Abstract
The moderately halotolerant cyanobacterium Synechocystis sp. strain PCC 6803 contains a plasma membrane aquaporin, AqpZ. We previously reported that AqpZ plays a role in glucose metabolism under photomixotrophic growth conditions, suggesting involvement of AqpZ in cytosolic osmolarity homeostasis. To further elucidate the physiological role of AqpZ, we have studied its gene expression profile and its function in Synechocystis. The expression level of aqpZ was regulated by the circadian clock. AqpZ activity was insensitive to mercury in Xenopus oocytes and in Synechocystis, indicating that the AqpZ can be categorized as a mercury-insensitive aquaporin. Stopped-flow light-scattering spectrophotometry showed that addition of sorbitol and NaCl led to a slower decrease in cell volume of the Synechocystis ΔaqpZ strain than the wild type. The ΔaqpZ cells were more tolerant to hyperosmotic shock by sorbitol than the wild type. Consistent with this, recovery of oxygen evolution after a hyperosmotic shock by sorbitol was faster in the ΔaqpZ strain than in the wild type. In contrast, NaCl stress had only a small effect on oxygen evolution. The amount of AqpZ protein remained unchanged by the addition of sorbitol but decreased after addition of NaCl. This decrease is likely to be a mechanism to alleviate the effects of high salinity on the cells. Our results indicate that Synechocystis AqpZ functions as a water transport system that responds to daily oscillations of intracellular osmolarity.
INTRODUCTION
The photoautotrophic cyanobacterium Synechocystis sp. strain PCC 6803 (henceforth referred to as Synechocystis) is a useful model organism not only for the study of photosynthesis but also for understanding the process of osmoadaptation (17, 20). Cells have developed different membrane transport systems to maintain cellular ion homeostasis and to secure their survival, despite being exposed to frequent changes in the osmolarity of their environment. Simultaneously with the transport of ions across the membrane or the change in intracellular concentration of compatible solutes, such as glucosylglycerol, carbamides, polyols, purines, pyrimidines, and glycerol, by de novo synthesis in response to the external salt concentration or osmolarity change, water flux occurs mainly through water-permeable channels called aquaporins (18, 19, 31, 32, 39, 40, 44, 47). Synechocystis contains in its genome a single-copy gene encoding an aquaporin homolog, aqpZ (1, 7, 42). The AqpZ protein resides in the plasma membrane in the cell (1). Deletion of Synechocystis aquaporin aqpZ resulted in an increase in cell volume in the presence of low concentrations (5 mM) of glucose (1). This indicates that AqpZ plays a crucial role in the cellular osmotic adaptation to fluctuation of cytosolic osmotic pressure due to production and consumption of the photosynthetic products synthesized during the day.
Some aspects of Synechocystis aquaporin function have been studied previously. Shapiguzov et al. conducted a microarray analysis to determine the expression profile of genes induced by hyperosmotic conditions in both the wild type (WT) and a ΔaqpZ strain (42). Azad et al. compared the water permeability of the Synechocystis aqpZ mutant and its knock-in cells with that of the wild type (7). Cyanobacterial aquaporins, including AqpZ, have been reported to exhibit hypersensitivity to oxidative reagents like mercury (7), and even before the isolation of the aqpZ gene from Synechocystis, oxidative reagents like mercury or p-chloromercuriphenyl-sulfonic acid have been used as water channel blockers in cyanobacteria (3). Since oxidative stress-related products, including reactive oxygen species, are elicited in response to metabolic changes and environmental stress (6, 25), the degree of sensitivity of AqpZ to sulfhydryl reagents may be crucial for the regulation of AqpZ function. Mercury-sensitive aquaporins like human AQP1 (hAQP1) and Arabidopsis γ-Tip (AtTIP1;1) contain cysteines which are the targets of inhibition of water permeability by mercury (12, 30, 31, 38). These cysteines in hAQP1 and AtTIP1;1 are located in close spatial distance to the Asn-Pro-Ala motif of the aquaporin filter (12, 38). Synechocystis AqpZ contains two cysteines, Cys 19 in the first transmembrane span and Cys 205 on the extracellular side (see Fig. S3A in the supplemental material), which are relatively far from the pore region. Hence, in this study, we reexamined the specificity of blockage of AqpZ-mediated water transport in a heterologous expression system as well as in Synechocystis.
Synechocystis belongs to the group of moderately halotolerant cyanobacteria (10, 41). Sodium is an essential element for cell division, photosynthesis, and pH regulation in cyanobacteria (34, 55), although excess amounts of sodium function as the causative element in salinity stress. Synechocystis contains several genes encoding Na+/H+ antiporters that mediate the exchange of Na+ and H+ across the plasma membrane and the thylakoid membrane (14, 21, 33, 48). The potassium transport system also contributes to maintaining the balance of Na+/K+ in the cell, which protects it from high-salinity stress and high osmolarity (8, 28). Water flux occurs mainly through water-permeable channels, including aquaporins, simultaneously with the transport of ions across the membrane and the change in concentration of the compatible solutes synthesized de novo in the cells. In Synechocystis, it has been reported that very little shrinkage of ΔaqpZ cells was observed after addition of large amounts of a nonionic compound, sorbitol, to the medium (7, 42). Unlike Na+, sorbitol shows very low permeation across the plasma membrane in the cyanobacterium Synechococcus sp. strain PCC 7942 (2).
The precise functional characterization of AqpZ is vital to understanding the molecular mechanism and physiological role of AqpZ-mediated cellular osmotic homeostasis in response to ionic compounds like NaCl and nonionic compounds like sorbitol. In the present study, we determined the circadian expression profile of aqpZ and the translational expression of AqpZ in response to the addition of sorbitol or NaCl. We also characterized the function of AqpZ in mediating the response of the cell to changes in high osmolarity and salinity in Synechocystis.
MATERIALS AND METHODS
Measurement of circadian rhythm and growth rate of Synechocystis cells.
A 371-bp DNA segment carrying the putative aqpZ promoter (PaqpZ) was fused to the bacterial luciferase gene set luxAB at the AflII and NdeI sites of p68TS1ΩLuxAB(+)PLNK (K. Onai and M. Ishiura, unpublished data), and this PaqpZ::luxAB reporter gene was inserted into the specific targeting site (TS1) of the Synechocystis genome (24). A PdnaK::luxAB bioluminescent reporter strain (4, 24) was used as a control. Cells were grown to colonies on solid BG11 medium (1, 42) at 30°C under white fluorescence lamps (42 μmol of photons m−2 s−1), placed in the dark for 12 h to synchronize the circadian clock, and then kept under constant light. Bioluminescence from colonies was measured automatically every hour as described previously (35, 36) using automated bioluminescence monitoring apparatuses (K. Okamoto, K. Onai, and M. Ishiura, unpublished data; models LL04-1 and CL24-W; Churitsu Electric Corp., Nagoya, Japan). The amplitude of the rhythms was calculated as the average ratios of the peak to the trough in each cycle. Circadian time (CT) was calculated by dividing the phase value by the period length and multiplying by 24. The growth rates of the cells were measured by a continuous culture system as described previously (24, 29). This system continuously monitored the optical density (OD) of the cultures with an optical sensor and automatically diluted the cultures with fresh medium to the preset OD. Cells were grown in liquid BG11 medium at 30°C under white fluorescent lights (34 μmol of photons m−2 s−1) with bubbling of air and stirring until the OD at 730 nm reached 0.50, exposed to 12 h of darkness to synchronize the clock, and then maintained at the same OD under constant light. Growth rates (volume of fresh medium added + culture volume/culture volume/hour) were calculated.
Bacterial strains and culture conditions.
The glucose-tolerant (GT) strain of Synechocystis sp. PCC 6803 and its derivatives were grown in BG11 medium (1, 43) supplemented with 20 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]-KOH (pH 8.0) under continuous white light (50 μmol of photons m−2 s−1; 400 to 700 nm) at 30°C in air.
