Abstract
We show here that NdgR, a known transcriptional activator of isopropylmalate dehydratase in actinomycetes, may have other targets in the cell. An in-frame deletion mutant of ndgR showed unexpectedly poor growth in defined minimal medium even in the presence of leucine. To our surprise, it was supplementation of cysteine and methionine that corrected the growth. Based on this, we propose that NdgR induces cysteine-methionine biosynthesis. Direct involvement of NdgR in the very last steps of methionine synthesis with methionine synthase (metH) and 5,10-methylenetetrahydrofolate reductase (metF) was examined. From a pulldown assay, it was seen that NdgR was enriched from crude cell lysates with a strong affinity to metH and metF upstream sequences. Direct physical interaction of NdgR with these targets was further examined with a gel mobility shift assay. ndgR, leuC, metH, and metF were inducible in M145 cells upon nutrient downshift from rich to minimal medium but were not induced in the ndgR knockout mutant. Taking these observations together, NdgR-dependent metH-metF expression would account for the abnormal growth phenotype of the ndgR mutant although there may be additional NdgR-dependent genes in the Cys-Met metabolic pathways. As the first transcriptional factor reported for regulating Cys-Met metabolism in Streptomyces, NdgR links two disparate amino acid families, branched-chain amino acids (BCAAs) and sulfur amino acids, at the transcriptional level. Considering that Cys-Met metabolism is connected to mycothiol and one-carbon metabolism, NdgR may have broad physiological impacts.
INTRODUCTION
With over 500 species and thousands of strains, streptomycetes are the most numerous and ubiquitous soil bacteria. Streptomyces strains have been widely used in the pharmaceutical industry over the last 80 years as they have the capacity to produce diverse bioactive materials from secondary metabolism, such as antibiotics, antitumor agents, and immune regulators. About 55% of commercially significant antibiotics are produced from the genus (4).
Since the first genome sequence was completed with Streptomyces coelicolor A3(2), more than 50 Streptomyces spp. genome sequences have been completed, and 28 of them are available in public (19). Still, S. coelicolor A3(2) is the most studied model organism, and the 8,667,507-bp genome has 7,825 predicted protein-encoding genes and more than 20 gene clusters for secondary metabolites. Of note is the abundance of transcriptional factors (TFs), totaling ca. 297 (1), meaning that the number of TFs per gene is twice that in Escherichia coli. Hence, additional layers and an increased number of interaction nodes in TF-mediated networks are anticipated.
With increased attention on connectivity in central and secondary metabolism, several TFs controlling central metabolism have been implicated in determining cellular developmental fate and antibiotic production. Multi-TF-mediated regulation would impart flexibility for adjusting metabolic and physiological needs to changing environmental conditions. The lack of information regarding central metabolism in Streptomyces compared to the level of understanding we have with E. coli is partly ascribed to the sensitive genetic response to physical and chemical conditions seen in the former. For instance, as filamentous Gram-positive bacteria, Streptomyces cultures on solid agar plates go through a developmental process proceeding from vegetative to aerial hyphal growth and further to sporulation. In contrast, most liquid submerged cultures display only vegetative growth although there are a few exceptional cases of sporulation, e.g., in Streptomyces griseus and Streptomyces venezuelae (5, 8). It is challenging to interpret this complex life cycle using methods appropriate for E. coli (which grows similarly on both agar and in suspension), and obtaining meaningful genetic network data depends entirely on the experimental setup.
Transcriptional regulation of amino acid metabolism is an old topic, but interest has been renewed because of its importance in Streptomyces spp. as active producers of amino acid-conjugated metabolites, such as siderophores, mycothiol and undecylprodigiosin (Red), calcium-dependent antibiotics (CDA), and other nonribosomal polypeptide (NRP) proteins in S. coelicolor. Branched-chain amino acids (BCAA) are of great interest as their degradation provides a significant portion of the precursors needed for polyketides (blue-pigmented actinorhordin [ACT]) and branched-chain fatty acids (BCFAs) (27, 29). About 70% of total fatty acids in Streptomyces are BCFAs, while only 30% are BCFAs in enteric bacteria (6). This biochemical difference suggests that there may also be a difference in the metabolic and regulatory activities related to BCAA.
With Streptomyces, three conserved TFs are characterized in the central metabolism of amino acids: ArgR, GlnR, and NdgR (17, 25, 31). GlnR, an OmpR-type regulator, has been studied mainly with respect to glutamine and glutamate biosynthesis. Recent chromatin immunoprecipitation coupled with DNA microarray (ChIP-chip) analysis identified 36 GlnR binding sites in the S. venezuelae genome. From this, its global regulatory activity was confirmed: a metabolic landscape that includes primary nitrogen metabolism and secondary metabolism (23). ArgR is a repressor for arginine biosynthesis and works by binding Arg boxes present upstream of several arginine biosynthesis genes. Arg boxes in Streptomyces clavuligerus are imperfect palindromes comprised of two 18-nucleotide Arg boxes separated by 2 bp (25). Derepression of arginine and pyrimidine biosynthesis genes by argR deletion was shown using a multi-omics approach; however, the change in gene expression was indifferent to arginine supplementation (22). NdgR is a recently identified TF. In the chromosome, ndgR is positioned adjacent to leuCD encoding isopropylmalate dehydrogenase subunits. Two independent studies with S. clavuligerus and S. coelicolor demonstrated that NdgR positively regulated leuCD expression (26, 31). However, knockout of ndgR and its ortholog had far-reaching impacts on the production of antibiotics such as ACT-undecylprodigiosin (Red) and clavulanic acid-cephamycin.
In an attempt to understand the regulatory function of NdgR, we found that abnormal growth of an ndgR knockout mutant could not be ascribed to leucine metabolism. That finding led us to the work described here in which we present evidence suggesting that NdgR is an activator of methionine (and likely cysteine) biosynthesis and discuss why this activity is important to S. coelicolor.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Strains and plasmids used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant feature(s) |
|---|---|
| Strains | |
| S. coelicolor | |
| M145 | Wild type |
| BG11 | M145 with a deletion of ndgR, Aprr |
| BG22 | M145 with a deletion of leuCD, Aprr |
| BG13 | BG11 ndgR complemented, Neor |
| E. coli | |
| ET12567(pUZ8002) | Methylation deficient; conjugation; Cmr Kmr |
| BL21 | Host for protein overexpression |
| Plasmids | |
| pET24ma | E. coli expression vector for histidine-tagged protein purification, Ampr |
| pET24ma-ndgR | pET24ma containing ndgR |
| pSET125 | Integration vector |
| pSETneo | pSET125 derivative, apramycin cassette replaced with Neor |
| pSETneo-ndgR | pSETneo with ndgR |
Spores of S. coelicolor A3(2) M145 and mutant strains were prepared using mannitol-soya flour (MS) agar plates. For shake flask cultures of S. coelicolor, 8 g of glass beads was added to 250-ml baffled flasks, and cells were grown at 30°C with vigorous shaking (200 rpm). Cell growth was monitored by the optical density at 450 nm (Multiskan Spectrum scanner; ThermoLabsystem).
