations were detected in several S. cerevisiae strains by genomic array hybridization followed by sequencing of the respective segments (141). Introgressions may confer novel phenotypic properties. For example, a ca. 65-kb subtelomeric region of wine yeast EC1118 chromosome XV that originated from another Saccharomyces species (157) contains, among other genes, the FSY1 gene, encoding a high-affinity fructose/H+ symporter susceptible to regulation during the wine fermentation process (56).
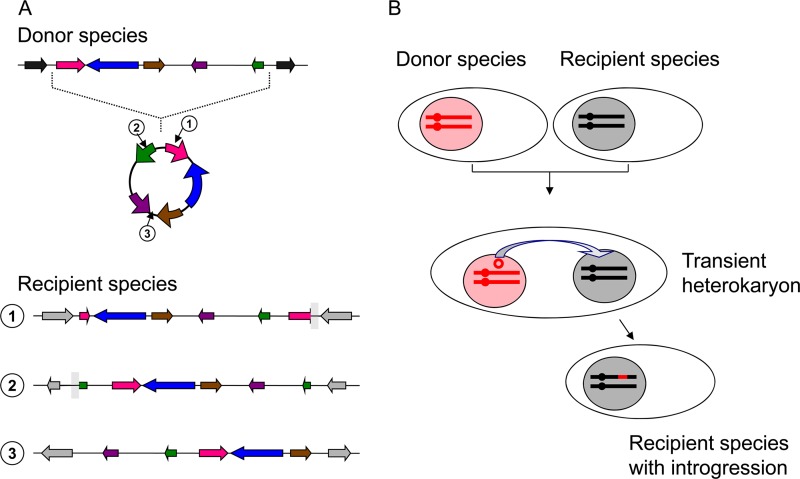
Although it has been studied much more extensively in the Saccharomyces sensu stricto species complex, introgression is not limited to this group of yeasts and can involve distantly related species. The majority of clinical or environmental strains of the pathogenic basidiomycetous yeast C. neoformans var. neoformans contain a 40-kb fragment with 14 genes that originate from C. neoformans var. grubii, a nearly syntenic variety sharing only 85 to 90% sequence identity with C. neoformans var. neoformans (95). More surprisingly, a 17-kb introgressive fragment from Zygosaccharomyces bailii was found in the genomes of many S. cerevisiae wine strains (157). Z. bailii is a common wine contaminant belonging to the Saccharomycetaceae family but not closely related to S. cerevisiae (202). Most interestingly, this 5-gene fragment was found with circular permutations at several different chromosomal locations in different wine strains (17, 55). This remarkable observation strongly suggests that a circular episome was formed out of a Z. bailii chromosome fragment and then, after transfer to a recipient S. cerevisiae strain by an as yet unknown mechanism, replicated and integrated several times independently in the chromosomes of the recipient (Fig. 4).
Fig 4.
Introgression by integration of circular episome. (A) A segment of chromosome for the donor species forms a self-replicating circular episome that, after transfer to another yeast, may amplify and eventually integrate at various locations within the chromosomes of the recipient species, resulting in circular permutations. This panel was inspired by previous references (17, 55). (B) Schematic representation of a possible transfer of a circular episome from one nucleus to the other during the transient heterokaryotic phase of an abortive hybridization.
Possible Mechanisms of Introgression
The mechanism of introgression between yeast species remains unknown. In plants, the frequently observed introgressions result from hybridizations followed by successive backcrosses. Assuming the same mechanism in yeasts would imply dozens of successive backcrosses with the same parent for each introgressive fragment observed, given their short average size (<0.3% of the total genome). Such a succession of unidirectional backcrosses seems unlikely for yeasts in nature, given the limited frequency of their sexual cycles relative to clonal divisions (184, 217, 237). In addition, the extensive sequence divergence between species can only increase the number of backcrosses needed, because it reduces the frequency of meiotic recombination. Other mechanisms should therefore be considered. One possible mechanism for accelerating the process is the segregation, from the initial allodiploid, of aneuploid ascospores containing only one or a few chromosomes from one parent and the complete genome of the other. Another possibility is the unidirectional transfer of a single chromosome from one nucleus to the next in newly formed hybrids prior to karyogamy. Such a situation has been observed in cytoduction experiments using mutants of S. cerevisiae (155), and also in S. cerevisiae-Lindnera jadinii hybrids (162). Alternatively, only a fragment of the chromosome may be transferred from one nucleus to the next in similar situations. The circularization of chromosome fragments observed in S. cerevisiae, forming autonomous episomes (110; A. Thierry et al., unpublished data), may facilitate this process. The circularly permuted Z. bailii fragments cited above (17, 55) support this hypothesis (Fig. 4).
Interspecific Exchanges of Nonchromosomal Elements
In addition to their nuclear and mitochondrial genomes, many yeast strains contain a variety of other, autonomous genetic elements made of DNA or RNA, such as plasmids or defective viruses. Among these, the best-characterized elements are the circular 2μm plasmids, irregularly found in Saccharomyces and a variety of related genera, and linear plasmids conferring killer properties and showing a broader phylogenetic distribution (54). Both types of plasmids are made of double-stranded DNA. Defective viruses made of double-stranded RNA (dsRNA) molecules also confer killer phenotypes (190). The irregular distribution of these autonomous genetic elements relative to the species phylogeny suggests a horizontal transfer, whose mechanism(s) remains unknown. Remarkably, the spontaneous transfer of short artificial plasmids was recently obtained experimentally by simple cocultivation of Candida glabrata with S. cerevisiae or S. bayanus (138). In this case, the transfer frequency was remarkably high (10−4 relative to recipient cells).
Interestingly, some autonomous elements can occasionally be integrated within chromosomes in a manner reminiscent of the integration of pieces of mitochondrial DNA (NUMTS) at chromosomal double-strand breaks (180, 236). The first evidence of this phenomenon was discovered accidentally in the genome of Vanderwaltozyma polyspora (187). In this case, a 10-kb chromosomal segment flanking a tRNA gene in a conserved syntenic region was found to contain 5 pseudogenes homologous in sequence to plasmid and viral genes from fungi. Subsequently, a systematic search within yeast and fungal genome sequences from public databases, using sequences of known extrachromosomal elements as queries, revealed that 42% of Saccharomycotina species (11 of 26 species tested) contain fragments of plasmid or viral origin (designated NUPAVs) integrated into their chromosomes (53). Most NUPAVs are pseudogenes, although one active gene, KHS1, encoding a killer factor, was also found in S. cerevisiae. This high frequency of NUPAVs among Saccharomycotina organisms confirms that interspecies genetic exchanges between these yeasts are much more frequent than usually imagined.
MEIOTIC FERTILITY OF HYBRID GENOMES AND HYBRID SPECIATION
Emergence of Fertile Lines from Sterile Hybrids
An important aspect of a successful contribution of interspecific hybridization to eukaryotic evolution is the degree of meiotic fertility of resulting hybrids. For organisms with obligate sexual reproduction, this parameter is obviously critical. For yeasts, its importance is reduced by their clonal propagation. For example, population genomics indicates that in nature, S. cerevisiae and S. paradoxus undergo ca. 100 mitotic divisions for 1 meiotic division (237) and that outcrossing is ca. 1,000 times rarer (184, 217). Under such conditions, the limited spore viability of interspecies hybrids—the main criterion used to genetically define the species (143)—is compensated by the considerable expansion that hybrid clones may undergo before entering meiosis.
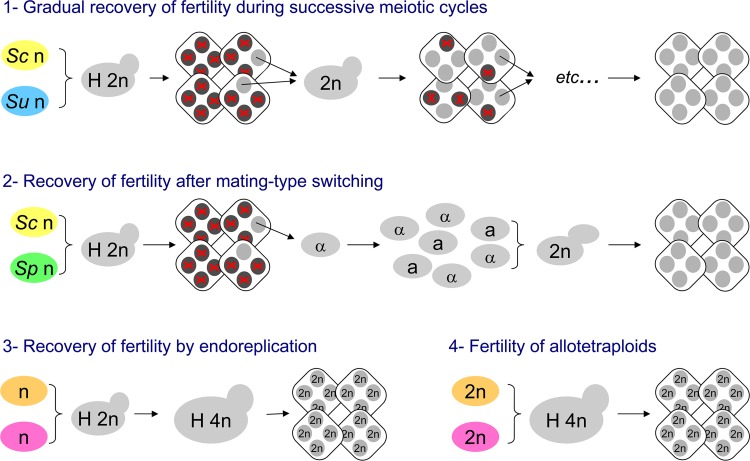
During clonal expansion, several processes permit the rapid emergence of sexually fertile lines from initially unfertile hybrids (Fig. 5). First, a gradual recovery of meiotic fertility follows genome stabilization of hybrid mitotic lines. This was demonstrated in an experimental study of an S. cerevisiae-S. uvarum hybrid line over four filial generations (6). During the successive generations, a gradual reduction of the S. uvarum genome occurred amid rearrangements with the S. cerevisiae genome, and a progressive increase of sporulation efficiency and ascospore viability was observed. Sexually fertile lines of hybrid ancestry were eventually obtained. Second, in yeast species with active mating-type switching, a haploid clone issued from a meiotic ascospore has a high probability of undergoing autofertilization, producing a homozygous diploid line. This property was used for an experimental study of an S. cerevisiae-S. paradoxus hybrid (71). The rare viable meiotic ascospores from this hybrid spontaneously formed homozygous diploid clones, showing an elevated degree of meiotic fertility. Interestingly, the set of fertile lines thus produced became reproductively isolated from one another as well as from their two parental species, i.e., the offspring of the original hybrid began a rapid speciation process. The same experiment repeated recently between the same two species, however, produced a higher frequency of tetrasomy, leading the authors to conclude that the genetic incompatibility between the two yeast species affects hybrid fertility more than their viability (233). Third, genome doubling can restore meiotic fertility. This was shown for a variety of allodiploid hybrids obtained between different Saccharomyces sensu stricto species (70, 198). In these cases, experimental inactivation of the MAT locus in diploids was used to construct allotetraploids that proved meiotically fertile. But spontaneous endomitotic events can also produce fertile allotetraploids from sterile allodiploids (192). Note the similarity of this process with the natural occurrence of autotetraploids in domesticated S. cerevisiae strains (2) and with the whole-genome duplication in the ancestry of Saccharomyces and related yeasts (230). The meiotic segregation of tetraploids, combined with the power of high-throughput sequencing, was recently applied to identify genetic determinants of xylose metabolism from a natural Saccharomyces hybrid (191).