Immunoblot analysis.
Cells were mechanically lysed with glass beads. The resulting protein extracts were subjected to SDS-PAGE (12.5% gels) and then transferred to polyvinylidene difluoride membranes. The membranes were probed with anti-AqpZ antibody and subsequently subjected to chemiluminescence detection as described previously (1).
Stopped-flow spectrophotometry on Synechocystis cell suspensions.
The water permeability of WT and ΔaqpZ cells was measured using a stopped-flow apparatus with a dead time of <3.95 ms (Unisoku, Hirakata, Japan) (52). For hyperosmotic shocks, 100 μl of Synechocystis cells was rapidly mixed with an equal volume of high-osmolarity medium (see the appropriate figure legend for final concentrations) at 30°C. The time course of changes in the 90° scattered light intensity was determined at 575 nm for 500 ms. For hypo-osmotic shocks, cells cultivated in BG11 medium were centrifuged and resuspended in BG11 medium containing 1 M sorbitol or 0.5 M NaCl for 2 h. To start the assay, the cell suspension (100 μl) was mixed in the stopped-flow device with an equal volume of H2O. To test the effect of the incubation medium, 80 mM HEPES-MES (morpholineethanesulfonic acid) buffer (pH 8.0) was used instead of BG11 medium. Averaged data from multiple determinations were fitted to single or double exponential curves. The fitting parameters were then used to determine osmotic water permeability (Pf) by first applying the linear conversion from relative fluorescence into relative volume and then iteratively solving the equation for Pf as follows: [dV(t)/dt]/[(SAV) (MVW) (Cin − Cout)], where V(t) is the relative intracellular volume as a function of time (t), SAV is the cell surface area-to-cell volume ratio, MVW is the molar volume of water (18 cm3 mol−1), and Cin and Cout are the initial concentrations of total solute inside and outside the cell, respectively (53, 54). For the test of Hg2+ effects, Synechocystis cells were incubated for 5 min in BG11 medium containing 300 μM HgCl2 before stopped-flow analysis.
Light microscopy.
An Eclipse E800 microscope (Nikon) equipped with a camera (KY-F1030; JVC) and the Diskus software package (Hilgers) were used for light microscopy. For cell size determination, pictures were analyzed using the ImageJ program. Beads of defined size (3.005 ± 0.027 μm) were used as a standard for the measurements.
Measurement of cell volume by EPR.
Synechocystis cell volume (cytoplasmic volume) was determined by electron paramagnetic resonance (EPR) spectrometry as described previously (9, 42). Cells were harvested and resuspended at 400 μg chlorophyll ml−1 in a solution containing 1 mM 2,2,6,6-tetramethyl-4-oxopiperidinooxy free radical (TEMPO), 20 mM K3[Fe(CN)6], and 75 mM Na2Mn-EDTA. TEMPO was used as the spin probe. When oxidized by Fe(CN)63−, TEMPO was able to penetrate the plasma membrane rapidly and reach an equilibrium throughout the cell suspension. The quenching agent Na2Mn-EDTA, which cannot cross the plasma membrane, broadened the external spin probe signal, and the remaining unbroadened spin probe signal was directly proportional to the cell volume. For the assay, the cells were enclosed in a sealed-glass capillary in a final volume of 40 μl. EPR spectra were recorded at room temperature in an EPR spectrometer (model ESP 300E; Bruker) under the following conditions: 100-kHz field modulation at a microwave frequency of 11.72 GHz, a modulation amplitude of 0.4 mT, microwave power of 10 mW, a time constant of 80 ms, and a scan rate of 0.4 G s−1 in the dark.
Oxygen evolution measurements.
Oxygen evolution of cells was measured in BG11 medium with a Clark-type electrode at a chlorophyll concentration of 5 μg ml−1. The medium was continuously stirred at 25°C and illuminated with saturating actinic light (1,000 μE m−2 s−1). Whole-cell photosynthetic activity was measured as oxygen evolution supported by 5 mM NaHCO3. Following methanol extraction, the chlorophyll contents of individual samples were determined using a Hitachi U-2010 spectrophotometer (37).
RESULTS
Expression of aqpZ is controlled by the circadian clock in Synechocystis.
To determine whether expression of aqpZ was regulated by the circadian clock and whether the circadian rhythm was influenced by deletion of the aqpZ gene, Synechocystis cells carrying a reporter construct consisting of the bacterial luciferase gene set under the control of the aqpZ promoter (PaqpZ::luxAB) were studied. Bioluminescence was measured every hour under continuous light following entrainment of the Synechocystis cells by a 12-h dark period (Fig. 1A and Table 1). Cells expressing luciferase driven by the dnaK promoter, known to show circadian rhythm, were used as controls (4, 29). The circadian period (wavelength of the cosine curve) of the cells expressing PdnaK::luxAB was 22.2 h for the WT cells and 22.5 h for the ΔaqpZ cells, indicating that the rhythm was not influenced by deletion of aqpZ. Peak expression of aqpZ was CT 15.3 h for WT cells and CT 15.5 h for the ΔaqpZ cells, corresponding to early subjective night.
Fig 1.
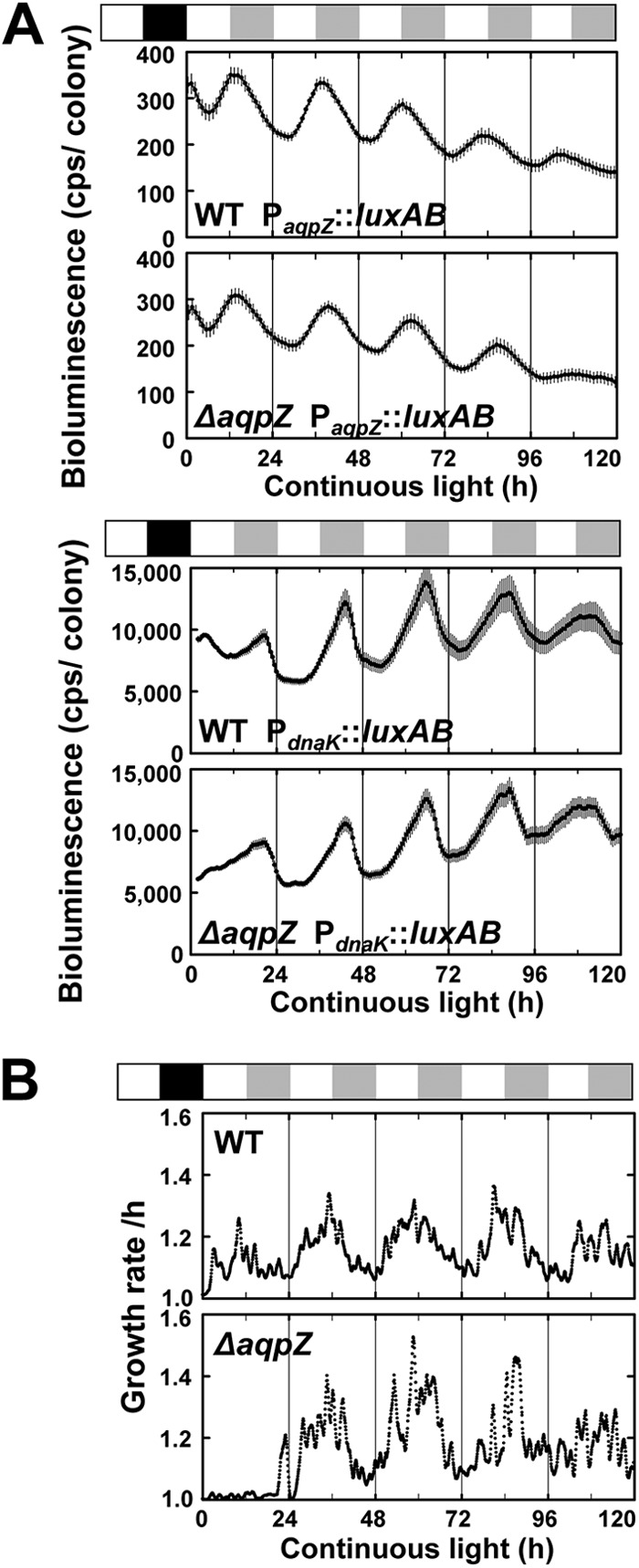
Circadian expression of aqpZ and effects of deletion of aqpZ on circadian rhythms in Synechocystis. (A) Synechocystis cells containing a bacterial luciferase reporter gene under the control of the aqpZ promoter (PaqpZ::luxAB) were entrained by a 12-h dark period (black box) to synchronize the circadian clock and then shifted to continuous light. The white and gray boxes above the graphs represent subjective day and night, respectively. The PdnaK::luxAB reporter strain was used as a control. The time scale represents the actual time after transfer to continuous light conditions. Data points show means ± SDs from 3 to 48 independent samples. Table 1 summarizes the results. We obtained essentially the same results in three independent experiments. (B) The growth rates of the cells were measured during continuous culture and were calculated on the basis of the optical density of cultures determined every 5 min with an optical sensor.
