Abstract
Summary: A wide spectrum of pathogenic bacteria and protozoa has adapted to an intracellular life-style, which presents several advantages, including accessibility to host cell metabolites and protection from the host immune system. Intracellular pathogens have developed strategies to enter and exit their host cells while optimizing survival and replication, progression through the life cycle, and transmission. Over the last decades, research has focused primarily on entry, while the exit process has suffered from neglect. However, pathogen exit is of fundamental importance because of its intimate association with dissemination, transmission, and inflammation. Hence, to fully understand virulence mechanisms of intracellular pathogens at cellular and systemic levels, it is essential to consider exit mechanisms to be a key step in infection. Exit from the host cell was initially viewed as a passive process, driven mainly by physical stress as a consequence of the explosive replication of the pathogen. It is now recognized as a complex, strategic process termed “egress,” which is just as well orchestrated and temporally defined as entry into the host and relies on a dynamic interplay between host and pathogen factors. This review compares egress strategies of bacteria, pathogenic yeast, and kinetoplastid and apicomplexan parasites. Emphasis is given to recent advances in the biology of egress in mycobacteria and apicomplexans.
INTRODUCTION
Intracellular pathogens display considerable differences in host cell preference, cell entry mechanisms, the establishment of the intracellular niche, and modes of replication. As a consequence, each pathogen resides in a particular environment, which, at the time of egress, determines specific requirements for a successful exit strategy. However, despite evolutionary adaptation to different breeding niches, similar egress strategies are used by even distantly related pathogens.
The subcellular localization of the pathogen within the host cell is a determining factor for egress. In most cases, entry into the host cell results in the pathogen, at least transiently, being enclosed in a vacuole within the host cell cytoplasm. One of the major hurdles that intracellular pathogens face is the progressive acidification of their vacuolar compartment, resulting from fusion with endolysosomes. With a few exceptions, such as the bacterium Coxiella and the kinetoplastid Leishmania, which are immune to harsh conditions and can replicate within this hostile environment, most pathogens evolved elegant strategies to evade this aspect of cell-intrinsic immunity. Apicomplexan parasites induce the formation of a nonfusogenic parasitophorous vacuole (PV). The pathogenic mycobacteria Mycobacterium tuberculosis and Mycobacterium marinum as well as Chlamydia, Salmonella, and Legionella manipulate the course of phagosomal maturation. All these pathogens establish a replication niche within the vacuole and may exit from the vacuole and from the host cell in a single step or in two steps that can be temporally spaced or happen in rapid succession. Other pathogens, such as Listeria monocytogenes, Shigella flexneri, and Trypanosoma cruzi, need to escape their vacuole and instead replicate in the host cell cytosol. This vacuolar escape can be considered the first step of egress, which needs to be perfectly controlled in order to lyse the vacuole but preserve host cell integrity. After replication, a second egress event then leads to the release of the progeny from the host cell. Importantly, both steps need to be individually regulated. This illustrates that the completion of replication must play a central role in triggering egress for vacuolar as well as cytosolic pathogens. The timing is likely controlled by intrinsic cues to optimize the number of progeny to be released and to ensure that the replication and maturation of the transmission forms have been completed.
Many but not all pathogens have coevolved with their hosts to achieve a state of latent infection with a limited impact on the host. The egress of T. cruzi trypomastigotes from the host cell is inhibited by one or more antibodies in sera of mice and humans, collectively termed antiegressin (39, 97). Interestingly, the generation of this activity during infection in mice coincides temporally with a decrease in parasitemia and the transition from the acute phase to the chronic phase (98). This indicates that the control of parasite egress by the host adaptive immune response might contribute to the establishment of a chronic infection.
Egress strategies have been designed to overcome one or more cellular membranes, the host cell cytoskeleton, and organelles. Proteases, lipases, and pore-forming proteins (PFPs) have been identified in various intracellular pathogens as molecular effectors of active egress. Proteases can degrade integral membrane proteins and host cell cytoskeletal elements or may contribute to egress by controlling the activation of additional factors. The digestion of membrane lipids by lipases can contribute to membrane disruption. Alternatively, lipases can also play a role in signaling leading to egress (102). PFPs are able to disrupt membrane integrity and to induce host cell death, and some, such as listeriolysin and leishporin, have the ability to polymerize into pores of increasing size dependent on time and/or monomer concentrations (103, 128). Such changes may determine whether these proteins are able to disrupt a membrane directly or mediate the release of effector molecules involved in this task. Many PFPs need to be activated (7, 63, 148), and insertion into the membrane depends on the recognition of membrane-associated lipids or proteins (reviewed in reference 119).
In addition, pathogens are known to use molecular mimicry to hijack host cellular functions, including host cytoskeleton dynamics (28, 36). These factors can induce actin cortex reorganization, F-actin polymerization for self-propulsion, and membrane fusion or fission mechanisms to achieve egress. In addition to these molecular effectors, extended rounds of microbial replication, exflagellation, and motility can exert considerable mechanical stress on membranes. Although mechanical stress alone does not seem to be sufficient to bring about egress in most organisms described here, it still remains an important contributor. Cumulative evidence suggests that a combination of factors operates in diverse strategies for egress from the host cell (Table 1).
Table 1.
Overview of egress strategiesa
| Organism | Pathogen | Category(ies) of egress strategy(ies) (reference[s]) |
|||
|---|---|---|---|---|---|
| No vacuole or cell lysis | Vacuole lysis and escape to the cytosol | Nonlytic egress from the cytosol | Vacuole and host cell lysis | ||
| Bacteria | Legionella | Exocytosis, LepA, LepB (35, 36) | Pyroptosis, flagellin, T4SS Dot/Icm, Nlrc4 (131) | ||
| Listeria | LLO, the phospholipases PI-PLC and PC-PLC, Mpl (5, 64, 68, 114, 127, 133) | Protrusion, actin tails, ActA, ezrin, CD44, Arp2/3 (25, 113) | Pyroptosis (61) | ||
| Porphyromonas | Exocytosis (142) | Protrusion (158) | |||
| Brucella | Autophagy related, Atg1, Atg6 (138) | ||||
| Francisella | Autophagy related? (34) | Lysis (124) | Pyroptosis (123, 125) | ||
| Mycobacteria | Autophagy related (37) | RD1 locus, T7SS ESX1 (43, 132, 136, 151) | Protrusion, actin tails, WASP, Arp2/3 (136, 137); ejection, RD1 locus, T7SS ESX1, actin, RacH (69); apoptosis | Necrosis, T7SS ESX1, Nlrp3 (42, 45, 155) | |
| Shigella | T3SS (76) | Protrusion, actin tails (76) | Pyroptosis, IpaB (61) | ||
| Salmonella | T3SS, SifA, PipB2, kinesin-1 (19, 24, 73) | Direct cell-to-cell spread (83, 141), flagellum dependent (121); apoptosis (154) | Pyroptosis, SipB, Nalp1 (57, 58, 84, 154); necrosis (154) | ||
| Chlamydia | Extrusion, actin (77) | Protease dependent (15, 77) | Protease dependent (15, 77) | ||
| Parasites | Plasmodium liver stage | Protease dependent, PbLISP1 (79, 126, 139, 140) | Budding and merosome formation (14, 66, 139, 140) | ||
| Plasmodium blood stage | Proteases and osmotic stress, (exoneme), PfSUB1, DPAP3, SERAs, PfCDPK5 (13, 23, 49, 65, 157) | Proteases and osmotic stress, calpains (23, 33, 65) | |||
| Plasmodium gametocyte stage | Proteases, (osmiophilic bodies), Pfg377, PbGEST, PbPEG3/MDV-1 (44, 112,135, 143) | Protease dependent (135) | |||
| Plasmodium mosquito stage | PbECP1, PbSIAP1, motility (10, 55) | ||||
| Toxoplasma tachyzoite stage | PFP mediated, (microneme), TgPLP1, motility (81) | PFP mediated, TgPLP1, motility, calpains (33, 81, 95, 111) | |||
| Toxoplasma bradyzoite stage | Protrusion; gliding motility (50) | ||||
| Trypanosoma | PFP mediated, TcTOX, TcLYT1 (12, 90, 93, 99) | Protrusion; motility, proteases (11, 40, 159) | |||
| Leishmania | Apoptosis (116) | ||||
| Yeast | Cryptococcus | Expulsion (9, 80, 92) | Lysis (150) | Direct cell-to-cell spread (8, 91) | Lysis (150) |
| Fungi | Candida | Outgrowth, Eed1 (87, 160) | |||
This table presents a synthetic overview of the egress strategies of pathogenic organisms described in this review. The rows indicate the organisms, while the columns present the various egress strategies. The mechanisms of egress, such as exocytosis or necrosis, are listed when known, as are the host and pathogen factors involved. Further information about abbreviations is given in the text or in the references indicated in the table.
Egress strategies can be destructive or nondestructive for the host cell. Egress has an important impact on the host organism, since any tissue damage will trigger the host's inflammatory response, resulting in the recruitment of immune cells to the site of infection. Despite this major drawback, numerous intracellular pathogens have opted for a lytic egress strategy, and many have been demonstrated to nevertheless subvert and modulate the inflammatory response. Detrimental damage can lead to a necrotic, apoptotic, or pyroptotic type of cell death. Necrosis is characterized by the breakdown of membranes, which releases intracellular pathogens. In contrast, apoptosis is a tightly controlled suicide that does not provoke inflammation, keeping the host plasma membrane intact with the pathogen enclosed. Compared to apoptosis, programmed cell death by pyroptosis involves different signaling pathways, triggers an inflammatory response, and culminates in cell lysis (48). Several nonlytic egress strategies are known to inflict damage on the host cell, but plasma membrane damage can be kept under control, for example, by housekeeping membrane repair mechanisms, such as lysosomal fusion, constriction, or sealing (78). Only a few strategies allow the pathogen to leave without any physical damage. In this case, the pathogen usually redirects itself into a constitutive or housekeeping exit process such as exocytosis. Examples of lytic and nonlytic exit routes exist for intravacuolar and cytosolic pathogens. Some pathogens are versatile in their egress strategies, such as Francisella, mycobacteria, Chlamydia, and Listeria. For example, Listeria cells commonly spread by a nonlytic protrusion mechanism from one cell to another (29) but can also induce necrotic death and thereby spread through the infected organism. Lytic and nonlytic strategies also exist for Cryptococcus; however, the determinants dictating the exit route remain unclear (9).