Complex R5 medium (31) or basic minimal medium (BMM) was used for culturing S. coelicolor. BMM was prepared by modifying the composition of a well-known supplemented liquid minimal medium (SMM) (28). One liter of BMM contains 50 g of polyethylene glycol (PEG) 6000, 5 mM MgSO4 · 7H2O, 25 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer, 3 mM (NH4)2SO4, 1 mM NaH2PO4, 1 mM K2HPO4, 1% glucose (wt/vol), 0.01% antifoam solution, and 1% (vol/vol) trace element solution (trace element solution contains 0.1 g · liter−1 each of ZnSO4 · 7H2O, FeSO4 · 7H2O, MnCl2 · 4H2O, CaCl2 · 6H2O, and NaCl). Sterile amino acid or Casamino Acid stock solution was separately prepared and added later to BMM. R5 sucrose-free medium was prepared by omitting sucrose from R5 medium, and glucose or N-acetylglucosamine content was adjusted to 0.8% (wt/vol). One liter of minimal medium (MM) agar plates was prepared with 10 g of Bacto agar, 0.5 g of K2HPO4, 0.2 g of MgSO4 · 7H2O, 0.01 g of FeSO4 · 7H2O, and 10 g of glucose. (NH4)2SO4 at 3 mM was used as a major nitrogen source (11).
For liquid BMM cultures, spores were pregerminated by heat (at 40°C for 20 min or at 50°C for 10 min) in double-strength germination buffer (1% yeast extract, 1% Casamino Acids, 0.01 M CaCl2). Nutrient carryover was minimized by thorough washing using centrifugation prior to inoculation in fresh medium. For plate cultures, spores were directly spread on agar plates without pregermination.
Escherichia coli DH5α and BL21 were used for cloning and protein overexpression, respectively, and were routinely grown in Luria-Bertani medium at 37°C. pET24ma vector has a C-terminal His tag sequence. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used for induction (Novagen, Merck). Escherichia coli BW25113 and ET12567 were used in constructing in-frame deletion mutants as described elsewhere (9).
Nutrient downshift assay.
For RNA extraction, S. coelicolor spores were seeded in complex R5 liquid medium. About 16 h later, cells were collected and washed three times in BMM washing buffer (without a C/N source) by brief centrifugation. Washed cells were resuspended in fresh medium. BMM, amino acid-supplemented BMM (containing 37 μg/ml of methionine and leucine or 0.2% [wt/vol] Casamino Acid solution), and R5 were used for comparisons. Resuspended cells were placed back on a rotary shaker set for 200 rpm at 30°C. At the time points indicated in the figures, cells were collected for RNA analysis by centrifugation (see the paragraph below on total RNA extraction).
For mycothiol analysis, cells at the early exponential phase in complex R5 liquid medium were thoroughly washed by centrifugation as described above. Washed cells were evenly spread on cellophane-sheeted MM agar plates. After ca. 16 h of incubation at 30°C, the cell mass was scraped from the plates, frozen immediately with liquid nitrogen, and stored at −80°C until further use.
Molecular cloning and DNA manipulation.
Standard procedures were followed for DNA cloning and transformation. Plasmids were extracted using DNA mini-columns by the alkaline lysis technique (GeneAll, South Korea). Genomic DNA (gDNA) extracts of S. coelicolor strains were obtained using G-Spin (iNtRon Biotechnology, Inc., South Korea). PCR amplification was done with a TaKaRa thermocycler (TaKaRa, Japan). Pfu polymerase was used for cloning (HanPfu; Genemed), and Taq polymerase was used for routine PCR quality checkup (Sp-Taq [GeneAll] and LA-Taq [TaKaRa]). PCR product gel purification was done using type-D mini-columns (GeneAll, South Korea).
PCR-mediated gene knockout was done following standard methods (9). S. coelicolor M145 was used as a parental strain. The leuCD knockout mutant strain was named BG22. While an ndgR knockout mutant, BG11, was previously described (31), chromosomal deletion of ndgR was repeated to ensure its phenotypic feature. A newly acquired isolate showed the same growth delay in minimal medium. Successful construction of knockout mutants was confirmed by acquired antibiotic resistance (Aprr Kms) and PCR amplification sequencing.
For ndgR genetic complementation, the coding sequence and the region upstream of the coding sequence of sco5552 were cloned into pSET152neo, a pSET152 derivative with a neomycin cassette. Successful genomic integration of the constructed vector was confirmed by acquired neomycin resistance (Neor) and by PCR amplification over the region surrounding the attB site (within sco3798).
Recombinant NdgR (rNdgR) was designed to have a carboxy-terminal polyhistidine tag (His6) by cloning the sco5552 coding sequence (CDS) into pET24ma using EcoRI and HindIII restriction enzyme sites.
All primers used in the study are listed in Table 2.
Table 2.