Table 1.
Characteristics of circadian parameters of expression of aqpZ gene of Synechocystis
| Strain | Period (h) | Phase (h) | Phase of CTb | Amplitude | No. of samples |
|---|---|---|---|---|---|
| WT PaqpZ::luxAB | 22.9 ± 0.1 | 14.6 ± 0.2 | 15.3 ± 0.2 | 1.78 ± 0.09 | 43 |
| ΔaqpZ PaqpZ::luxAB | 23.4 ± 0.2 | 15.1 ± 0.2 | 15.5 ± 0.2 | 1.75 ± 0.08 | 38 |
| WT Pdnak::luxABa | 22.2 ± 0.4 | 20.0 ± 0.1 | 21.6 ± 0.1 | 1.44 ± 0.09 | 3 |
| ΔaqpZ Pdnak::luxABa | 22.5 ± 0.2 | 20.4 ± 0.2 | 21.8 ± 0.2 | 1.45 ± 0.03 | 9 |
The circadian parameters of the expression of dnaK are shown as a control (4).
Phases are represented by circadian time (CT), which was calculated by dividing the phase value by the period length and multiplying by 24. In cyanobacteria, subjective daytime and nighttime are CT 0 to 12 h and CT 12 to 24 h (CT 0 h = CT 24 h), respectively.
Since AqpZ may be involved in cell growth, we evaluated the effect of aqpZ on cell growth in liquid cultures under continuous light. Under these conditions, the growth rates of WT and ΔaqpZ cells were similar (Fig. 1B). The average cell growth rate was increased from early subjective day and was relatively high from late subjective day through early subjective night (Fig. 1B), following the normal period of photosynthesis. The peak of AqpZ expression appeared to be timed late in the high-cell-growth-rate phase.
The ΔaqpZ strain displayed slower shrinkage under high-osmolarity conditions.
To evaluate the role of AqpZ in the cells, the volume loss of Synechocystis cells in response to high osmolarity was measured by stopped-flow light-scattering spectrophotometry. Figures 2A and B show representative time courses of light scattering of Synechocystis WT and ΔaqpZ cells in response to 1 M sorbitol (Fig. 2A) or 0.5 M NaCl (Fig. 2B) in BG11 medium (1,236 mosM and 1,015 mosM, respectively). In response to both treatments, WT cells exhibited a rapid and strong increase in light scattering, indicating a decrease in cell size. The response in ΔaqpZ cells was much slower (Fig. 2A and B). Changes in light scattering were also determined in response to treatments with increasing osmolarities of sorbitol or NaCl (0.25 to 1.0 M in BG11 medium), and Pf was calculated (Fig. 2C and D). In these experiments, the light-scattering profiles and values for Pf showed distinct differences between cells treated with sorbitol and cells treated with NaCl (Fig. 2A to D).
Fig 2.
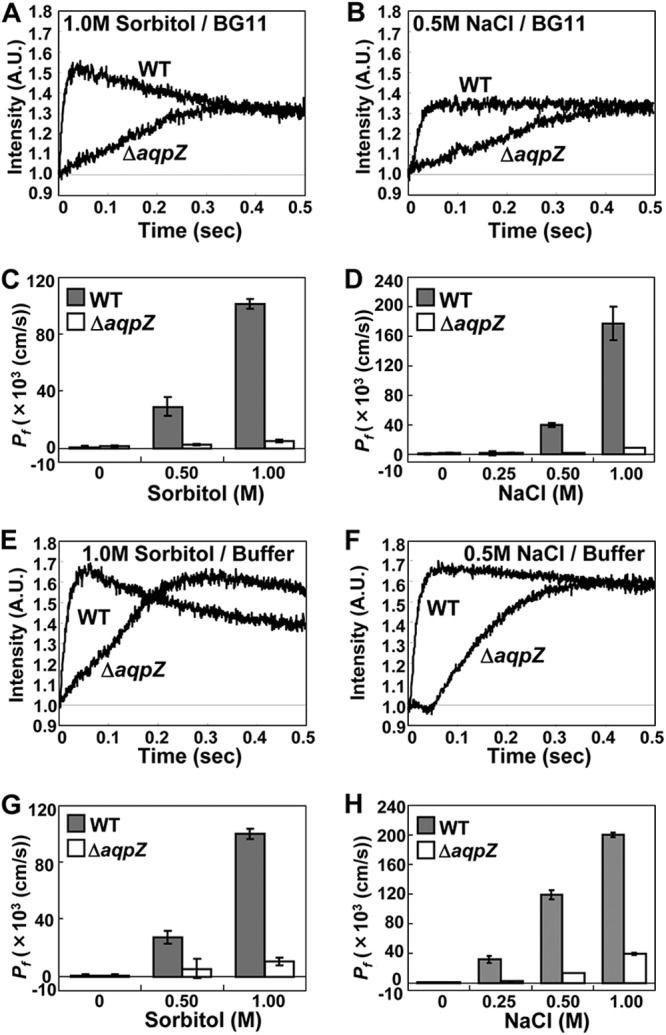
Characterization of AqpZ-mediated water permeability in Synechocystis cells subjected to hyperosmotic stress. (A and B) Time course of light scattering of Synechocystis WT and ΔaqpZ cells moved to BG11 medium containing 1.0 M sorbitol (A) or 0.5 M NaCl (B). (C and D) Pf of WT and ΔaqpZ cells exposed to various concentrations of sorbitol (C) or NaCl (D) in BG11 medium. (E to H) The same experiments shown in panels A to D were repeated with cells incubated in 80 mM HEPES-MES (pH 8.0) instead of BG11 medium. A.U., absorbance units.
To exclude the possibility that the cellular response may have been influenced by the ingredients of the BG11 medium, the experiments were repeated with HEPES-MES buffer instead of BG11 medium (Fig. 2E to H). In the case of treatment with sorbitol, there was not much difference between incubation in buffer or BG11 medium (Fig. 2E and G). However, in the case of treatment with NaCl, the Pf values of both WT and mutant cells were increased more than 3-fold by addition of 0.25 and 0.5 M NaCl in the buffer (Fig. 2F and H) compared with the results obtained with NaCl in BG11 medium (Fig. 2B and D). No additional increase in the Pf value was seen with 1 M NaCl in the buffer compared to that obtained with 1 M NaCl in BG11 medium, probably because at 1 M NaCl cells may have reached the maximum rate of shrinkage. Overall, cells responded more strongly to NaCl in buffer than in BG11 medium. Components of the BG11 medium likely contribute to the cells' response to high NaCl.