Fig 5.
Processes of emergence of fertile lines from newly formed sterile hybrids. The figure illustrates the major processes leading from a meiotically unfertile hybrid (H 2n) to the rapid emergence of fertile hybrid lines (see the text). Tetrads are represented by diamonds with four ascospores (gray circles, live ascospores able to form haploid clones and/or mate; black crossed circles, dead ascospores). The ratio of gray to black crossed circles grossly illustrates the degree of meiotic fertility. In reality, this degree is measured from the average for many tetrads. (1) Gradual recovery of fertility can be obtained by successive mating/sporulation cycles, corresponding to gradual genome stabilization of the hybrids (6). (2) Hybrid speciation can be obtained by mating-type switching in the haploid clones issued from live ascospores, followed by the formation of homozygous diploids (71). (3) Endoreplication transforms an unfertile diploid hybrid (H 2n) into a meiotically fertile allotetraploid (H 4n) that is able to give rise to diploid ascospores (192). (4) The same is true for allotetraploids formed directly by mating between diploids of distinct species (70).
Mechanisms of Infertility
Contrary to classical expectations, the major cause of reproductive isolation between Saccharomyces species is not the genetic incompatibility between the two parental genomes but must be sought for in the combination of other molecular mechanisms (69). Among these are the chromosomal rearrangements leading to abnormal gene segregation after regular meiotic crossovers. The chromosomal translocations observed between the different Saccharomyces sensu stricto genomes do not play a major role in the meiotic infertility of hybrids (51). Although a partial increase in meiotic fertility is obtained after their experimental elimination (33), such translocations are insufficient to explain natural speciation. Instead, the large sequence divergence observed between these essentially syntenic genomes can be suspected, by analogy to prokaryotes, to trigger the mismatch repair system (25). This system lowers meiotic recombination between chromosome pairs and consequently increases the frequency of chromosomal nondisjunction (Fig. 6A). This idea, which is consistent with the frequent aneuploidy observed in meiotic segregants, is directly supported by experimental results (87). Mutations in the mismatch repair genes PMS1 and MSH2 were shown to increase meiotic recombination between parental chromosomes in S. cerevisiae-S. paradoxus hybrids and to reduce aneuploidy in their meiotic products. The same mechanism can also explain the reduction of fertility observed after intraspecific crosses between strains showing a high degree of sequence polymorphism (72). In general, a smooth monotonic relationship is observed between meiotic fertility and the degree of sequence divergence between the parents of a cross (113).
Fig 6.
Mechanisms of infertility. (A) Recombination-dependent chromosome loss. (Left) Low sequence divergence between parental genomes in the diploid allows normal crossover and chromosomal segregation at meiosis. (Right) High sequence divergence between parental genomes in the diploid prevents crossovers and results in abnormal chromosome segregation at meiosis. (B) Effect of reciprocal loss of ohnologs. Two species derived from a common ancestor having undergone whole-genome duplication may exhibit reciprocal loss (triangles) of the initially duplicated gene copies (blue and yellow arrows). Meiosis of their hybrid will produce gene-lacking ascospores owing to the independent assortment of chromosomes. (Panel A adapted from reference 87 by permission from Macmillan Publishers Ltd., copyright 1996; panel B inspired by reference 186.)
The mechanism of recombination-dependent chromosome loss is expected to apply to all yeast lineages, predicting the low fertility of interspecies hybrids. But in the Saccharomyces sensu stricto and related yeasts that inherited their genomes from the ancestral whole-genome duplication, another mechanism may also contribute to reduced ascospore viability (186). This mechanism is based on reciprocal gene loss after whole-genome duplication (Fig. 6B). In this case, postspeciation loss of one or the other member of a gene pair (called “ohnologs” to distinguish them from other classical paralogs) leaves the remaining active gene copy on different chromosomes in each of the two species. When the two species subsequently hybridize, half of their meiotic progeny are expected to be geneless after normal chromosomal segregation. Considering this phenomenon across the entire genome predicts that only a limited fraction of ascospores will inherit a complete gene set, hence a low meiotic viability.
FUNCTIONAL INCOMPATIBILITIES
Viability of Hybrids
According to the Bateson-Dobzhansky-Muller incompatibility model (10, 37, 140), referred to here as DM incompatibility, the number of deleterious epistatic interactions between gene products should rapidly increase with the divergence time between the parental species of a hybrid. Inviability, however, appears astonishingly rarely in yeasts, as judged from the large variety of viable hybrids obtained experimentally between species separated by very long evolutionary distances (Fig. 2). Therefore, dominant-negative DM interactions do not seem frequent between yeast genomes. Hybrid lines are not necessarily as fit as their parents, but they can reproduce mitotically and form viable clones, even if this propagation is often accompanied by important genomic changes during successive generations.
In contrast, meiotic infertility is observed very frequently, even after crosses between species of the same genus. In a recent analysis of many hybrid lines issued from an S. cerevisiae-S. paradoxus cross, the strong reduction of meiotic fertility observed contrasts with the near absence of fitness reduction (233). Mapping of hybrid incompatibility genes for fertility (speciation genes) should therefore be possible. In theory, their deleterious interactions could be either dominant or recessive, although the former case has not been reported so far for yeasts. The fact that, among Saccharomyces sensu stricto species, allotetraploids issued from genome doubling of sterile diploid hybrids recover fertility (69, 70) argues against the existence of any dominant-negative interactions, at least for this group of yeasts. Similarly, no clear-cut case of a recessive deleterious gene interaction, leading to a dead gamete in a normal tetrad, has been identified so far. The various combinations of parental chromosomal subsets observed in the rare viable ascospores from allodiploids (71, 87) could perhaps be used to map the possible cases of recessive DM interactions. But in two recent genomewide analyses, no DM incompatibility pairs of speciation genes could be found between the S. cerevisiae and S. paradoxus nuclear genomes (68, 93). Note that the limited role of DM incompatibility in yeast hybrids explains ancient results showing that proteins can be replaced by their orthologs from distinct yeast species in large macromolecular complexes, such as the ribosome (1) or the RNA polymerase I or II complex (169, 181, 194, 195).
Nucleomitochondrial Genetic Incompatibilities
Surprisingly, against this background, a nucleomitochondrial interaction provided the first example of a DM gene interaction between yeast genomes. This finding required a systematic approach in which all chromosomes of S. bayanus were successively placed (one or two at a time) into an S. cerevisiae genomic background, followed by a systematic complementation of all genes by use of a complete S. cerevisiae mutant library and subsequent candidate gene testing. It was eventually found that the S. bayanus allele of AEP2 on chromosome 13 is incompatible with the mitochondrial genome of S. cerevisiae, because its product is unable to replace the S. cerevisiae protein in the translation of the OLI1 mRNA of the S. cerevisiae mitochondria (102). It was speculated that the nuclear gene AEP2, encoding a mitochondrial translation factor, and the 5′-untranslated region (5′-UTR) of the mitochondrial gene OLI1, encoding a subunit of the Fo ATP synthase complex, evolved differently during adaptation of S. cerevisiae and S. bayanus to the respiration of nonfermentable carbon sources.