Oligonucleotides used in this study
| Primer function and name | Sequence (5′→3′) |
|---|---|
| DACA | |
| DACA_MetE_ up | ACCTACGACCGGGACTGAG |
| DACA_MetE_down (5′biotin) | CTCGATCGCCTTCTTCAGTT |
| DACA_MetH _up | GTCGTCCTGCTCCACGAAC |
| DACA_MetH_down (5′biotin) | TGAAAGGGCTCCCTAGGAT |
| DACA_metF_up | ACACGGGTAGCCGCACCT |
| DACA_metF_down (5′biotin) | CGGTGGCGAGGATGTCAC |
| EMSA | |
| FAM_ndgR_48for | TCTGTGAAACGCAAGTTCAATTTTCCATGGAACGCGCCACCCTGGACG |
| FAM_metH_48for | GGTGACCGCGAACGTTCCGTACGAGGCGTGCGCGCCCCACACGTGCGC |
| FAM_metF_48for | GTGGACAACAACTCGACAATGTGGACAAAAGTCCCGCATCCGGTTGGG |
| ndgR_48for | TCTGTGAAACGCAAGTTCAATTTTCCATGGAACGCGCCACCCTGGACG |
| metH_48for | GGTGACCGCGAACGTTCCGTACGAGGCGTGCGCGCCCCACACGTGCGC |
| metF_48for | GTGGACAACAACTCGACAATGTGGACAAAAGTCCCGCATCCGGTTGGG |
| ndgR_48rev | CGTCCAGGGTGGCGCGTTCCATGGAAAATTGAACTTGCGTTTCACAGA |
| metH_48rev | GCGCACGTGTGGGGCGCGCACGCCTCGTACGGAACGTTCGCGGTCACC |
| rmetF_48rev | CCCAACCGGATGCGGGACTTTTGTCCACATTGTCGAGTTGTTGTCCAC |
| ndgR_38for | GTGGCGCGTTCCATGGAAAATTGAACTTGCGTTTCACA |
| ndgR_38rev | TGTGAAACGCAAGTTCAATTTTCCATGGAACGCGCCAC |
| metH_38for | GTGACCGCGAACGTTCCGTACGAGGCGTGCGCGCCCCA |
| metH_38rev | TGGGGCGCGCACGCCTCGTACGGAACGTTCGCGGTCAC |
| metF38a_for | AAAAGTCCCGCATCCGGTTGGGTGACCGCCGCACCCTG |
| metF38a_rev | CAGGGTGCGGCGGTCACCCAACCGGATGCGGGACTTTT |
| metF38b (predict)_for | AAGGGTGGACAACAACTCGACAATGTGGACAAAAGTCC |
| metF38b (predict)_rev | GGACTTTTGTCCACATTGTCGAGTTGTTGTCCACCCTT |
| Cloning | |
| ndgR_EcoRI_pET24ma | AATAGAATTCATGGACAACAGTAGCGGCGT |
| ndgR_HindIII_pET24ma | ATATAAGCTTACCGTTGCGGCGCAGCGCCT |
| ndgR_pSET_BamHI | AATAGGATCCGGCCGTTGTGACTTCCTG |
| ndgR_pSET_EcoRV | ATATGATATCAGTGTCCTGGGACGGTCA |
| PCR knockout | |
| leuCD_PCRdetect_left | GGCAGGAAGTCACAACGG |
| leuCD_PCRdetec_right | AGTTGTGCGTCACGTCCG |
| leuCD_PCRKO _up | TGGGTCGGCGCCTCGTCGCCGACCGAAGGGAAAGCGATGTGTAGGCTGGAGCTGCTTC |
| leuCD_PCRKO_down | CTCGATCGACATGTTGCAGATGGTCATGCGGGCCTCCATATTCCGGGGATCCGTCGACC |
| attB_detect complementationsco_up | CCGTGACCGTCGAGAACC |
| attB_detect complementation_down | GCCCCTTCTGGAAATCCTC |
| S1 mapping | |
| S1_metF_for | AGCAATACACGGGTAGCCGCACCT |
| S1_metH_for | AATCCGACCGAACACTACCGGTCC |
| S1_metF_rev | CGGTGGCGAGGATGTCAC |
| S1_metH_rev | CGAGCTGCTGGAAGTCGT |
Total RNA preparation from S. coelicolor cultures and quantitative reverse transcription-PCR and S1 mapping.
Cells were harvested and washed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4) by brief centrifugation for immediate processing. Alternatively, RNAlater solution was used for long-term sample storage (Sigma-Aldrich).
Extraction of total RNA extracts was obtained using Ribospin columns (GeneAll, South Korea) or by using a modified Kirby mix solution (14). After DNase treatment to eliminate DNA contamination (Fermentas/Thermo Fisher Scientific), samples were repurified by the phenol-chloroform-ethanol precipitation method and stored at −80°C. RNA concentration was measured by UV absorbance (NanoDrop ND-1000 spectrophotometer; Thermo Fisher Scientific). RNA integrity was checked by formaldehyde-agarose gel electrophoresis (1.2% agarose).
For reverse transcription, Moloney murine leukemia virus (M-MLV) reverse transcriptase (M-Biotech, South Korea) was used with 1 μg of total RNA template. Real-time PCR was carried out with a LightCycler instrument and SYBR PCR mixture solution (Roche Diagnostics). Duplicate sample mixtures were prepared for each PCR reading. ΔΔCT (where CT is threshold cycle) values (16) were obtained by averaging data from multiple (at least three) independent experiments.
S1 mapping was performed as described in Practical Streptomyces Genetics (13). A total of 40 μg of RNA sample was hybridized with a 32P-labeled DNA probe for 4 h at 45°C. One hundred units of S1 nuclease was used (TaKaRa, Japan).
DACA.
S. coelicolor cells were flask cultured in R5 and in BMM liquid media. BMM-leucine (37 μg/ml) was used in culturing BG22 cells. At the exponential phase, cells were collected and washed in phosphate-buffered saline (PBS) buffer by centrifugation. Cells were sonicated and ruptured in lysis buffer. Crude soluble protein extracts were obtained by centrifugation (for 20 min at 4°C). Detailed procedures of the DNA affinity capture assay (DACA) have been described previously (21). Protein extracts obtained from the pulldown assay were tryptic digested and subjected to nano-high-performance liquid chromatography (HPLC)/tandem mass spectrometry (MS/MS) using a QSTAR Elite (Applied Biosystems) system at the National Instrument Center for Environmental Management (NICEM), Seoul National University, South Korea. PEAKS Studio, version 5.3, software was used for protein identification (33). The PEAKS DB program uses linear discriminative function (LDF) scores in matching the peptide spectrum for the most likely correct peptide from a database search. Converted to P value, the probability of false identification is reported in log10 scale; 10lgP is the International Organization of Standardization (ISO)-reserved notation for log10.
Bioinformatics tools for identifying NdgR protein binding sites.
DNA sequence and annotation for the S. coelicolor A3(2) genome were obtained from StrepDB. The MEME suite Web server (http://meme.sdsc.edu/meme) and SeSiMCMC were used for sequence homology comparison (http://favorov.imb.ac.ru/cgi-bin/gibbslfm/gibbslfm.pl?action=form). Predicted NdgR binding sites were aligned with CLUSTALW (http://www.ebi.ac.uk/Tools/msa/clustalw2).
Electrophoresis mobility shift assay (EMSA).
Purification of recombinant NdgR was done as previously described (31). DNA probes modified at the 5′ end with 6-carboxyfluorescein (FAM) in one strand were used for visualization (Cosmo Genetech, South Korea). Mixtures of forward and reverse DNA strands were heated to 94°C for 5 min and then cooled at room temperature for annealing. Fluorescent probes longer than 60 bp (including metH 394-bp) were obtained by PCR amplification.
For protein and DNA binding complex formation, 2 pmol of FAM-labeled double-stranded DNA (dsDNA) probe and ∼0.1 μg of recombinant NdgR (rNdgR) were routinely added to a mixture containing 1 μg of sheared salmon sperm DNA (as nonspecific competitor DNA), 5 mM MgCl2, 0.5% (vol/vol) glycerol, and 1× Tris-borate-EDTA (TBE) buffer (pH 7.6). Each assay mixture was incubated at 37°C for 30 min. Specific competitor DNAs are label-free, double-stranded oligonucleotides. Specific competitors were routinely added at 50 times the fluorescent probe amount unless otherwise indicated. Acrylamide gel electrophoresis was conducted with 4% nondenaturing gel and TBE running buffer (0.5× TBE buffer, pH 7.6). After a 30-min electrophoresis run (constant voltage, 100 V), fluorescent bands were separated and visualized with a Typhoon 8600 scanner (GE Healthcare). All primers used for EMSA are listed in Table 2.
Metabolite analysis.