Overall, our understanding of host cell egress and cell-to-cell transmission by intracellular pathogens is still limited. The inherent difficulties in the cultivation and manipulation of many microbes account for these gaps. In addition, the complexity of species-specific life cycles comprising various stages in different host organisms makes it necessary to examine each stage individually. The identification and characterization of novel egress strategies continue, and some exotic mechanisms, such as the egress of microsporidia from intestinal cells in Caenorhabditis elegans (149), might be more widespread than assumed. In the following sections, the various exit strategies known to date and examples from the recent literature are presented in order of increasing damage inflicted to the host cell, from nonlytic to death-inflicting processes. A schematic overview of the various egress strategies is presented in Fig. 1.
Fig 1.
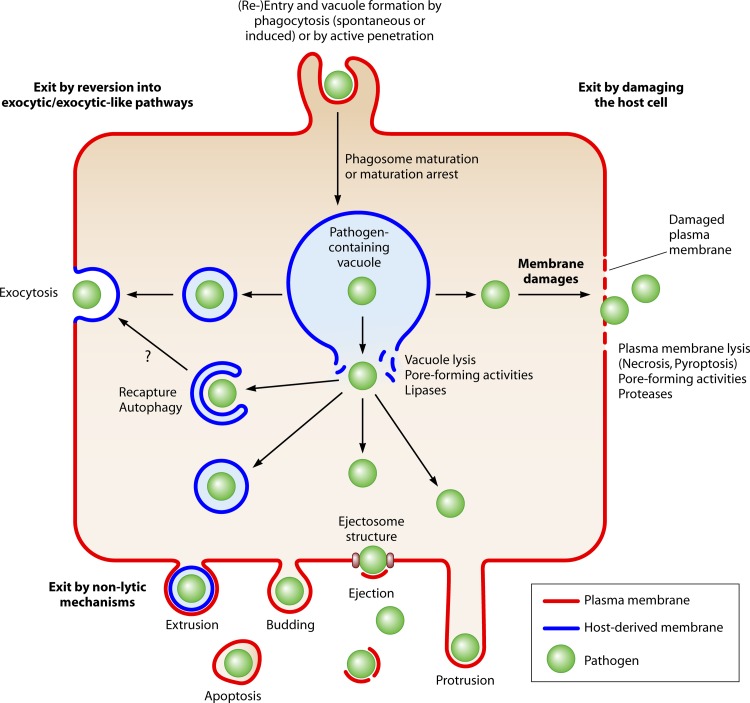
Egress strategies. Various strategies for egress from a host cell are depicted. After uptake, pathogens are present in a membranous compartment. The pathogens can then either egress from the cell while remaining within a vacuole, e.g., by reverting the fate of the compartment to an exocytic or an exocytic-like pathway (left side) (egress without membrane damage) or leave the vacuole and translocate into the cytosol (vacuole lytic activity). Cytosolic pathogens have been described to leave the host cell in both nonlytic (e.g., protrusion, ejection, and apoptosis) and lytic (necrosis and pyroptosis) manners by disrupting the plasma membrane. In addition, cytosolic pathogens have been shown to be recaptured into membranous compartments and enter the autophagic pathway, which in turn allows egress via an exocytic-like route.
EGRESS STRATEGIES WITH NEITHER VACUOLE LYSIS NOR CELL LYSIS
Exocytosis and Autophagy-Related Mechanisms
Arguably, the simplest mechanistic exit strategy for an intracellular pathogen is to remain in a vacuole during replication and induce the fusion of that vacuole with the host plasma membrane when the time has come for transmission. This strategy has the advantage of perfectly preserving the host cell. For example, Legionella can reemerge from cells of its primary amoeba hosts, Dictyostelium discoideum and Acanthamoeba castellani, via exocytosis (35, 36). Two pathogen factors, LepA and LepB, were identified, which, based on structural similarities, could mimic the SNARE function and steer the pathogen into the exocytic pathway. In the case of the common periodontal pathogen Porphyromonas gingivalis, the endocytic recycling pathway leads to the exocytosis of bacteria from epithelial cells (142). However, depending on the host cell type, P. gingivalis not only localizes to endosomes but also may be found in the cytosol or autophagosomes. Therefore, the authors of that study suggested that other exit mechanisms are likely to exist. Indeed, another group proposed the existence of a protrusion-like strategy without the exposure of the pathogen to the extracellular milieu (158).
The facultative intracellular pathogenic yeast Cryptococcus neoformans proliferates inside the phagosome of macrophages and monocytes. The exit of progeny from phagolysosomes has been described to occur by either lytic egress or a nonlytic exocytosis-like expulsion or “vomocytosis” mechanism. Whereas lytic egress appears to be the result of intracellular replication and is preceded by the permeabilization of the phagosomal membrane (150), expulsion seems to depend on a yeast factor, indicating that it might be an active process (9, 92). Those studies also noted an inhibitory effect of the host cell actin. More recently, actin flashes were observed around the phagosome prior to expulsion (80), likely in coordination with a WASH-mediated V-ATPase retrieval mechanism (31). Indeed, the Arp2/3 activator WASH localizes to Dictyostelium endosomes and phagosomes during the reneutralization phase that follows the (phago)lysosomal acidic stage and leads to a near-neutral postlysosomal compartment. WASH-driven actin polymerization was shown to be necessary for V-ATPase retrieval (31). However, whether the actin flashes play a direct role in the Cryptococcus exit mechanism remains to be defined. In addition, a direct cell-to-cell spreading mechanism between macrophages without an exposure of the pathogen to the extracellular milieu was proposed, but its details and significance remain unclear (8, 91).
A particular way to use an exocytosis-like mechanism was recently reported for Brucella and may also apply to Francisella, which might exploit an autophagy-like process to gain access to the cell exterior and reinvade naïve bystanders (34, 138). Interestingly, this newly revealed strategy has gained interest at the same time as another (maybe distinct or not) autophagy-related pathway has been implicated in unconventional protein secretion (26, 110). In both cases, cytoplasmic (including vacuolar and cytosolic) bacteria and proteins are sequestered in a membrane compartment that fuses with the plasma membrane and releases them outside.
Brucella usually spends much energy and a good share of its virulence program to establish a replication niche that acquires most features of the endoplasmic reticulum (ER) (153). The steps from entry to the establishment of this replication compartment are well understood, but how Brucella finally escapes its primary host cell at later stages and spreads infection has long been a neglected field of study. Recently, the transformation of the Brucella-containing vacuole into an autophagosome-like compartment was described, and it was revealed that this strategy somehow contributes to dissemination (138). Indeed, the formation of infection foci in epithelial cell monolayers was strongly promoted by the capacity of Brucella to stimulate the generation of that late autophagy-dependent compartment. Strikingly, in a way similar to the recapture of cytosolic M. marinum mentioned below, this mechanism also seems to be independent of Atg5, although other classical autophagy factors, such as BECLIN1 (Atg6) and ULK1 (Atg1), are necessary (138).
In the case of Francisella, it is well documented that the bacterium first escapes its vacuole, a phagosome with features of a late endosomal compartment, within the first 30 min of infection (124). It then multiplies in the cytosol of its host macrophage, of mammalian or arthropod origin (122), and is able to trigger pyroptosis and cell lysis, possibly as a primary strategy for dissemination (123, 125). However, at least in murine macrophages, it is subsequently taken up again into a membranous compartment by autophagy. In principle, from this compartment, Francisella could reemerge from the host cell by a mechanisms related to exocytosis and start a new cycle of infection. For unknown reasons, this pathway does not seem to prevail in arthropod phagocytic cells (4). A possible similar recapture mechanism was reported for cytosolic M. marinum (see “Vacuole Lysis and Escape to the Cytosol” for a detailed presentation of mycobacterial vacuole escape). That pathway was described to be similar to autophagy and leads to the sequestration of ubiquitinated M. marinum in a vacuolar compartment by a process independent of Atg5 (37). The dependence of the recapture of Francisella on Atg5 has not yet been reported, despite intense study of the cytosolic host factors that modulate its fate in the cytosol (4). These strategies and host species-specific differences acutely highlight the difficulties of classifying intracellular bacteria dogmatically into vacuolar or cytosolic pathogens.
Extrusion from the Cell Surface
Chlamydia replicates within a membrane-bound inclusion, from where the bacteria escape by two mutually exclusive strategies. Whereas lytic egress had been described previously, Chlamydia is also known to use an extrusion mechanism at an almost equal frequency (77). Lytic egress proceeds by a temporally well-defined two-step process (15, 77). The lysis of the inclusion vacuole is a cysteine protease-dependent event, but the factor responsible remains unknown. Subsequently, the host cell plasma membrane ruptures, triggering the influx of extracellular calcium and the release of cytosolic bacteria. To prevent complete lysis, the host cell responds with the activation of the lysosome fusion-mediated plasma membrane repair mechanism (15). In contrast, extrusion is a nonlytic process in which protruding Chlamydia inclusions are partially pinched off from the host cell in an actin-dependent manner. The resulting extracellular inclusion bodies are surrounded by the host cell cytosol and the host plasma membrane. Both the host cell and the residual inclusion remain intact (77), which might play an important role in the persistence of infection.
VACUOLE LYSIS AND ESCAPE TO THE CYTOSOL
Vacuole lysis can be part of an exit strategy that is either nonlytic or lytic for the host cell (see Strategies for Nonlytic Egress from the Cytosol as well as Egress Strategies Involving Lysis of the Vacuole and Host Cell). Controlled vacuolar escape is crucial for pathogens replicating in the cytosol but can also be necessary for intravacuolar progeny to pursue a particular egress strategy (for example, the budding of Plasmodium liver-stage parasites). Shigella replicates in the cytosol, and vacuolar escape depends on a functional type III secretion system (T3SS) along with its translocon (105). While the exact mechanism of vacuolar lysis remains unknown, this event is accompanied by the recruitment of cytosolic galectins, possibly to glycosylations decorating the exposed bacteria but surely to membranes of the ruptured phagosome (105, 115, 144). This phenomenon has been observed not only for Gram-negative Shigella and Salmonella spp. but also for the Gram-positive bacterium Listeria monocytogenes as well as during sterile damage and therefore appears to be a generic cytosolic defense response to phagosomal damage (105, 144). In particular, galectin-8 is able to restrict Salmonella proliferation by binding to specific carbohydrates on the inner leaflet of the breaking vacuole, leading to the activation of antibacterial autophagy (144).
