Abstract
Enhancers are regulatory DNA sequences that activate transcription over long distances. Recent studies revealed a widespread role of distant activation in eukaryotic gene regulation and in development of various human diseases, including cancer. Genomic and gene-targeted studies of enhancer action revealed novel mechanisms of transcriptional activation over a distance. They include formation of stable, inactive DNA-protein complexes at the enhancer and target promoter before activation, facilitated distant communication by looping of the spacer chromatin-covered DNA, and promoter activation by mechanisms that are different from classic recruiting. These studies suggest the similarity between the looping mechanisms involved in enhancer action on DNA in bacteria and in chromatin of higher organisms.
INTRODUCTION
Enhancers (Es) are short (20- to 400-bp) DNA sequences that can activate transcription from target promoters (P) in trans and over various distances (more than 100 kb) (7). Enhancers operate in pro- and eukaryotes; in the majority of cases, action of Es involves direct E-P interaction through proteins bound at the E and P, accompanied by formation of an intervening chromatin loop (7, 24, 38). Recent genomic studies using various versions of the 3C approach revealed widespread use of gene regulation by enhancers (see reference 32 for a review). In parallel, genomic studies identified specific signatures (histone modifications and associated proteins) of enhancers that greatly facilitated analysis of the databases (32).
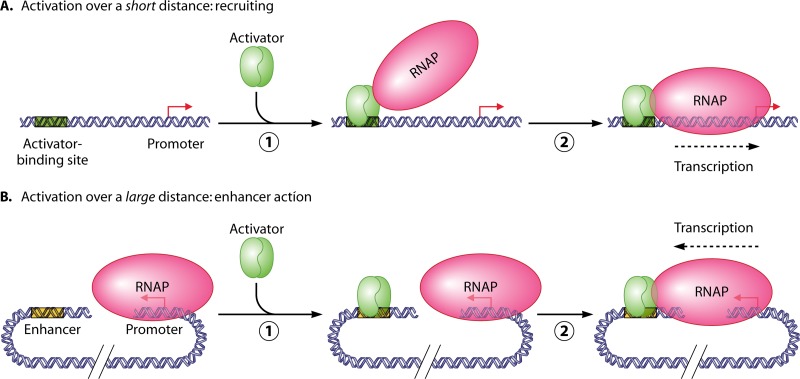
At the same time, understanding of mechanistic aspects of enhancer action trails behind, primarily due to the lack of in vitro systems faithfully recapitulating distant activation. The enhancer field remains driven by the concept of recruiting that was proposed to explain short-distance activation of transcription in prokaryotes (Fig. 1A) (44). During recruiting, an activator protein increases the local concentration of another protein/protein complex (e.g., RNA polymerase [RNAP]) in the vicinity of its binding site. The local increase of protein concentration results in relief of a step limiting the rate of initiation (usually binding of RNAP to a promoter nearby) and induces transcription. During distant action, even if a protein complex was recruited to the enhancer, its concentration at the target would not necessarily be increased because E/P do not typically colocalize. Furthermore, enhancers typically activate preformed complexes already recruited to DNA (Fig. 1B; also see below). Thus, the concept of recruiting cannot explain some principal aspects of enhancer action; instead, the presence of preformed enhancer targets raises questions about efficient E-P communication and activation of transcription (Fig. 1B). In this review, we focus primarily on mechanistic aspects of enhancer action; other recent studies were covered in several excellent reviews (7, 24, 32, 38).
Fig 1.
Mechanisms of transcriptional activation over short and long distances: recruiting versus DNA looping. (A) Recruiting. As an activator binds to DNA (step 1), it recruits (increases the local concentration of) another protein (e.g., RNA polymerase), the binding of which is a rate-limiting step during transcription initiation (step 2). (B) Enhancer action. Binding of an activator (or a recruited protein, not shown) to the enhancer (step 1) does not automatically increase its local concentration at the target promoter because the promoter and enhancer do not colocalize. Thus, enhancer-bound protein has to (i) efficiently explore surrounding DNA/chromatin regions, (ii) identify the target promoter (e.g., marked by RNAP), and (iii) interact with and activate the promoter by a mechanism that is different from recruiting (step 2). This interaction is typically accompanied by looping of the spacer DNA or chromatin.