Thiol compounds were fluorescence labeled with monobromobimane (mBBr) for detection (20). A reverse-phase analytic column (SunFire C18; 5-μm particle size; 4.6 by 150 mm) was used, and metabolites were separated (Shimazu HPLC; Japan). A step gradient was made by mixing solvent A (water plus 0.1% trifluoroacetic acid [TFA]) and solvent B (99.9% acetonitrile [ACN] plus 0.1% TFA). Fluorescence was monitored by setting the wavelength at 380 nm for excitation and at 480 nm for emission. Details of sample preparation are as described below.
For reduced mycothiol, cells were sonicated in 40 mM HEPES (pH 7.6) and mixed with an equal volume of ACN solution containing 4 mM mBBr (Fluka). The mixtures were incubated at 60°C for 25 min in the dark. Control samples, blocked of mBBr conjugation, were prepared by N-ethylmaleimide (NEM; 5 mM) treatment prior to ACN-mBBr addition.
For detecting oxidized mycothiols, each cell lysate was mixed with an equal volume of ACN with 0.5 mM NEM, incubated for 10 min at 60°C, and cooled on ice. Cell debris was removed by centrifugation (at 12,000 rpm for 15 min). Excess NEM was removed with 1 mM β-mercaptoethanol. After a 10-min incubation at room temperature, dithiothreitol (DTT) was added to the mixture (2 mM) to oxidize all thiol compounds. After 15 min, 100 mM mBBr was added at 1/10 of the sample volume, and the mixture was kept at room temperature for another 15 min.
All samples, treated as above, were acidified with 5 M methanesulfonic acid. After centrifugation (12,000 rpm for 5 min), cleared samples were injected to the HPLC system at a flow rate of 1 ml · min−1.
RESULTS
Normal growth of BG11 depends on cysteine and methionine.
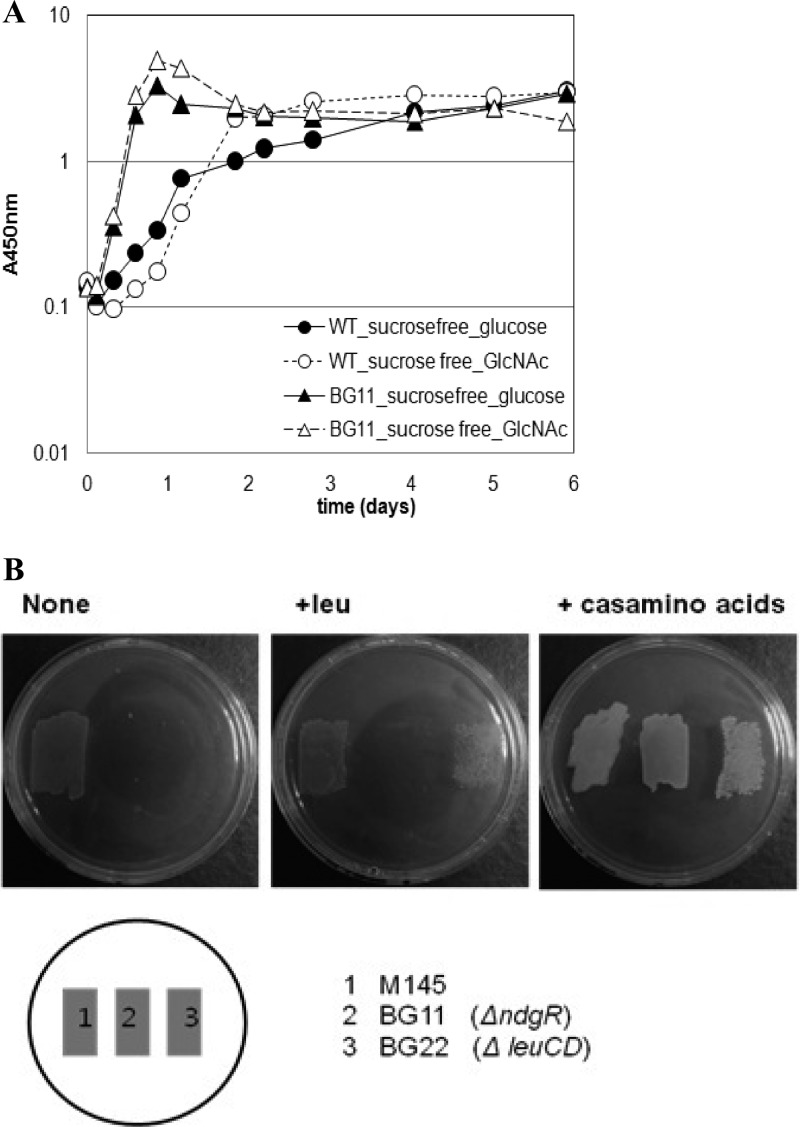
Growth of the ndgR knockout mutant, BG11, was affected by nitrogen components in the medium. In complex medium containing yeast extracts and Casamino Acids, BG11 grew as well as or even better than its parental strain, M145 (Fig. 1A). In minimal defined medium, however, BG11 grew very poorly even with leucine supplementation. Slow growth was consistently noticeable after about a 2-week incubation in liquid BMM (made only with glucose and NH4+); growth was affected by the identity of the amino acid(s) added to the medium, and in some instances BG11 failed to grow.
Fig 1.
Leucine-independent growth defect of ndgR knockout mutant. (A) Growth comparison of M145 (circles) and BG11 (ΔndgR; triangles) in complex liquid media. R5 sucrose-free media were prepared as described in Materials and Methods. Glucose-medium is marked with closed symbols, while GlcNAc-medium is marked with open symbols. (B) Growth comparison of M145, BG11, and BG22 on three different agar plates. Growth on basic minimal medium (BMM) made of glucose and (NH4)2SO4 was used as a control (None). Growth was compared on BMM supplemented with leucine (+leu) or Casamino Acids (right). WT, wild type.
The growth defect was less severe on agar plates than in liquid medium. An approximately 2-day delay in growth was seen on the leucine-supplemented agar plates, while other supplements such as glutamate made the mutant grow much more slowly. Since leuCD is a major target of NdgR, it was odd and unclear whether BCAA metabolism could be complicated as such physiological outcomes in Streptomyces. When a leuCD in-frame deletion mutant (BG22) was constructed as an extreme measure and BCAA metabolism was blocked, leucine (37 μg/ml) restored the growth without any delay (Fig. 1B). The difference in growth rates with leucine between the BG11 and BG22 cells made it clear that the abnormal growth of BG11 was not due to BCAA metabolic complication, if any. Growth of BG11 could also be restored by chromosomal reintegration of ndgR (Fig. 2A); therefore, the observation should be an NdgR-dependent phenomenon.