Other cases of nucleomitochondrial incompatibility between Saccharomyces species were reported previously, but without identification of the genes involved (158, 207, 208). Another example of precise nucleomitochondrial incompatibility is illustrated by the MRS1 gene. This gene was discovered long ago in S. cerevisiae as encoding a factor required for the RNA splicing of some group I introns. Upon crosses with S. douglasii, which is closely related to S. cerevisiae, it was observed that the S. cerevisiae MRS1 gene product was unable to help the splicing of the first intron of the mitochondrial COX1 gene of S. douglasii (81). The same phenomenon was rediscovered recently in a systematic screening of nucleomitochondrial incompatibilities between S. cerevisiae and S. paradoxus or S. bayanus (28). It was shown that the S. cerevisiae MRS1 allele is incompatible with a COX1 intron present in S. paradoxus, S. bayanus, and several other species but absent in the S. cerevisiae mitochondrial gene. The functional change between the S. cerevisiae and S. paradoxus MRS1 alleles is determined mainly by three nonsynonymous mutations. In the same recent screen, a third nuclear gene of S. cerevisiae, AIM22, confirmed the importance of nucleomitochondrial incompatibility in yeast speciation (29). AIM22 encodes a ligase involved in mitochondrial protein lipoylation. Interestingly, in the case of both AEP2-OLI1 and MRS1-COX1, the incompatibility was observed in only one direction, consistent with earlier observations on mitochondrial replacement by crosses and cytoductions (208). For example, the S. bayanus allele of AEP2 is unable to induce protein translation of the S. cerevisiae mitochondrial transcript OLI1, but the S. cerevisiae allele acts on the S. bayanus cognate transcript. Similarly, the S. cerevisiae MRS1 allele is unable to activate splicing of the S. paradoxus mitochondrial intron, but the S. paradoxus MRS1 allele acts on S. cerevisiae group I introns. If it is not coincidental (only two examples have been described), this unidirectionality suggests that DM incompatibility results from adaptive sequence changes in one or the other species, not both.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
The numerous examples reported above demonstrate that viable hybrids, albeit often genetically unstable ones, can be formed in the laboratory between a large variety of yeast species. This variety includes very distantly related species, as can now be measured from their important sequence divergence and differences in genomic architectures. In contrast, all natural hybrids observed so far involve parental genomes that remain highly syntenic despite their high sequence divergence. This situation corresponds to an initial step of evolutionary separation between yeast lineages during which a very high degree of sequence divergence can accumulate without numerous gene order alterations (183). Note that a similar situation is also observed for insect genomes, albeit at a lower scale. This stage of evolution results in the formation of “species complexes,” such as the Saccharomyces sensu stricto complex and others. Within each “species complex,” distinct lineages develop postzygotic barriers based on sequence divergence and other mechanisms—hence the species definition—but no prezygotic barriers—hence the presence of many natural hybrids. In recovering normal meiotic fertility by one of the above-mentioned mechanisms (Fig. 5), novel lines of mixed ancestry can be formed that will be biologically indistinguishable from lines of single ancestry. On an evolutionary time scale, interspecies hybridizations therefore superimpose stochastic events of major importance on top of gradual sequence divergence. After each hybridization event, the novel lineages that suddenly emerge extensively reshuffle the gene pools of their parental lineages. Whether the differences between these parental pools are adaptive or not has been discussed elsewhere (167), but the very limited number of DM incompatibility genes presently identified in yeasts and other organisms (127) suggests that this reshuffling may be extensive and that, at least in yeasts, hybridization may play a greater role in the formation of novel species than generally considered. Presently, this role is not precisely quantified, but given the fact that even some type strains (used to define species in classical taxonomy) are actually complex hybrids emerged from successive ancestral hybridization events, it is likely important and largely overlooked. The accelerated sequencing of novel yeast genomes allowed by new technologies should help to answer this question rapidly.
But even with more data available, the basic problem of species definition in yeasts and fungi (214), and hence the definition of hybrids themselves, may remain difficult to solve. To what extent does an autodiploid differ from an allodiploid when the degree of sequence diversity increases within the population of a defined sexual species? For large figures, the level of heterozygosity of regular autodiploids may mimic that of allodiploids. For example, figures as high as ca. 5% of single nucleotide polymorphism (SNP) divergence are observed between different isolates of S. paradoxus, and diploids made between them show a partially reduced meiotic fertility (113). Therefore, only a quantitative difference separates these diploids from described interspecies hybrids such as M. sorbitophila, with 15% sequence divergence between the two parents. Further investigations of natural yeast populations are needed to determine whether clear-cut discontinuities in sequence divergence exist, upon which species could be defined more clearly. In the meantime, only classical operational definitions can be used (101), with the risk that the biological species concept defined by Mayr (136) becomes blurred for yeasts.
The viability of artificial hybrids between very different yeast species, including those using distinct genetic codes, shows that dominant genetic incompatibilities are rare between yeasts, even over very long evolutionary distances. A few cases of recessive genetic incompatibilities were identified, but this question was not addressed systematically because most long-distance hybrids show an absence of meiotic fertility, or even sporulation (although a few cases were reported). The reduction of each parental contribution during stabilization of hybrid clones could partly replace the absence of meiotic segregation for such studies. But if one excepts the few natural hybrids recently studied by genomic methods, most published experiments are older and do not precisely describe the genetic reassortments between parental genomes in hybrids. The application of modern genomic technologies to study the evolution of well-selected hybrid clones is needed.
Despite the fact that the DM incompatibility model has provided the major theoretical framework to interpret speciation for almost a century, very few precise cases of deleterious epistatic interactions postulated by this model have been identified so far for any eukaryotic organisms (127). The model predicts, however, that the number of genes involved in postzygotic incompatibilities should increase faster than linearly with the divergence time between species. This prediction was recently verified with Drosophila (135). With yeasts, only two examples of DM incompatibility were identified recently, and both concern nucleomitochondrial interactions, not interactions between nuclear genes. Again, application of modern genomic technologies should rapidly change this situation, especially combined with recent studies on the evolution of gene expression networks (45, 215, 239). In this regard, yeasts offer the advantage that basic epistatic interactions and network rewiring can be studied at the cellular level, in the absence of interference by developmentally based incompatibilities. For this reason, as well as their high level of evolutionary diversity, their clonal propagation in addition to sexual reproduction, and the facility of their experimental manipulation, we believe that yeasts have much to offer to deepen our understanding of speciation and evolutionary processes in all eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues from the Génolevures Consortium (GDR2354 CNRS) and the Unité de Génétique Moléculaire des Levures (Institut Pasteur, UMR3525 CNRS, and UFR927, University Pierre and Marie Curie) for stimulating discussions.
This work was supported in part by contract DYGEVO from the ANR (2011 SVSE6). L.M. is a fellow of the Pasteur-Paris University (PPU) International Ph.D. program. B.D. is a member of the Institut Universitaire de France.
Biographies

Lucia Morales received her undergraduate degree in genomics and computational biology from the National Autonomous University of Mexico (UNAM), Cuernavaca, Morelos, Mexico. She then studied molecular genetics in the laboratory of Karla Neugebauer at the Max Planck Institute for Molecular Cell Biology and Genetics, Dresden, Germany, where she investigated the endogenous target genes of SR proteins in cycling and neural cells. She is currently pursuing a Ph.D. in molecular genetics and genomics at the University Pierre and Marie Curie, Paris, France, working in the laboratory of B. Dujon at the Institut Pasteur to study the role of genomic rearrangements in the evolution of natural yeast genomes and artificially constructed hybrids.

Bernard Dujon is a Professor of Molecular Genetics at the University Pierre and Marie Curie and the Institut Pasteur, Paris, France. He received his doctorate degree from the University Pierre and Marie Curie, working on mitochondrial genetics in yeast in the laboratory of P. Slonimski at CNRS, Gif sur Yvette, France. He conducted postdoctoral work in the laboratory of W. Gilbert at Harvard University, Cambridge, MA, studying the molecular nature of group I introns of the yeast mitochondrial genome and discovering the homing endonucleases that they encode. He was appointed Head of the Laboratory at the Institut Pasteur in 1987. Dr. Dujon's research interests are focused on eukaryotic genome structure, dynamics, and evolution, using yeasts as models.
Footnotes
Supplemental material for this article may be found at http://mmbr.asm.org/.