In contrast to Shigella, Salmonella usually replicates within the vacuole. Salmonella secretes factors similar to those secreted by Shigella via its T3SS but in addition releases SifA and PipB2, which interfere with kinesin-mediated vacuolar dynamics, delay vacuolar lysis, and, hence, ensure intravacuolar replication (24, 73). A Salmonella sifA mutant rapidly becomes cytosolic, and its virulence is strongly attenuated in mice (19). While vacuolar lysis most probably occurs during lytic egress (see Egress Strategies Involving Lysis of the Vacuole and Host Cell), it might not be required for a mechanism of direct cell-to-cell transfer, relying on the kinesin-dependent repositioning of the Salmonella-containing vacuole from the juxtanuclear region to the periphery (83, 141).
Listeria secretes the well-characterized PFP listeriolysin O (LLO). LLO, like T. cruzi TcTOX, is nonfunctional in the pH-neutral cytosol but is activated at a low pH (12, 93, 127), as occurs in the course of phagosome maturation. The pH dependence confines these molecules to a specific role in pathogen escape from the vacuole into the host cell cytosol while keeping the host plasma membrane intact (90). The dysregulation of LLO activity impacts the virulence of Listeria in mice (64). LLO activity might also be affected by a perturbation of host cell ion homeostasis, which inhibits phagosomal escape and also results in decreased virulence (114). Listeria resides in single-membrane primary vacuoles when taken up by professional or nonprofessional phagocytic cells or in double-membrane secondary vacuoles when resulting from cell-to-cell spread by protrusion. Although not required in several human epithelial cell lines, LLO appears to be essential for vacuolar escape in most other cell types by acting on primary vacuoles and the outer membrane of secondary vacuoles (5). The early secretion of LLO after the uptake of bacteria by macrophages leads to the permeabilization of the primary vacuole, the breakdown of ion gradients, and the inhibition of phagolysosome fusion, preserving an environment from which the bacteria can escape efficiently (72, 128). Membrane disruption by LLO is assisted by two phospholipases (phosphatidylinositol-phospholipase C [PI-PLC] and phosphatidylcholine-phospholipase C [PC-PLC]). PC-PLC is activated through proteolytic cleavage by the bacterial acid-dependent metalloprotease Mpl, which also cleaves the actin nucleator ActA that is necessary for protrusion (see Strategies for Nonlytic Egress from the Cytosol) and could be responsible for delaying bacterial motility in the cytoplasm (94, 117). The two lipases have overlapping functions in the dissolution of primary and secondary vacuoles and appear to be particularly important for the disruption of the inner membrane of secondary vacuoles (5, 133). Moreover, in epithelial cells, where LLO expression is not required, PC-PLC activity was shown to be necessary for the lysis of these single- and double-membrane compartments (68).
Mycobacteria from the tuberculosis group are classically considered vacuolar intracellular pathogens, but recent evidence has seriously challenged this view. The first blow to this dogma came from studies of M. marinum, the closest relative of the tuberculosis group, a pathogen responsible for fish tuberculosis and for opportunistic skin infections of humans. A mycobacterium was observed to escape from its vacuole to the cytosol of its host macrophage and even to move around the cytoplasm, powered by an actin tail, much like Listeria and Shigella (136). Further work demonstrated the involvement of the Arp2/3 complex in this motility, and this mechanism was also proposed to play a role in the spread of infection (see “Protrusion at the Plasma Membrane”). A potential but still hotly debated breakthrough was reported in studies of M. tuberculosis and Mycobacterium leprae in myeloid cells. Careful morphometric quantitations indeed revealed that a sizable fraction of these mycobacteria can escape their compartment and invade the cytosol of their hosts (151). Interestingly, the attenuated vaccine strain Mycobacterium bovis BCG was apparently incapacitated in this vacuole escape, raising speculations about the role of cytosolic mycobacteria in the immune response mounted during various stages of a tuberculosis infection. The RD1 locus is the major region of difference between virulent M. tuberculosis and BCG (75) and was shown to encode the type VII secretion system ESX1 as well as its secreted factors, such as early secreted antigen target 6 (ESAT-6) (reviewed in reference 1). The ESX1 machinery localizes to and secretes at the new poles of growing bacteria (30). It was hypothesized that ESAT-6 plays a key role in vacuole escape, as it was shown to possess membranolytic activity (43) and induces membrane pores of approximately 4.5 nm in diameter in a contact-dependent manner (134). The perforation of and exit from vacuoles by M. marinum and M. tuberculosis were recently confirmed by a very elegant experimental strategy (132). Using an approach similar to the one already validated for Shigella (115), those authors monitored the exposure of a fluorescence resonance energy transfer (FRET) substrate loaded into the cytosol of the host to M. tuberculosis cell wall-associated β-lactamase activity. That study also confirmed the requirement for a functional ESX1 secretion system for vacuole escape.
The genetic dissection of host and pathogen factors involved in vacuolar escape and egress from the host cell made rapid progress thanks to the establishment of a mycobacterium-host system in an alternative model organism, Dictyostelium discoideum. This social amoeba naturally grazes on soil bacteria by efficient phagocytosis; it interacts with a large variety of bacteria and fungi and has developed defense processes to survive in such complex environments, which are precursors to the cell-intrinsic resistance mechanisms further refined in phagocytes of the innate immune system (38). As described above for macrophages, within Dictyostelium, M. marinum also modifies the composition of the phagosome. The association of M. marinum with the vacuolar H+-ATPase is decreased compared to that of nonpathogenic mycobacteria and is practically undetectable at 6 h postinfection (70). Furthermore, during the ensuing proliferation phase, M. marinum avoids the delivery of cathepsin D, a lysosomal protease, to the phagosomal lumen. Surprisingly, Dictyostelium vacuolin (a flotillin homologue and a raft and late phagolysosomal marker) strongly accumulates around the niche, making it possible to observe and quantitate the dynamics of escape from the vacuole. Even more striking, upon infection of Dictyostelium, M. tuberculosis was also able to escape to the cytosol to a certain extent. That study not only confirmed the evolutionary conservation of tubercular mycobacterium virulence mechanisms and host processes but also elaborated the crucial role of ESAT-6. Indeed, M. marinum RD1 mutants (equivalent to the BCG strain) showed a greatly reduced vacuole escape efficiency, which was partially rescued by trans-complementation, namely, the expression of the bacterial virulence factor directly in the cytosol of the host Dictyostelium (69).
As mentioned above, the vacuolar escape of T. cruzi depends on the PFP TcTOX. While the molecular identity of TcTOX remains unknown, TcLYT1, a protein with similar characteristics and suspected to be identical to TcTOX, appears to contribute to the hemolytic activity. A TcLYT1−/− strain showed diminished infectivity in cell cultures (93) and attenuated virulence in the mouse model (159). The vacuolar escape of T. cruzi may depend on the differentiation of metacyclic trypomastigotes into amastigotes (93, 99), as hemolytic activity has been detected for only some developmental stages. A trans-sialidase localized on the surface of T. cruzi trypomastigotes facilitates exit from the PV (120), but it remains uncertain if and how this relates to the action of TcTOX. The sialidase catalyzes the transfer of host cell sialic acid residues onto mucin-like proteins on the parasite surface. Parasites exit from sialic acid-deficient CHO cells earlier, suggesting that sialic acid inside the PV might constitute a barrier for the parasite.
STRATEGIES FOR NONLYTIC EGRESS FROM THE CYTOSOL
Protrusion at the Plasma Membrane
Protrusion mechanisms play an important role in the egress of prokaryotic intracellular pathogens (28, 76). Protrusion induced by Listeria and Shigella has been extensively described and relies on the polymerization of host cell actin on the bacterial surface. For example, cytosolic Listeria uses its factor ActA to stimulate F-actin nucleation by mimicking the host WASP family proteins (25). Through the formation of actin tails, the bacteria move and induce filopodia extending from the host plasma membrane. Filopodia contain the bacterium at their tip and are engulfed by neighboring cells. This transmission process does not seem to involve the death of the initially infected cell (62). Actin filaments in filopodia are relatively stable (117). The formation of the protrusion and effective spreading rely on the ezrin-radixin-moesin (ERM) family of proteins, which link the actin cytoskeleton to transmembrane proteins, and in particular, ezrin and CD44 were shown to be important (113). A similar transmission process was proposed for the fish and frog pathogen M. marinum in macrophages. The motility of cytosolic M. marinum by WASP-induced actin polymerization has been described (136, 137); however, M. marinum has not yet been observed in double-membrane compartments, which would result from such a transmission strategy.
Despite several eukaryotic pathogens carrying their own machinery for motility, there is so far surprisingly little documentation of protrusion mechanisms for eukaryotic pathogens. One exception might be Toxoplasma gondii bradyzoites, the parasite form characteristic of chronic infections, contrasting with the lytic egress of acute-stage T. gondii tachyzoites (see “Concerted Egress from the Vacuole and the Host Cell”). Visual observations suggested that bradyzoites use a nonlytic strategy reminiscent of protrusion mechanisms involving the parasites' gliding motility, allowing the invasion of neighboring cells without exposure to the external medium (50).
Another way of exploiting cytoskeleton dynamics to spread from cell to cell is the stabilization of direct, membranous, and F-actin-containing connections between an infected and a noninfected target cell. Physical connections, such as nanotubes or filopodium bridges, are used by bacteria (3, 104, 158) to cross a distance to neighboring cells. Conceptually, this transfer can occur intracellularly, within the tubes in a one-dimensional diffusion model; however, its mechanism is not yet understood.
