Abstract
Transcription of the Escherichia coli hydrogenase-1 operon (hyaABCDEF) is increased by the transcription factors ArcA and AppY under anaerobic growth conditions. However, IscR, which represses transcription of the hyaA promoter (PhyaA) under aerobic conditions, was not known to repress transcription of this promoter under anaerobic conditions. Here, we report that ArcA and AppY increase PhyaA expression under anaerobic conditions by antagonizing IscR binding at PhyaA, since IscR repression is observed when either ArcA or AppY is eliminated. The ability of ArcA and AppY to act as antirepressors of IscR repression of PhyaA depended on IscR levels, suggesting that IscR competes with ArcA and/or AppY for binding. In support of this competition model, electrophoretic mobility shift assays and DNase I footprinting showed that the ArcA and IscR binding sites overlap and that binding of ArcA and IscR is mutually exclusive. Unexpectedly, IscR with a C92A mutation (IscR-C92A), which mimics the clusterless form of the protein that is present predominantly under aerobic conditions, was a better repressor under anaerobic conditions of both PhyaA and a constitutive promoter containing the IscR binding site from PhyaA than wild-type IscR, which is predominantly in the [2Fe-2S] form under anaerobic conditions. This observation could not be explained by differences in DNA binding affinities or IscR levels, so we conclude that [2Fe-2S]-IscR is a weaker repressor of PhyaA than clusterless IscR. In sum, a combination of ArcA and AppY antirepression of IscR function, lower levels of IscR, and weak repression by [2Fe-2S]-IscR leads to increased PhyaA expression under anaerobic conditions.
INTRODUCTION
Hydrogenases, which catalyze the reversible oxidation of H2, are widespread throughout the prokaryotic world (36). Despite a detailed biochemical and structural understanding of these enzymes (11, 15), the importance of individual isoforms of hydrogenases to cellular physiology is often not known. In particular, hydrogenase-1 (Hyd-1), encoded by hyaAB of the hyaABCDEF operon, is one of two hydrogenases that function in H2 oxidation under anaerobic conditions in Escherichia coli (30). Recent studies have demonstrated that, unlike most hydrogenases, Hyd-1 is O2 tolerant (20), raising the question of whether it might also have a role under O2-limiting conditions. Expression of E. coli hya is also subject to various physiological inputs (i.e., anaerobiosis, stationary phase, nitrate, and phosphate starvation) (2, 5, 31), suggesting that it could have specialized roles.
It is well established that expression of the hya operon is increased under anaerobic fermentative conditions and decreased under aerobic or anaerobic respiratory conditions with nitrate, consistent with the proposal that Hyd-1 functions in fermentation, not respiration (30). NarL and NarP appear to be responsible for repression of hya expression in response to nitrate (5, 31), whereas the anaerobic transcription factors ArcA and AppY are required for the increased expression of the hya operon in response to anaerobiosis (5, 31). Under anaerobic conditions, the activity of the response regulator ArcA is increased through phosphorylation by the sensor kinase ArcB (1), while the activity of AppY is presumably increased by changes in appY transcription (6). More recently, another transcription factor, IscR, has been shown to directly repress the hyaA promoter (PhyaA) but only under aerobic conditions when ArcA and AppY are not active (14, 28). The finding that PhyaA was repressed by IscR under aerobic conditions was surprising since activation of PhyaA under anaerobic conditions by ArcA and AppY would make an aerobic repressor unnecessary.
DNase I footprinting showed that IscR binds to a site from −55 to −29 relative to the more-downstream of two hyaA transcription start sites (−2 and +1), both of which are repressed by IscR in in vitro transcription assays with RNA polymerase σ70 (14). The sequence of the protected site indicated that it belongs to the type 2 class of IscR binding sites, which binds with similar affinities to either the [2Fe-2S] cluster or the apo (clusterless) form of IscR (28). Although recent data indicate that the [2Fe-2S] cluster occupancy of IscR is decreased under aerobic conditions (13), we did not expect any differences in IscR function between aerobic and anaerobic conditions for promoters containing type 2 sites since the affinities of the two forms of IscR for the hyaA promoter region were similar. Despite this, we found that under anaerobic growth conditions when IscR is predominantly in the [2Fe-2S] form, IscR did not regulate most promoters with type 2 sites, including PhyaA (14).
In this study, we examined the roles of IscR, ArcA, and AppY in regulating the expression of the hyaA promoter under aerobic and anaerobic conditions. Using various combinations of mutant alleles, we investigated the role of ArcA and AppY in antagonizing IscR repression of PhyaA expression under anaerobic growth conditions. DNA binding assays were used to determine whether IscR and ArcA bind simultaneously to the hya promoter region. To test whether increasing the concentration of IscR could overcome antirepression by ArcA and AppY, we varied the levels of both the wild-type and a clusterless mutant of IscR and monitored the expression of the hya promoter under anaerobic conditions. Our studies revealed that IscR repressor function is diminished under anaerobic conditions because of antirepression by ArcA and AppY, decreased IscR levels, and decreased repressor function of [2Fe-2S]-IscR.
MATERIALS AND METHODS
Strain construction.
In-frame deletions of arcA and lacY were constructed by replacing the coding region with the cat cassette flanked by FLP recognition target (FRT) sites from pKD32 as described previously (8). Transduction with P1vir was used to move arcA::cat, lacY::cat, or appY::kan (from the Keio JW0553-1) (3) to the appropriate strain background(s) with selection for either 20 μg/ml chloramphenicol or 40 μg/ml kanamycin. In some instances, the antibiotic cassette was removed by transforming strains with pCP20, which encodes FLP recombinase (8), and screening for antibiotic sensitivity. In addition, transduction with P1vir was used to introduce kan-promoter-lacZ constructs into the appropriate strain background(s). Genetic manipulations were confirmed by colony PCR and, in some cases, DNA sequencing.
The chromosomal construct containing iscR under the control of Ptac and the isc operon under the control of PBAD (bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS) (13) was the starting point to construct a variant with a C-to-A mutation at position 92 encoded by iscR [iscR(C92A)]. The region encompassing bla-Ptac-iscR was amplified by colony PCR and subsequently cloned into the HindIII and BamHI sites of pACYClacIq-tet (4) to form pPK9156 (Table 1). Quikchange (Agilent) was subsequently used to create pPK9157 containing bla-Ptac-iscR(C92A). The bla-Ptac-iscR(C92A) region was amplified and electroporated into strain PK9133 as described previously (13) to create strain PK9175. Both bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS from strain PK9520 and bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS from strain PK9175 were moved to strains PK8153 (PhyaA-lacZ ΔlacY::FRT) and PK9176 (PhyaA-lacZ ΔlacY::FRT ΔarcA::FRT ΔappY::FRT) by transduction with P1vir by selecting for Apr and Cmr with 10 mM arabinose to derepress PBAD-iscS. After verification by DNA sequencing, the strains were transformed with pACYClacIq-tet.