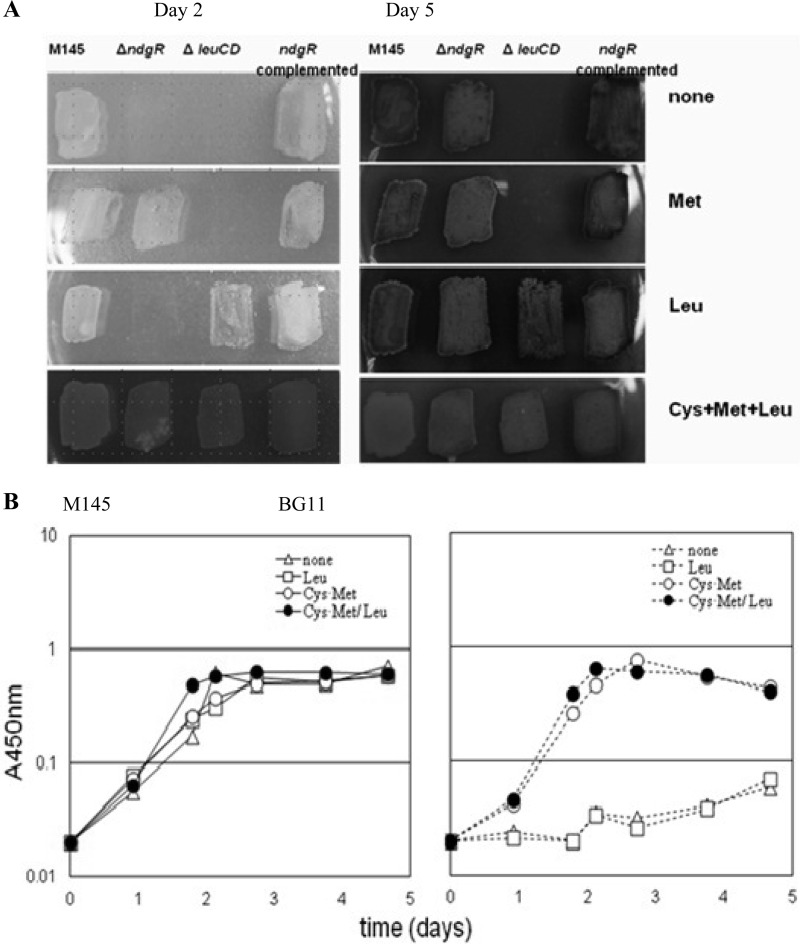
Fig 2.
Restoration of BG11 growth with cysteine and methionine supplementation. (A) Growth of M145, BG11, BG22, and BG13 was compared on MM agar plates. MM without amino acids was used as a control (none). Images show results for MM supplemented with methionine, leucine, and cysteine-methionine-leucine. Images were acquired on the day 2 and day 5 after spore inoculation. (B) Growth comparison by amino acid in minimal liquid medium. The left panel shows the growth of M145, and the right panel shows the growth of BG11. Open triangles represent the growth in BMM (none). Results of growth in BMM supplemented with leucine (37 μg/ml), cysteine and methionine (18 μg/ml each), and cysteine-methionine-leucine (at 18, 18, and 37 μg/ml, respectively) are presented as indicated.
Based on the NdgR requirement for proper growth in minimal medium, we looked into the possibility of NdgR involvement in the metabolism of other amino acids. It was a reasonable speculation since LtbR (the NdgR ortholog in Corynebacterium glutamicum) was shown to affect sets of genes for biosynthesis of tryptophan besides BCAA. Amino acid-dependent growth was not characterized in that study, however (3). Adopting an empirical approach, we examined 20 individual l-amino acids as nutritional supplements to minimal medium (see Fig. S1 in the supplemental material). As minimal medium has 1% glucose and 3 mM (NH4)2SO4, addition of each amino acid at a final concentration of 37 μg/ml was regarded as a minor C/N source in the catabolic process.
Growth on agar plates was monitored by an endpoint, defined as the time when cells were discernibly amassed. The growth delay disappeared with methionine and/or cysteine or both on agar plates (Fig. 2A) and in liquid BMM (at 18 μg/ml final concentration of each equivalent to 150 μM Cys and 124 μM Met). In contrast to the little benefit of leucine for growth (37 μg/ml final concentration; 282 μM Leu) in liquid BMM (Fig. 2B), the striking positive effects of Cys-Met indicated that deficiency of the Cys-Met supply should be the main reason for the growth defect.
Activation of the Met biosynthetic pathway by NdgR via direct protein-DNA interaction.
Genes in the Cys-Met biosynthetic pathway are scattered in the genome, and some are present as multiple forms in S. coelicolor. Initial determination of candidates was aided by measuring mRNA expression, which showed an overall reduction of gene expression (ca. 2-fold) in BG11 compared to that in M145 in minimal medium (data not shown).
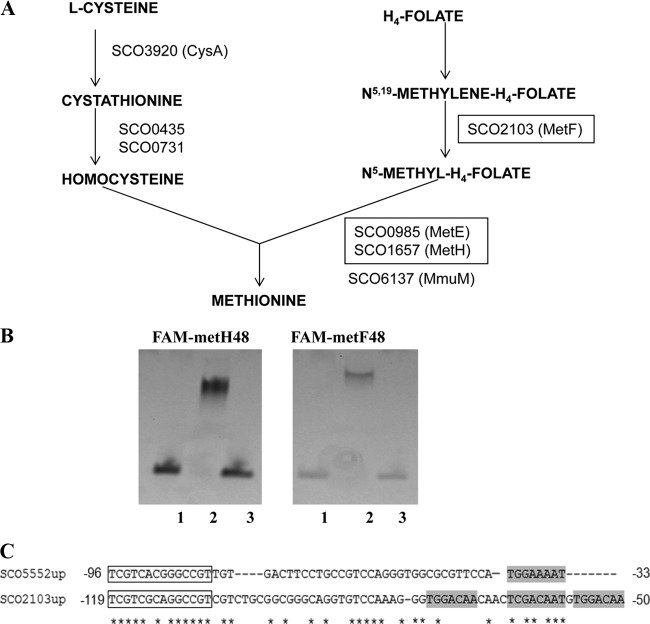
Direct involvement of NdgR in gene expression was examined by considering the genes involved in the very last steps of methionine synthesis (Fig. 3A): metF and metE-metH. Two metabolic branches converge to make methionine: a branch to produce the homocysteine moiety from acetyl-serine and the other to provide the methyl-donor from folate. MetF is methylenetetrahydrofolate reductase for converting N5,N10-methylenetetrahydrofolate to N5-methyltetrahydrofolate (H4-folate). MetE and MetH use the methyl group from H4-folate to convert methylate homocysteine to methionine.
Fig 3.
Direct interaction of NdgR on target genes. (A) Biosynthetic pathway of methionine. The metF and metH-metE genes tested with DACA are boxed. (B) Gel shift assay. FAM-labeled probe sequences for metH and metF upstream were used. Lane 1, probe only; lane 2, probe with rNdgR; lane 3, rNdgR with labeled and competitor DNAs. ndgR-38 was used as a specific competitor for FAM-metH-48 and FAM-metF-48. (C) Comparison of sco5552 (ndgR) and sco2103 (metF) upstream sequences. Relative position from the translational start codon is indicated, and conserved short motifs are boxed. Asterisks indicate identical residues.