In addition, the cellular recovery response from hyperosmotic stress was examined (Fig. 3). For this, Synechocystis WT and ΔaqpZ cells that had been preincubated in BG11 medium containing 1 M sorbitol (Fig. 3A) or 0.5 M NaCl (Fig. 3B) for 2 h were rapidly mixed with an equal volume of water to decrease the osmolarity, and the change in light scattering was recorded. The size of ΔaqpZ cells increased much more slowly than the size of WT cells, indicated by the higher level of light scattering in the mutant (Fig. 3C). These data demonstrate that AqpZ mediated water movement across the membrane in response to both osmotic upshock and osmotic downshock (Fig. 2 and 3).
Fig 3.
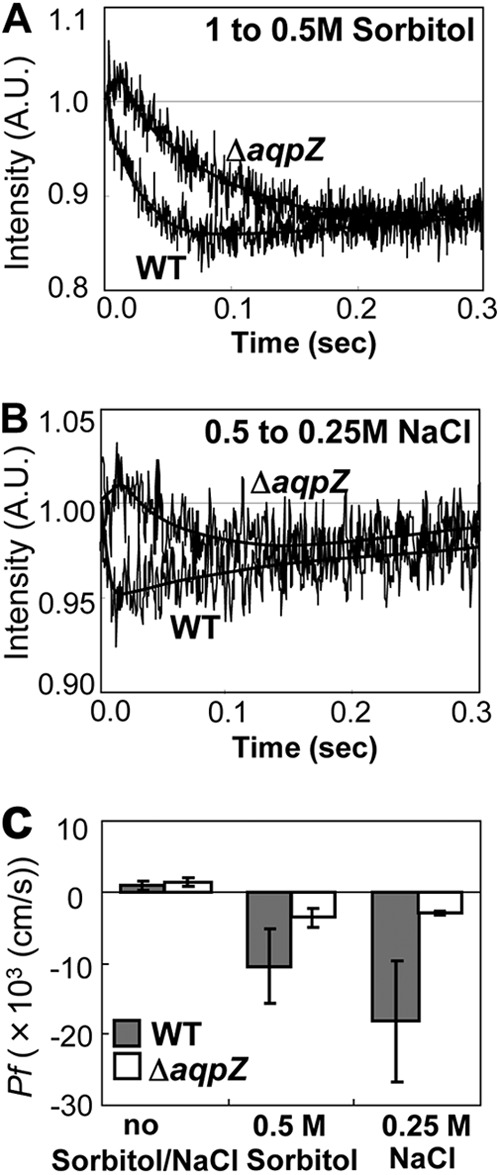
Recovery from hyperosmotic stress on Synechocystis wild-type and ΔaqpZ cells. (A and B) Representative time courses of light scattering of Synechocystis WT and ΔaqpZ cells exposed to osmotic downshock. Cells precultured for 2 h in BG11 medium with 1 M sorbitol (A) or 0.5 M NaCl (B) were diluted with an equal volume of water to shift the final concentration to 0.5 M sorbitol (A) or 0.25 M NaCl (B). (C) Calculated Pf of the cells. Values are expressed as means ± SDs calculated for three independent experiments.
AqpZ is insensitive to inhibition by mercury.
Although cyanobacterial aquaporins have been reported to be hypersensitive to sulfhydryl reagents (3, 7, 42), no target residues have been identified in Synechocystis AqpZ. We performed oocyte swelling assays to test the sensitivity of AqpZ to potential regulatory compounds, including oxidative reagents (1). Addition of HgCl2 (300 μM), a known inhibitor of some aquaporins, had no effect on the water permeability of Synechocystis cells expressing AqpZ, whereas the human aquaporin hAQP1 was about 50% inhibited under these conditions (Fig. 4A) (38). The protein kinase C (PKC) activator phorbol-12-myristate-13-acetate (PMA) increased the water permeability of oocytes expressing soybean nodulin 26 (16, 45, 51). When PMA was applied to oocytes expressing AqpZ, the osmotic water permeability did not change compared to that of untreated oocytes (Fig. 4A). There was also no change in water permeability when the cells were incubated at lower pH (pH 6; Fig. 4A).
Fig 4.
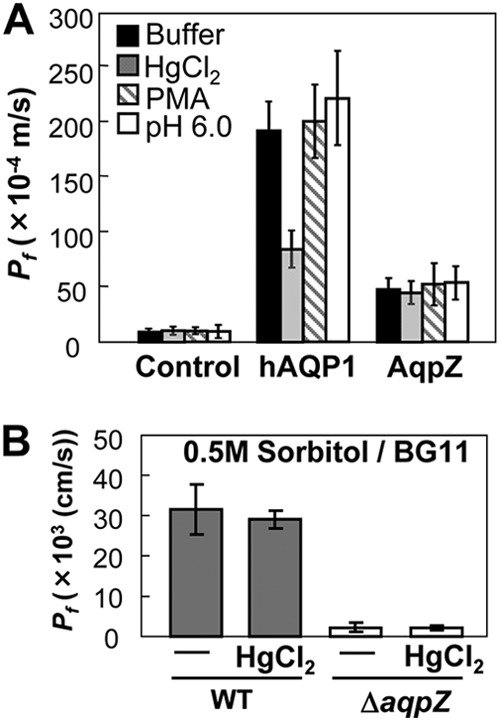
AqpZ is insensitive to inhibition by mercury in Xenopus oocytes and in Synechocystis. (A) Effects of mercury, the protein kinase C activator PMA, and pH on water permeability in Xenopus oocytes expressing AqpZ. HgCl2 (300 μM) or PMA (10 μM) was added to the incubation medium 5 min before the start of the swelling assay. The mercury-sensitive human aquaporin hAQP1 was used as a control. (B) Test of inhibition of water permeability by HgCl2. Synechocystis WT and ΔaqpZ cells were pretreated with BG11 medium containing 300 μM HgCl2 for 5 min before subjecting them to hyperosmotic shock (BG11 medium containing 0.5 M sorbitol). Values are expressed as the mean Pf ± SD calculated for three independent experiments.
We also tested whether this insensitivity to mercury could be reproduced in vivo (Fig. 4B). When 300 μM HgCl2 was applied to Synechocystis cells in BG11 medium before performing the light-scattering assay, no obvious decrease of water efflux was observed in the WT (Fig. 4B). This insensitivity of AqpZ to 300 μM HgCl2 in vivo is consistent with the data from our heterologous expression experiment (Fig. 4A). Therefore, we concluded that Synechocystis AqpZ can be categorized as a mercury-tolerant aquaporin.
The ΔaqpZ strain is less sensitive to high osmolarity.
To evaluate the physiological function of AqpZ in Synechocystis, we compared the growth of WT and ΔaqpZ cells under conditions of high osmolarity (Fig. 5). No difference in growth was found between WT and ΔaqpZ cells on standard solid BG11 medium or on medium containing 0.2 M sorbitol or 0.2 M NaCl, whereas on BG11 medium containing 0.4 M sorbitol, ΔaqpZ cells grew better than WT cells (Fig. 5A). In contrast, on BG11 medium containing 0.4 M NaCl, ΔaqpZ cells grew slightly less well than WT cells. In liquid culture, similar results were seen (Fig. 5B to D). The ΔaqpZ cells were more tolerant to 0.5 M sorbitol than WT cells (Fig. 5C). When NaCl (0.25 to 0.5 M) was added to the medium, there was little difference in growth between WT and ΔaqpZ cells (Fig. 5D). When exponentially growing cells were subjected to hyperosmotic shock by the addition of 0.5 M sorbitol, the WT was more severely delayed in its growth than the ΔaqpZ strain (Fig. 5E). When WT and ΔaqpZ cells were subjected to either hypo-osmotic or drought stress, no difference in their viability was detected (see Fig. S1 in the supplemental material). Therefore, a lack of AqpZ enhanced the tolerance of the cells to high osmotic stress but not to increased NaCl concentration (salt stress).