ENHANCER ACTION ON DNA
In prokaryotes, there are two types of transcriptional enhancers using tracking and looping mechanisms for enhancer-promoter communication (EPC) (7). Only the looping mechanism is shared between pro- and eukaryotic enhancers and is considered here. In Escherichia coli, the looping mechanism is employed by NtrC-dependent transcriptional enhancers activating σ54-dependent promoters; it has been extensively studied using the glnAp2 promoter as a model (see reference 5 for a review). NtrC is an activator protein complex that binds to the enhancer, and, after phosphorylation and oligomerization, it interacts with the Eσ54 RNAP (bound as a stable, inactive, closed complex at the target promoters), and it also activates conversion of the inactive complex into the productive open initiation complex. As the RNAP leaves the promoter, the σ54 subunit dissociates. During the transient enhancer-promoter interaction, the intervening DNA is looped out.
While the concept of DNA looping explains how enhancer- and promoter-bound proteins interact, it does not automatically explain the high efficiency of E-P communication. In fact, DNA sequences separated by more than 1 kb do not communicate efficiently on linear DNA in vitro (4); therefore, enhancer action over a long distance requires use of special facilitating mechanisms (30). Thus, a short distance is typically <1 kb; efficient EPC within this range does not necessarily require special facilitating mechanisms.
Computer modeling studies suggest that the average distance between linearly separated DNA regions could be considerably decreased on supercoiled DNA (53). Indeed, DNA supercoiling greatly facilitates EPC over a long, but not over a short (<1 kb), distance through a mechanism that involves a global change of DNA conformation (5, 30). Computer simulations and experimental studies suggest that DNA supercoiling results in formation of DNA branches and thus increases the probability of juxtaposition between linearly separated DNA sites (Fig. 2) (17, 43), provided that DNA branches are dynamic structures. Indeed, it has been computationally predicted (17) and experimentally established (6) that communication within each DNA branch occurs by slithering (fast movement of intertwined DNA helices within and along the DNA branches on supercoiled DNA) (Fig. 2). The slithering model, in contrast to the more traditional tracking model of enhancer action (see reference 7 for a review), does not require directional movement of the enhancer toward the promoter or involvement of a special protein moving along DNA.
Fig 2.
Slithering mechanism of facilitated distant communication on DNA (6). Two identical promoters (P1 and P2) are differently positioned relative to an enhancer (E) on a plasmid and are separated by a protein bridge (insulator) formed by the lac repressor (lacI). Sliding of intertwined DNA helices within branches formed on supercoiled DNA (slithering; dashed arrows) greatly increases the probability of E juxtaposition with the promoter P1 positioned within the same topological DNA domain (6, 43). In contrast, the P2 promoter positioned on a different domain of the same DNA molecule cannot efficiently communicate with the E because slithering through the protein bridge is impossible.
To experimentally evaluate the contribution of this mechanism to the high rate of E-P communication on negatively supercoiled DNA, slithering was prevented by introducing two lac operators surrounding the glnAp2 promoter (Fig. 2) (6). The lac repressor formed a protein bridge that placed the promoter and the enhancer on topologically isolated DNA loops and prevented DNA slithering between the loops. The presence of the lac repressor results in selective and strong inhibition of the promoter positioned on the topologically isolated DNA loop; an identical promoter positioned within the same loop with the enhancer remains fully active, suggesting that EPC within each DNA loop is permitted. The data support the slithering model of enhancer action and indicate that the lac repressor can strongly inhibit transcription over a long distance (>1.5 kb from the promoter) via formation of DNA loops that topologically isolate the promoter and enhancer. These features make lac operators formally similar to eukaryotic insulators blocking enhancer action over a distance. Further experiments revealed that the protein bridge assayed in the prokaryotic system in vitro has all of the enhancer-blocking properties of eukaryotic insulators described in vivo (6).