Table 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Strains | ||
| MG1655 | λ− F− rph-1 | Laboratory stock |
| PK4854 | MG1655 ΔiscR | 33 |
| BW25993/pKD46 | lacIq hsdR514 ΔaraBADAH33 ΔrhaBADLD78 pKD46 | 8 |
| PK7599 | BL21(λ)DE ΔhimA::tet pPK6161 | 33 |
| PK7510 | BW25993 ΔarcA::cat | This study |
| PK7514 | MG1655 ΔarcA::cat | This study |
| PK7521 | MG1655 ΔarcA::FRT | This study |
| JW0553-1 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔappY773::kan rph-1 Δ(rhaD-rhaB)568 hsdR514 | Keio Collection |
| PK8713 | MG1655 ΔappY::FRT | This study |
| PK8714 | PK4854 ΔappY::FRT | This study |
| PK6881 | BW25993 PhyaA-lacZ | 14 |
| PK7573 | MG1655 PhyaA-lacZ | 14 |
| PK6886 | PK4854 PhyaAA-lacZ | 14 |
| PK8716 | PK8713 PhyaA-lacZ | This study |
| PK7525 | PK7521 PhyaA-lacZ | This study |
| PK8717 | PK8714 PhyaA-lacZ | This study |
| PK8776 | PK6886 ΔarcA::cat | This study |
| PK8767 | PK8716 ΔarcA::cat | This study |
| PK8768 | PK8717 ΔarcA::cat | This study |
| PK8758 | MG1655 PhyaA(−50G)-lacZ | 28 |
| PK8759 | PK4854 PhyaA(−50G)-lacZ | 28 |
| PK9282 | PK8713 PhyaA(−50G)-lacZ | This study |
| PK9283 | PK8714 PhyaA(−50G)-lacZ | This study |
| PK9285 | PK7514 PhyaA(−50G)-lacZ | This study |
| PK9517 | PK8759 ΔarcA::cat | This study |
| PK9518 | PK9283 ΔarcA::cat | This study |
| PK9519 | PK9282 ΔarcA::cat | This study |
| PK9520 | bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS | 13 |
| PK8137 | PK6881 pKD46 | This study |
| PK8139 | PK8137 ΔlacY::cat | This study |
| PK8148 | MG1655 PhyaA-lacZ ΔlacY::cat | This study |
| PK8153 | MG1655 PhyaA-lacZ ΔlacY::FRT | This study |
| PK8170 | PK4854 PhyaA-lacZ ΔlacY::FRT | This study |
| PK9522 | PK8153 bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS | This study |
| PK9524 | PK9522 pACYClacIq-tet | This study |
| PK9133 | FRT-cat-FRT-araC-PBAD | 13 |
| PK9175 | PK9133 bla-Ptac-iscR(C92A) | This study |
| PK9529 | PK8153 bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS pACYClacIq-tet | This study |
| PK9527 | PK8713ΔappY ΔarcA::FRT | This study |
| PK9176 | PK9527 PhyaA-lacZ ΔlacY::FRT | This study |
| PK9178 | PK9176 bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS | This study |
| PK9179 | PK9178 pACYClacIq-tet | This study |
| PK9184 | PK9176 bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS | This study |
| PK9188 | PK9184 pACYClacIq-tet | This study |
| PK10519 | MG1655 Psyn(hyaA)-lacZ ΔlacY | This study |
| PK10529 | PK4854 Psyn(hyaA)-lacZ ΔlacY | This study |
| PK10554 | PK10519 bla-Ptac iscR-araC-PBAD-iscS pACYClacIq-tet | This study |
| PK10556 | PK10519 bla-Ptac-iscR(C92A)-araC-PBAD-iscS pACYClacIq-tet | This study |
| Plasmids | ||
| pKD46 | Phage λ gam-bet-exo genes under ParaB control | 8 |
| pKD32 | FRT-cat-FRT | 8 |
| pCP20 | Apr | 8 |
| pACYClacIq-tet | lacIq in pACYC184, Tetr | 4 |
| pPK9156 | bla-Ptac-iscR cloned into HindIII/BamHI sites of pACYClacIq-tet | This study |
| pPK9157 | pPK9156 bla-Ptac-iscR(C92A) | This study |
| pPK6842 | PhyaA cloned into pUC19-spf with XhoI site replacing SalI site | 14 |
| pPK9003 | pRZ7411 based vector with bla from pBR322 at AflII site and Ptac from pPK7332 at AflII and NdeI sites | 13 |
| pPK7035 | pBR322 with kan from pHP45Ω and BamHI-NdeI fragment from pRS1553 | 17 |
| pPK9054 | pPK7035 with Ptac from pPK9003 cloned into BamHI and XhoI sites | This study |
| pPK6161 | iscR in pET11a | 33 |
A hybrid Psyn(hyaA) promoter, a synthetic promoter in which the IscR binding site from PhyaA was incorporated into a constitutive variant of Ptac, was constructed. Ptac from pPK9003 was first cloned into the BamHI and XhoI sites of pPK7035 to generate pPK9054. The Psyn(hyaA) promoter was constructed by Quikchange (Agilent) of pPK9054, in which the region from −52 to −28 relative to the transcription start site of Ptac in pPK9054 was replaced with the IscR binding site from PhyaA (−55 to −31 relative to the downstream-most transcription start site [14]). The DNA fragment containing kan-Psyn(hyaA)-lacZ was electroporated and recombined onto the chromosome of BW25993/pKD46 using a previously described method (8) with selection for Kmr. Subsequently, an in-frame deletion of lacY was constructed by replacing the coding region with the cat cassette flanked by FLP recognition target (FRT) sites from pKD32, as described previously, and the antibiotic cassette was removed by transforming the strain with pCP20, which encodes FLP recombinase (8). Transduction with P1vir was used to introduce kan-Psyn(hya)-lacZ with ΔlacY into PK4854 and MG1655, selecting for Kmr; the strains were then transformed with pACYClacIq-tet. Finally, bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS and bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS were introduced into PK10519 using P1vir transduction, selecting for Apr. The resulting strains were confirmed using colony PCR and sequencing.
β-Galactosidase assays.