Ejection at the Plasma Membrane
Apart from cell death-mediated mechanisms (see “Apoptosis as a Nonlytic Exit Strategy” and “Unconcerted Egress from the Vacuole and the Host Cell”), there is evidence for an alternative strategy of intercellular spreading for cytosolic M. marinum and M. tuberculosis in epithelial cell layers (27, 32) as well as in amoebae (69). RD1, the major virulence locus of pathogenic mycobacteria that is absent in attenuated strain M. bovis BCG, has been implicated in cell lysis, egress, and dissemination (42, 75, 82, 152). The exact mechanism behind this plethora of effects is unclear, but effectors associated with the ESX1 secretion system are secreted at the bacterial poles (30) and might be ideally positioned to initiate an egress strategy when they contact the host cell cortex. This view is supported by observations of M. marinum escape from the amoeba Dictyostelium. In this system, the nonlytic ejection of cytosolic bacteria across the host plasma membrane was observed to take place via a structure of dense F-actin, termed the ejectosome (69). A bacterial mutant lacking the RD1 locus was not able to escape from the host cell, and this inhibition could be partially trans-complemented by expressing ESAT-6 directly in the host cell cytosol (69). Interestingly, a host mutant lacking the small GTPase RacH was also deficient in forming the ejectosome.
It remains to be shown whether other pathogenic mycobacteria use an ejectosome-based egress strategy in addition to cell death-based mechanisms. However, because virulent M. tuberculosis and M. leprae strains, but not the avirulent strain M. bovis BCG, can translocate into the cytosol for replication like M. marinum (151), at least under some conditions, we speculate that transmission will have to involve some form of protrusion or ejection. Sustaining this hypothesis, M. marinum was observed to induce long filopodium-like structures reminiscent of Listeria-induced protrusions in infected macrophages. These structures were observed to serve cell-to-cell transmission between macrophages in vitro (137) and inside infected zebrafish at early stages of infection (41). Recently, microsporidian obligate intracellular parasites have been shown to follow an actin-dependent nonlytic egress strategy to exit intestinal epithelial cells of the worm Caenorhabditis elegans (149). While the details of the egress process are not fully clarified, it appears to be preceded by the remodeling of a dense layer of actin filaments, followed by the egress itself, which is nonlytic and does not appear to damage the cells (55a).
Budding from the Plasma Membrane
Plasmodium sporozoites invade hepatocytes, where thousands of exoerythrocytic merozoites are generated to initiate the blood-stage cycle. Merozoites egress from the vacuole and from the host cell in two temporally distinct steps (Fig. 2). The rupture of the parasitophorous vacuolar membrane (PVM) occurs first (14, 66). In vitro studies indicated that PVM permeabilization is protease dependent (126, 139, 140). In addition, Plasmodium berghei liver-stage protein 1 (PbLISP1) plays an important role in the breakdown of the PVM. LISP1 localizes to the PVM, but its exact function remains unknown. LISP1-deficient parasites remain trapped inside hepatocytes, and infected mice display decreased levels of blood parasitemia (79). Intravital imaging of the rodent parasite P. berghei revealed that merozoites are subsequently released from the cytosol into hepatic blood vessels (sinusoids) by a budding mechanism (139). The released vesicles have been termed merosomes and are estimated to contain up to thousands of merozoites as well as host cell organelles and undefined vesicular structures (14). The merosome membrane is derived from the host cell (66). During this process, the parasite induces host cell death different from classical apoptosis or necrosis, leading to the detachment of the hepatocyte from neighboring cells (66). Remarkably, the asymmetric distribution of phosphatidylserine, a hallmark of viable cells, is maintained at the surrounding membrane of the hepatocyte and merosomes (14, 139). While the remnants of the hepatocyte are eventually phagocytosed by liver-resident macrophages (Kupffer cells), the capacity to delay the exposure of phosphatidylserine helps merosomes to evade this fate. Infectious merozoites are finally released from the merosomes in the pulmonary microvasculature, as was shown for Plasmodium yoelii in mice (14).
Fig 2.
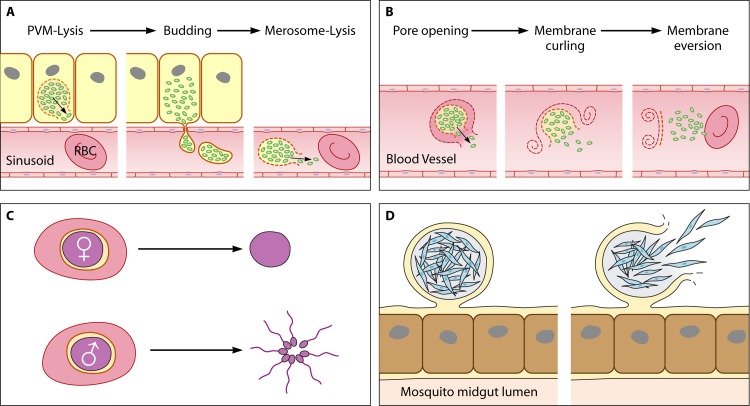
Life cycle stage-specific egress strategies for Plasmodium. The complex life cycle of the malaria parasite comprises four stages requiring egress. (A) First, hepatocytes release merozoites (green) to initiate the blood stage. This occurs by an unconcerted egress strategy involving parasitophorous vacuolar membrane (PVM) lysis, the release of merosomes into the bloodstream by budding, and, finally, merosome lysis. (B) Second, merozoites proliferate in a lytic cycle in red blood cells (RBCs). The lysis of the PVM and the destabilization of the RBC cytoskeleton culminates in a rapid succession of events, namely, pore formation, the curling of the RBC membrane, and the projection of the progeny by the buckling/eversion of the same membrane into the bloodstream. (C) Third, gametocytes (purple) inside RBCs are taken up by a mosquito and egress into the midgut for fertilization. Egress results in the release of one female macrogamete or eight flagellated male microgametes. (D) Fourth, sporozoites (blue) escape from the oocyst in the mosquito midgut epithelium in order to migrate to the salivary glands. The oocyst stage is the only replicative phase of the parasite that does not take place within a host cell. Instead, the cyst is surrounded by an inner layer of parasite origin and by an outer layer derived from the basal lamina. Interestingly, apart from the lytic cycle in RBCs, all other egress events are preceded by the differentiation of the parasite into a new form specifically adapted to meet the respective requirements for successful egress and to initiate the next stage of the life cycle.
Apoptosis as a Nonlytic Dissemination Strategy
Most, if not all, intracellular pathogens modulate host cell death responses in order to counteract these innate defense mechanisms and establish themselves a replication niche. Aspects relating to the persistence of an infection will not be discussed here. However, some pathogens are able to exploit cell death pathways to facilitate spreading.
The modulation of host macrophage death by mycobacteria is now recognized as an important determinant for infection (reviewed in reference 16). It appears that, while macrophages infected with nonvirulent M. bovis BCG undergo apoptosis, virulent M. tuberculosis strains can induce necrosis (see “Unconcerted Egress from the Vacuole and the Host Cell”). The potential role of the ESX1 secretion system, the ESAT-6 membranolytic activity, and the strategy for escape from the vacuole in this important difference is currently being studied intensively. Nevertheless, the live imaging of M. marinum-infected, translucent zebrafish by time-lapse fluorescence microscopy suggested an active role for intragranuloma apoptotic events in the spread of infection by virulent M. marinum (42). Cell death attracted macrophages, which engulfed bacteria, spreading infection and inducing new granulomatous structures. Granulomas can persist over long periods of time and have traditionally been associated with the containment of infection in mammals. Interestingly, M. avium, which is classified as a ubiquitous nontuberculous mycobacterium that can cause pulmonary diseases in immunocompromised individuals, was reported to use apoptotic macrophages for spread (51). While some M. avium strains appear to use the apoptotic bodies as a vehicle to infect naïve macrophages that engulf them, others seem to be able to lyse both the vacuole and the apoptotic body to reinfect neighboring macrophages. Therefore, apoptosis can act as an innate defense mechanism against mycobacteria but might play different roles in different hosts.
EGRESS STRATEGIES INVOLVING LYSIS OF THE VACUOLE AND HOST CELL
Vacuolar lysis and host cell lysis often take place simultaneously or in rapid succession, but there are also examples where they are spaced in time. According to this difference in the timing of the two steps, we distinguish concerted from unconcerted lytic egress. Unconcerted egress can, for example, result from pathogen replication in the cytoplasm or from mechanistically different strategies for lysing the vacuole and the host cell. In principle, a pathogen could also trigger host cell lysis without previously exiting the vacuole. However, to our knowledge, there is no example of such an egress strategy to date.
Perhaps the most striking example for a (superficially) “uncontrolled” egress strategy is the one used by the dimorphic fungus Candida albicans to exit from mucosal epithelial cells. Microscopic observations suggested that the simple physical penetration of membrane barriers driven by outgrowth might result in “egress” and the spread of infection. The fungus' ability to undergo a morphological switch from spherical yeast to filamentous hyphae leads to high mechanical stress on the host cell and has been linked to its virulence (87). A Δeed1 (epithelial escape and dissemination) mutant incapable of hyphal formation in response to a diverse range of stimuli is strongly attenuated in causing the tissue damages characteristic of advanced infections (160). In reconstituted human oral epithelial cells, spherical forms of this strain were endocytosed and proliferated within cells but remained trapped inside.
Unconcerted Egress from the Vacuole and the Host Cell
Many intracellular pathogens have been shown to subvert host cell death pathways for exit from the host cell and dissemination. Pyroptosis is a caspase-1-dependent programmed cell death resulting in cell lysis and the release of proinflammatory cytokines. It is therefore considered a potent innate defense mechanism. Despite this, some pathogens, such as Francisella (123, 125) and Legionella (131), exit host cells that have undergone this type of cell death. As another example, Salmonella enterica induces the extrusion of infected cells from the intestinal epithelium for dissemination into the gut and the environment. Homeostatic extrusion involves apoptosis, but extrusion triggered by the bacteria is accompanied by pyroptosis (84). That work also showed the existence of a subpopulation of flagellated, motile bacteria that reside in the cytosol and, in contrast to intravacuolar bacteria, express invasion factors such as T3SS1. Interestingly, in this context, another study demonstrated the existence of a nonlytic, flagellum-dependent egress mechanism in oncotic macrophages (121).