We screened TFs that can bind upstream sequences of metF and metE-metH using a DNA affinity capture assay (DACA) (21). For this, upstream intergenic DNA probe sequences, biotin end labeled, were PCR amplified. Proteins attached to the probes were pulled down from total crude cell lysates using streptavidin-coupled DynaBeads (Invitrogen). Tandem MS analysis was performed to identify them. Two sets of experiments were conducted as described below.
In the first experiment, cells grown in complex R5 medium were used to test metE and metH as target baits. NdgR was identified as the most likely protein bound to the metH probe with 13 unique peptide peaks from MS/MS scanning (−10lgP = 160.9) (see Fig. S2 and Table S3 in the supplemental material). Of note, the metH gene was reported as essential to aerobic hyphal formation in S. coelicolor (7). As the biotin-metE probe did not succeed in pulling down NdgR, metE was not investigated further. Other top-ranked proteins obtained from the metH probe are listed in Table S3 in the supplemental material. There are three characterized TFs (SCO0608-SlbR, SCO4118-AtrA, and SCO5231-GntR) (10, 24, 32) and one uncharacterized TF (SCO3198) in the list. A PEAKS −10lgP score of 100 was used as a cutoff.
A second set of experiments was carried out testing metH and metF as target baits. Cells were grown in minimal medium to prepare crude cell extracts for the assay. We used BMM-grown cells as we knew by then that NdgR is inducible by nutritional downshift (see below). By this modification, much simpler protein profiles were obtained singling out NdgR as the most highly scored and abundant protein on both probes (Table 3).
Table 3.
Transcriptional regulators bound to the biotinylated metH and metF probesa
| Probe and cell line | Accession no. or name | Score | Coverge (%) | No. of peptides | Locus and description |
|---|---|---|---|---|---|
| metH | |||||
| M145 | SC1C2.33c | 263.45 | 53 | 10 | SCO5552, putative transcriptional regulator |
| SCF55.32 | 44.21 | 4 | 1 | SCO0608, putative transcriptional regulator | |
| SCD84.25c | 25.89 | 4 | 1 | SCO4158, putative LacI-family regulator | |
| SCI46.03 | 21.77 | 6 | 1 | SCO1658, glycerol operon regulator, gylR | |
| 2SCG61.35 | 21.52 | 4 | 1 | SCO1353, putative transcriptional regulator | |
| SCE34.23 | 28.51 | 2 | 1 | SCO3042, putative transcriptional regulator | |
| SCM1.10 | 22.67 | 1 | 1 | SCO0877, putative transcriptional regulator | |
| SCF1.04 | 20.83 | 4 | 1 | SCO0262, putative LysR-family transcriptional regulator | |
| BG22 | SC1C2.33c | 206.07 | 20 | 5 | SCO5552, putative transcriptional regulator |
| SCC121.21c | 21.47 | 2 | 1 | SCO2518, putative two-component sensor kinase | |
| SCJ1.15 | 20.88 | 3 | 1 | SCO0166, putative regulator | |
| metF | |||||
| M145 | SC1C2.33c | 225.09 | 34 | 7 | SCO5552, putative transcriptional regulator |
| SCF55.32 | 204.24 | 10 | 4 | SCO0608, putative transcriptional regulator | |
| SCE34.23 | 28.51 | 2 | 1 | SCO3042, putative transcriptional regulator | |
| SCJ12.15c | 25.78 | 2 | 1 | SCO0203, putative two-component sensor | |
| lipR | 34.07 | 2 | 1 | SCO0712, putative transcriptional activator | |
| BG22 | SC1C2.33c | 239.23 | 32 | 8 | SCO5552, putative transcriptional regulator |
| SC6G9.13 | 22.85 | 2 | 1 | SCO5320, whiE protein | |
| SCH44.04 | 22.68 | 4 | 1 | SCO3664, putative regulator | |
| 2SCG4.20 | 21.65 | 4 | 1 | SCO1104, putative TetR family transcriptional regulator |
Whole-cell lysates were obtained from M145 and BG22 cultures from the basic minimal liquid medium (leucine was supplemented for culturing BG22). With PEAKS Studio, version 5.3, MS/MS spectra of the captured proteins were analyzed, and proteins were identified.
Additionally, electrophoretic mobility shift assays (EMSAs) were performed with purified His6-tagged rNdgR. We carried out a series of independent experiments using different nucleotide sequences, starting with long PCR probes covering the whole metH upstream intergenic sequence and later using shorter ones. An iterative sequence homology search on intergenic sequences of ndgR-leuC, metH-glyR, and metF upstream sequences was carried out. By this method, the probe size required for TF binding in vitro was reduced, and 48-bp FAM-labeled probes were routinely used (Fig. 3B). There was little difference in the binding affinity of rNdgR with the three 48-bp probes.
Further trimming was possible by competitive EMSA (cEMSA) employing label-free, double-stranded DNA sequences differing in size and sequence. As an alternative method to DNA footprinting, cEMSA provides rough but still useful information on a DNA-protein binding region. Specific competitors were assessed for their ability to dissociate an rNdgR–FAM–48-mer complex, and a size of 39 bp was enough to dissociate the protein-DNA complex. The chosen 38-bp specific competitors for ndgR, metH, and metF were interchangeable (Fig. 3B).
There was notably high sequence homology between upstream sequences of ndgR and metF, with multiple short motifs, e.g., TGGAA(C)AA (Fig. 3C). A bioinformatics-based approach predicted two sequences upstream of metF, and they differed in dissociating FAM-metF-48 complex (see Fig. S4A in the supplemental material).
Oligomerization or multiple binding of NdgR on target regions in vivo is assumed. Increasing the protein concentration made the protein-DNA complex bigger in vitro. Increase in the FAM-labeled probe size from 48 bp to 394 bp also appeared to strengthen DNA binding to rNdgR. A routine ratio of specific competitor to labeled probe (50×) for the FAM-labeled 394-bp probe was not as good as with the FAM-labeled 48-bp probe for complete dissociation but showed rather gradual dissociation (see Fig. S4B to D).
Inducible expression of ndgR and its target genes by nutritional downshift.