In summary, analysis of NtrC-dependent enhancer action revealed several principles that are often used during action over a distance (see below), including the following: (i) formation of a stable, inactive protein-DNA complex at the enhancer and target promoter before activation; (ii) use of special mechanisms facilitating distant communication; (iii) promoter activation via a mechanism that is different from that of recruiting; and (iv) reestablishing the looping after each activation event.
ENHANCER ACTION IN CHROMATIN
Prokaryotic enhancers typically work over distances of up to several kb (7), while eukaryotic enhancers often communicate over a >100-kb range and in trans (28, 33). Additional challenges faced by eukaryotic enhancers include constant competition between binding of sequence-specific proteins and chromatin formation at the enhancers and promoters and the need to communicate over chromatin-covered DNA. The molecular mechanisms of action of eukaryotic enhancers are not well studied, primarily because of the limitations of the in vitro systems poorly supporting distant enhancer action (3, 26, 47).
Inactive chromatin in interphase eukaryotic cell nuclei is in a highly condensed state and is localized within distinct chromosome territories (12). In order to be activated, a gene has to be relocated outside the chromosome territory and its chromatin structure decondensed to a level consistent with the existence of the 30-nm chromatin fiber (35). Poised (potentially active in transcription) and active chromatin domains likely exist in the form of the 30-nm fibers (13 and 37; but also see reference 51). Thus, the spacer DNA separating communicating eukaryotic enhancers and promoters is likely organized into the 30-nm chromatin fiber.
As in prokaryotes, the inactive enhancer and the target promoter are preset before activation. In this case, potentially active promoters are marked by the presence of specific patterns of histone methylation (H3K4me3 and H3K27me3), histone variants H3.3 and H2A.Z, and elongating (paused) RNA polymerase II (Pol II) making short transcripts (15, 18, 22, 31). Elongating Pol II is one of the most stable DNA-protein complexes in eukaryotic nuclei and likely serves two functions: it protects poised promoters against formation of inactive chromatin structure (14) and constitutes a highly stable target for enhancer action. Poised enhancers are marked by H3K4me1/2, H3.3, and H2A.Z, as well as by formation of specific, functionally inactive DNA-protein complexes (different for different enhancers) (22, 31, 50). During cell differentiation these inactive, stable complexes are often replaced by different, tissue-specific DNA-protein complexes that then activate the target promoters (see reference 39 for a review). Active enhancers are specifically marked by H3K27ac (45).
Similar to the NtrC-dependent enhancers, all well-studied eukaryotic enhancers work by looping: in the active state the enhancer and target promoter are in physical proximity (see references 7, 24, and 38 for reviews). How can chromatin looping occur efficiently? The simplest, most straightforward model suggests that chromatin-compacted DNA supports efficient EPC. Functionally active genomic regions usually contain E-P spacer DNA that is compacted up to ∼30-fold into the 30-nm chromatin fiber (35). Therefore, the range of action of the recruiting mechanism could be increased up to 30-fold (to ∼20- to 30-kb range), provided that the chromatin structure is dynamic. Indeed, recent studies suggest that chromatin structure maintains efficient EPC in vivo (46). Furthermore, chromatin structure can greatly facilitate intramolecular ligation of distantly spaced DNA ends (49) and support efficient distant EPC in vitro by the looping mechanism (26, 47). The high efficiency of EPC cannot be explained only by chromatin-induced DNA compaction (25, 47). Interestingly, DNA supercoiling does not affect the high rate of EPC in chromatin, suggesting that chromatin structure per se can support highly efficient communication over a distance and functionally mimic the supercoiled state characteristic for prokaryotic DNA (47). To explain the efficient communication in chromatin, it has been proposed that spontaneous uncoiling of the ends of nucleosomal DNA from the histones provide dynamic DNA flexibility that facilitates distant EPC (Fig. 3A) (47).