As different host cell types undergoing different types of cell death may be involved during the course of an infection, it is sometimes difficult to establish which events are beneficial to the host or to the pathogen during infection in vivo. This has been discussed for systemic infections of Salmonella, and new tools to investigate the population dynamics of pathogens in the host should help resolve these questions (reviewed in reference 154). Salmonella enterica serovar Typhimurium induces apoptosis in intestinal epithelial cells (which might be of importance to cross this barrier and initiate infection) and in a subset of infected macrophages. However, pyroptosis and necrosis appear to be numerically dominant in systemic infections (154). S. Typhimurium induces pyroptosis in macrophages and is able to delay host cell death to improve replication and systemic dissemination (58). The activation of pyroptosis by Salmonella is similar to but distinct from the pathway induced by the anthrax lethal toxin. The former seems to act via Ipaf, whereas the latter is known to require the inflammasome adaptor Nalp1 (57). Interestingly, Salmonella induces both caspase-1 activation and the release of inflammatory cytokines, associated with or possibly triggering massive (phago)lysosome exocytosis (18). It is not yet clear whether the microbicidal activities released from lysosomes participate in the antimicrobial nature of pyroptosis (18). Shigella spp. also induce pyroptosis of their host macrophages (61). Similarly to Salmonella, a pathogenic factor, Shigella IpaB or Salmonella SipB, is secreted via a T3SS and can directly bind to and activate caspase-1. The pyroptosis of Shigella-infected macrophages recruits polymorphonuclear leukocytes, which can be infected by capturing these dying cells (61). Interestingly, while Listeria induces apoptosis in a variety of cell types, in macrophages, the bacterium appears to make use of pyroptosis to spread the infection (61).
As mentioned in “Apoptosis as a Nonlytic Exit Strategy,” the modulation of host macrophage death by mycobacteria is emerging as an important determinant of infection (reviewed in reference 16). Virulent M. tuberculosis cells induce necrosis in a complex pathway that depends on the ESX1 secretion system. The manipulation of the host eicosanoid pathways leads to the inhibition of cellular membrane repair, affecting mitochondria and the plasma membrane and possibly affecting other organelles, such as the replication vacuole. This results in the steering of the macrophage into necrosis (45). The release of bacteria by this mechanism not only avoids apoptosis as an innate defense mechanism but also delays the establishment of adaptive immunity in mice, as necrosis prevents the cross-presentation of antigens by dendritic cells (46). The induction of necrosis by virulent mycobacteria might also be important for the outcome of infection in other hosts, because susceptibility has been linked to a mutation affecting eicosanoid biosynthesis in zebrafish and humans (145, 146). The exact mechanisms of the induction of necrosis are under study, but a recent report signaled a critical role for the NLRP3 inflammasome in this process (155). A contact-dependent hemolysin activity linked to a functional ESX1 system induces Ca2+-dependent lysosome secretion that is concurrent with the release of proinflammatory cytokines from infected macrophages (85). ESAT-6 appears to generate vacuole damage that is sensed by the host and recruits various signaling molecules, such as galectin-3, leading to the Syk tyrosine kinase-dependent activation of NLRP3 (85, 155). This pathway is reminiscent of Syk-dependent NLRP3 activation during fungal and malarial infections (67, 130). Recently, such a link between M. tuberculosis vacuole damage and/or complete lysis and host cell death by necrosis was confirmed independently (132).
Legionella infects Acanthamoeba but is also an opportunistic human pathogen found within an intracellular vacuole in alveolar epithelial cells and macrophages. At later stages of infection, bacterial flagellin induces pyroptosis in macrophages in a mechanism dependent on the bacterial type IVB secretion system Dot/Icm and on the host Nlrc4 inflammasome (131). As part of the pyroptotic process, a host-derived pore-forming activity is induced, the action of which precedes host cell lysis and the release of the bacteria (131). These findings might have a general significance for various intracellular pathogens inducing host cell pyroptosis. Legionella mutants that do not induce this host-derived pore-forming activity are able to replicate but are severely delayed in exit from the host cell (6). Similar observations were made for amoeba hosts (60). However, it remains unknown whether pore-forming activity is critical for bacterial release, because other factors of the pyroptotic response may contribute.
Although it is unclear whether it occurs as a concerted or unconcerted strategy, there is another example illustrating the importance of host cell death pathways in Leishmania major infection. L. major is a parasite that usually replicates within the phagosome. After a bite by a sand fly, neutrophils phagocytose the transmitted parasites (108). Neutrophils undergoing apoptosis appear to promote the release and subsequent transfer of the parasite into macrophages and, in addition, are critical for the modulation of macrophage and dendritic cell functions (discussed in reference 109). The efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early antileishmania response (116).
Other unconcerted lytic egress strategies unrelated to cell death-mediated mechanisms do exist. In T. cruzi, for example, motility appears to be an important contributor to egress. After replication, nonmotile amastigotes redifferentiate in the cytosol into flagellated trypomastigotes. Based on visual observations, the latter breach the host plasma membrane shortly after becoming motile (11). Trypomastigotes are capable of protrusion from cytochalasin D-treated host cells immediately after uptake (156). Therefore, under normal conditions, a potential protrusion mechanism would probably require the destabilization of the host cell actin cytoskeleton by parasite factors. These may include a cysteine protease activity that has been implicated in the egress of T. cruzi trypomastigotes from host cells (40).
Concerted Egress from the Vacuole and the Host Cell
Concerted egress strategies are common among apicomplexan parasites, although there are some notable exceptions, such as the budding of Plasmodium liver-stage parasites (see “Budding from the Plasma Membrane”). This illustrates that in organisms with complex life cycles, the mechanisms of exit from the host cell can be stage specific (Fig. 2). The egress of blood-stage Plasmodium parasites from red blood cells (RBCs) is governed by the release of parasite proteases and a calcium-dependent kinase (49). Merozoites are very short lived in the blood circulation, and hence, egress needs to be tightly controlled to optimize dissemination. The lysis of the PVM and the lysis of the host plasma membrane require different effectors and occur in rapid succession (reviewed in reference 23). The rupture of RBCs also involves osmotic stress (65). Other classes of effectors, such as PFPs (52) and lipases (20), might also be involved, but there is currently no direct support for this hypothesis. Modifications of the RBC membrane and cytoskeleton appear central for the efficient dispersal of the nonmotile progeny, enabling the RBC membrane to undergo a sequence of events described as curling-buckling-eversion-vesiculation (2). Indeed, the removal of adaptor proteins and cytoskeletal elements from the RBC membrane has been reported (96). At least in vitro, the subversion of host calpain proteases facilitates the egress of Plasmodium falciparum and T. gondii by cleaving several cytoskeletal elements (33). However, the reticulocyte-invading rodent malaria parasite P. yoelii develops equally in calpain−/− mice compared to wild-type mice (71).
The subtilisin-like serine protease SUB1 is essential for the egress of P. falciparum from RBCs (157). PfSUB1 is released into the parasitophorous vacuole (PV) from a novel type of parasite secretory organelle, called the exoneme, just prior to egress, but nothing is known about the stimulus that leads to its secretion. PfSUB1 is a Ca2+-dependent protease, and the low-Ca2+ environment of the PV space could represent a second level of control in addition to regulated exoneme discharge. PfSUB1 and the cysteine protease dipeptidyl aminopeptidase 3 (DPAP3) process papain-like proteins of the serine repeat antigen (SERA) family, which is crucial for parasite egress (13, 157). The existence of a proteolytic cascade has been postulated; however, evidence for protease activity has so far been obtained only for PfSERA5 (74). One P. berghei SERA homologue, called egress cysteine protease 1 (PbECP1), was shown to be essential for the egress of the mosquito stage of the parasite (10). Despite the activation of parasite motility, PbECP1-negative sporozoites are unable to leave the oocyst. Nevertheless, motility does contribute to efficient egress in this stage, because sporozoites lacking productive gliding locomotion also display a partial defect in oocyst egress (55).
The exit of Plasmodium gametocytes from RBCs occurs concomitantly with differentiation into gametes. Gametogenesis and egress depend on activation by external stimuli (temperature drop and xanthurenic acid) in the mosquito midgut and on parasite protease activities (135). Parasite organelles called osmiophilic bodies, found predominantly in female gametocytes, were postulated to be functionally equivalent to exonemes in the blood stage (44). Pfg377 is a protein located in the osmiophilic bodies that plays a pivotal role in the biogenesis of these organelles, and parasites deficient in Pfg377 showed a reduced emergence of female gametes from RBCs (but no effect on male gametes) and reduced infectivity toward mosquitoes. PbGEST and the protein of early gametocyte 3 (PEG3) (also called male development 1 [MDV-1]) are released from osmiophilic bodies into the PV and are important for the egress of both male and female P. berghei gametocytes (112, 143). Besides its role in vertebrate-mosquito transmission, PbGEST also functions in cell traversal by sporozoites, which is required for productive transmission back to the vertebrate host (143).
In the apicomplexan model organism T. gondii, perforin-like protein 1 (TgPLP1) plays a key role in the egress of tachyzoites, the form characteristic of acute infections (81). The protein is secreted into the PV space from parasite organelles called micronemes, presumably just prior to egress. TgPLP1-deficient parasites are avirulent in mice and display severely delayed and temporally heterogeneous egress in cell culture, which suggested that parasite gliding motility is able to force exit from the host cell eventually. TgPLP1 destabilizes the PVM and possibly also attacks other host cell membranes (81). The fact that TgPLP1 is released from micronemes has important implications not only for T. gondii but potentially also for other apicomplexan parasites. Microneme secretion in apicomplexans is thought to resemble Ca2+-mediated exocytosis in other organisms and has traditionally been associated with the activation of gliding motility and host cell invasion (21, 102). Microneme secretion and egress can be artificially induced by Ca2+ ionophores in a variety of parasites (17, 54). Importantly, T. gondii ionophore-induced-egress mutants show a defect in the establishment of in vivo infections (89). Both induced and noninduced egress requires the release of TgPLP1 (81). In addition, induced egress relies on parasite motility (95, 100, 111). In contrast, noninduced egress involves increased vacuolar pressure but does not require gliding motility (88). In addition to parasite factors, the release of Ca2+ from the ER leads to the activation of host cell calpains that participate in parasite egress by destabilizing the host cell cytoskeleton (33). The action of TgPLP1 likely results in the breakdown of ion gradients in the host cell and was proposed to trigger a feedback-loop mechanism leading to the amplification of microneme secretion (118).
While the replication of T. gondii by successive rounds of binary fission is compatible with induced/premature egress, this is not necessarily the case for other parasites. For example, Plasmodium multiplies by schizogony, which means that premature egress could be fatal due to incomplete cell division.