Fig 5.
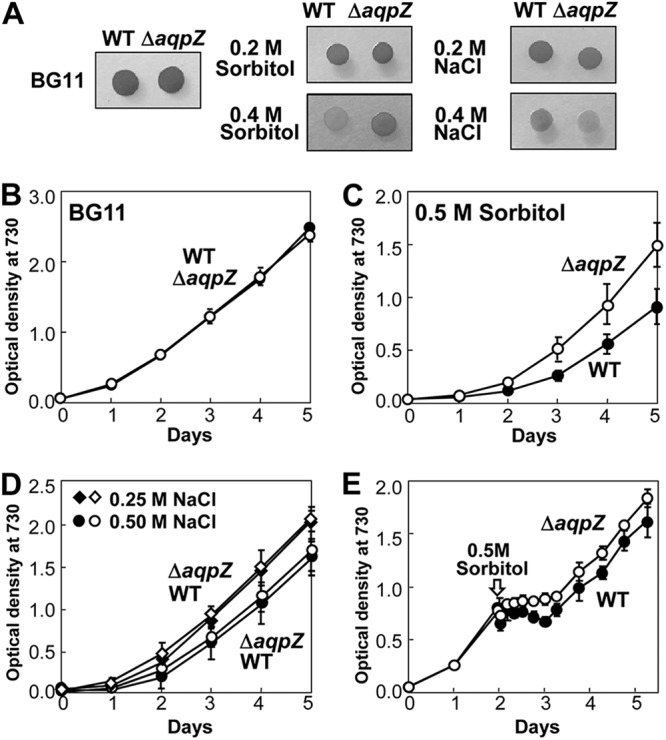
Growth of Synechocystis wild-type and ΔaqpZ cells under hyperosmotic conditions. (A) Growth of WT and ΔaqpZ cells on solid BG11 medium containing the indicated concentrations of NaCl or sorbitol. (B to D) Growth curves of WT (filled symbols) and ΔaqpZ (open symbols) cells growing in liquid culture. Cells were grown either in BG11 medium (B) or in BG11 medium supplemented with 0.5 M sorbitol (C) or 0.5 M NaCl (D). (E) Growth curves of WT and ΔaqpZ cells. Sorbitol (0.5 M) was added to the exponentially growing cultures at 48 h (indicated by the arrow).
Comparison of oxygen evolution between wild-type and ΔaqpZ cells.
We evaluated the effect of the loss of aqpZ on photosynthetic activity by monitoring the oxygen evolution of the cells under different conditions (Fig. 6). To examine the immediate effect of hyperosmotic shock on the oxygen evolution of WT and ΔaqpZ cells, sorbitol or NaCl was directly added to BG11 medium-suspended cells in a Clark-type oxygen electrode cuvette (see Fig. S2 in the supplemental material). The amount of oxygen evolution was similar in both ΔaqpZ and WT strains in standard medium (Fig. 6A and B). When 0.5 M sorbitol or 0.5 M NaCl was added to the BG11 medium, the oxygen evolution rate of ΔaqpZ cells was less inhibited than that of WT cells (Fig. 6A and B). Figures 6C and D show the rate of oxygen evolution from both WT and ΔaqpZ cells during extended culture. The rate decreased to its lowest level (40 to 50% of the initial value) at 5 min, and then recovered in both the WT and the ΔaqpZ cultures. The recovery of the rate of oxygen evolution of the ΔaqpZ strain was faster than that of the WT in the 0.5 M sorbitol-containing cultures (Fig. 6C), but it was similar for both strains in the 0.5 M NaCl-containing cultures (Fig. 6D). The finding that the ΔaqpZ strain was less affected by high sorbitol was consistent with the results in Fig. 5.
Fig 6.
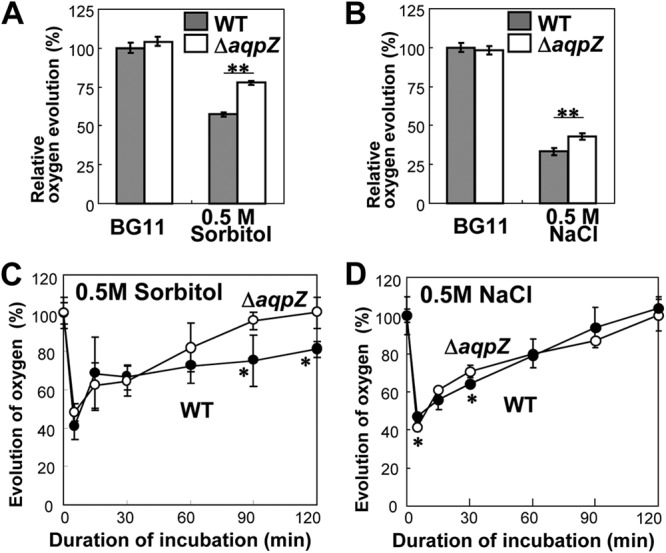
Photosynthetic oxygen evolution in intact cells during incubation with sorbitol or NaCl. (A and B) Rapid effects of hyperosmotic shock on the oxygen evolution of WT and ΔaqpZ cells. Dissolved oxygen evolution was monitored, and 0.5 M sorbitol (A) or NaCl (B) was directly added to BG11 medium-suspended cells in a Clark-type oxygen electrode cuvette. The relative oxygen evolution was calculated from the data before and after the addition of sorbitol or NaCl. (C and D) Oxygen evolution of cells incubated in BG11 medium containing 0.5 M sorbitol or 0.5 M NaCl over an extended time period. Cells were incubated in the presence of 0.5 M sorbitol (C) or 0.5 M NaCl (D). At designated times, aliquots were withdrawn and whole-chain-mediated oxygen evolution was measured at 25°C after addition of 5 mM NaHCO3. Each point represents the average ± SE from four independent experiments. Statistical significance was assessed using Student's t test: *, P < 0.05; **, P < 0.01.
Deletion of aqpZ affects the response of Synechocystis cells exposed to hyperosmotic shock.
To assess the difference in the effect of high concentrations of sorbitol and NaCl, we observed and recorded changes in the size of Synechocystis WT and ΔaqpZ cells exposed to hyperosmotic stress directly (Fig. 7A). When 0.5 M sorbitol was added, the cell size of both strains decreased significantly within 5 min. In contrast, cells exposed to 0.5 M NaCl showed less shrinkage, even though the osmolarity of 0.5 M NaCl is twice as high as that of 0.5 M sorbitol. When a single cell was observed over time, the volume of both WT and ΔaqpZ cells had strongly decreased by 2 min after the addition of high concentrations of sorbitol (Fig. 7B). It has previously been reported that ΔaqpZ cells did not shrink in response to upshock when measured by electron paramagnetic resonance (EPR) (42). We used the same method to measure the cytoplasmic volume changes of WT and ΔaqpZ cells over an extended period of time (see Materials and Methods) during incubation in 0.5 M sorbitol or 0.5 M NaCl. After addition of 0.5 M sorbitol, the cell volume of the WT had decreased to 41% at 5 min and 36% at 15 min. After that, the cell volume gradually increased again, reaching approximately 60% of the initial volume after 60 min. The cell volume of the ΔaqpZ strain was reduced to 54% after 5 min and remained at this level after 60 min (Fig. 7C). The shrinkage rate of cells exposed to 0.5 M NaCl (Fig. 7D) was generally less than that of cells exposed to sorbitol (Fig. 7C). The maximum shrinkage of ΔaqpZ cells was less than that of WT cells (Fig. 7C). Taken together, both WT and ΔaqpZ cells shrank under our experimental conditions, but the overall decrease in size was smaller for ΔaqpZ cells than for WT cells and sorbitol had a stronger effect on cell shrinkage than NaCl.
Fig 7.