Fig 3.
Proposed mechanisms of highly efficient EPC in chromatin. (A) DNA uncoiling mechanism. Partial spontaneous uncoiling of the ends of nucleosomal DNA from the octamer occurs at a high rate (27, 42). Thus, each nucleosome in an array can provide two points of dynamic histone-induced DNA flexibility at the positions where DNA enters and exits nucleosomes. (B) The hypothetical mechanisms of long-range EPC involving histone N-tails. In mechanism 1, termed brachiation, transient internucleosomal interactions mediated by histone tails could keep chromatin arrays or fibers in close proximity and allow relocation of the interacting partners relative to each other at a high rate. In mechanism 2, termed transient collapse, multiple intrafiber interactions mediated by histone tails could be fully disrupted and reestablished in a different register (36).
Further studies have suggested that the mechanisms of communication on supercoiled DNA and in chromatin are even more similar. Thus, the protein bridge that blocks distant action of bacterial enhancers in vitro (Fig. 2) has all the enhancer-blocking properties of eukaryotic insulators described in vivo (6, 55). Furthermore, a very similar protein bridge formed using the tetracycline-controlled ttA-TetO system has a strong enhancer-blocking insulator activity in transiently transfected mammalian cells (2), suggesting that formation of a chromatin loop topologically isolating the enhancer from the target promoter is sufficient to block EPC in vivo. These experiments suggest that similar mechanistic principles could be utilized during EPC in eukaryotic chromatin and on supercoiled DNA of prokaryotes. Recent data obtained using assembled chromatin arrays suggest that internucleosomal interactions involving the histone tails are essential for highly efficient, long-range EPC in chromatin in vitro (25). We speculate that long-range, transient internucleosomal interactions through the histone N-terminal tails (16, 21) keep chromatin fibers in close proximity and allow relocation of the interacting fibers relative to one another (Fig. 3B). These relocations could occur by a mechanism involving sliding of interacting chromatin fibers (termed brachiation; Fig. 3B, mechanism 1) or by reestablishing internucleosomal interactions de novo (Fig. 3B, mechanism 2) (36).
At the same time, recent data suggest that chromatin structure per se is not sufficient for distant EPC, and some additional facilitating mechanisms are used in higher organisms. Genomic studies show that many enhancers and the spacer DNA are transcribed, producing noncoding RNA (ncRNA) molecules with an average size of 1 kb (23, 40); the transcripts and/or ongoing transcription are required for enhancer action (23, 40, 54, 57). Two models have been proposed to explain these observations. For some enhancers, the enhancer DNA together with Pol II and TATA binding protein (TBP) tracks along the intervening DNA (57), presumably forming unstable chromatin loops before reaching the promoter and forming the activation loop. In this case, the process of transcription itself is essential, since a terminator or an insulator inserted between the enhancer and the promoter traps Pol II and blocks long-range enhancer function (29, 57). Since Pol II movement is not highly processive, the putative intermediate loops may need to be stabilized. At the same time, genomic studies have revealed that the presence of ncRNA per se is required for enhancer action (23, 40, 54), suggesting a second model where RNA stabilizes the E-P chromatin loop, either during or after communication. It is also possible that ncRNA transcription locally affects chromatin structure or the pattern of histone modifications.