Interestingly, in the case of Eimeria tenella infection, the host might rely on immune-mediated mechanisms (cytokines and antibodies) to force the premature exit of sporozoites before schizogony starts (47). For T. gondii, it was hypothesized that induced/premature egress reflects the parasite's ability to react to environmental changes, whereas noninduced egress is the response to an intrinsic stimulus presumably coupled to replication. The significance of proliferation-dependent versus premature egress in in vivo infection was investigated in a recent study in mice. During acute infection, T. gondii undergoes a rapid turnover of egress and reinvasion in macrophages (147). Activated macrophages were suspected to be involved in the external trigger for premature egress, but it remains to be determined if the parasite or the host ultimately benefits from this rapid turnover. Proliferation-dependent egress was also observed in vivo in mesothelial cells in a way similar to what is known from cell culture conditions (147).
STIMULI AND SIGNALING LEADING TO EGRESS
To date, the stimuli and the ensuing signaling leading to egress remain largely unknown for most intracellular pathogens. An exception is the apicomplexan parasite T. gondii. The phytohormone abscisic acid (ABA) acts as a natural agonist of egress in T. gondii tachyzoites (101). ABA induces the formation of the secondary messenger cyclic ADP-ribose (cADPR), which in turn triggers Ca2+-dependent protein secretion from micronemes. The successful completion of replication appears to be coupled to parasite ABA biosynthesis (101). Conversely, a metabolic block of ABA biosynthesis severely delays egress and results in the differentiation of parasites into the semidormant bradyzoites characteristic of chronic infection (101). In addition to this intrinsic egress stimulus, the T. gondii tachyzoite is able to monitor the state of the host cell and reacts to extrinsic stimuli with egress from its host cell. Such premature egress can be an ultimate solution in response to a life-threatening situation. The egress of T. gondii can be triggered by cytotoxic T lymphocytes through the induction of death receptor-mediated apoptosis (107) and possibly by activated macrophages (147). The means by which the pathogen senses environmental changes are only beginning to be understood. Although not essential for egress, a drop in the host cell cytosolic K+ concentration is sufficient to trigger egress through the activation of a parasite PI-PLC, which in turn leads to an increase in the intraparasitic Ca2+ concentration (59, 88, 100). In accordance with this, PFPs secreted by cytotoxic T or NK cells were shown to induce egress (106, 107). Alternatively, an insufficient maintenance of the K+ gradient across the host plasma membrane due to an energy exhaustion of the host cell could cause a decrease in the concentration of host cell cytosolic K+ and thus trigger egress.
CONCLUSIONS AND PERSPECTIVES
Intracellular pathogens comprise a very diverse collection of organisms. Egress from their host cell is a vital event in their life cycle. The release of progeny is important for the spread and establishment of infection in the host organism and for transmission to a new host. Failure to egress has been associated with considerably reduced virulence in a variety of organisms, indicating that the targeting of effectors of egress may have therapeutic potential.
The analysis of egress has been technically challenging: it is difficult to investigate factors that are involved in the release of the pathogen from a vacuolar compartment and/or exit from the host cell independently, as the first is a prerequisite of the second. To address this issue, a number of elegant methods have been developed. Inducible expression systems have proven to be a valuable tool in this regard (5, 68, 105). Recently, fluorescence microscopy using a FRET-based approach and/or galectin-3 as a marker for vacuolar lysis was established as a new tool to identify host cellular factors implicated in vacuolar egress by Shigella, Listeria, Salmonella, mycobacteria, and potentially other intracellular pathogens (105, 115, 132). High spatiotemporal resolution also allows the localization of these factors during the event, as was demonstrated for RhoA and Rac1, which were recruited to the site of vacuole rupture by Shigella (115). Another useful technique is a hypotonic shock protocol, which allows the artificial delivery of mycobacteria from the vacuole into the host cell cytosol (134). Furthermore, the establishment of powerful forward genetic screens in T. gondii for the isolation of egress mutants is promising to shed light on signaling pathways leading to egress (53). Most importantly, the increasing use of alternative host model systems has significantly advanced our understanding of host-pathogen interactions and, specifically, of egress strategies. Translucent zebrafish embryos as a model host organism make it possible to monitor mycobacterial infections over time in a living organism. Furthermore, amoebae are gaining attention as single-cell host model systems. Environmental amoebae are a natural breeding ground for pathogenic bacteria, and the concept is emerging that pathogenic strategies, including egress, emerged from these very early interactions (35, 36, 69).
Although host cell egress by different pathogens sometimes needs to meet very different requirements, the various strategies share some common principles. From what is known to date, it appears that most eukaryotic intracellular parasites have opted for a lytic egress strategy, which might simply be due to their relatively large sizes compared to the sizes of bacteria, for which nonlytic egress strategies are more common (76). As a consequence, these pathogens need to be prepared to face the hostile extracellular environment. In order to minimize exposure to the host immune system, many of these pathogens have developed rapid and highly controlled exit mechanisms that ensure the fast release of progeny that are ready for the immediate invasion of neighboring cells. In fact, it has become clear that, at least in some apicomplexans, egress and subsequent invasion are linked (22, 86). In T. gondii tachyzoites, the connection between invasion and egress has been achieved by the storage of molecular effectors for invasion and egress in the same set of secretory organelles, the micronemes (21, 22, 81, 102). Furthermore, Ca2+ signaling appears to connect the control of microneme discharge with the activation of the glideosome machinery functioning in invasion and egress (21, 95, 100, 102, 111). Similarly, exonemes in Plasmodium play an important role in egress and invasion. PfSUB1 not only is essential for egress but also contributes directly to the efficient invasion of parasites into RBCs (86). Plasmodium also harbors micronemes, but in contrast to T. gondii, these appear to be dispensable for egress, at least for blood-stage parasites (56). It remains to be established whether corresponding and/or different secretory organelles operate in related organisms, as has been proposed for rhoptries and microspheres in the escape of the Theileria sporozoite from its vacuole into the lymphocyte cytosol (129).
With new assays and model systems and the improvements in pathogen gene expression analyses and regulated gene expression systems, we are well equipped to deepen our understanding of egress as a pathogen-controlled process. From future work, we can expect to see a broader picture of the implications of egress in host-pathogen interactions and virulence mechanisms.
ACKNOWLEDGMENTS
We thank V. B. Carruthers and K. Matuschewski for critical reading of earlier versions of the manuscript.
This work was supported by the Swiss National Foundation (grant FN3100A0-116722 to D.S.-F. and grant 31003A_132995 to T.S.) and is part of the activities of the BioMalPar European Network of Excellence, supported by a European grant (LSHP-CT-2004-503578) from the Priority 1 Life Sciences, Genomics, and Biotechnology for Health in FP6.
Biographies

Nikolas Friedrich obtained his Ph.D. in 2009 from the University of Geneva for his work in the laboratory of Professor Dominique Soldati-Favre and Dr. Mike Blackman (National Institute for Medical Research, London, United Kingdom). Currently, he is a postdoctoral fellow in the group of Professor Alexandra Trkola at the University of Zürich.

Monica Hagedorn has been a group leader in the Parasitology department (group cell biology) at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany, since April 2010. After conducting her Ph.D. at the University of Ulm, she joined the laboratory of Thierry Soldati in Geneva, Switzerland, as a postdoctoral fellow from 2004 until 2010.

Dominique Soldati-Favre is full professor at the Department of Microbiology and Molecular Medicine at the Faculty of Medicine of the University of Geneva since 2004. She carried out her doctoral work at the University of Zurich prior to undertaking postdoctoral training at the Department of Microbiology and Immunology at Stanford Medical School. In 1995, she joined the Center for Molecular Biology at the University of Heidelberg (ZMBH) as a junior group leader. In 2001, she took a Senior Lecturer followed by a Reader position at Imperial College London.

Thierry Soldati has been associate professor at the Department of Biochemistry, University of Geneva, since 2004. He carried out his doctoral work at the ETH in Zurich and was then a postdoctoral fellow at Stanford University Medical School. In 1995, he joined the Max Planck Institute for Medical Research in Heidelberg, Germany, as a group leader. In 2001, he was appointed Lecturer at the Department of Biological Sciences of Imperial College London. He is also vice president of the Union of the Swiss Societies for Experimental Biology.