REFERENCES
- 1. Adoutte-Panvier A, Davies JE, Gritz LR, Littlewood BS. 1980. Studies of ribosomal proteins of yeast species and their hybrids: gel electrophoresis and immunochemical cross-reactions. Mol. Gen. Genet. 179: 273–282 [DOI] [PubMed] [Google Scholar]
- 2. Albertin W, et al. 2009. Evidence for autotetraploidy associated with reproductive isolation in Saccharomyces cerevisiae: towards a new domesticated species. J. Evol. Biol. 22: 2157–2170 [DOI] [PubMed] [Google Scholar]
- 3. Aminnejad M, et al. 2011. Identification of novel hybrids between Cryptococcus neoformans var. grubii VNI and Cryptococcus gattii VGII. Mycopathologia 173: 337–346 [DOI] [PubMed] [Google Scholar]
- 4. Andersen MP, Nelson ZW, Hetrick ED, Gottschling DE. 2008. A genetic screen for increased loss of heterozygosity in Saccharomyces cerevisiae. Genetics 179: 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antunovics Z, Irinyi L, Sipiczki M. 2005. Combined application of methods to taxonomic identification of Saccharomyces strains in fermenting botrytized grape must. J. Appl. Microbiol. 98: 971–979 [DOI] [PubMed] [Google Scholar]
- 6. Antunovics Z, Nguyen Gaillardin H-VC, Sipiczki M. 2005. Gradual genome stabilisation by progressive reduction of the Saccharomyces uvarum genome in an interspecific hybrid with Saccharomyces cerevisiae. FEMS Yeast Res. 5: 1141–1150 [DOI] [PubMed] [Google Scholar]
- 7. Arnold ML, Martin NH. 2010. Hybrid fitness across time and habitats. Trends Ecol. Evol. 25: 530–536 [DOI] [PubMed] [Google Scholar]
- 8. Arroyo-López FN, Orlić S, Querol A, Barrio E. 2009. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 131: 120–127 [DOI] [PubMed] [Google Scholar]
- 9. Arroyo-López FN, Pérez-Torrado R, Querol A, Barrio E. 2010. Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 27: 628–637 [DOI] [PubMed] [Google Scholar]
- 10. Bateson W. 1909. Heredity and variation in modern lights, p 85–101 In Seward AC. (ed), Darwin and modern science. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 11. Belloch C, Orlic S, Barrio E, Querol A. 2008. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int. J. Food Microbiol. 122: 188–195 [DOI] [PubMed] [Google Scholar]
- 12. Belloch C, et al. 2009. Chimeric genomes of natural hybrids of Saccharomyces cerevisiae and Saccharomyces kudriavzevii. Appl. Environ. Microbiol. 75: 2534–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellon J, et al. 2011. Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl. Microbiol. Biotechnol. 91: 603–612 [DOI] [PubMed] [Google Scholar]
- 14. Boekhout T, et al. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147: 891–907 [DOI] [PubMed] [Google Scholar]
- 15. Bon E, et al. 2000. Genomic exploration of the hemiascomycetous yeasts. 5. Saccharomyces bayanus var. uvarum. FEBS Lett. 487: 37–41 [DOI] [PubMed] [Google Scholar]
- 16. Bond U, Neal C, Donnelly D, James TC. 2004. Aneuploidy and copy number breakpoints in the genome of lager yeasts mapped by microarray hybridisation. Curr. Genet. 45: 360–370 [DOI] [PubMed] [Google Scholar]
- 17. Borneman AR, et al. 2011. Whole-genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 7: e1001287 doi:10.1371/journal.pgen.1001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borneman AR, et al. 2012. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 12: 88–96 [DOI] [PubMed] [Google Scholar]
- 19. Børsting C, et al. 1997. Saccharomyces carlsbergensis contains two functional genes encoding the acyl-CoA binding protein, one similar to the ACB1 gene from S. cerevisiae and one identical to the ACB1 gene from S. monacensis. Yeast 13: 1409–1421 [DOI] [PubMed] [Google Scholar]
- 20. Bovers M, et al. 2006. Unique hybrids between the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii. FEMS Yeast Res. 6: 599–607 [DOI] [PubMed] [Google Scholar]
- 21. Bovers M, et al. 2008. AIDS patient death caused by novel Cryptococcus neoformans × C. gattii hybrid. Emerg. Infect. Dis. 14: 1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradbury JE, et al. 2006. A homozygous diploid subset of commercial wine yeast strains. Antonie Van Leeuwenhoek 89: 27–37 [DOI] [PubMed] [Google Scholar]
- 23. Butler G, et al. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459: 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casaregola S, Nguyen HV, Lapathitis G, Kotyk A, Gaillardin C. 2001. Analysis of the constitution of the beer yeast genome by PCR, sequencing and subtelomeric sequence hybridization. Int. J. Syst. Evol. Microbiol. 51: 1607–1618 [DOI] [PubMed] [Google Scholar]
- 25. Chambers SR, Hunter N, Louis EJ, Borts RH. 1996. The mismatch repair system reduces meiotic homologous recombination and stimulates recombination-dependent chromosome loss. Mol. Cell. Biol. 16: 6110–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng M, Chang R, Dent D, Hsieh P-C. 2011. Breeding an amylolytic yeast strain for alcoholic beverage production. Appl. Biochem. Biotechnol. 163: 693–706 [DOI] [PubMed] [Google Scholar]
- 27. Chibana H, Magee PT. 2009. The enigma of the major repeat sequence of Candida albicans. Future Microbiol. 4: 171–179 [DOI] [PubMed] [Google Scholar]
- 28. Chou J-Y, Hung Y-S, Lin K-H, Lee H-Y, Leu J-Y. 2010. Multiple molecular mechanisms cause reproductive isolation between three yeast species. PLoS Biol. 8: e1000432 doi:10.1371/journal.pbio.1000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chou J-Y, Leu J-Y. 2010. Speciation through cytonuclear incompatibility: insights from yeast and implications for higher eukaryotes. Bioessays 32: 401–411 [DOI] [PubMed] [Google Scholar]
- 30. Cliften P, Fulton R, Wilson R, Johnston M. 2006. After the duplication: gene loss and adaptation in Saccharomyces genomes. Genetics 172: 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cogliati M, Barchiesi F, Spreghini E, Tortorano AM. 2011. Heterozygosis and pathogenicity of Cryptococcus neoformans AD-hybrid isolates. Mycopathologia 173: 347–357 [DOI] [PubMed] [Google Scholar]
- 32. de Barros Lopes M, Bellon JR, Shirley NJ, Ganter PF. 2002. Evidence for multiple interspecific hybridization in Saccharomyces sensu stricto species. FEMS Yeast Res. 1: 323–331 [DOI] [PubMed] [Google Scholar]
- 33. Delneri D, et al. 2003. Engineering evolution to study speciation in yeasts. Nature 422: 68–72 [DOI] [PubMed] [Google Scholar]
- 34. de-Richard MS, Broock M. 1984. Protoplast fusion between a petite strain of Candida utilis and Saccharomyces cerevisiae respiratory-competent cells. Curr. Microbiol. 10: 117–120 [Google Scholar]
- 35. Dhamija SS, Gera R, Singh DK, Singh D, Marchant R. 1997. Physiological and biochemical characterization of intergeneric hybrids of thermotolerant and non-thermotolerant yeasts. J. Basic Microbiol. 37: 307–312 [DOI] [PubMed] [Google Scholar]
- 36. Diogo D, Bouchier C, D'enfert C, Bougnoux M-E. 2009. Loss of heterozygosity in commensal isolates of the asexual diploid yeast Candida albicans. Fungal Genet. Biol. 46: 159–168 [DOI] [PubMed] [Google Scholar]
- 37. Dobzhansky T. 1937. Genetic nature of species difference. Am. Nat. 71: 404–420 [Google Scholar]
- 38. Doniger SW, et al. 2008. A catalog of neutral and deleterious polymorphism in yeast. PLoS Genet. 4: e1000183 doi:10.1371/journal.pgen.1000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D'Souza CA, et al. 2011. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2: e00342–10 doi:10.1128/mBio.00342-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dujon B. 2010. Yeast evolutionary genomics. Nat. Rev. Genet. 11: 512–524 [DOI] [PubMed] [Google Scholar]
- 41. Dujon B, et al. 2004. Genome evolution in yeasts. Nature 430: 35–44 [DOI] [PubMed] [Google Scholar]
- 42. Dunn B, Levine RP, Sherlock G. 2005. Microarray karyotyping of commercial wine yeast strains reveals shared, as well as unique, genomic signatures. BMC Genomics 6: 53 doi:10.1186/1471-2164-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunn B, Sherlock G. 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 18: 1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dziuba E, Chmielewska J. 2002. Fermentative activity of somatic hybrids of Saccharomyces cerevisiae and Candida shehatae or Pachysolen tannophilus. Electron. J. Pol. Agric. 5: 1 http://www.ejpau.media.pl/volume5/issue1/biotechnology/art-01.html [Google Scholar]
- 45. Emerson JJ, et al. 2010. Natural selection on cis and trans regulation in yeasts. Genome Res. 20: 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erny C, et al. 2012. Ecological success of a group of Saccharomyces cerevisiae/Saccharomyces kudriavzevii hybrids in the Northern European wine-making environment. Appl. Environ. Microbiol. 78: 3256–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esberg A, Muller L, McCusker J. 2011. Genomic structure of and genome-wide recombination in the Saccharomyces cerevisiae S288C progenitor isolate EM93. PLoS One 6: e25211 doi:10.1371/journal.pone.0025211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Evans C, Conrad D. 1987. An improved method for protoplast formation and its application in the fusion of Rhodotorula rubra with Saccharomyces cerevisiae. Arch. Microbiol. 148: 77–82 [DOI] [PubMed] [Google Scholar]
- 49. Ferenczy L, Maráz A. 1977. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature 268: 524–525 [DOI] [PubMed] [Google Scholar]
- 50. Fernández-Espinar T, Barrio E, Querol A. 2003. Analysis of the genetic variability in the species of the Saccharomyces sensu stricto complex. Yeast 20: 1213–1226 [DOI] [PubMed] [Google Scholar]
- 51. Fischer G, James SA, Roberts IN, Oliver SG, Louis EJ. 2000. Chromosomal evolution in Saccharomyces. Nature 405: 451–454 [DOI] [PubMed] [Google Scholar]