Three independent isolates of each strain were grown to saturation in morpholinepropanesulfonic acid (MOPS) minimal medium with either 0.2% glucose (27) or, for strains containing bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS or bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS, 20 mM arabinose (35) with the indicated concentrations of isopropyl-β-d-thiogalactopyranoside (IPTG), 0.2% Casamino Acids (in Fig. 4) and tetracycline (10 μg/liter). The cultures were diluted 1:250 into the same medium containing the same additives and grown at 37°C under aerobic conditions to an optical density at 600 nm (OD600) of ∼0.2 or under anaerobic conditions to an OD600 of ∼0.1. The cultures were subsequently assayed for β-galactosidase activity (26). For comparison of aerobically and anaerobically grown cultures in Fig. 1, differences in cell numbers per OD600 unit were accounted for by multiplying the Miller units from aerobic cultures by 1.55, as described previously (25). The average of three isolates repeated on at least three independent occasions is presented. In Fig. 3 and 4, fold repression was calculated by dividing the promoter activity in a strain lacking IscR by the promoter activity in the indicated strain. Standard error of the fold repression was calculated as described previously (18).
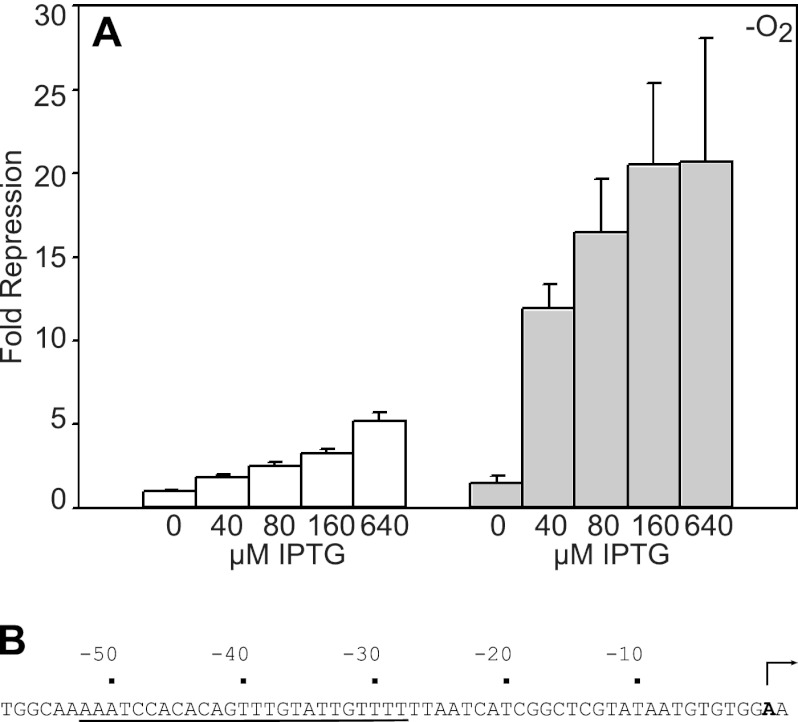
Fig 4.
(A) Expression levels of Psyn(hyaA) fused to lacZ in strains containing either bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS (white bars) or bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS (shaded bars). Cells were grown under anaerobic conditions in MOPS minimal medium containing 20 mM arabinose, tetracycline (10 μg/liter), 0.2% Casamino Acids, and the indicated concentration of IPTG and were assayed for β-galactosidase activity. Error bars for fold repression are given for the data from three strain isolates repeated on at least three independent occasions. (B) Sequence of Psyn(hyaA) promoter fused to lacZ. The IscR binding site from PhyaA (underlined) was incorporated into a construct containing a constitutively active variant of Ptac fused to lacZ. An arrow represents the position of the transcription initiation site.
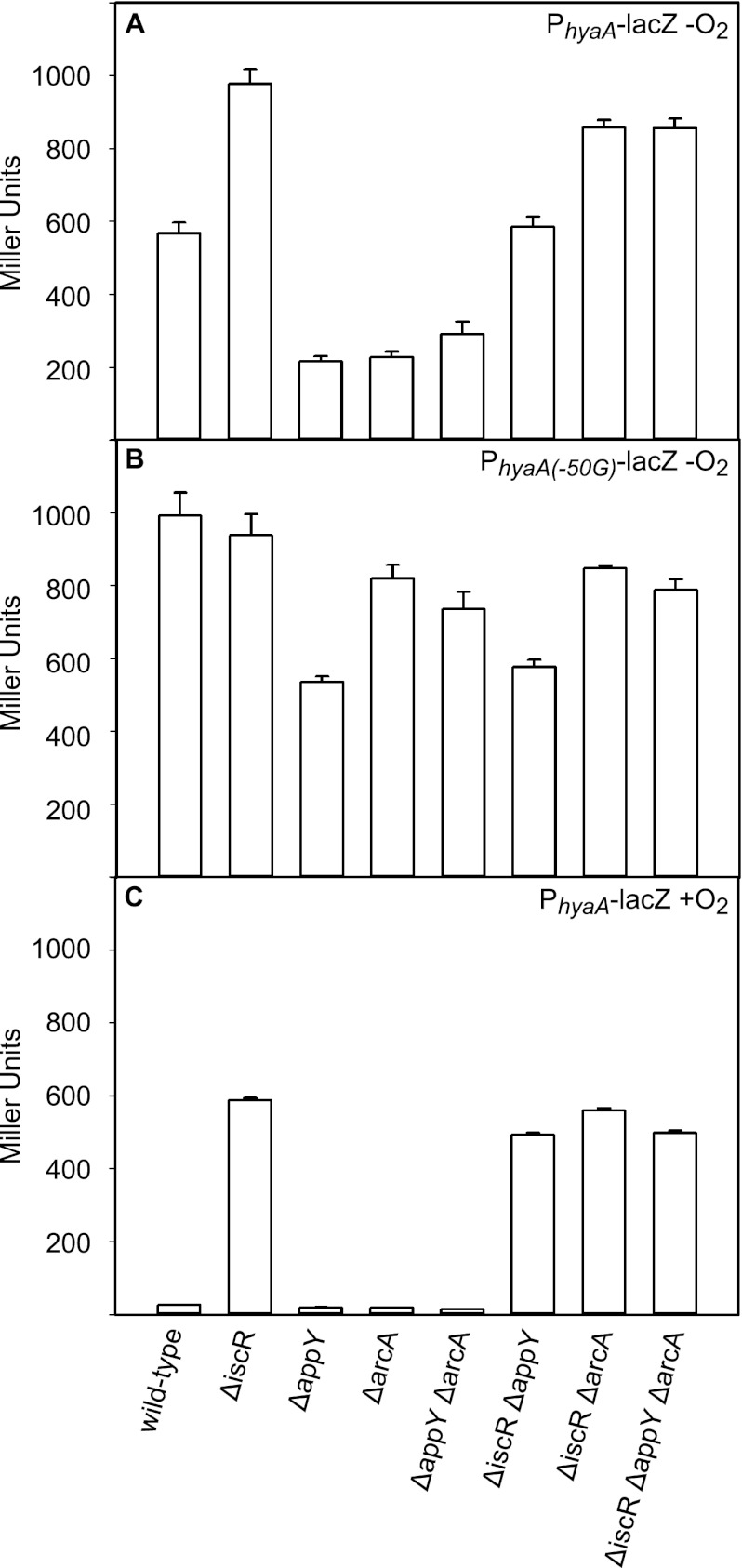
Fig 1.