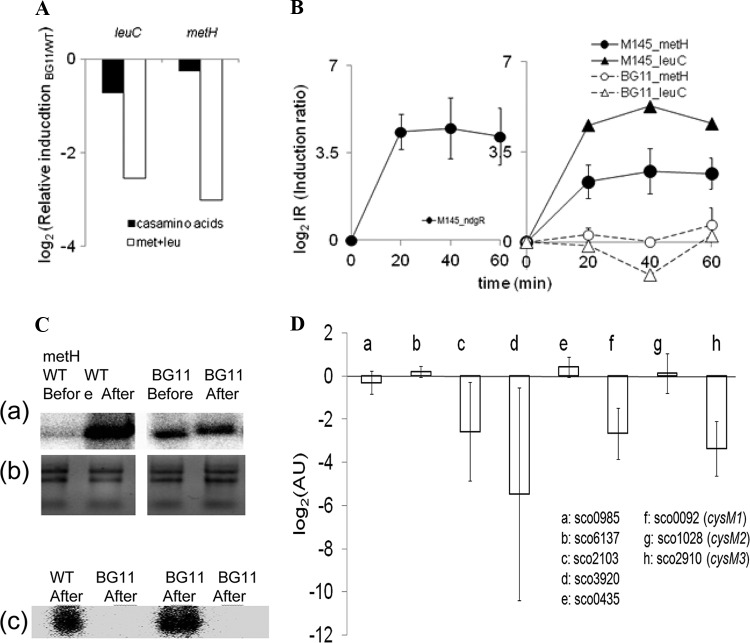
NdgR-dependent differential gene expression was examined by culturing M145 and BG11 in liquid BMM supplemented with Casamino Acids or with Met-Leu. Total RNA extracts were obtained from cells at the logarithmic phase approximately on the third day of culturing (optical density at 450 nm [OD450] of ∼0.5). Quantitative real-time RT-PCR was carried out, and the expression of each gene was normalized using hrdB as an internal control. Induction of metH and metF by the presence of NdgR was validated by comparing M145 and BG11 cells. A differential ratio of mRNA expression was more pronounced in the Met-Leu-supplemented medium than in the Casamino Acid-supplemented one (Fig. 4A). The availability of specific amino acids is assumed to be sensed by an unknown mechanism in controlling gene expression.
Fig 4.
NdgR-dependent gene expression. (A) Expression of leuC and metH was reduced in BG11 compared to M145 (y axis. Real-time qRT-PCR was performed using cells grown in minimal medium supplemented with Casamino Acids or Met-Leu (at 37 μg/ml). (B) Relative fold changes were compared, before and after medium exchange (log2 relative induction ratio of fold change after exchange/fold change before exchange. Instant induction of ndgR was noted (left). The mRNA expression of leuC-metH increased upon nutrient downshift in the wild type (filled symbols) but not in BG11 (open symbols) (right). (C) Low-resolution S1 mapping for metH and metF. Expression of metH was examined, comparing before and after nutrient downshift (panel a). The integrity of total RNA used in the assay was examined (panel b). A dramatic difference in metH and metF expression upon nutrient downshift was observed (panel c). RNA samples from 40 min after nutrient downshift were used. (D) The relative gene expression of BG11 over M145 by medium exchange was compared (AU, arbitrary units; log2 scale). The relative induction level (induction at 40 min/induction at time zero) was calculated for each strain and used in comparing the expression in BG11 with that in M145 (AU, in log2 scale). Error bars represent standard deviations of three independent experiments.
Supporting this assertion, medium exchange from rich medium to minimal medium altered gene expression. After nutrient downshift, mRNA expression of ndgR immediately increased along with expression of metH-metF-leuC in M145. There was no such sudden induction of these genes in BG11, confirming NdgR to be pivotal for this (Fig. 4B and C).
The possibility remains high that additional genes, particularly in cysteine biosynthesis, are ndgR dependent. Cysteine synthase, for instance, showed NdgR-dependent induction similar to that of metF-metH upon nutrient downshift. The S. coelicolor genome has three annotated genes for the specific function, namely, sco0092 (possible cysteine synthase, cysM1), sco1028 (possible lyase, cysM2), and sco2910 (cysM3). Among them, sco2910 (cysM3) showed the most striking induction by NdgR, and there was moderate induction of the others (Fig. 4D).
DISCUSSION
Here, we have identified methionine biosynthesis as another major metabolic target of NdgR and confirmed two key genes, metH and metF, to be under NdgR regulation in transcription. NdgR consists of two domains, a helix-turn-helix DNA binding N-terminal domain and an effector binding C-terminal domain. Although high sequence similarity is found in the substrate-recognizing domain with IclR-type regulators, most of the other part of the amino acid sequence of NdgR differs from the sequences of typical IclR-type proteins (26).
Besides the pathway-specific regulatory role with respect to leuCD, no other direct activity of NdgR has been confirmed to date. This study provides strong evidence proving the unexpected NdgR targets. It also suggests a way to improve the affinity-based protein search method (DACA) to boost TF expression in vivo by optimizing cell culture conditions, which in turn increases the chance of protein-DNA complex formation. From a biotechnological standpoint, NdgR regulation may be associated with amino acid-conjugated secondary metabolites such as thiopeptides. It would be exciting to see if NdgR has a role in antibiotic production (31) and to test if NdgR activity and its interaction with target genes can be manipulated to increase this production.
Liquid minimal medium-based cell culture is a good method for highlighting the pivotal role of NdgR in growth through its control of essential genes such as methionine synthase (metH) and tetrahydrofolate reductase (metF) (2, 7). However, the possibility remains high that there are additional target genes of NdgR in related pathways, as shown with cysteine synthase and its differential expression. Hence, further systemic approaches are needed to fully understand NdgR regulation and global regulatory networks.
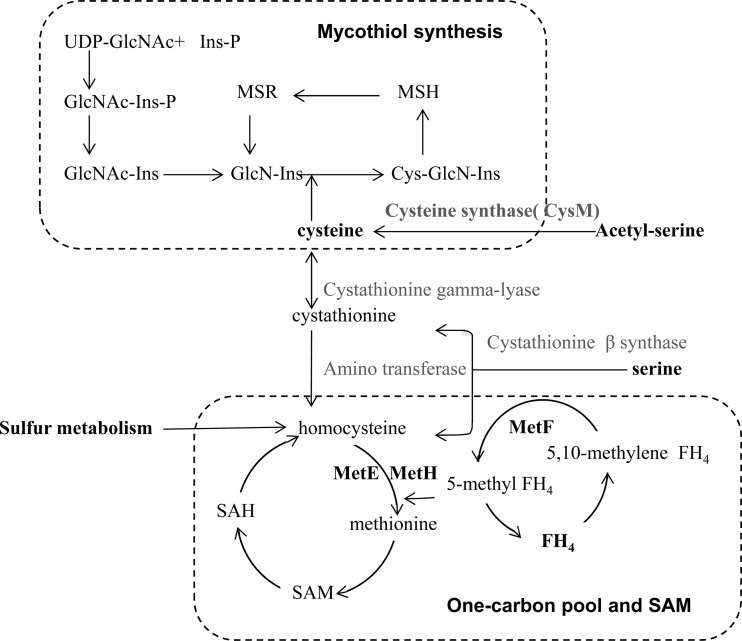
Closely linked to Cys-Met, mycothiol and S-adenosylmethionine (SAM) are worth mentioning in regard to cell growth. First, mycothiol is the major cysteine reserve in Actinobacteria and works as low-molecular-weight thiol redox buffer in place of glutathione. Demands on mycothiol would increase in liquid cultures as additional stresses are imposed on soil-living bacteria (30). Induction of cysteine biosynthesis genes by osmotic shock (15) and oxidative stress-dependent induction of methionine metabolism were noted in S. coelicolor (12). Supporting this, the intracellular content of thiol compounds in BG11 were reduced to 50% of that in M145 after nutrient downshift (see Fig. S5 in the supplemental material).