- 52. Forche A, May G, Magee PT. 2005. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot. Cell 4: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frank AC, Wolfe KH. 2009. Evolutionary capture of viral and plasmid DNA by yeast nuclear chromosomes. Eukaryot. Cell 8: 1521–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fukuhara H. 1995. Linear DNA plasmids of yeasts. FEMS Microbiol. Lett. 131: 1–9 [DOI] [PubMed] [Google Scholar]
- 55. Galeote V, Bigey F, Beyne E, Novo M, Legras Casaregola J-LS, Dequin S. 2011. Amplification of a Zygosaccharomyces bailii DNA segment in wine yeast genomes by extrachromosomal circular DNA formation. PLoS One 6: e17872 doi:10.1371/journal.pone.0017872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Galeote V, et al. 2010. FSY1, a horizontally transferred gene in the Saccharomyces cerevisiae EC1118 wine yeast strain, encodes a high-affinity fructose/H+ symporter. Microbiology 156: 3754–3761 [DOI] [PubMed] [Google Scholar]
- 57. Gangl H, et al. 2009. Exceptional fermentation characteristics of natural hybrids from Saccharomyces cerevisiae and S. kudriavzevii. N. Biotechnol. 25: 244–251 [DOI] [PubMed] [Google Scholar]
- 58. Gautam SP, Gupta AK, Pandey S. 1993. Hybrid construction by fusion of protoplasts of Endomycopsis fibuligera and Saccharomyces cerevisiae. Cytobios 73: 183–188 [PubMed] [Google Scholar]
- 59. Gillece JD, et al. 2011. Whole genome sequence analysis of Cryptococcus gattii from the Pacific Northwest reveals unexpected diversity. PLoS One 6: e28550 doi:10.1371/journal.pone.0028550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giudici P, Caggia C, Pulvirenti A, Zambonelli C, Rainieri S. 1998. Electrophoretic profile of hybrids between cryotolerant and non-cryotolerant Saccharomyces strains. Lett. Appl. Microbiol. 27: 31–34 [DOI] [PubMed] [Google Scholar]
- 61. Goffeau A, et al. 1996. Life with 6000 genes. Science 274: 563–567 [DOI] [PubMed] [Google Scholar]
- 62. González SS, Barrio E, Gafner J, Querol A. 2006. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 6: 1221–1234 [DOI] [PubMed] [Google Scholar]
- 63. González SS, Barrio E, Querol A. 2008. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 74: 2314–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. González SS, Gallo L, Climent MAD, Barrio E, Querol A. 2007. Enological characterization of natural hybrids from Saccharomyces cerevisiae and S. kudriavzevii. Int. J. Food Microbiol. 116: 11–18 [DOI] [PubMed] [Google Scholar]
- 65. Goodey A, Bevan EA. 1983. Production and genetic analysis of yeast hybrids. Curr. Genet. 7: 69–72 [DOI] [PubMed] [Google Scholar]
- 66. Gordon JL, Byrne KP, Wolfe KH. 2009. Additions, losses, and rearrangements on the evolutionary route from a reconstructed ancestor to the modern Saccharomyces cerevisiae genome. PLoS Genet. 5: e1000485 doi:10.1371/journal.pgen.1000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gordon JL, Wolfe KH. 2008. Recent allopolyploid origin of Zygosaccharomyces rouxii strain ATCC 42981. Yeast 25: 449–456 [DOI] [PubMed] [Google Scholar]
- 68. Greig D. 2007. A screen for recessive speciation genes expressed in the gametes of F1 hybrid yeast. PLoS Genet. 3: e21 doi:10.1371/journal.pgen.0030021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greig D. 2009. Reproductive isolation in Saccharomyces. Heredity 102: 39–44 [DOI] [PubMed] [Google Scholar]
- 70. Greig D, Borts RH, Louis EJ, Travisano M. 2002. Epistasis and hybrid sterility in Saccharomyces. Proc. Biol. Sci. 269: 1167–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greig D, Louis E, Borts R, Travisano M. 2002. Hybrid speciation in experimental populations of yeast. Science 298: 1773–1775 [DOI] [PubMed] [Google Scholar]
- 72. Greig D, Travisano M, Louis EJ, Borts RH. 2003. A role for the mismatch repair system during incipient speciation in Saccharomyces. J. Evol. Biol. 16: 429–437 [DOI] [PubMed] [Google Scholar]
- 73. Groth C, Hansen J, Piskur J. 1999. A natural chimeric yeast containing genetic material from three species. Int. J. Syst. Bacteriol. 49: 1933–1938 [DOI] [PubMed] [Google Scholar]
- 74. Groves D, Oliver S. 1984. Formation of intergeneric hybrids of yeast by protoplast fusion of Yarrowia and Kluyveromyces species. Curr. Genet. 8: 49–55 [DOI] [PubMed] [Google Scholar]
- 75. Gunge N, Sakaguchi K. 1981. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J. Bacteriol. 147: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupthar AS. 1992. Segregation of altered parental properties in fusions between Saccharomyces cerevisiae and the d-xylose fermenting yeasts Candida shehatae and Pichia stipitis. Can. J. Microbiol. 38: 1233–1237 [DOI] [PubMed] [Google Scholar]
- 77. Hansen J, Cherest H, Kielland-Brandt M. 1994. Two divergent MET10 genes, one from Saccharomyces cerevisiae and one from Saccharomyces carlsbergensis, encode the alpha subunit of sulfite reductase and specify potential binding sites for FAD and NADPH. J. Bacteriol. 176: 6050–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hawthorne D, Philippsen P. 1994. Genetic and molecular analysis of hybrids in the genus Saccharomyces involving S. cerevisiae, S. uvarum and a new species, S. douglasii. Yeast 10: 1285–1296 [DOI] [PubMed] [Google Scholar]
- 79. Hellborg L, Piskur J. 2009. Complex nature of the genome in a wine spoilage yeast, Dekkera bruxellensis. Eukaryot. Cell 8: 1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Heluane H, Spencer J, Spencer D. 1993. Characterization of hybrids obtained by protoplast fusion, between Pachysolen tannophilus and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 40: 98–100 [Google Scholar]
- 81. Herbert CJ, Macadre C, Bécam AM, Lazowska J, Slonimski PP. 1992. The MRS1 gene of S. douglasii: co-evolution of mitochondrial introns and specific splicing proteins encoded by nuclear genes. Gene Expr. 2: 203–214 [PMC free article] [PubMed] [Google Scholar]
- 82. Hittinger CT, et al. 2010. Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature 464: 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hockney R, Freeman R. 1980. Construction of polysaccharide-degrading yeasts by protoplast fusion, p 139–144 In Ferenczy L, Farkas GL. (ed), Proceedings of the 5th International Protoplast Symposium. Pergamon Press, Oxford, United Kingdom [Google Scholar]
- 84. Horitsu H, et al. 1989. Characterization of an interspecific fusant between Candida utilis and Candida lipolytica. Agric. Biol. Chem. 53: 389–394 [Google Scholar]
- 85. Hou X, Yao S. 2011. Improved inhibitor tolerance in xylose-fermenting yeast Spathaspora passalidarum by mutagenesis and protoplast fusion. Appl. Microbiol. Biotechnol. 93: 2591–2601 [DOI] [PubMed] [Google Scholar]
- 86. Hu G, et al. 2008. Comparative hybridization reveals extensive genome variation in the AIDS-associated pathogen Cryptococcus neoformans. Genome Biol. 9: R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hunter N, Chambers SR, Louis EJ, Borts RH. 1996. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 15: 1726–1733 [PMC free article] [PubMed] [Google Scholar]
- 88. Imanishi Y, Ueda-Nishimura K, Mikata K. 2007. Two new species of Kazachstania that form ascospores connected by a belt-like intersporal body: Kazachstania zonata and Kazachstania gamospora. FEMS Yeast Res. 7: 330–338 [DOI] [PubMed] [Google Scholar]
- 89. James SA, Bond CJ, Stratford M, Roberts IN. 2005. Molecular evidence for the existence of natural hybrids in the genus Zygosaccharomyces. FEMS Yeast Res. 5: 747–755 [DOI] [PubMed] [Google Scholar]
- 90. James TC, Usher J, Campbell S, Bond U. 2008. Lager yeasts possess dynamic genomes that undergo rearrangements and gene amplification in response to stress. Curr. Genet. 53: 139–152 [DOI] [PubMed] [Google Scholar]
- 91. Johannsen E, Halland L, Opperman A. 1984. Protoplast fusion within the genus Kluyveromyces van der Walt emend. van der Walt. Can. J. Microbiol. 30: 540–552 [Google Scholar]
- 92. Jones T, et al. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 101: 7329–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kao KC, Schwartz K, Sherlock G. 2010. A genome-wide analysis reveals no nuclear Dobzhansky-Muller pairs of determinants of speciation between S. cerevisiae and S. paradoxus, but suggests more complex incompatibilities. PLoS Genet. 6: e1001038 doi:10.1371/journal.pgen.1001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kavanagh K, Whittaker PA. 1996. Application of protoplast fusion to the nonconventional yeast. Enzyme Microb. Technol. 18: 45–51 [Google Scholar]
- 95. Kavanaugh LA, Fraser JA, Dietrich FS. 2006. Recent evolution of the human pathogen Cryptococcus neoformans by intervarietal transfer of a 14-gene fragment. Mol. Biol. Evol. 23: 1879–1890 [DOI] [PubMed] [Google Scholar]
- 96. Kielland-Brandt MC, Nilsson-Tillgren T. 1995. Genetics of brewing yeasts, p 223–254 In Rose AH, Wheals E, Harrison JS. (ed), The yeasts. Academic Press, London, United Kingdom [Google Scholar]
- 97. Kishida M, Muguruma T, Sakanaka K, Katsuragi T, Sakai T. 1996. Chromosomal deletion or rearrangement in chimeric hybrids of Saccharomycopsis fibuligera and Saccharomyces diastaticus obtained by cell fusion. J. Ferment. Bioeng. 81: 281–285 [Google Scholar]
- 98. Kucsera J, Pfeiffer I, Ferenczy L. 1998. A novel method for hybridization of Saccharomyces species without genetic markers. Can. J. Microbiol. 44: 959–964 [PubMed] [Google Scholar]
- 99. Kunicka-Styczyńska A, Rajkowska K. 2011. Physiological and genetic stability of hybrids of industrial wine yeasts Saccharomyces sensu stricto complex. J. Appl. Microbiol. 110: 1538–1549 [DOI] [PubMed] [Google Scholar]
- 100. Reference deleted.