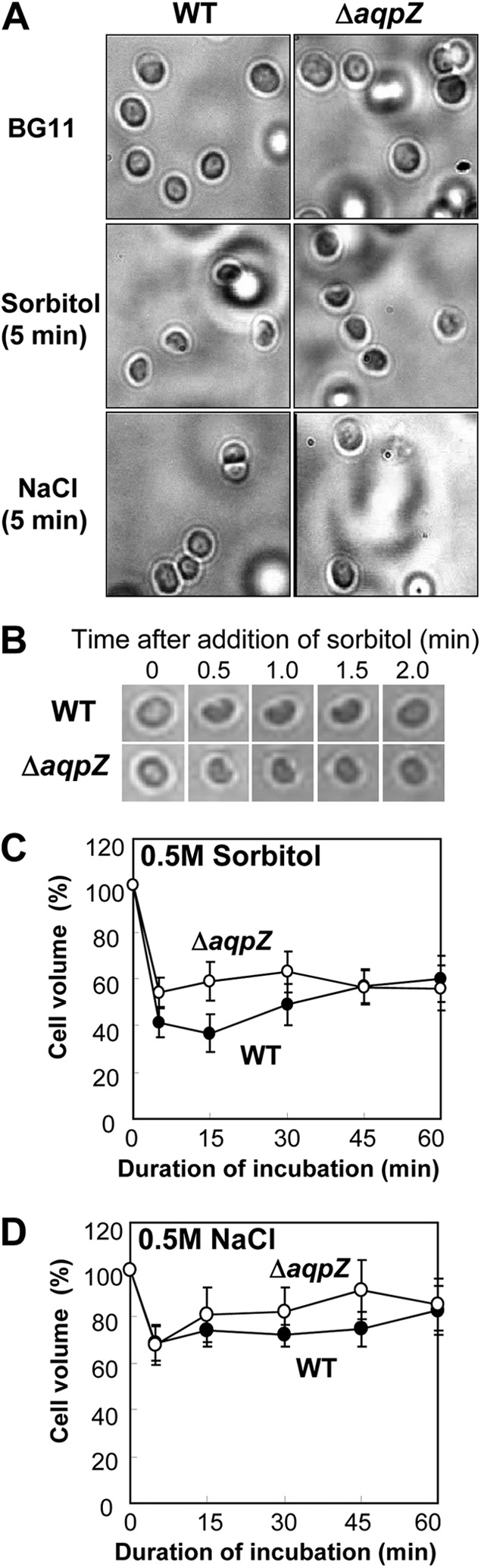
Changes in cell volume of Synechocystis wild-type and ΔaqpZ cells in response to hyperosmotic shock. (A) Light microscopy photographs of Synechocystis WT and ΔaqpZ cells before and 5 min after the start of osmotic stress by addition of 0.5 M sorbitol or 0.5 M NaCl. (B) Time course of morphological changes of single Synechocystis WT and ΔaqpZ cells after the addition of sorbitol. (C and D) Determination of changes in cell volume by EPR. Cells were treated with 0.5 M sorbitol (C) or 0.5 M NaCl (D) at time zero. At designated times, samples were withdrawn from each cell suspension and the cell volume was determined by EPR. Each point represents the average ± SE from four independent experiments.
Change of expression of AqpZ protein due to hyperosmotic shock.
To evaluate the reason for the difference in the effect of high concentrations of sorbitol and NaCl, we examined the expression of AqpZ at the translational level in the WT (Fig. 8). High concentrations of sorbitol (0.5 M) had no effect on the amount of AqpZ protein in Synechocystis. In contrast, 4 h after the addition of 0.5 M NaCl, the amount of AqpZ protein had decreased by about 50% and remained at this level over the course of the experiment (Fig. 8C). The decrease in the amount of AqpZ protein in response to high NaCl likely contributed to alleviation of the salt stress caused by water loss. In contrast, the lack of change in the amount of AqpZ protein seen under high-sorbitol conditions explained the observed hypersensitivity of Synechocystis to the high osmolarity in the medium.
Fig 8.
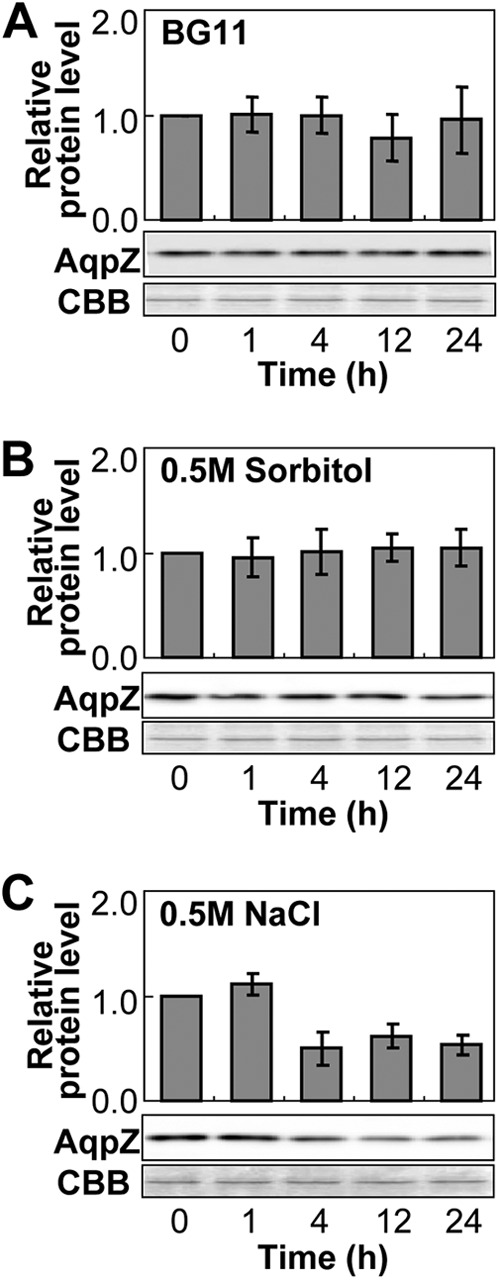
Expression of AqpZ protein in Synechocystis cells after hyperosmotic shock. Synechocystis cells were treated with BG11 medium only (A) or with BG11 medium supplemented with 0.5 M sorbitol (B) or 0.5 M NaCl (C). Cell samples taken from the cultures at the indicated times were subjected to Western blot analysis using anti-AqpZ antibodies. The expression level of AqpZ protein was determined by densitometry and is expressed as the level of protein in relation to the level at the beginning of the experiment before addition of sorbitol or NaCl (time zero). Total protein stained with Coomassie brilliant blue R-250 (CBB) served as a loading control.
DISCUSSION
In the moderately halotolerant cyanobacterium Synechocystis sp. strain PCC 6803, water permeability across the membrane is closely correlated with the dynamic processes of physiological responses to daily as well as unexpected environmental changes (17). We have shown here that AqpZ expression levels showed diurnal and circadian oscillations (Fig. 1 and Table 1). Cells lacking aqpZ lost less water than WT cells when they were exposed to hyperosmotic or salt stress (Fig. 2). This decrease in water loss coincided with an increase in tolerance to hyperosmotic stress (but not to salt stress) of the ΔaqpZ cells, demonstrated by an increased growth rate (Fig. 5) and oxygen evolution compared to those for the WT cells (Fig. 6). Therefore, Synechocystis AqpZ is important for control of the cell volume in response to osmolarity changes by nonionic compounds like sorbitol but less so in response to salt stress by NaCl.