Expression levels of PhyaA (A and C) or PhyaA(−50G) (B) fused to lacZ in wild-type, ΔiscR, ΔappY, ΔarcA, ΔappY ΔarcA, ΔiscR ΔappY, ΔiscR ΔarcA, or ΔiscR ΔappY ΔarcA strain backgrounds grown under anaerobic (A and B) or aerobic (C) conditions in MOPS minimal medium containing 0.2% glucose. Strains were assayed for β-galactosidase activity and normalized for cell number, as explained in Materials and Methods. Error bars are for the activity from three strain isolates repeated on at least three independent occasions.
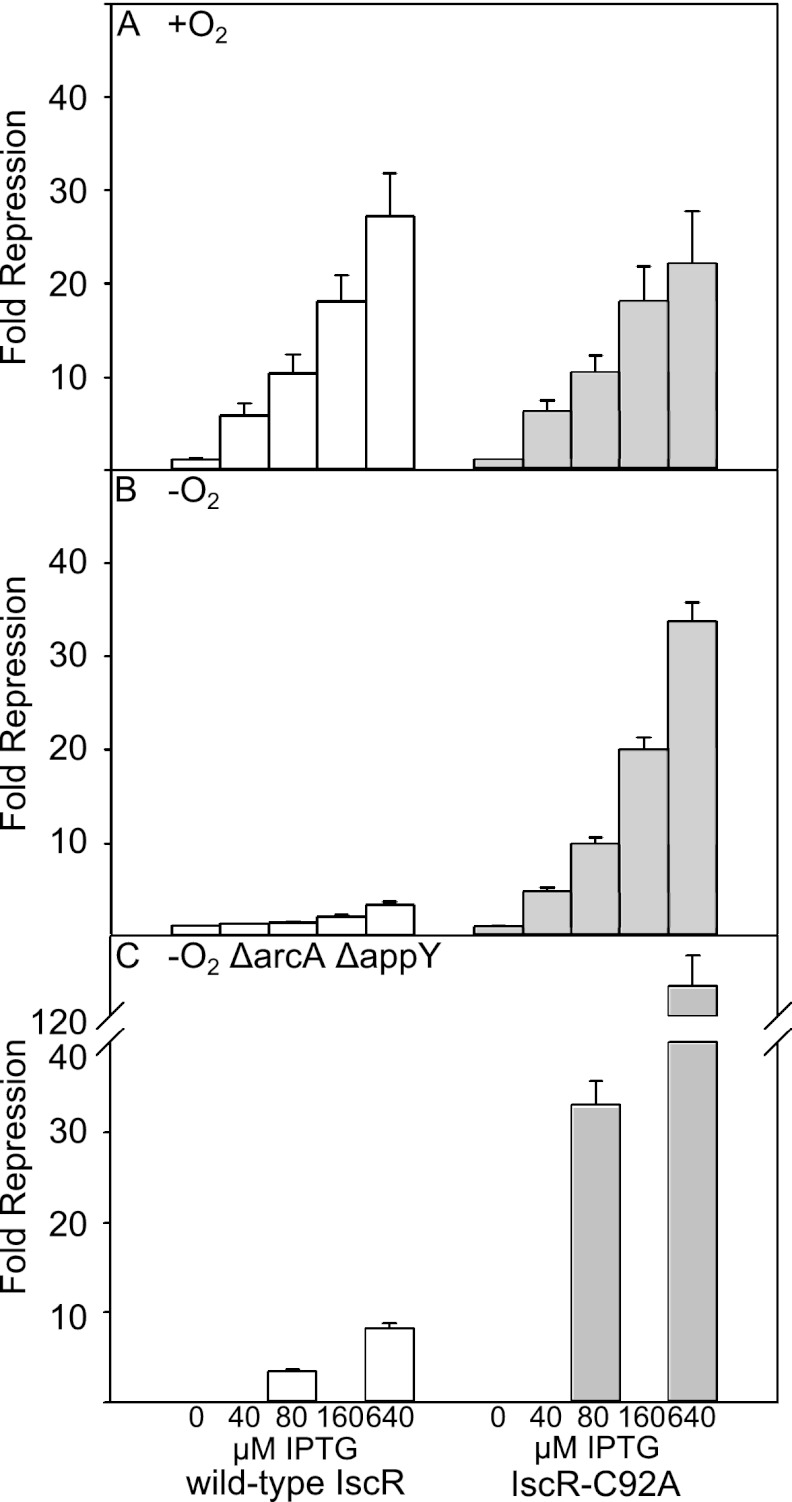
Fig 3.
Expression levels of PhyaA fused to lacZ in strains containing either bla-Ptac-iscR-FRT-cat-FRT-araC-PBAD-iscS (white bars) or bla-Ptac-iscR(C92A)-FRT-cat-FRT-araC-PBAD-iscS (shaded bars) in either a wild-type strain (A and B) or in a strain lacking AppY and ArcA (C). Cells were grown under aerobic (+O2) or anaerobic (−O2) conditions in MOPS minimal medium containing 20 mM arabinose, tetracycline (10 μg/liter), and the indicated concentration of IPTG and were assayed for β-galactosidase activity. Error bars represent data from three independent isolates repeated on at least three independent occasions.
Purification of IscR and ArcA.
Wild-type IscR was purified from PK7599 as described previously (14). N-terminal His6-tagged ArcA was expressed from a pET21d (Novagen) derivative in BL21(λDE3). The protein was isolated by passage over a Ni-nitrilotriacetic acid (NTA) matrix column (GE Healthcare) at 4°C and elution with 50 mM Tris-Cl, pH 7.0, 0.4 M NaCl, 1 mM EDTA, and 500 mM imidazole, followed by dialysis against 50 mM Tris-Cl, pH 7.0, 0.1 M NaCl, and 1 mM EDTA. The His6 tag was removed from ArcA by overnight incubation with tobacco etch virus (TEV) protease at 4°C and passage over a Ni2+-agarose column (Qiagen). The protein concentrations of ArcA and IscR and the iron and sulfide content of IscR were determined colorimetrically, as described previously (33).