- 101. Kurtzman CP, Fell JW, Boekhout T. 2010. The yeasts: a taxonomic study, 5th ed, p 2080 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 102. Lee H-Y, et al. 2008. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135: 1065–1073 [DOI] [PubMed] [Google Scholar]
- 103. Leelavatcharamas V, Boonyakamola A, Kishida M, Kawasaki H. 2006. Growth characteristics of fusants by protoplast fusion between the thermotolerant yeast, Kluyveromyces marxianus, and the starch-assimilating yeast, Schwanniomyces occidentalis. Biocontrol Sci. 11: 81–84 [DOI] [PubMed] [Google Scholar]
- 104. Legmann R, Margalith P. 1983. Interspecific protoplast fusion of Saccharomyces cerevisiae and Saccharomyces mellis. Appl. Microbiol. Biotechnol. 18: 320–322 [Google Scholar]
- 105. Leh-Louis V, et al. 2012. Pichia sorbitophila, an interspecies yeast hybrid, reveals early steps of genome resolution after polyploidization. G3 (Bethesda) 2: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Le Jeune C, et al. 2007. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res. 7: 540–549 [DOI] [PubMed] [Google Scholar]
- 107. Lengeler KB, Cox GM, Heitman J. 2001. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect. Immun. 69: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li W, et al. 2012. Genetic diversity and genomic plasticity of Cryptococcus neoformans AD hybrid strains. G3 (Bethesda) 2: 83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Libkind D, et al. 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. U. S. A. 108: 14539–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Libuda DE, Winston F. 2006. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature 443: 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lin CC, Hsieh PC, Mau JL, Teng DF. 2005. Construction of an intergeneric fusion from Schizosaccharomyces pombe and Lentinula edodes for xylan degradation and polyol production. Enzyme Microb. Technol. 36: 107–117 [Google Scholar]
- 112. Lin X, et al. 2007. αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 3: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liti G, Barton DBH, Louis EJ. 2006. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174: 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liti G, et al. 2009. Population genomics of domestic and wild yeasts. Nature 458: 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liti G, Schacherer J. 2011. The rise of yeast population genomics. C. R. Biol. 334: 612–619 [DOI] [PubMed] [Google Scholar]
- 116. Litvintseva AP, Lin X, Templeton I, Heitman J, Mitchell TG. 2007. Many globally isolated AD hybrid strains of Cryptococcus neoformans originated in Africa. PLoS Pathog. 3: e114 doi:10.1371/journal.ppat.0030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Llorente B, et al. 2000. Genomic exploration of the hemiascomycetous yeasts. 18. Comparative analysis of chromosome maps and synteny with Saccharomyces cerevisiae. FEBS Lett. 487: 101–112 [DOI] [PubMed] [Google Scholar]
- 118. Llorente B, Smith CE, Symington LS. 2008. Break-induced replication: what is it and what is it for? Cell Cycle 7: 859–864 [DOI] [PubMed] [Google Scholar]
- 119. Loftus BJ, et al. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307: 1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lopandic K, et al. 2007. Genetically different wine yeasts isolated from Austrian vine-growing regions influence wine aroma differently and contain putative hybrids between Saccharomyces cerevisiae and Saccharomyces kudriavzevii. FEMS Yeast Res. 7: 953–965 [DOI] [PubMed] [Google Scholar]
- 121. Lopes CA, Barrio E, Querol A. 2010. Natural hybrids of S. cerevisiae × S. kudriavzevii share alleles with European wild populations of Saccharomyces kudriavzevii. FEMS Yeast Res. 10: 412–421 [DOI] [PubMed] [Google Scholar]
- 122. Loray M, Spencer J, Spencer D, De Figueroa L. 1995. Hybrids obtained by protoplast fusion with a salt-tolerant yeast. J. Ind. Microbiol. Biotechnol. 14: 508–513 [DOI] [PubMed] [Google Scholar]
- 123. Lorenz A, Fuchs J, Trelles-Sticken E, Scherthan H, Loidl J. 2002. Spatial organisation and behaviour of the parental chromosome sets in the nuclei of Saccharomyces cerevisiae × S. paradoxus hybrids. J. Cell Sci. 115: 3829–3835 [DOI] [PubMed] [Google Scholar]
- 124. Lucca M, Spencer J, De Figueroa L. 2002. Glycerol and arabitol production by an intergeneric hybrid, PB2, obtained by protoplast fusion between Saccharomyces cerevisiae and Torulaspora delbrueckii. Appl. Microbiol. Biotechnol. 59: 472–476 [DOI] [PubMed] [Google Scholar]
- 125. Maclean CJ, Greig D. 2008. Prezygotic reproductive isolation between Saccharomyces cerevisiae and Saccharomyces paradoxus. BMC Evol. Biol. 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Magwene PM, et al. 2011. Outcrossing, mitotic recombination, and life-history trade-offs shape genome evolution in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 108: 1987–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Maheshwari S, Barbash DA. 2011. The genetics of hybrid incompatibilities. Annu. Rev. Genet. 45: 331–355 [DOI] [PubMed] [Google Scholar]
- 128. Mallet J. 2007. Hybrid speciation. Nature 446: 279–283 [DOI] [PubMed] [Google Scholar]
- 129. Maráz A, Ferenczy L. 1980. Selective transfer of fungal cytoplasmic genetic elements by protoplast fusion. Curr. Microbiol. 4: 343–345 [Google Scholar]
- 130. Marinoni G, Lachance M-A. 2004. Speciation in the large-spored Metschnikowia clade and establishment of a new species, Metschnikowia borealis comb. nov. FEMS Yeast Res. 4: 587–596 [DOI] [PubMed] [Google Scholar]
- 131. Marinoni G, et al. 1999. Horizontal transfer of genetic material among Saccharomyces yeasts. J. Bacteriol. 181: 6488–6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Martín-Rendón E, Jiménez J, Benítez T. 1989. Ethanol inhibition of Saccharomyces and Candida enzymes. Curr. Genet. 15: 7–16 [DOI] [PubMed] [Google Scholar]
- 133. Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D. 1998. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Environ. Microbiol. 64: 3887–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Masneuf I, Murat M, Naumov G, Tominaga T, Dubourdieu D. 2002. Hybrids Saccharomyces cerevisiae × Saccharomyces bayanus var. uvarum having a high liberating ability of some sulfur varietal aromas of Vitis vinifera sauvignon blanc wines. J. Int. Sci. Vigne Vin 36: 205–212 [Google Scholar]
- 135. Matute DR, Butler IA, Turissini DA, Coyne JA. 2010. A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science 329: 1518–1521 [DOI] [PubMed] [Google Scholar]
- 136. Mayr E. 1942. Systematics and the origin of species, from the viewpoint of a zoologist. Harvard University Press, Cambridge, MA [Google Scholar]
- 137. McMurray MA, Gottschling DE. 2003. An age-induced switch to a hyper-recombinational state. Science 301: 1908–1911 [DOI] [PubMed] [Google Scholar]
- 138. Mentel M, Spírek M, Jørck-Ramberg D, Piskur J. 2006. Transfer of genetic material between pathogenic and food-borne yeasts. Appl. Environ. Microbiol. 72: 5122–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Morgan A, Brunner A, Whittaker PA. 1980. Protoplast fusion in a petite-negative yeast, Kluyveromyces lactis. Curr. Genet. 2: 87–93 [DOI] [PubMed] [Google Scholar]
- 140. Muller HJ. 1942. Isolating mechanisms, evolution and temperature. Biol. Symp. 6: 71–125 [Google Scholar]
- 141. Muller L, McCusker J. 2009. A multispecies-based taxonomic microarray reveals interspecies hybridization and introgression in Saccharomyces cerevisiae. FEMS Yeast Res. 9: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nakao Y, et al. 2009. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16: 115–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Naumov G. 1987. Genetic basis for classification and identification of the ascomycetous yeasts. Stud. Mycol. 30: 469–475 [Google Scholar]
- 144. Naumov G. 1996. Genetic identification of biological species in the Saccharomyces sensu stricto complex. J. Ind. Microbiol. Biotechnol. 17: 295–302 [Google Scholar]
- 145. Naumov G, James S, Naumova E, Louis E, Roberts I. 2000. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int. J. Syst. Evol. Microbiol. 50: 1931–1942 [DOI] [PubMed] [Google Scholar]
- 146. Naumov GI, et al. 2000. Natural polyploidization of some cultured yeast Saccharomyces sensu stricto: auto- and allotetraploidy. Syst. Appl. Microbiol. 23: 442–449 [DOI] [PubMed] [Google Scholar]
- 147. Naumov GI, Naumova ES, Masneuf-Pomarède I. 2010. Genetic identification of new biological species Saccharomyces arboricolus Wang et Bai. Antonie Van Leeuwenhoek 98: 1–7 [DOI] [PubMed] [Google Scholar]
- 148. Naumova E, Naumov G, Masneuf-Pomarède I, Aigle M. 2005. Molecular genetic study of introgression between Saccharomyces bayanus and S. cerevisiae. Yeast 22: 1099–1115 [DOI] [PubMed] [Google Scholar]