REFERENCES
- 1. Abdallah AM, et al. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5: 883–891 [DOI] [PubMed] [Google Scholar]
- 2. Abkarian M, Massiera G, Berry L, Roques M, Braun-Breton C. 2011. A novel mechanism for egress of malarial parasites from red blood cells. Blood 117: 4118–4124 [DOI] [PubMed] [Google Scholar]
- 3. Abounit S, Zurzolo C. 2012. Wiring through tunneling nanotubes—from electrical signals to organelle transfer. J. Cell Sci. 125: 1089–1098 [DOI] [PubMed] [Google Scholar]
- 4. Akimana C, Al-Khodor S, Abu Kwaik Y. 2010. Host factors required for modulation of phagosome biogenesis and proliferation of Francisella tularensis within the cytosol. PLoS One 5: e11025 doi:10.1371/journal.pone.0011025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberti-Segui C, Goeden KR, Higgins DE. 2007. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell. Microbiol. 9: 179–195 [DOI] [PubMed] [Google Scholar]
- 6. Alli OA, et al. 2000. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68: 6431–6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almeida-Campos FR, Horta MF. 2000. Proteolytic activation of leishporin: evidence that Leishmania amazonensis and Leishmania guyanensis have distinct inactive forms. Mol. Biochem. Parasitol. 111: 363–375 [DOI] [PubMed] [Google Scholar]
- 8. Alvarez M, Casadevall A. 2007. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 8: 16 doi:10.1186/1471-2172-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarez M, Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16: 2161–2165 [DOI] [PubMed] [Google Scholar]
- 10. Aly AS, Matuschewski K. 2005. A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J. Exp. Med. 202: 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrade LO, Andrews NW. 2005. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat. Rev. Microbiol. 3: 819–823 [DOI] [PubMed] [Google Scholar]
- 12. Andrews NW, Abrams CK, Slatin SL, Griffiths G. 1990. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell 61: 1277–1287 [DOI] [PubMed] [Google Scholar]
- 13. Arastu-Kapur S, et al. 2008. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat. Chem. Biol. 4: 203–213 [DOI] [PubMed] [Google Scholar]
- 14. Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U. 2007. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 3: e171 doi:10.1371/journal.ppat.0030171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beatty WL. 2007. Lysosome repair enables host cell survival and bacterial persistence following Chlamydia trachomatis infection. Cell. Microbiol. 9: 2141–2152 [DOI] [PubMed] [Google Scholar]
- 16. Behar SM, Divangahi M, Remold HG. 2010. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 8: 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behrendt JH, Taubert A, Zahner H, Hermosilla C. 2008. Studies on synchronous egress of coccidian parasites (Neospora caninum, Toxoplasma gondii, Eimeria bovis) from bovine endothelial host cells mediated by calcium ionophore A23187. Vet. Res. Commun. 32: 325–332 [DOI] [PubMed] [Google Scholar]
- 18. Bergsbaken T, Fink SL, den Hartigh AB, Loomis WP, Cookson BT. 2011. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J. Immunol. 187: 2748–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beuzon CR, et al. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19: 3235–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhanot P, Schauer K, Coppens I, Nussenzweig V. 2005. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J. Biol. Chem. 280: 6752–6760 [DOI] [PubMed] [Google Scholar]
- 21. Billker O, Lourido S, Sibley LD. 2009. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 5: 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black MW, Arrizabalaga G, Boothroyd JC. 2000. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol. Cell. Biol. 20: 9399–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blackman MJ. 2008. Malarial proteases and host cell egress: an ‘emerging’ cascade. Cell. Microbiol. 10: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boucrot E, Henry T, Borg JP, Gorvel JP, Meresse S. 2005. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science 308: 1174–1178 [DOI] [PubMed] [Google Scholar]
- 25. Boujemaa-Paterski R, et al. 2001. Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry 40: 11390–11404 [DOI] [PubMed] [Google Scholar]
- 26. Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V. 2011. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 195: 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byrd TF, Green GM, Fowlston SE, Lyons CR. 1998. Differential growth characteristics and streptomycin susceptibility of virulent and avirulent Mycobacterium tuberculosis strains in a novel fibroblast-mycobacterium microcolony assay. Infect. Immun. 66: 5132–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlsson F, Brown EJ. 2006. Actin-based motility of intracellular bacteria, and polarized surface distribution of the bacterial effector molecules. J. Cell. Physiol. 209: 288–296 [DOI] [PubMed] [Google Scholar]
- 29. Carlsson F, Brown EJ. 2009. Cell biology. The art of making an exit. Science 323: 1678–1679 [DOI] [PubMed] [Google Scholar]
- 30. Carlsson F, Joshi SA, Rangell L, Brown EJ. 2009. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog. 5: e1000285 doi:10.1371/journal.ppat.1000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carnell M, et al. 2011. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J. Cell Biol. 193: 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castro-Garza J, King CH, Swords WE, Quinn FD. 2002. Demonstration of spread by Mycobacterium tuberculosis bacilli in A549 epithelial cell monolayers. FEMS Microbiol. Lett. 212: 145–149 [DOI] [PubMed] [Google Scholar]
- 33. Chandramohanadas R, et al. 2009. Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells. Science 324: 794–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U. S. A. 103: 14578–14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J, et al. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303: 1358–1361 [DOI] [PubMed] [Google Scholar]
- 36. Chen J, Reyes M, Clarke M, Shuman HA. 2007. Host cell-dependent secretion and translocation of the LepA and LepB effectors of Legionella pneumophila. Cell. Microbiol. 9: 1660–1671 [DOI] [PubMed] [Google Scholar]
- 37. Collins CA, et al. 2009. Atg5-independent sequestration of ubiquitinated mycobacteria. PLoS Pathog. 5: e1000430 doi:10.1371/journal.ppat.1000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cosson P, Soldati T. 2008. Eat, kill or die: when amoeba meets bacteria. Curr. Opin. Microbiol. 11: 271–276 [DOI] [PubMed] [Google Scholar]
- 39. Costales J, Rowland EC. 2005. Human chagasic serum contains antibodies capable of inhibiting Trypanosoma cruzi egress from tissue culture cells. J. Parasitol. 91: 950–953 [DOI] [PubMed] [Google Scholar]
- 40. Costales J, Rowland EC. 2007. A role for protease activity and host-cell permeability during the process of Trypanosoma cruzi egress from infected cells. J. Parasitol. 93: 1350–1359 [DOI] [PubMed] [Google Scholar]
- 41. Davis JM, et al. 2002. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17: 693–702 [DOI] [PubMed] [Google Scholar]
- 42. Davis JM, Ramakrishnan L. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Jonge MI, et al. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J. Bacteriol. 189: 6028–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Koning-Ward TF, et al. 2008. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 67: 278–290 [DOI] [PubMed] [Google Scholar]
- 45. Divangahi M, et al. 2009. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 10: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. 2010. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 11: 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dong X, et al. 2011. Enhanced egress of intracellular Eimeria tenella sporozoites by splenic lymphocytes from coccidian-infected chickens. Infect. Immun. 79: 3465–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. 2009. Major cell death pathways at a glance. Microbes Infect. 11: 1050–1062 [DOI] [PubMed] [Google Scholar]
- 49. Dvorin JD, et al. 2010. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 328: 910–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dzierszinski F, Nishi M, Ouko L, Roos DS. 2004. Dynamics of Toxoplasma gondii differentiation. Eukaryot. Cell 3: 992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Early J, Fischer K, Bermudez LE. 2011. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb. Pathog. 50: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ecker A, Pinto SB, Baker KW, Kafatos FC, Sinden RE. 2007. Plasmodium berghei: plasmodium perforin-like protein 5 is required for mosquito midgut invasion in Anopheles stephensi. Exp. Parasitol. 116: 504–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eidell KP, Burke T, Gubbels MJ. 2010. Development of a screen to dissect Toxoplasma gondii egress. Mol. Biochem. Parasitol. 171: 97–103 [DOI] [PubMed] [Google Scholar]
- 54. Ellison SP, Greiner E, Dame JB. 2001. In vitro culture and synchronous release of Sarcocystis neurona merozoites from host cells. Vet. Parasitol. 95: 251–261 [DOI] [PubMed] [Google Scholar]
- 55. Engelmann S, Silvie O, Matuschewski K. 2009. Disruption of Plasmodium sporozoite transmission by depletion of sporozoite invasion-associated protein 1. Eukaryot. Cell 8: 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a. Estes KA, Szumowski SC, Troemel ER. 2011. Non-lytic, actin-based exit of intracellular parasites from C. elegans intestinal cells. PLoS Pathog. 7: e1002227 doi:10.1371/journal.ppat.1002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farrell A, et al. 2012. A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science 335: 218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fink SL, Bergsbaken T, Cookson BT. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 105: 4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fink SL, Cookson BT. 2007. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 9: 2562–2570 [DOI] [PubMed] [Google Scholar]
- 59. Fruth IA, Arrizabalaga G. 2007. Toxoplasma gondii: induction of egress by the potassium ionophore nigericin. Int. J. Parasitol. 37: 1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gao LY, Kwaik YA. 2000. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ. Microbiol. 2: 79–90 [DOI] [PubMed] [Google Scholar]
- 61. Gao LY, Kwaik YA. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8: 306–313 [DOI] [PubMed] [Google Scholar]
- 62. Gedde MM, Higgins DE, Tilney LG, Portnoy DA. 2000. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68: 999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Geoffroy C, Gaillard JL, Alouf JE, Berche P. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect. Immun. 55: 1641–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Glomski IJ, Decatur AL, Portnoy DA. 2003. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect. Immun. 71: 6754–6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glushakova S, Yin D, Li T, Zimmerberg J. 2005. Membrane transformation during malaria parasite release from human red blood cells. Curr. Biol. 15: 1645–1650 [DOI] [PubMed] [Google Scholar]
- 66. Graewe S, et al. 2011. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 7: e1002224 doi:10.1371/journal.ppat.1002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gross O, et al. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459: 433–436 [DOI] [PubMed] [Google Scholar]
- 68. Grundling A, Gonzalez MD, Higgins DE. 2003. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J. Bacteriol. 185: 6295–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hagedorn M, Rohde KH, Russell DG, Soldati T. 2009. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323: 1729–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hagedorn M, Soldati T. 2007. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cell. Microbiol. 9: 2716–2733 [DOI] [PubMed] [Google Scholar]
- 71. Hanspal M, Goel VK, Oh SS, Chishti AH. 2002. Erythrocyte calpain is dispensable for malaria parasite invasion and growth. Mol. Biochem. Parasitol. 122: 227–229 [DOI] [PubMed] [Google Scholar]
- 72. Henry R, et al. 2006. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell. Microbiol. 8: 107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Henry T, et al. 2006. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc. Natl. Acad. Sci. U. S. A. 103: 13497–13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hodder AN, et al. 2003. Enzymic, phylogenetic, and structural characterization of the unusual papain-like protease domain of Plasmodium falciparum SERA5. J. Biol. Chem. 278: 48169–48177 [DOI] [PubMed] [Google Scholar]
- 75. Hsu T, et al. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U. S. A. 100: 12420–12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hybiske K, Stephens RS. 2008. Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 6: 99–110 [DOI] [PubMed] [Google Scholar]