DNase I footprinting and electrophoretic mobility shift assays.
A DNA fragment containing the hyaA region from −200 to +40 relative to the transcription start site was excised by BamHI and HindIII digestion of pPK6842, as described previously (14). The fragment was radiolabeled using Klenow fragment (New England BioLabs) with [α-32P]dGTP (GE Healthcare), and the DNA containing the hyaA region was isolated from a 5% nondenaturing gel, followed by purification via an Elutip-d column (Schleicher and Schuell). ArcA was phosphorylated by incubating ArcA with 50 mM disodium carbamyl phosphate in a buffer composed of 50 mM Tris, pH 7.9, 150 mM NaCl, and 10 mM MgCl2 for 1 h at 30°C (21) and immediately used in the binding assays.
DNase I footprinting was performed at 37°C by incubating protein with 4 nM hyaA DNA, 40 mM Tris, pH 7.9, 70 mM KCl, and 0.1 mg/ml bovine serum albumin (BSA) for 10 min before the addition of 2 μg/ml DNase I and 10 mM MgCl2. Assays were terminated after 30 s by addition of 300 mM sodium acetate (NaOAc) and 20 mM EDTA, pH 7.9; DNA was ethanol precipitated and resuspended in stop solution (USB Scientific) before being heated to 95°C for 30 s and loaded onto an 8% polyacrylamide gel with 7 M urea. An A+G ladder was made by formic acid modification of the radiolabeled DNA, followed by piperidine cleavage (23). The products were visualized using a Storm PhosphorImager (Molecular Dynamics).
Electrophoretic mobility shift assays were performed by incubating either 60% [2Fe-2S]-occupied as-purified wild-type IscR and/or phosphorylated ArcA with 5 nM radiolabeled DNA fragment containing the region from −200 to +40 of the hyaA promoter in 5% glycerol, 100 mM potassium glutamate (pH 7.5), 10 mM potassium phosphate (pH 7.5), 50 μg/ml BSA, 3 mM Tris-Cl (pH 7.9), and 10 mM MgCl2. After a 10-min incubation at 37°C, samples were loaded onto a 10% nondenaturing acrylamide (38:2, acrylamide/bis-acrylamide) gel and run at 150 V for 2 h. The gels were visualized using a Storm PhosphorImager (Molecular Dynamics).
RESULTS
AppY and ArcA prevent IscR repression of the hyaA promoter under anaerobic conditions.
In agreement with previous findings, deletion of arcA or appY or both arcA and appY resulted in a 3- to 4-fold decrease in expression of PhyaA-lacZ, whereas deletion of iscR alone showed only a slight increase in expression (5, 14, 31) (Fig. 1A). However, elimination of all three transcription factors (IscR, AppY, and ArcA) resulted in an unexpectedly high level of expression under anaerobic conditions, which was slightly greater than the level of expression in the parent strain (Fig. 1A). Therefore, the decreased expression in strains lacking ArcA and/or AppY under anaerobic conditions does not appear to result from a defect in activation by ArcA or AppY but, rather, from unmasking IscR repression in the absence of these other transcription factors. The repression by IscR in the absence of either ArcA or AppY appears to be a direct result of IscR binding, since the increase in expression observed from a mutation that is known to disrupt the IscR binding site (C-to-G mutation at position −50 in PhyaA [PhyaA(−50G)]) (28) mimicked the increase in expression of the ΔiscR strains (Fig. 1B).
These data also show that both AppY and ArcA must be present to prevent IscR repression of PhyaA under anaerobic conditions, since loss of either transcription factor is sufficient to permit repression by IscR. For example, the decreased expression of PhyaA that arises when strains lack ArcA (AppY+ and IscR+) or AppY (ArcA+ and IscR+) is reversed by the further elimination of IscR from either strain (Fig. 1A). ArcA alone appears to slightly repress PhyaA under anaerobic conditions, since a strain containing deletions of appY and iscR (ArcA+) showed an intermediate expression level (Fig. 1A). However, ArcA repression is not observed when all three transcription factors are present, suggesting that the major effect of ArcA under anaerobic conditions is to increase expression of PhyaA by preventing IscR repression.
ArcA and AppY had no effect on PhyaA expression under aerobic conditions, as expected (Fig. 1C), since ArcA is not active aerobically and since AppY is poorly expressed under these growth conditions (1, 6). In contrast, IscR was able to repress PhyaA-lacZ expression under aerobic conditions, as previously reported (14, 28). Consistent with the notion that PhyaA expression is regulated by IscR repression aerobically and by ArcA and AppY antirepression of IscR anaerobically, PhyaA-lacZ expression levels in the absence of any transcription factors were similar under anaerobic and aerobic conditions (Fig. 1A and C).
ArcA competes for binding with IscR at the hyaA promoter.
To examine how ArcA interferes with IscR repression of PhyaA, in vitro DNA binding experiments were carried out with isolated ArcA and IscR (Fig. 2A and B). The location of the ArcA binding site within the hya promoter region was determined by DNase I footprinting, which showed that phosphorylated ArcA bound hyaA DNA from position −32 to +3 relative to the transcription start site (Fig. 2A). As expected, no footprint was detected with similar concentrations of unphosphorylated ArcA (data not shown). Comparison of the DNA sequence within the bound region to the predicted ArcA consensus (5′-GTTAATTAAATGTTA-3′) (10, 24) shows a match from position −17 to +1 on the bottom strand (Fig. 2C). The location of the ArcA footprint overlapped the predicted −10 promoter hexamer for binding RNA polymerase σ70.
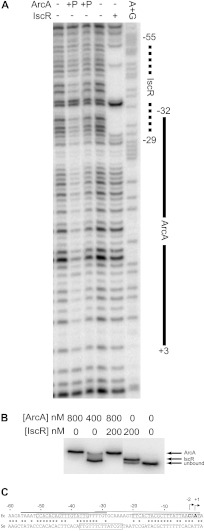
Fig 2.