- 149. Naumova ES, Naumov GI, Michailova YV, Martynenko NN, Masneuf-Pomarède I. 2011. Genetic diversity study of the yeast Saccharomyces bayanus var. uvarum reveals introgressed subtelomeric Saccharomyces cerevisiae genes. Res. Microbiol. 162: 204–213 [DOI] [PubMed] [Google Scholar]
- 150. Nga B, et al. 1992. Intergeneric hybrids between Saccharomycopsis fibuligera and Yarrowia lipolytica. J. Gen. Microbiol. 138: 223–227 [Google Scholar]
- 151. Nguyen HV, Gaillardin C. 2005. Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res. 5: 471–483 [DOI] [PubMed] [Google Scholar]
- 152. Nguyen HV, Legras J, Neuvéglise C, Gaillardin C. 2011. Deciphering the hybridisation history leading to the lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380. PLoS One 6: e25821 doi:10.1371/journal.pone.0025821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nguyen HV, Lepingle A, Gaillardin CA. 2000. Molecular typing demonstrates homogeneity of Saccharomyces uvarum strains and reveals the existence of hybrids between S. uvarum and S. cerevisiae, including the S. bayanus type strain CBS 380. Syst. Appl. Microbiol. 23: 71–85 [DOI] [PubMed] [Google Scholar]
- 154. Nilsson-Tillgren T, et al. 1981. Genetic differences between Saccharomyces carlsbergensis and S. cerevisiae. Analysis of chromosome III by single chromosome transfer. Carlsberg Res. Commun. 46: 65–76 [Google Scholar]
- 155. Nilsson-Tillgren T, Petersen J, Holmberg S, Kielland-Brandt MC. 1980. Transfer of chromosome III during kar mediated cytoduction in yeast. Carlsberg Res. Commun. 45: 113–117 [Google Scholar]
- 156. Nolte AW, Tautz D. 2010. Understanding the onset of hybrid speciation. Trends Genet. 26: 54–58 [DOI] [PubMed] [Google Scholar]
- 157. Novo M, et al. 2009. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc. Natl. Acad. Sci. U. S. A. 106: 16333–16338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Osuský M, Kissová J, Kovác L. 1997. Interspecies transplacement of mitochondria in yeasts. Curr. Genet. 32: 24–26 [DOI] [PubMed] [Google Scholar]
- 159. Paques F, Haber JE. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pasha C, Kuhad R, Rao LV. 2007. Strain improvement of thermotolerant Saccharomyces cerevisiae VS3 strain for better utilization of lignocellulosic substrates. J. Appl. Microbiol. 13: 1480–1489 [DOI] [PubMed] [Google Scholar]
- 161. Peberdy J. 1979. Fungal protoplasts: isolation, reversion, and fusion. Annu. Rev. Microbiol. 33: 21–39 [DOI] [PubMed] [Google Scholar]
- 162. Perez C, Benítez J. 1986. Defective karyogamy in meiotic segregants of a Candida utilis-Saccharomyces cerevisiae hybrid. Curr. Genet. 10: 639–642 [DOI] [PubMed] [Google Scholar]
- 163. Perez C, Vallin C, Benitez J. 1984. Hybridization of Saccharomyces cerevisiae with Candida utilis through protoplast fusion. Curr. Genet. 8: 575–580 [DOI] [PubMed] [Google Scholar]
- 164. Peris D, et al. 2012. The molecular characterization of new types of Saccharomyces cerevisiae × S. kudriavzevii hybrid yeasts unveils a high genetic diversity. Yeast 29: 81–91 [DOI] [PubMed] [Google Scholar]
- 165. Peris D, Lopes CA, Belloch C, Querol A, Barrio E. 2012. Comparative genomics among Saccharomyces cerevisiae × Saccharomyces kudriavzevii natural hybrid strains isolated from wine and beer reveals different origins. BMC Genomics 13: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pina A, Calderon I, Benitez T. 1986. Intergeneric hybrids of Saccharomyces cerevisiae and Zygosaccharomyces fermentati obtained by protoplast fusion. Appl. Environ. Microbiol. 51: 995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Presgraves DC. 2010. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 11: 175–180 [DOI] [PubMed] [Google Scholar]
- 168. Presgraves DC. 2010. Darwin and the origin of interspecific genetic incompatibilities. Am. Nat. 176(Suppl 1): S45–S60 [DOI] [PubMed] [Google Scholar]
- 169. Proshkina GM, et al. 2006. Ancient origin, functional conservation and fast evolution of DNA-dependent RNA polymerase III. Nucleic Acids Res. 34: 3615–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Provost A, Bourguignon C, Fournier P, Ribet A, Heslot H. 1978. Intergeneric hybridization in yeasts through protoplast fusion. FEMS Microbiol. Lett. 3: 309–312 [Google Scholar]
- 171. Pujol C, et al. 2004. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot. Cell 3: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Pulvirenti A, Caggia C, Restuccia C, Giudici P, Zambonelli C. 2000. Inheritance of mitochondrial DNA in interspecific Saccharomyces hybrids. Ann. Microbiol. 50: 61–64 [Google Scholar]
- 173. Pulvirenti A, et al. 2000. Saccharomyces uvarum, a proper species within Saccharomyces sensu stricto. FEMS Microbiol. Lett. 192: 191–196 [DOI] [PubMed] [Google Scholar]
- 174. Querol A, Bond U. 2009. The complex and dynamic genomes of industrial yeasts. FEMS Microbiol. Lett. 293: 1–10 [DOI] [PubMed] [Google Scholar]
- 175. Rainieri S, et al. 2006. Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl. Environ. Microbiol. 72: 3968–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rainieri S, Kodama Y, Nakao Y, Pulvirenti A, Giudici P. 2008. The inheritance of mtDNA in lager brewing strains. FEMS Yeast Res. 8: 586–596 [DOI] [PubMed] [Google Scholar]
- 177. Rainieri S, Zambonelli C, Hallsworth JE, Pulvirenti A, Giudici P. 1999. Saccharomyces uvarum, a distinct group within Saccharomyces sensu stricto. FEMS Microbiol. Lett. 177: 177–185 [DOI] [PubMed] [Google Scholar]
- 178. Replansky T, Koufopanou V, Greig D, Bell G. 2008. Saccharomyces sensu stricto as a model system for evolution and ecology. Trends Ecol. Evol. 23: 494–501 [DOI] [PubMed] [Google Scholar]
- 179. Rhind N, et al. 2011. Comparative functional genomics of the fission yeasts. Science 332: 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ricchetti M, Fairhead C, Dujon B. 1999. Mitochondrial DNA repairs double-strand breaks in yeast chromosomes. Nature 402: 96–100 [DOI] [PubMed] [Google Scholar]
- 181. Riva M, Buhler JM, Sentenac A, Fromageot P, Hawthorne DC. 1982. Natural variation in yeast RNA polymerase A. Formation of a mosaic RNA polymerase A in a meiotic segregant from an interspecific hybrid. J. Biol. Chem. 257: 4570–4577 [PubMed] [Google Scholar]
- 182. Rodrigues de Miranda L, Appel K, Seyfarth H. 1980. Pichia sorbitophila sp nov. Antonie Van Leeuwenhoek 46: 157–159 [DOI] [PubMed] [Google Scholar]
- 183. Rolland T, Dujon B. 2011. Yeasty clocks: dating genomic changes in yeasts. C. R. Biol. 334: 620–628 [DOI] [PubMed] [Google Scholar]
- 184. Ruderfer D, Pratt S, Seidel H, Kruglyak L. 2006. Population genomic analysis of outcrossing and recombination in yeast. Nat. Genet. 38: 1077–1081 [DOI] [PubMed] [Google Scholar]
- 185. Santos MAS, Gomes AC, Santos MC, Carreto LC, Moura GR. 2011. The genetic code of the fungal CTG clade. C. R. Biol. 334: 607–611 [DOI] [PubMed] [Google Scholar]
- 186. Scannell DR, Byrne KP, Gordon JL, Wong S, Wolfe KH. 2006. Multiple rounds of speciation associated with reciprocal gene loss in polyploid yeasts. Nature 440: 341–345 [DOI] [PubMed] [Google Scholar]
- 187. Scannell DR, et al. 2007. Independent sorting-out of thousands of duplicated gene pairs in two yeast species descended from a whole-genome duplication. Proc. Natl. Acad. Sci. U. S. A. 104: 8397–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Scannell DR, et al. 2011. The awesome power of yeast evolutionary genetics: new genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3 (Bethesda) 1: 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Schacherer J, Shapiro JA, Ruderfer DM, Kruglyak L. 2009. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 458: 342–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Schmitt MJ, Breinig F. 2006. Yeast viral killer toxins: lethality and self-protection. Nat. Rev. Microbiol. 4: 212–221 [DOI] [PubMed] [Google Scholar]
- 191. Schwartz K, Wenger JW, Dunn B, Sherlock G. 2012. APJ1 and GRE3 homologs work in concert to allow growth in xylose in a natural Saccharomyces sensu stricto hybrid yeast. Genetics 191: 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sebastiani F, Barberio C, Casalone E, Cavalieri D, Polsinelli M. 2002. Crosses between Saccharomyces cerevisiae and Saccharomyces bayanus generate fertile hybrids. Res. Microbiol. 153: 53–58 [DOI] [PubMed] [Google Scholar]
- 193. Serra A, Strehaiano P, Taillandier P. 2005. Influence of temperature and pH on Saccharomyces bayanus var. uvarum growth; impact of a wine yeast interspecific hybridization on these parameters. Int. J. Food Microbiol. 104: 257–265 [DOI] [PubMed] [Google Scholar]