After formation of the activation chromatin loop, it is likely stabilized by additional protein factors, such as CTCF and cohesin (see references 10, 11, and 56 for reviews). CTCF is a sequence-specific insulator-binding protein, and cohesin can form large complexes that could incorporate two DNA molecules and thus stabilize the chromatin loop. In some cases, regulatory E-P chromatin loops are formed with the mediator and cohesin (20). Formation of chromatin loops can occur in cis and in trans; in fact, looping interactions only rarely occur with the nearest gene (48). It is unclear whether the chromatin loop has to be reestablished after each round of transcription, as it occurs in prokaryotes. It is tempting to propose that EPC over a long distance in higher organisms requires special mechanisms preventing complete disruption of the loops after each round of transcription.
Like in bacteria, activation in eukaryotes occurs via mechanisms that are different from those of classic recruiting. Two mechanisms of enhancer action have been studied in detail. Transcriptional activation facilitating formation of the preinitiation complex involves recruiting of transcription factor IID (TFIID) on the promoter and its activation by TFIIA (8). TFIIA-TFIID interaction results in a change of TFIID conformation leading to a more stable interaction with the promoter and formation of functionally active, committed preinitiation complex (41). More recently, genomic studies have revealed that many enhancer-activated genes contain paused Pol II (9, 14, 15, 34) that most likely protect these poised promoters from inactivation by competing chromatin assembly (14). Since elongating Pol II complex is one of the most stable DNA-protein complexes in the nuclei, paused Pol II provides a very stable target for enhancer action. Some poised genes also contain stably bound, paused Pol II (19). Thus, in all studied cases, poised promoters contain stably bound DNA-protein complexes and are enhancer activated by the mechanisms that are different from classic recruiting.
CONCLUSIONS
In summary, recent studies identified striking mechanistic similarities between the bacterial and eukaryotic enhancers, revealing fundamental principles of action over a distance. In the majority of cases, inactive, stable protein-DNA complexes are formed at the enhancer and target promoter before activation. This indicates that enhancers are unable to establish a promoter de novo, since in this case it is unclear how the target promoter is identified. On activation, EPC is accompanied by chromatin looping and involves use of special mechanisms facilitating distant communication. Promoter activation occurs via a mechanism that is different from recruiting, likely because recruiting does not work over long distances.
The widespread use of enhancers in gene regulation dictates their involvement in development of human diseases (see reference 52 for a review). Indeed, recent studies revealed thousands of variant enhancer loci (VEL) that comprise a signature that is predictive of colon cancer (1). Without doubt, future studies of enhancers will reveal new mechanisms of development of human diseases and provide new targets for their treatment.
ACKNOWLEDGMENTS
This work was supported by NSF MCB-1050470 and Government of the Russian Federation (order number 220) grants to V.M.S.
Footnotes
Published ahead of print 8 October 2012
REFERENCES
- 1. Akhtar-Zaidi B, et al. 2012. Epigenomic enhancer profiling defines a signature of colon cancer. Science 336:736–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ameres SL, et al. 2005. Inducible DNA-loop formation blocks transcriptional activation by an SV40 enhancer. EMBO J. 24:358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bagga R, Michalowski S, Sabnis R, Griffith JD, Emerson BM. 2000. HMG I/Y regulates long-range enhancer-dependent transcription on DNA and chromatin by changes in DNA topology. Nucleic Acids Res. 28:2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellomy GR, Record MT., Jr 1990. Stable DNA loops in vivo and in vitro: roles in gene regulation at a distance and in biophysical characterization of DNA. Prog. Nucleic Acid Res. Mol. Biol. 39:81–128 [DOI] [PubMed] [Google Scholar]