- 77. Hybiske K, Stephens RS. 2007. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. U. S. A. 104: 11430–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Idone V, Tam C, Andrews NW. 2008. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 18: 552–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ishino T, et al. 2009. LISP1 is important for the egress of Plasmodium berghei parasites from liver cells. Cell. Microbiol. 11: 1329–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johnston SA, May RC. 2010. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 6: e1001041 doi:10.1371/journal.ppat.1001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kafsack BF, et al. 2009. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323: 530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kaku T, Kawamura I, Uchiyama R, Kurenuma T, Mitsuyama M. 2007. RD1 region in mycobacterial genome is involved in the induction of necrosis in infected RAW264 cells via mitochondrial membrane damage and ATP depletion. FEMS Microbiol. Lett. 274: 189–195 [DOI] [PubMed] [Google Scholar]
- 83. Kaniuk NA, et al. 2011. Salmonella exploits Arl8B-directed kinesin activity to promote endosome tubulation and cell-to-cell transfer. Cell. Microbiol. 13: 1812–1823 [DOI] [PubMed] [Google Scholar]
- 84. Knodler LA, et al. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. U. S. A. 107: 17733–17738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koo IC, et al. 2008. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10: 1866–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koussis K, et al. 2009. A multifunctional serine protease primes the malaria parasite for red blood cell invasion. EMBO J. 28: 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kumamoto CA, Vinces MD. 2005. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 7: 1546–1554 [DOI] [PubMed] [Google Scholar]
- 88. Lavine MD, Arrizabalaga G. 2008. Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryot. Cell 7: 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lavine MD, Knoll LJ, Rooney PJ, Arrizabalaga G. 2007. A Toxoplasma gondii mutant defective in responding to calcium fluxes shows reduced in vivo pathogenicity. Mol. Biochem. Parasitol. 155: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ley V, Robbins ES, Nussenzweig V, Andrews NW. 1990. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J. Exp. Med. 171: 401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ma H, Croudace JE, Lammas DA, May RC. 2007. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 8: 15 doi:10.1186/1471-2172-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ma H, Croudace JE, Lammas DA, May RC. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16: 2156–2160 [DOI] [PubMed] [Google Scholar]
- 93. Manning-Cela R, et al. 2001. LYT1 protein is required for efficient in vitro infection by Trypanosoma cruzi. Infect. Immun. 69: 3916–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marquis H, Hager EJ. 2000. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol. Microbiol. 35: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meissner M, Schluter D, Soldati D. 2002. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298: 837–840 [DOI] [PubMed] [Google Scholar]
- 96. Millholland MG, et al. 2011. The malaria parasite progressively dismantles the host erythrocyte cytoskeleton for efficient egress. Mol. Cell. Proteomics 10: M111.010678 doi:10.1074/mcp.M111.010678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moore-Lai D, Rowland E. 2004. Discovery and characterization of an antibody, anti-egressin, that is able to inhibit Trypanosoma cruzi egress in vitro. J. Parasitol. 90: 524–530 [DOI] [PubMed] [Google Scholar]
- 98. Moore-Lai D, Rowland EC. 2004. Antiegressin acts late in the intracellular growth cycle of Trypanosoma cruzi to inhibit parasite egress. J. Parasitol. 90: 85–91 [DOI] [PubMed] [Google Scholar]
- 99. Mortara RA, et al. 2005. Mammalian cell invasion and intracellular trafficking by Trypanosoma cruzi infective forms. An. Acad. Bras. Cienc. 77: 77–94 [DOI] [PubMed] [Google Scholar]
- 100. Moudy R, Manning TJ, Beckers CJ. 2001. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J. Biol. Chem. 276: 41492–41501 [DOI] [PubMed] [Google Scholar]
- 101. Nagamune K, et al. 2008. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 451: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nagamune K, Moreno SN, Chini EN, Sibley LD. 2008. Calcium regulation and signaling in apicomplexan parasites. Subcell. Biochem. 47: 70–81 [DOI] [PubMed] [Google Scholar]
- 103. Noronha FS, Cruz JS, Beirao PS, Horta MF. 2000. Macrophage damage by Leishmania amazonensis cytolysin: evidence of pore formation on cell membrane. Infect. Immun. 68: 4578–4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Onfelt B, et al. 2006. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177: 8476–8483 [DOI] [PubMed] [Google Scholar]
- 105. Paz I, et al. 2009. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12: 530–544 [DOI] [PubMed] [Google Scholar]
- 106. Persson CM, et al. 2009. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infect. Immun. 77: 970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Persson EK, et al. 2007. Death receptor ligation or exposure to perforin trigger rapid egress of the intracellular parasite Toxoplasma gondii. J. Immunol. 179: 8357–8365 [DOI] [PubMed] [Google Scholar]
- 108. Peters NC, et al. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321: 970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Peters NC, Sacks DL. 2009. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell. Microbiol. 11: 1290–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pfeffer SR. 2010. Unconventional secretion by autophagosome exocytosis. J. Cell Biol. 188: 451–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Plattner F, et al. 2008. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3: 77–87 [DOI] [PubMed] [Google Scholar]
- 112. Ponzi M, et al. 2009. Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cell. Microbiol. 11: 1272–1288 [DOI] [PubMed] [Google Scholar]
- 113. Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. 2005. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 24: 1287–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Radtke AL, et al. 2011. Listeria monocytogenes exploits cystic fibrosis transmembrane conductance regulator (CFTR) to escape the phagosome. Proc. Natl. Acad. Sci. U. S. A. 108: 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ray K, et al. 2010. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell. Microbiol. 12: 545–556 [DOI] [PubMed] [Google Scholar]
- 116. Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. 2012. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 8: e1002536 doi:10.1371/journal.ppat.1002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Robbins JR, et al. 1999. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146: 1333–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Roiko MS, Carruthers VB. 2009. New roles for perforins and proteases in apicomplexan egress. Cell. Microbiol. 11: 1444–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rosado CJ, et al. 2008. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 10: 1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rubin-de-Celis SS, Uemura H, Yoshida N, Schenkman S. 2006. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell. Microbiol. 8: 1888–1898 [DOI] [PubMed] [Google Scholar]
- 121. Sano G, et al. 2007. Flagella facilitate escape of Salmonella from oncotic macrophages. J. Bacteriol. 189: 8224–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Santic M, et al. 2009. Intracellular fate of Francisella tularensis within arthropod-derived cells. Environ. Microbiol. 11: 1473–1481 [DOI] [PubMed] [Google Scholar]
- 123. Santic M, Al-Khodor S, Abu Kwaik Y. 2010. Cell biology and molecular ecology of Francisella tularensis. Cell. Microbiol. 12: 129–139 [DOI] [PubMed] [Google Scholar]
- 124. Santic M, Molmeret M, Klose KE, Abu Kwaik Y. 2006. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 14: 37–44 [DOI] [PubMed] [Google Scholar]
- 125. Santic M, Pavokovic G, Jones S, Asare R, Kwaik YA. 2010. Regulation of apoptosis and anti-apoptosis signalling by Francisella tularensis. Microbes Infect. 12: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schmidt-Christensen A, Sturm A, Horstmann S, Heussler VT. 2008. Expression and processing of Plasmodium berghei SERA3 during liver stages. Cell. Microbiol. 10: 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schuerch DW, Wilson-Kubalek EM, Tweten RK. 2005. Molecular basis of listeriolysin O pH dependence. Proc. Natl. Acad. Sci. U. S. A. 102: 12537–12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. 2006. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell. Microbiol. 8: 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shaw MK. 2003. Cell invasion by Theileria sporozoites. Trends Parasitol. 19: 2–6 [DOI] [PubMed] [Google Scholar]
- 130. Shio MT, et al. 2009. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 5: e1000559 doi:10.1371/journal.ppat.1000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Silveira TN, Zamboni DS. 2010. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect. Immun. 78: 1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Simeone R, et al. 2012. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 8: e1002507 doi:10.1371/journal.ppat.1002507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Smith GA, et al. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63: 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Smith J, et al. 2008. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect. Immun. 76: 5478–5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sologub L, et al. 2011. Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell. Microbiol. 13: 897–912 [DOI] [PubMed] [Google Scholar]
- 136. Stamm LM, et al. 2003. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 198: 1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Stamm LM, et al. 2005. Role of the WASP family proteins for Mycobacterium marinum actin tail formation. Proc. Natl. Acad. Sci. U. S. A. 102: 14837–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Starr T, et al. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11: 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sturm A, et al. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313: 1287–1290 [DOI] [PubMed] [Google Scholar]
- 140. Sturm A, et al. 2009. Alteration of the parasite plasma membrane and the parasitophorous vacuole membrane during exo-erythrocytic development of malaria parasites. Protist 160: 51–63 [DOI] [PubMed] [Google Scholar]
- 141. Szeto J, Namolovan A, Osborne SE, Coombes BK, Brumell JH. 2009. Salmonella-containing vacuoles display centrifugal movement associated with cell-to-cell transfer in epithelial cells. Infect. Immun. 77: 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Takeuchi H, Furuta N, Morisaki I, Amano A. 2011. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell. Microbiol. 13: 677–691 [DOI] [PubMed] [Google Scholar]
- 143. Talman AM, et al. 2011. PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol. Microbiol. 82: 462–474 [DOI] [PubMed] [Google Scholar]
- 144. Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. 2012. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482: 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tobin DM, et al. 2012. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148: 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tobin DM, et al. 2010. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tomita T, Yamada T, Weiss LM, Orlofsky A. 2009. Externally triggered egress is the major fate of Toxoplasma gondii during acute infection. J. Immunol. 183: 6667–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Trent MS, Worsham LM, Ernst-Fonberg ML. 1998. The biochemistry of hemolysin toxin activation: characterization of HlyC, an internal protein acyltransferase. Biochemistry 37: 4644–4652 [DOI] [PubMed] [Google Scholar]
- 149. Troemel ER, Felix MA, Whiteman NK, Barriere A, Ausubel FM. 2008. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 6: 2736–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tucker SC, Casadevall A. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 99: 3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. van der Wel N, et al. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129: 1287–1298 [DOI] [PubMed] [Google Scholar]
- 152. Volkman HE, et al. 2010. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327: 466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. von Bargen K, Gorvel JP, Salcedo SP. 2012. Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol. Rev. 36: 533–562 [DOI] [PubMed] [Google Scholar]
- 154. Watson KG, Holden DW. 2010. Dynamics of growth and dissemination of Salmonella in vivo. Cell. Microbiol. 12: 1389–1397 [DOI] [PubMed] [Google Scholar]
- 155. Wong KW, Jacobs WR., Jr 2011. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 13: 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Woolsey AM, Burleigh BA. 2004. Host cell actin polymerization is required for cellular retention of Trypanosoma cruzi and early association with endosomal/lysosomal compartments. Cell. Microbiol. 6: 829–838 [DOI] [PubMed] [Google Scholar]
- 157. Yeoh S, et al. 2007. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 131: 1072–1083 [DOI] [PubMed] [Google Scholar]
- 158. Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. 2006. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect. Immun. 74: 703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zago MP, et al. 2008. Impairment of infectivity and immunoprotective effect of a LYT1 null mutant of Trypanosoma cruzi. Infect. Immun. 76: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zakikhany K, et al. 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 9: 2938–2954 [DOI] [PubMed] [Google Scholar]