- 194. Shpakovski GV, et al. 2000. Functional conservation of RNA polymerase II in fission and budding yeasts. J. Mol. Biol. 295: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 195. Shpakovski GV, Shematorova EK. 1999. Rpc19 and Rpc40, two alpha-like subunits shared by nuclear RNA polymerases I and III, are interchangeable between the fission and budding yeasts. Curr. Genet. 36: 208–214 [DOI] [PubMed] [Google Scholar]
- 196. Sicard D, Legras J-L. 2011. Bread, beer and wine: yeast domestication in the Saccharomyces sensu stricto complex. C. R. Biol. 334: 229–236 [DOI] [PubMed] [Google Scholar]
- 197. Sipiczki M. 1979. Interspecific protoplast fusion in fission yeasts. Curr. Microbiol. 3: 37–40 [Google Scholar]
- 198. Sipiczki M. 2008. Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res. 8: 996–1007 [DOI] [PubMed] [Google Scholar]
- 199. Sipiczki M, Ferenczy L. 1977. Protoplast fusion of Schizosaccharomyces pombe auxotrophic mutants of identical mating-type. Mol. Gen. Genet. 151: 77–81 [DOI] [PubMed] [Google Scholar]
- 200. Solieri L, Antúnez O, Pérez-Ortín JE, Barrio E, Giudici P. 2008. Mitochondrial inheritance and fermentative:oxidative balance in hybrids between Saccharomyces cerevisiae and Saccharomyces uvarum. Yeast 25: 485–500 [DOI] [PubMed] [Google Scholar]
- 201. Solieri L, Cassanelli S, Croce MA, Giudici P. 2008. Genome size and ploidy level: new insights for elucidating relationships in Zygosaccharomyces species. Fungal Genet. Biol. 45: 1582–1590 [DOI] [PubMed] [Google Scholar]
- 202. Souciet J-L, et al. 2009. Comparative genomics of protoploid Saccharomycetaceae. Genome Res. 19: 1696–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Spata L, Weber H. 1980. A study on protoplast fusion and parasexual hybridization of alcane utilizing yeast, p 131–137 In Ferenczy L, Farkas GL. (ed), Proceedings of the 5th International Protoplast Symposium. Pergamon Press, Oxford, United Kingdom [Google Scholar]
- 204. Spencer J, Bizeau C, Reynolds N, Spencer D. 1985. The use of mitochondrial mutants in hybridization of industrial yeast strains. Curr. Genet. 9: 649–652 [Google Scholar]
- 205. Spencer J, Spencer D. 1981. The use of mitochondrial mutants in hybridization of industrial yeasts. Curr. Genet. 4: 177–180 [DOI] [PubMed] [Google Scholar]
- 206. Spencer J, Spencer D, Whittington-Vaughan P, Miller R. 1983. Use of mitochondrial mutants in the isolation of hybrids involving industrial yeast strains. Curr. Genet. 7: 159–164 [DOI] [PubMed] [Google Scholar]
- 207. Spírek M, Horváth A, Piskur J, Sulo P. 2000. Functional co-operation between the nuclei of Saccharomyces cerevisiae and mitochondria from other yeast species. Curr. Genet. 38: 202–207 [DOI] [PubMed] [Google Scholar]
- 208. Sulo P, Spírek M, SoltésovÁ A, Marinoni G, Piskur J. 2003. The efficiency of functional mitochondrial replacement in Saccharomyces species has directional character. FEMS Yeast Res. 4: 97–104 [DOI] [PubMed] [Google Scholar]
- 209. Svoboda A. 1978. Fusion of yeast protoplast induced by polyethylene glycol. J. Gen. Microbiol. 109: 169–175 [Google Scholar]
- 210. Svoboda A. 1980. Intergeneric fusion of yeast protoplast: Saccharomyces cerevisiae + Schizosaccharomyces pombe, p 119–123 In Ferenczy L, Farkas GL. (ed), Proceedings of the 5th International Protoplast Symposium. Pergamon Press, Oxford, United Kingdom [Google Scholar]
- 211. Tamaki H. 1982. Genetic properties of abortive products resulting from the protoplast fusion in yeasts. Mol. Gen. Genet. 187: 177–179 [Google Scholar]
- 212. Tamaki H. 1986. Genetic analysis of intergeneric hybrids obtained by protoplast fusion in yeasts. Curr. Genet. 10: 491–494 [DOI] [PubMed] [Google Scholar]
- 213. Taya M, Honda H, Kobayashi T. 1984. Lactose-utilizing hybrid strain derived from Saccharomyces cerevisiae and Kluyveromyces lactis by protoplast fusion. Agric. Biol. Chem. 48: 2239–2243 [Google Scholar]
- 214. Taylor JW, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31: 21–32 [DOI] [PubMed] [Google Scholar]
- 215. Tirosh I, Reikhav S, Levy AA, Barkai N. 2009. A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324: 659–662 [DOI] [PubMed] [Google Scholar]
- 216. Tronchoni J, Gamero A, Arroyo-López FN, Barrio E, Querol A. 2009. Differences in the glucose and fructose consumption profiles in diverse Saccharomyces wine species and their hybrids during grape juice fermentation. Int. J. Food Microbiol. 134: 237–243 [DOI] [PubMed] [Google Scholar]
- 217. Tsai I, Bensasson D, Burt A, Koufopanou V. 2008. Population genomics of the wild yeast Saccharomyces paradoxus: quantifying the life cycle. Proc. Natl. Acad. Sci. U. S. A. 105: 4957–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Usher J, Bond U. 2009. Recombination between homologous chromosomes of lager yeasts leads to loss of function of the hybrid GPH1 gene. Appl. Environ. Microbiol. 75: 4573–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Van het Hoog M, et al. 2007. Assembly of the Candida albicans genome into sixteen supercontigs aligned on the eight chromosomes. Genome Biol. 8: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Van Solingen P, Plaat J. 1977. Fusion of yeast spheroplasts. J. Bacteriol. 130: 946–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Vaughan-Martini A, Kurtzman C. 1985. Deoxyribonucleic acid relatedness among species of the genus Saccharomyces sensu stricto. Int. J. Syst. Bacteriol. 35: 508–511 [Google Scholar]
- 222. Vaughan-Martini A, Martini A. 1987. Three newly delimited species of Saccharomyces sensu stricto. Antonie Van Leeuwenhoek 53: 77–84 [DOI] [PubMed] [Google Scholar]
- 223. Viviani MA, et al. 2006. Molecular analysis of 311 Cryptococcus neoformans isolates from a 30-month ECMM survey of cryptococcosis in Europe. FEMS Yeast Res. 6: 614–619 [DOI] [PubMed] [Google Scholar]
- 224. Vondrejs V, et al. 1983. The use of a killer factor in the selection of hybrid yeast strains. Folia Biol. 29: 372–384 [PubMed] [Google Scholar]
- 225. Wang S-A, Bai F-Y. 2008. Saccharomyces arboricolus sp. nov., a yeast species from tree bark. Int. J. Syst. Evol. Microbiol. 58: 510–514 [DOI] [PubMed] [Google Scholar]
- 226. Wang Y, Song L, Zhou Y. 1992. Selection of strains capable of utilizing d-xylose and cellobiose to produce ethanol by electric field-induced protoplast fusion. Chin. J. Biotechnol. 8: 51–56 [PubMed] [Google Scholar]
- 227. Wei W, et al. 2007. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc. Natl. Acad. Sci. U. S. A. 104: 12825–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Whittaker P, Leach S. 1978. Interspecific hybrid production between the yeasts Kluyveromyces lactis and Kluyveromyces fragilis by protoplast fusion. FEMS Microbiol. Lett. 4: 31–34 [Google Scholar]
- 229. Witte V, Grossmann B, Emeis CC. 1989. Molecular probes for the detection of Kluyveromyces marxianus chromosomal DNA in electrophoretic karyotypes of intergeneric protoplast fusion products. Arch. Microbiol. 152: 441–446 [DOI] [PubMed] [Google Scholar]
- 230. Wolfe KH, Shields DC. 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387: 708–7139192896 [Google Scholar]
- 231. Xu J, Luo G, Vilgalys RJ, Brandt ME, Mitchell TG. 2002. Multiple origins of hybrid strains of Cryptococcus neoformans with serotype AD. Microbiology 148: 203–212 [DOI] [PubMed] [Google Scholar]
- 232. Xu J, Vilgalys R, Mitchell TG. 2000. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 9: 1471–1481 [DOI] [PubMed] [Google Scholar]
- 233. Xu M, He X. 2011. Genetic incompatibility dampens hybrid fertility more than hybrid viability: yeast as a case study. PLoS One 6: e18341 doi:10.1371/journal.pone.0018341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Yamamoto M, Fukui S. 1977. Fusion of yeast protoplast. Agric. Biol. Chem. 41: 1829–1830 [Google Scholar]
- 235. Yoshida K. 1979. Interspecific and intraspecific mitochondria-induced cytoplasmic transformation in yeasts. Plant Cell Physiol. 20: 851–856 [Google Scholar]
- 236. Yu X, Gabriel A. 1999. Patching broken chromosomes with extranuclear cellular DNA. Mol. Cell 4: 873–881 [DOI] [PubMed] [Google Scholar]
- 237. Zeyl C. 2009. The role of sex in fungal evolution. Curr. Opin. Microbiol. 12: 592–598 [DOI] [PubMed] [Google Scholar]
- 238. Zhang H, Skelton A, Gardner RC, Goddard MR. 2010. Saccharomyces paradoxus and Saccharomyces cerevisiae reside on oak trees in New Zealand: evidence for migration from Europe and interspecies hybrids. FEMS Yeast Res. 10: 941–947 [DOI] [PubMed] [Google Scholar]
- 239. Zill OA, Rine J. 2008. Interspecies variation reveals a conserved repressor of alpha-specific genes in Saccharomyces yeasts. Genes Dev. 22: 1704–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zimmermann M, Hoffmann-Hintz M, Kolvenbach M, Emeis CC. 1988. OFAGE banding patterns of different yeast genera and of intergeneric hybrids. J. Basic Microbiol. 28: 241–247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.