- 5. Bondarenko V, Liu Y, Ninfa A, Studitsky VM. 2002. Action of prokaryotic enhancer over a distance does not require continued presence of promoter-bound sigma54 subunit. Nucleic Acids Res. 30:636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bondarenko VA, Jiang YI, Studitsky VM. 2003. Rationally designed insulator-like elements can block enhancer action in vitro. EMBO J. 22:4728–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bondarenko VA, Liu YV, Jiang YI, Studitsky VM. 2003. Communication over a large distance: enhancers and insulators. Biochem. Cell Biol. 81:241–251 [DOI] [PubMed] [Google Scholar]
- 8. Burley SK, Roeder RG. 1996. Biochemistry and structural biology of transcription factor IID (TFIID). Annu. Rev. Biochem. 65:769–799 [DOI] [PubMed] [Google Scholar]
- 9. Cheng B, et al. 2012. Functional association of Gdown1 with RNA polymerase II poised on human genes. Mol. Cell 45:38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorsett D, Strom L. 2012. The ancient and evolving roles of cohesin in gene expression and DNA repair. Curr. Biol. 22:R240–R250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Felsenfeld G, Dekker J. 2012. Genome architecture and expression. Curr. Opin. Genet. Dev. 22:59–61 [DOI] [PubMed] [Google Scholar]
- 12. Fraser P, Bickmore W. 2007. Nuclear organization of the genome and the potential for gene regulation. Nature 447:413–417 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert N, et al. 2004. Chromatin architecture of the human genome: gene-rich domains are enriched in open chromatin fibers. Cell 118:555–566 [DOI] [PubMed] [Google Scholar]
- 14. Gilchrist DA, et al. 2010. Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell 143:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golob JL, et al. 2011. Evidence that gene activation and silencing during stem cell differentiation requires a transcriptionally paused intermediate state. PLoS One 6:e22416 doi:10.1371/journal.pone.0022416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon F, Luger K, Hansen JC. 2005. The core histone N-terminal tail domains function independently and additively during salt-dependent oligomerization of nucleosomal arrays. J. Biol. Chem. 280:33701–33706 [DOI] [PubMed] [Google Scholar]
- 17. Huang J, Schlick T, Vologodskii A. 2001. Dynamics of site juxtaposition in supercoiled DNA. Proc. Natl. Acad. Sci. U. S. A. 98:968–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin C, et al. 2009. H3.3/H2A.Z double variant-containing nucleosomes mark “nucleosome-free regions” of active promoters and other regulatory regions. Nat. Genet. 41:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jishage M, et al. 2012. Transcriptional regulation by Pol II(G) involving mediator and competitive interactions of Gdown1 and TFIIF with Pol II. Mol. Cell 45:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kagey MH, et al. 2010. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kan PY, Lu X, Hansen JC, Hayes JJ. 2007. The H3 tail domain participates in multiple interactions during folding and self-association of nucleosome arrays. Mol. Cell. Biol. 27:2084–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kellner WA, Ramos E, Van Bortle K, Takenaka N, Corces VG. 2012. Genome-wide phosphoacetylation of histone H3 at Drosophila enhancers and promoters. Genome Res. 22:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim TK, et al. 2010. Widespread transcription at neuronal activity-regulated enhancers. Nature 465:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krivega I, Dean A. 2012. Enhancer and promoter interactions-long distance calls. Curr. Opin. Genet. Dev. 22:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulaeva OI, et al. 2012. Internucleosomal interactions mediated by histone tails allow distant communication in chromatin. J. Biol. Chem. 287:20248–20257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laybourn PJ, Kadonaga JT. 1992. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science 257:1682–1685 [DOI] [PubMed] [Google Scholar]
- 27. Li G, Levitus M, Bustamante C, Widom J. 2005. Rapid spontaneous accessibility of nucleosomal DNA. Nat. Struct. Mol. Biol. 12:46–53 [DOI] [PubMed] [Google Scholar]
- 28. Li G, et al. 2012. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ling J, et al. 2004. HS2 enhancer function is blocked by a transcriptional terminator inserted between the enhancer and the promoter. J. Biol. Chem. 279:51704–51713 [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Bondarenko V, Ninfa A, Studitsky VM. 2001. DNA supercoiling allows enhancer action over a large distance. Proc. Natl. Acad. Sci. U. S. A. 98:14883–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marks H, et al. 2012. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149:590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maston GA, Landt SG, Snyder M, Green MR. 2012. Characterization of enhancer function from genome-wide analyses. Annu. Rev. Genomics Hum. Genet. 13:29–57 [DOI] [PubMed] [Google Scholar]