The finding that AqpZ was insensitive to mercury both in Synechocystis and when heterologously expressed in oocytes (Fig. 4) is significant because mercurial sulfhydryl reagents have often been used as potent inhibitors to block aquaporins in living cells (23). Indeed, HgCl2 and p-chloromercuriphenyl-sulfonic acid at concentrations ranging from 100 μM to the millimolar level have been used to block aquaporins in Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7942 (2, 7). Although Synechocystis AqpZ has two cysteines which may act as target sites for the interaction with these reagents, we considered that mercurial sulfhydryl reagents might lead to nonspecific interactions with cellular components other than AqpZ proteins. HgCl2 at concentrations higher than 1 mM had additional deleterious effects, when applied to whole cells (data now shown), similar to those reported for blockage of Synechocystis AqpZ by 2 mM HgCl2 (7). In contrast, addition of 300 μM HgCl2 had no effect on AqpZ function in oocytes or in the Synechocystis WT and ΔaqpZ mutant (Fig. 4); under those conditions, the human AqpZ used as a control in oocytes was clearly inhibited. Therefore, we concluded that these two cysteines are not the target of inhibition of AqpZ activity by mercury, and it is likely that the deleterious effects of such high HgCl2 concentrations on cells were not due to specific blockage of water transport through AqpZ but rather were due to indirect effects of mercury toxicity. According to the phylogenetic tree, Synechocystis AqpZ is most similar to Escherichia coli AqpZ, which, like Synechocystis AqpZ, is insensitive to inhibition by mercury (38) (see Fig. S3B in the supplemental material). In both organisms, the aquaporins have very short cytosolic N- and C-terminal domains (see Fig. S3A in the supplemental material). The insensitivity of the AqpZ proteins from both Synechocystis and E. coli to mercurial sulfhydryl reagents may provide an advantage to organisms that are exposed to oxidative environments.
Circadian control of many biological events occurs in a wide range of living organisms. Vasopressin is an antidiuretic hormone in mammals which triggers the movement of aquaporin 2 into the plasma membrane of the epithelial cells of the collecting duct to reabsorb water from the urine into the bloodstream. A recent study demonstrated that the release of vasopressin is enhanced by an intrinsic biological clock during the sleep period when water uptake is suppressed in the kidney in mammals (46). Here we found that the expression of Synechocystis AqpZ was similarly regulated by the circadian clock (Fig. 1), which in cyanobacteria consists of the endogenous oscillators (5, 22). This was confirmed by an experiment where the pattern of the rhythm of aqpZ expression showed a proportional relationship to the shorter circadian time in Synechocystis carrying point mutations in kaiC (M. Akai, K. Onai, M. Morishita, M. Ishiura, and N. Uozumi, unpublished data). The daily oscillation of aqpZ gene expression was coordinated with the circadian rhythm of cell growth, which requires carbon fixation and glucose metabolism (1) (Fig. 1 and Table 1). Interestingly, the peak of the expression of aqpZ corresponded to early subjective night, which is similar to the circadian profile of the Na+/H+ antiport system NhaS3 in Synechocystis, which is known to be involved in osmoregulation (48).
An important finding of this study is that loss of aqpZ resulted in tolerance to high osmolarity, which was shown by several different experimental approaches (Fig. 5 to 7). Interestingly, hyperosmotic stress due to nonionic (sorbitol) versus ionic (NaCl) solutes had different effects in Synechocystis with respect to the oxygen evolution rate and cell volume change (Fig. 6 and 7) (49). This difference may be the result of the Na+ adaptive system in Synechocystis. It has been reported that Synechocystis regulates Na+ influx and efflux across the plasma membrane and the thylakoid membrane via an Na+ transport system, e.g., an Na+/H+ antiporter, in order to control ion homeostasis in the cytosol (11, 21, 33, 48). In addition to the Na+ cycling system, NaCl also induces synthesis of the osmolyte glucosylglycerol in Synechocystis (27). A reduction in the amount of AqpZ protein was observed during salt stress (Fig. 8) and may also help to protect the cell from water loss due to high external NaCl. The downregulation of AqpZ protein expression by high NaCl (Fig. 8) was consistent with previous results from DNA microarray analysis in Synechocystis (26). Similarly, in the ice plant, the transcript levels of all three aquaporin genes (MipA, MipB, and MipC) decreased initially during salt stress and later recovered to pretreatment levels (50). As an immediate response prior to the accumulation of osmoprotectants in the cytosol, cells take up K+ through an Na+-activated Ktr-type K+ uptake system (8, 28) in order to restore cell volume (18, 19, 26, 42, 44). Together, these adaptive mechanisms to Na+ stress are likely to be the reason why Synechocystis showed a relatively high tolerance to Na+ toxicity (Fig. 5 to 7).
In contrast to Na+, sorbitol is probably not transported across the cellular membrane in Synechococcus sp. PCC 7942 (2), although in one study, a limited amount of sorbitol was taken up by the cells (15). In Synechocystis, hyperosmotic shock due to sorbitol triggered strong deformation of the cell envelope in both WT and ΔaqpZ cells; this was not seen in cells subjected to hyperosmotic stress by NaCl (Fig. 7). In E. coli, the large efflux of water driven by the osmotic gradient causes visible shrinkage of the cytoplasm, leading to a separation of the cytoplasmic membrane from the wall and, consequently, to the formation of plasmolysis spaces (13). In Synechocystis, it is plausible that the physical force caused by the shrinkage of the intracellular space adversely affected the function of membrane proteins in both the plasma membrane and the thylakoid membrane, including the proteins of the photosynthetic electron transport system (Fig. 7). An earlier study (42) as well as a recent report by Azad et al. (7), detected almost no shrinkage of ΔaqpZ cells in response to osmotic stress. In our study, the difference in the rate of cellular shrinkage between WT and ΔaqpZ cells was small but detectable by microscopic observations (Fig. 7A and B) and EPR measurements (Fig. 7C), and both the WT and the ΔaqpZ strain responded to osmotic stress by a decrease in cell volume. The fact that we observed some shrinkage of ΔaqpZ cells suggests that even though water can move rapidly through AqpZ (Fig. 2 to 4), it can also permeate the plasma membrane by another, yet unknown water transport system different from AqpZ or by simple diffusion.
ACKNOWLEDGMENTS
We thank Hideyuki Matsumoto (Tohoku University) for measurement of cell volume and Anke Reinders (University of Minnesota) for critical reading of our manuscript.