(A) DNase I footprinting of ArcA and IscR binding to the hyaA promoter region. The labeled DNA is the top strand from −200 to +40 relative to the hyaA transcription start site. Regions protected by IscR (dashed line) or phosphorylated ArcA (solid line) are marked, and numbers are relative to the downstream transcription start site. Lanes 1 and 4 lack IscR or ArcA; lanes 2 and 3 contain 800 nM and 400 nM phosphorylated ArcA, respectively; lane 5 contains 800 nM as-purified IscR; lane 6 is a Maxam and Gilbert A+G ladder made from the same DNA. (B) Electrophoretic mobility shift assay using the same radiolabeled hyaA DNA as the DNase I footprinting assay. Lanes 1 and 2 contain 800 nM and 400 nM phosphorylated ArcA, respectively; lane 3 contains 200 nM as-purified IscR and 800 nM phosphorylated ArcA; lane 4 contains 200 nM as-purified IscR; lane 5 contains no protein. (C) The regulatory region of the hya promoter from E. coli (Ec) and S. enterica (Se). The upstream IscR and downstream ArcA sites are boxed in the E. coli sequence (top). A predicted FNR site is boxed in the S. enterica sequence (bottom). Asterisks indicate conserved residues. The thick line is the IscR footprinted region, and the thin line is the ArcA footprinted region. Arrows indicate the positions of the two transcription initiation sites (31).
Notably, the region protected by ArcA (−32 to +3) was also found to overlap the region protected by IscR (−55 to −29) (Fig. 2A and C) (14), suggesting that binding of ArcA and IscR to hyaA promoter DNA may be mutually exclusive. To test whether IscR and ArcA can bind simultaneously, electrophoretic mobility shift assays were performed with radiolabeled hyaA DNA, IscR, and/or phosphorylated ArcA. The protein-DNA complex observed with phosphorylated ArcA was larger than the complex with [2Fe-2S]-IscR. However, no complex with a size larger than the ArcA-DNA complex was observed when both proteins were present in the binding assay, indicating that binding of ArcA and IscR to hyaA promoter DNA is mutually exclusive (Fig. 2B). Furthermore, preincubation of hyaA DNA with phosphorylated ArcA followed by addition of IscR or vice versa did not produce an additional complex, suggesting that the order of addition does not affect binding (data not shown). Thus, ArcA and IscR appear to compete for binding to the hyaA promoter.
No binding site information is available for AppY, and attempts to carry out binding studies with AppY were unsuccessful due to an inability to express amounts sufficient for its isolation. Therefore, while the role of AppY in preventing repression by IscR is unclear, the simplest model from the combined results is that both AppY and ArcA act as antirepressors, competing with the repressor IscR for binding to PhyaA.
Lower IscR levels also contribute to decreased IscR regulation under anaerobic conditions.
Since ArcA competes with IscR for binding to PhyaA, we examined whether the proposed antirepression mechanism is dependent on the low IscR protein levels observed under anaerobic conditions compared to levels observed under aerobic conditions (13). Therefore, a strain containing both iscR under the control of Ptac and iscSUA hscBA fdx under the control of PBAD was grown aerobically (Fig. 3A) or anaerobically (Fig. 3B) in the presence of different IPTG concentrations to determine the effect of various IscR levels within the range normally produced under either condition. In addition, the levels of the Isc pathway were maintained at levels similar to aerobic conditions by the addition of 20 mM arabinose (data not shown). We found that as the levels of IscR were increased (by increasing the IPTG concentration) under both conditions, there was a corresponding increase in repression by IscR (Fig. 3). However, much greater repression was observed aerobically than anaerobically at the same concentrations of IPTG, consistent with direct IscR repression at PhyaA without competition from ArcA and AppY aerobically. Furthermore, by similarly increasing the levels of IscR in a strain lacking ArcA and AppY, we found that less IscR was required for repression anaerobically (Fig. 3C) than in strains containing AppY and ArcA (Fig. 3B). Therefore, downregulation of IscR levels under anaerobic conditions appears to be important for proper regulation of PhyaA, such that IscR competition with ArcA and AppY for PhyaA binding is maintained at a low level, keeping PhyaA from being repressed anaerobically.
[2Fe-2S]-IscR exhibits decreased repressor function at the hyaA promoter.
Despite the fact that we observed more repression of the hyaA promoter with increasing levels of IscR, the extent of PhyaA repression under anaerobic conditions was never as great as that measured under aerobic conditions, even in the absence of AppY and ArcA (Fig. 1 and 3). The observed difference in repression between anaerobic and aerobic growth conditions was not due to insufficient IscR concentrations since the highest IPTG concentrations resulted in IscR protein levels well above those observed in aerobic cells (determined by Western blotting) (data not shown). Since we have previously observed a difference in IscR cluster occupancy between anaerobic and aerobic conditions (13), we examined the possibility that [2Fe-2S]- and apo-IscR might function differently in their abilities to repress PhyaA. To determine whether cluster occupancy affects the ability of IscR to repress under anaerobic conditions, we measured PhyaA-lacZ expression while increasing the levels of IscR with the C92A mutation (IscR-C92A), which lacks the [2Fe-2S] cluster. We found that under anaerobic conditions, more repression of PhyaA was observed with IscR-C92A than with wild-type IscR at the same IPTG concentrations (Fig. 3B), despite the fact that protein levels were similar for wild-type IscR and IscR-C92A (data not shown). A further, but small, increase in repression of PhyaA was observed with IscR-C92A when ArcA and AppY were eliminated (Fig. 3C), indicating that the ability of apo-IscR to repress PhyaA under anaerobic conditions is largely indifferent to the presence of ArcA and AppY. Hence, [2Fe-2S]-IscR appears to be a weaker repressor of PhyaA than apo-IscR.
In order to test IscR repression of the hyaA site in the absence of other factors that might unknowingly affect PhyaA regulation anaerobically, we incorporated the IscR binding site from PhyaA into a constitutively active promoter [Psyn(hya)]. In this synthetic promoter construct, the 25-bp IscR binding site from PhyaA was aligned in the same register relative to the −35 σ70 promoter recognition element of Ptac. When we tested the ability of different levels of wild-type IscR (containing the [2Fe-2S] cluster) and IscR-C92A (apo mutant) to repress the synthetic promoter under anaerobic conditions, we found that the apo mutant repressed more efficiently than the wild-type IscR (Fig. 4). Since removing the IscR binding site from the context of PhyaA results in a similar preference for promoter repression by the apo form of IscR, it is unlikely that there is another factor dictating the cluster dependence of PhyaA repression by IscR. Thus, in addition to decreased IscR protein levels under anaerobic conditions, the poor repressing function of the [2Fe-2S] form of IscR, which predominates under anaerobic conditions, contributes to overall regulation of PhyaA under anaerobic conditions.