- 33. McCord RP, Zhou VW, Yuh T, Bulyk ML. 2011. Distant cis-regulatory elements in human skeletal muscle differentiation. Genomics 98:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Min IM, et al. 2011. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 25:742–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morey C, Da Silva NR, Kmita M, Duboule D, Bickmore WA. 2008. Ectopic nuclear reorganisation driven by a Hoxb1 transgene transposed into Hoxd. J. Cell Sci. 121:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mukhopadhyay S, Schedl P, Studitsky VM, Sengupta AM. 2011. Theoretical analysis of the role of chromatin interactions in long-range action of enhancers and insulators. Proc. Natl. Acad. Sci. U. S. A. 108:19919–19924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naughton C, Sproul D, Hamilton C, Gilbert N. 2010. Analysis of active and inactive X chromosome architecture reveals the independent organization of 30 nm and large-scale chromatin structures. Mol. Cell 40:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ong CT, Corces VG. 2011. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat. Rev. Genet. 12:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ong CT, Corces VG. 2012. Enhancers: emerging roles in cell fate specification. EMBO Rep. 13:423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orom UA, et al. 2010. Long noncoding RNAs with enhancer-like function in human cells. Cell 143:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papai G, et al. 2010. TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation. Nature 465:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poirier MG, Bussiek M, Langowski J, Widom J. 2008. Spontaneous access to DNA target sites in folded chromatin fibers. J. Mol. Biol. 379:772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Polikanov YS, et al. 2007. Probability of the site juxtaposition determines the rate of protein-mediated DNA looping. Biophys. J. 93:2726–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ptashne M. 1986. Gene regulation by proteins acting nearby and at a distance. Nature 322:697–701 [DOI] [PubMed] [Google Scholar]
- 45. Rada-Iglesias A, et al. 2011. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470:279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ringrose L, Chabanis S, Angrand PO, Woodroofe C, Stewart AF. 1999. Quantitative comparison of DNA looping in vitro and in vivo: chromatin increases effective DNA flexibility at short distances. EMBO J. 18:6630–6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubtsov MA, Polikanov YS, Bondarenko VA, Wang YH, Studitsky VM. 2006. Chromatin structure can strongly facilitate enhancer action over a distance. Proc. Natl. Acad. Sci. U. S. A. 103:17690–17695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanyal A, Lajoie BR, Jain G, Dekker J. 2012. The long-range interaction landscape of gene promoters. Nature 489:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stein A, Dalal Y, Fleury TJ. 2002. Circle ligation of in vitro assembled chromatin indicates a highly flexible structure. Nucleic Acids Res. 30:5103–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taberlay PC, et al. 2011. Polycomb-repressed genes have permissive enhancers that initiate reprogramming. Cell 147:1283–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tremethick DJ. 2007. Higher-order structures of chromatin: the elusive 30 nm fiber. Cell 128:651–654 [DOI] [PubMed] [Google Scholar]
- 52. Visel A, Rubin EM, Pennacchio LA. 2009. Genomic views of distant-acting enhancers. Nature 461:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vologodskii A, Cozzarelli NR. 1996. Effect of supercoiling on the juxtaposition and relative orientation of DNA sites. Biophys. J. 70:2548–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang KC, et al. 2011. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. West AG, Gaszner M, Felsenfeld G. 2002. Insulators: many functions, many mechanisms. Genes Dev. 16:271–288 [DOI] [PubMed] [Google Scholar]
- 56. Yang J, Corces VG. 2012. Insulators, long-range interactions, and genome function. Curr. Opin. Genet. Dev. 22:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu X, et al. 2007. A facilitated tracking and transcription mechanism of long-range enhancer function. Nucleic Acids Res. 35:5532–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]