This work was supported by grants-in-aid for scientific research (22380056, 24246045, and 24658090) from the Ministry of Education, Culture, Sports, Science and Technology and from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 5 October 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Akai M, et al. 2011. Plasma membrane aquaporin AqpZ protein is essential for glucose metabolism during photomixotrophic growth of Synechocystis sp. PCC 6803. J. Biol. Chem. 286:25224–25235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N. 2000. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 123:1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N. 2000. Inactivation of photosystems I and II in response to osmotic stress in Synechococcus. Contribution of water channels. Plant Physiol. 122:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoki S, Kondo T, Ishiura M. 1995. Circadian expression of the dnaK gene in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 177:5606–5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aoki S, Onai K. 2009. Circadian clocks of Synechocystis sp. strain PCC 6803, Thermosynechococcus elongatus, Prochlorococcus spp., Trichodesmium spp. and other species, p 259–282 In Ditty JL, Mackey SR. (ed), Bacterial circadian programs. Springer, Berlin, Germany [Google Scholar]
- 6. Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azad AK, et al. 2011. Functional characterization and hyperosmotic regulation of aquaporin in Synechocystis sp. PCC 6803. Plant Sci. 180:375–382 [DOI] [PubMed] [Google Scholar]
- 8. Berry S, et al. 2003. Potassium uptake in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 mainly depends on a Ktr-like system encoded by slr1509 (ntpJ). FEBS Lett. 548:53–58 [DOI] [PubMed] [Google Scholar]
- 9. Blumwald E, Mehlhorn RJ, Packer L. 1983. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc. Natl. Acad. Sci. U. S. A. 80:2599–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blumwald E, Tel-Or E. 1984. Salt adaptation of the cyanobacterium Synechococcus 6311 growing in a continuous culture (turbidostat). Plant Physiol. 74:183–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blumwald E, Wolosin JM, Packer L. 1984. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem. Biophys. Res. Commun. 122:452–459 [DOI] [PubMed] [Google Scholar]
- 12. Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ. 1996. Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell 8:587–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delamarche C, et al. 1999. Visualization of AqpZ-mediated water permeability in Escherichia coli by cryoelectron microscopy. J. Bacteriol. 181:4193–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elanskaya IV, Karandashova IV, Bogachev AV, Hagemann M. 2002. Functional analysis of the Na+/H+ antiporter encoding genes of the cyanobacterium Synechocystis PCC 6803. Biochemistry (Mosc.) 67:432–440 [DOI] [PubMed] [Google Scholar]
- 15. Froger A, et al. 2001. Functional characterization of a microbial aquaglyceroporin. Microbiology 147:1129–1135 [DOI] [PubMed] [Google Scholar]
- 16. Guenther JF, et al. 2003. Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 15:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagemann M. 2011. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol. Rev. 35:87–123 [DOI] [PubMed] [Google Scholar]
- 18. Hagemann M, Erdmann N. 1994. Activation and pathway of glucosylglycerol synthesis in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 140:1427–1431 [Google Scholar]
- 19. Hagemann M, Richter S, Mikkat S. 1997. The ggtA gene encodes a subunit of the transport system for the osmoprotective compound glucosylglycerol in Synechocystis sp. strain PCC 6803. J. Bacteriol. 179:714–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haimovich-Dayan M, et al. 2011. Cross-talk between photomixotrophic growth and CO2-concentrating mechanism in Synechocystis sp. strain PCC 6803. Environ. Microbiol. 13:1767–1777 [DOI] [PubMed] [Google Scholar]
- 21. Inaba M, Sakamoto A, Murata N. 2001. Functional expression in Escherichia coli of low-affinity and high-affinity Na+ Li+/H+ antiporters of Synechocystis. J. Bacteriol. 183:1376–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishiura M, et al. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281:1519–1523 [DOI] [PubMed] [Google Scholar]
- 23. Iwabuchi K, Kaneko T, Kikuyama M. 2008. Mechanosensitive ion channels in chara: influence of water channel inhibitors, HgCl2 and ZnCl2, on generation of receptor potential. J. Membr. Biol. 221:27–37 [DOI] [PubMed] [Google Scholar]
- 24. Kucho K, et al. 2005. Global analysis of circadian expression in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 187:2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Latifi A, Ruiz M, Zhang CC. 2009. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 33:258–278 [DOI] [PubMed] [Google Scholar]
- 26. Marin K, et al. 2004. Gene expression profiling reflects physiological processes in salt acclimation of Synechocystis sp. strain PCC 6803. Plant Physiol. 136:3290–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marin K, Stirnberg M, Eisenhut M, Kramer R, Hagemann M. 2006. Osmotic stress in Synechocystis sp. PCC 6803: low tolerance towards nonionic osmotic stress results from lacking activation of glucosylglycerol accumulation. Microbiology 152:2023–2030 [DOI] [PubMed] [Google Scholar]
- 28. Matsuda N, et al. 2004. Na+-dependent K+ uptake Ktr system from the cyanobacterium Synechocystis sp. PCC 6803 and its role in the early phases of cell adaptation to hyperosmotic shock. J. Biol. Chem. 279:54952–54962 [DOI] [PubMed] [Google Scholar]
- 29. Matsuo T, et al. 2008. A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes Dev. 22:918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. 1993. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 12:2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maurel C, Reizer J, Schroeder JI, Chrispeels MJ, Saier MH., Jr 1994. Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 269:11869–11872 [PubMed] [Google Scholar]
- 32. Mikkat S, Effmert U, Hagemann M. 1997. Uptake and use of the osmoprotective compounds trehalose, glucosylglycerol, and sucrose by the cyanobacterium Synechocystis sp. PCC6803. Arch. Microbiol. 167:112–118 [PubMed] [Google Scholar]
- 33. Mikkat S, Milkowski C, Hagemann M. 2000. The gene sll0273 of the cyanobacterium Synechocystis sp. strain PCC6803 encodes a protein essential for growth at low Na+/K+ ratios. Plant Cell Environ. 23:549–559 [Google Scholar]
- 34. Miller AG, Turpin DH, Canvin DT. 1984. Na+ requirement for growth, photosynthesis, and pH regulation in the alkalotolerant cyanobacterium Synechococcus leopoliensis. J. Bacteriol. 159:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okamoto K, Onai K, Furusawa T, Ishiura M. 2005. A portable integrated automatic apparatus for the real-time monitoring of bioluminescence in plants. Plant Cell Environ. 28:1305–1315 [Google Scholar]
- 36. Onai K, Morishita M, Itoh S, Okamoto K, Ishiura M. 2004. Circadian rhythms in the thermophilic cyanobacterium Thermosynechococcus elongatus: compensation of period length over a wide temperature range. J. Bacteriol. 186:4972–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975:384–394 [Google Scholar]
- 38. Preston GM, Jung JS, Guggino WB, Agre P. 1993. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 268:17–20 [PubMed] [Google Scholar]
- 39. Reed RH, Stewart WDP. 1985. Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from fresh-water and marine habitats. Mar. Biol. (Berl.) 88:1–9 [Google Scholar]
- 40. Reed RH, Warr SRC, Richardson DL, Moore DJ, Stewart WDP. 1985. Multiphasic osmotic adjustment in a euryhaline cyanobacterium. FEMS Microbiol. Lett. 28:225–229 [Google Scholar]
- 41. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 42. Shapiguzov A, et al. 2005. Osmotic shrinkage of cells of Synechocystis sp. PCC 6803 by water efflux via aquaporins regulates osmostress-inducible gene expression. Microbiology 151:447–455 [DOI] [PubMed] [Google Scholar]
- 43. Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. 1971. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35:171–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stirnberg M, et al. 2007. A membrane-bound FtsH protease is involved in osmoregulation in Synechocystis sp. PCC 6803: the compatible solute synthesizing enzyme GgpS is one of the targets for proteolysis. Mol. Microbiol. 63:86–102 [DOI] [PubMed] [Google Scholar]
- 45. Tournaire-Roux C, et al. 2003. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425:393–397 [DOI] [PubMed] [Google Scholar]
- 46. Trudel E, Bourque CW. 2010. Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat. Neurosci. 13:467–474 [DOI] [PubMed] [Google Scholar]
- 47. Tsukaguchi H, et al. 1998. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 273:24737–24743 [DOI] [PubMed] [Google Scholar]
- 48. Tsunekawa K, et al. 2009. Identification and characterization of the Na+/H+ antiporter NhaS3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem. 284:16513–16521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waditee R, Hibino T, Nakamura T, Incharoensakdi A, Takabe T. 2002. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc. Natl. Acad. Sci. U. S. A. 99:4109–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamada S, Katsuhara M, Kelly WB, Michalowski CB, Bohnert HJ. 1995. A family of transcripts encoding water channel proteins: tissue-specific expression in the common ice plant. Plant Cell 7:1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yasui M, et al. 1999. Rapid gating and anion permeability of an intracellular aquaporin. Nature 402:184–187 [DOI] [PubMed] [Google Scholar]
- 52. Yukutake Y, et al. 2008. Mercury chloride decreases the water permeability of aquaporin-4-reconstituted proteoliposomes. Biol. Cell 100:355–363 [DOI] [PubMed] [Google Scholar]
- 53. Zeidel ML, Ambudkar SV, Smith BL, Agre P. 1992. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31:7436–7440 [DOI] [PubMed] [Google Scholar]
- 54. Zeidel ML, et al. 1994. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry 33:1606–1615 [DOI] [PubMed] [Google Scholar]
- 55. Zhao JD, Brand JJ. 1988. Sequential effects of sodium depletion on photosystem II in Synechocystis. Arch. Biochem. Biophys. 264:657–664 [DOI] [PubMed] [Google Scholar]