DISCUSSION
By examining the expression of the hya operon encoding Hyd-1 in E. coli K-12, we have uncovered a new antirepression mechanism that results in O2-regulated gene expression. We found that under anaerobic conditions two transcription factors, ArcA and AppY, work together to overcome the repression of PhyaA by IscR that occurs under aerobic conditions. Antirepression by the anaerobic transcription factors ArcA and AppY, in addition to a combination of decreased levels of wild-type IscR and the weak repressing function of [2Fe-2S]-IscR, resulted in the lack of repression by IscR under anaerobic conditions. Thus, these data suggest a new mechanism by which promoters can be regulated by O2 availability.
ArcA, AppY, and IscR act combinatorially to control hyaA promoter expression levels under anaerobic conditions.
Previous studies (5, 31) showed that deletion of ArcA or AppY results in decreased expression of PhyaA under anaerobic conditions, which led to the hypothesis that ArcA and AppY activated PhyaA. However, the mechanism by which activation occurred was unknown. Our data indicate that the decrease in expression upon elimination of ArcA or AppY is not due to activation by either transcription factor, but, rather, to repression by IscR in the absence of ArcA and/or AppY. Based on the DNase I footprint and electrophoretic mobility shift assays presented in this study, we propose that ArcA antagonizes IscR repression by partially occluding IscR binding. Although isolation of AppY will be required before we can elucidate how this protein inhibits IscR repression at PhyaA, the simplest explanation is that both AppY and ArcA block IscR binding. Taken together, these data show how multiple transcription factors can act combinatorially to achieve an effect not possible by a single transcription factor.
Linking of expression of Hyd-1 with the cytochrome bd-II oxidase.
Immediately downstream of the hya operon are the genes cbdAB (also known as appCB) encoding subunits of the cytochrome bd-II oxidase. Previous in vivo studies indicated that cbdAB expression is also decreased in strains lacking AppY and ArcA (6). Furthermore, transcription of both cbdAB and hya operons is regulated by similar growth conditions, including stationary phase, phosphate starvation, and anaerobiosis (2). The similar regulation of these two operons suggests that they may function in a common pathway. Since cytochrome bd-II oxidase functions to reduce O2 to H2O (34), it is reasonable to speculate that under low-O2 conditions, H2 oxidation via Hyd-1 might provide reducing equivalents for the cytochrome bd-II oxidase and explain the O2 tolerance of Hyd-1. If this is indeed the case, then the combinatorial control that we have observed of PhyaA may also facilitate expression under low-O2 conditions.
Novel features of IscR regulation.
The lack of repression of PhyaA under anaerobic conditions appears to be linked to the lower anaerobic levels of IscR (10-fold less than aerobic protein levels [13]) since artificially increasing the levels of wild-type IscR to those found under aerobic conditions resulted in increased repression of PhyaA by IscR, even in the presence of ArcA and AppY. Hence, the concentration of IscR, in addition to regulation by ArcA and AppY, is important for determining the anaerobic expression levels of PhyaA.
Surprisingly, we also found that even in the absence of AppY and ArcA, wild-type IscR could not repress PhyaA anaerobically as well as an IscR mutant that lacked the Fe-S cluster or as well as wild-type IscR aerobically at the same protein concentrations. The only reported difference regarding IscR between aerobic and anaerobic conditions is that under anaerobic conditions IscR appears to be more highly occupied with the [2Fe-2S] cluster than its aerobic counterpart (13). Since in vitro DNA binding experiments have shown that [2Fe-2S]-IscR and apo-IscR bind the hyaA DNA site with the same affinities (16, 28), our data imply that [2Fe-2S]-IscR is a weaker repressor than the apo form at PhyaA. The differential repressor activities of [2Fe-2S]- and apo-IscR are reminiscent of [2Fe-2S]-SoxR, which binds DNA in both an active and inactive state but regulates transcription only in the active state (12, 16). However, additional experiments will be required to explain this observation.
Interestingly, the mechanism described here for the regulation of the hyaA promoter may be relevant to the IscR-dependent regulation of other promoters that contain type 2 IscR binding sites and are regulated by multiple transcription factors, including the sufA, hybO, and sodA promoters (7, 14, 31, 37). However, the combination of transcription factors active at each promoter varies, suggesting that the mechanism of regulation of these promoters is different. How IscR interacts with other transcription factors to regulate this subset of promoters is therefore in need of further study.
ArcA- and IscR-dependent regulation of the hyaA promoter is not conserved in a close relative.
Surprisingly, the mechanism of O2 regulation of PhyaA described here for E. coli K-12 is not conserved in the close relative Salmonella enterica serovar Typhimurium. Rather, hya expression in S. enterica is regulated not by IscR or ArcA but, rather, by FNR under anaerobic conditions (38). However, comparison of the hyaA upstream promoter regions from the two organisms suggests that while the IscR binding site is conserved, a single base pair mismatch to the ArcA site may decrease DNA binding, thus preventing ArcA regulation (Fig. 2C). Further work is necessary to understand the significance of this finding, including mapping the transcription start site for the hya operon from S. enterica, since it is not known whether the positions of the promoter elements are conserved. However, the sequence similarities between the S. enterica and E. coli promoter regions may suggest a recent loss in the regulatory control by ArcA and IscR, with a gain of FNR regulation in this closely related enterobacterium.
Role of ArcA as an antirepressor.
Phosphorylated ArcA directly regulates many genes, including those encoding aerobic or anaerobic respiratory enzymes, and typically functions as a repressor (19, 32). Therefore, the role of ArcA in antirepression of IscR at PhyaA described in this study is more consistent with its typical function as a repressor than the previously suggested role as an activator at this promoter. However, the role of ArcA as an anaerobic antirepressor of IscR at PhyaA raises the question of whether the decreased expression of other promoters in strains lacking ArcA is due to the loss of ArcA activation or to the unmasking of repression by another transcription factor. Several other promoters that show decreased expression in strains lacking ArcA (erpA, cydA, ndh, caiT, and focA promoters) (9, 19, 32) are also regulated by other transcription factors, so antirepression by ArcA might be possible at other targets. Alternatively, these transcripts may be targets of the small RNA ArcZ (22) although transcript analysis of an S. enterica arcZ mutant did not show regulation of these genes by ArcZ (29). Because ArcA has not yet been shown to activate transcription in vitro, it remains to be seen whether ArcA functions as an activator or as an antirepressor at other promoters, whose expression decreases upon deletion of arcA.
In sum, we have found that a combinatorial strategy leads to ArcA and AppY overcoming IscR repression at PhyaA. Thus, we have uncovered a new antirepression mechanism to increase gene expression under anaerobic conditions that does not require direct activation of RNA polymerase. Additional studies will be needed to determine the extent to which an antirepression mechanism is used to control the expression of other O2-regulated genes in E. coli K-12 and other facultative bacteria.
ACKNOWLEDGMENTS
This work was supported by the NIH grant GM45844 to P.J.K. A.D.N. is a trainee of the NIH Molecular Biosciences Predoctoral Training Grant GM07215 to the University of Wisconsin—Madison, and A.S.F. is funded by Ruth L. Kirschstein NIH postdoctoral fellowship F32GM085987.
We thank James Keck for kindly providing TEV protease, Sohail Akhtar for providing purified His6-ArcA, Lee Meredith for assistance with the β-galactosidase activity assays, and the Kiley lab for comments on the manuscript.
Footnotes
Published ahead of print 12 October 2012
REFERENCES
- 1. Alvarez AF, Georgellis D. 2010. In vitro and in vivo analysis of the ArcB/A redox signaling pathway. Methods Enzymol. 471:205–228 [DOI] [PubMed] [Google Scholar]
- 2. Atlung T, Knudsen K, Heerfordt L, Brondsted L. 1997. Effects of σS and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J. Bacteriol. 179:2141–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd D, Manoil C, Beckwith J. 1987. Determinants of membrane protein topology. Proc. Natl. Acad. Sci. U. S. A. 84:8525–8529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brondsted L, Atlung T. 1994. Anaerobic regulation of the hydrogenase 1 (hya) operon of Escherichia coli. J. Bacteriol. 176:5423–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brondsted L, Atlung T. 1996. Effect of growth conditions on expression of the acid phosphatase (cyx-appA) operon and the appY gene, which encodes a transcriptional activator of Escherichia coli. J. Bacteriol. 178:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Compan I, Touati D. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 175:1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drapal N, Sawers G. 1995. Purification of ArcA and analysis of its specific interaction with the pfl promoter-regulatory region. Mol. Microbiol. 16:597–607 [DOI] [PubMed] [Google Scholar]
- 10. Favorov AV, et al. 2005. A Gibbs sampler for identification of symmetrically structured, spaced DNA motifs with improved estimation of the signal length. Bioinformatics 21:2240–2245 [DOI] [PubMed] [Google Scholar]
- 11. Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. 2007. Structure/function relationships of [NiFe]- and [FeFe]-hydrogenases. Chem. Rev. 107:4273–4303 [DOI] [PubMed] [Google Scholar]
- 12. Gaudu P, Weiss B. 1996. SoxR, a 2Fe-2S transcription factor, is active only in its oxidized form. Proc. Natl. Acad. Sci. U. S. A. 93:10094–10098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giel JL, et al. 17 October 2012, posting date Regulation of iron-sulfur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol. Microbiol. doi:10.1111/mmi.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. 2006. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol. 60:1058–1075 [DOI] [PubMed] [Google Scholar]
- 15. Hexter SV, Grey F, Happe T, Climent V, Armstrong FA. 2012. Electrocatalytic mechanism of reversible hydrogen cycling by enzymes and distinctions between the major classes of hydrogenases. Proc. Natl. Acad. Sci. U. S. A. 109:11516–11521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hidalgo E, Leautaud V, Demple B. 1998. The redox-regulated SoxR protein acts from a single DNA site as a repressor and an allosteric activator. EMBO J. 17:2629–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang Y, Weber KD, Qiu Y, Kiley PJ, Blattner FR. 2005. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 187:1135–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ku HH. 1966. Notes on the use of propagation of error formulas. J. Res. Natl. Bur. Stand. C Eng. Instrum. 70C:263–273 [Google Scholar]
- 19. Liu X, De Wulf P. 2004. Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J. Biol. Chem. 279:12588–12597 [DOI] [PubMed] [Google Scholar]
- 20. Lukey MJ, et al. 2010. How Escherichia coli is equipped to oxidize hydrogen under different redox conditions. J. Biol. Chem. 285:3928–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch AS, Lin EC. 1996. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol. 178:6238–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandin P, Gottesman S. 2010. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 29:3094–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maxam AM, Gilbert W. 1980. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 65:499–560 [DOI] [PubMed] [Google Scholar]
- 24. McGuire AM, De Wulf P, Church GM, Lin EC. 1999. A weight matrix for binding recognition by the redox-response regulator ArcA-P of Escherichia coli. Mol. Microbiol. 32:219–221 [DOI] [PubMed] [Google Scholar]
- 25. Mettert EL, Kiley PJ. 2007. Contributions of [4Fe-4S]-FNR and integration host factor to fnr transcriptional regulation. J. Bacteriol. 189:3036–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nesbit AD, Giel JL, Rose JC, Kiley PJ. 2009. Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J. Mol. Biol. 387:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papenfort K, et al. 2009. Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved, Hfq-dependent small RNA. Mol. Microbiol. 74:139–158 [DOI] [PubMed] [Google Scholar]
- 30. Pinske C, McDowall JS, Sargent F, Sawers RG. 2012. Analysis of hydrogenase 1 levels reveals an intimate link between carbon and hydrogen metabolism in Escherichia coli K-12. Microbiology 158:856–868 [DOI] [PubMed] [Google Scholar]
- 31. Richard DJ, Sawers G, Sargent F, McWalter L, Boxer DH. 1999. Transcriptional regulation in response to oxygen and nitrate of the operons encoding the [NiFe] hydrogenases 1 and 2 of Escherichia coli. Microbiology 145:2903–2912 [DOI] [PubMed] [Google Scholar]
- 32. Salmon KA, et al. 2005. Global gene expression profiling in Escherichia coli K12: effects of oxygen availability and ArcA. J. Biol. Chem. 280:15084–15096 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz CJ, et al. 2001. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc. Natl. Acad. Sci. U. S. A. 98:14895–14900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shepherd M, Sanguinetti G, Cook GM, Poole RK. 2010. Compensations for diminished terminal oxidase activity in Escherichia coli: cytochrome bd-II-mediated respiration and glutamate metabolism. J. Biol. Chem. 285:18464–18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stewart V, Bledsoe PJ, Chen LL, Cai A. 2009. Catabolite repression control of napF (periplasmic nitrate reductase) operon expression in Escherichia coli K-12. J. Bacteriol. 191:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vignais PM, Billoud B. 2007. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107:4206–4272 [DOI] [PubMed] [Google Scholar]
- 37. Yeo WS, Lee JH, Lee KC, Roe JH. 2006. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol. Microbiol. 61:206–218 [DOI] [PubMed] [Google Scholar]
- 38. Zbell AL, Benoit SL, Maier RJ. 2007. Differential expression of NiFe uptake-type hydrogenase genes in Salmonella enterica serovar Typhimurium. Microbiology 153:3508–3516 [DOI] [PubMed] [Google Scholar]