Second, SAM is a methionine-derived active methyl donor and works on diverse substrates including nucleic acids and lipids (Fig. 5). Availability of SAM does matter for cells growing on fatty acids and BCAA as a sole carbon source as utilization of such substrates generates propionyl-coenzyme A (CoA) that is toxic to cells when it accumulates (18, 26). SAM-dependent methylation converts propionyl-CoA to succinate and drives it forward to the tricarboxylic acid (TCA) cycle. Consistent with the previous observation with S. clavuligerus (26), BG11 grew poorly on leucine as a sole carbon source, which again could be overcome by methionine/cysteine supplementation (unpublished data). Since we could observe similar positive effects of Cys-Met in the Streptomyces peucetius model system (data not shown), regulation of NdgR and its orthologs on Cys-Met biosynthesis is regarded as a conserved feature in Streptomyces spp.
Fig 5.
Overview of Cys-Met biosynthesis associated with mycothiol and SAM. SAM and SAH recycling pathway (one-carbon metabolism) and mycothiol production pathway are connected to Cys-Met biosynthesis. FH4, 5,10-methylenetetrahydrofolate; MSH, 1-d-myo-inosityl-2-(N-acetyl-l-cysteinyl) amido-2-deoxy-a-d-glucopyranoside; MSR, MSH conjugate of the electrophile; GlcN-Ins, 1-d-myo-inosityl-2-amino-2-deoxy-a-d-glucopyranoside; Cys-GlcN-Ins, 1-d-myo-inosityl 2-(l-cysteinyl)amido-2-deoxy-α-d-glucopyranoside; Ins, 1-d-myo-inositol; GlcNAc, 2-amino-2-deoxy-α-d-glucopyranoside; SAH, S-adenosyl homocysteine.
Metabolite-mediated modulation may affect assembly or disassembly of higher-order protein-DNA complexes. As the protein-DNA complex shown in our gel shift assay did not require any metabolite in the reaction mixture, we attempted to see if metabolite-mediated interference may occur in NdgR-DNA complex formation, but we failed to see any such specific interference. Should NdgR interact with genomic DNA as a dimer or as dimers of dimers in vivo, the 48-bp probe DNA used in our EMSA might have been too short and inappropriate for achieving such a larger complex (see Fig. S4 in the supplemental material).
Understanding of amino acid metabolism and genetic regulation in Streptomyces is still at its early stage, and only limited numbers of transcriptional factors are characterized. To the best of our knowledge, NdgR is the first reported transcriptional regulator working directly for sulfur amino acid biosynthesis. Simultaneous action of NdgR on such disparate amino acid families as BCAA and Cys-Met is quite exciting and asks for further studies to elucidate the connectivity among genetic components and the interaction for metabolic flows including fatty acids and/or one-carbon metabolic pools. Now, with the expanded regulatory scope of NdgR, many questions remain to be explored at the molecular level regarding its working mechanism and the issues of synchronous and flexible gene regulation and cooperation with other TFs.
ACKNOWLEDGMENTS
We appreciate the scientific comments and advice from John C. March, Cornell University, and David J. McGee, LSU Health Sciences Center.
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (2011-0031388) and by the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Education, Science and Technology (2011-0031960).
Footnotes
Published ahead of print 12 October 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Bentley SD, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147 [DOI] [PubMed] [Google Scholar]
- 2. Blanco J, Coque JJ, Martin JF. 1998. The folate branch of the methionine biosynthesis pathway in Streptomyces lividans: disruption of the 5,10-methylenetetrahydrofolate reductase gene leads to methionine auxotrophy.J. Bacteriol. 180:1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brune I, et al. 2007. The IclR-type transcriptional repressor LtbR regulates the expression of leucine and tryptophan biosynthesis genes in the amino acid produce Corynebacterium glutamicum. J. Bacteriol. 189:2720–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Champness EC. 2000. Prokaryotic development. ASM Press, Washington, DC [Google Scholar]
- 5. Daza A, Martin JF, Dominguez A, Gil JA. 1989. Sporulation of several species of Streptomyces in submerged cultures after nutritional downshift. J. Gen. Microbiol. 135:2483–2491 [DOI] [PubMed] [Google Scholar]
- 6. De Rossi E, Leva R, Gusberti L, Manachini PL, Riccardi G. 1995. Cloning, sequencing and expression of the ilvBNC gene cluster from Streptomyces avermitilis. Gene 166:127–132 [DOI] [PubMed] [Google Scholar]
- 7. Gehring AM, Wang ST, Kearns DB, Storer NY, Losick R. 2004. Novel genes that influence development in Streptomyces coelicolor. J. Bacteriol. 186:3570–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glazebrook MA, Doull JL, Stuttard C, Vining LC. 1990. Sporulation of Streptomyces venezuelae in submerged cultures. J. Gen. Microbiol. 136:581–588 [DOI] [PubMed] [Google Scholar]
- 9. Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U. S. A. 100:1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirano S, Tanaka K, Ohnishi Y, Horinouchi S. 2008. Conditionally positive effect of the TetR-family transcriptional regulator AtrA on streptomycin production by Streptomyces griseus. Microbiology 154:905–914 [DOI] [PubMed] [Google Scholar]
- 11. Hopwood DA. 1967. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol. Rev. 31:373–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kallifidas D, Thomas D, Doughty P, Paget MS. 2010. The σR regulon of Streptomyces coelicolor A32 reveals a key role in protein quality control during disulphide stress. Microbiology 156:1661–1672 [DOI] [PubMed] [Google Scholar]
- 13. Kieser T, Bibb M, Buttner MJ, Chater K, Hopwood D. 2000. Practical Streptomyces Genetics. Crowes, Norwich, England [Google Scholar]
- 14. Kirby KS, Fox-Carter E, Guest M. 1967. Isolation of deoxyribonucleic acid and ribosomal ribonucleic acid from bacteria. Biochem. J. 104:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee EJ, et al. 2005. A master regulator σB governs osmotic and oxidative response as well as differentiation via a network of sigma factors in Streptomyces coelicolor. Mol. Microbiol. 57:1252–1264 [DOI] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 17. Malm S, et al. 2009. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology 155:1332–1339 [DOI] [PubMed] [Google Scholar]
- 18. Massey LK, Sokatch JR, Conrad RS. 1976. Branched-chain amino acid catabolism in bacteria. Bacteriol. Rev. 40:42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell W. 2011. Natural products from synthetic biology. Curr. Opin. Chem. Biol. 15:505–515 [DOI] [PubMed] [Google Scholar]
- 20. Newton GL, et al. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SS, et al. 2009. Mass spectrometric screening of transcriptional regulators involved in antibiotic biosynthesis in Streptomyces coelicolor A3(2). J. Ind. Microbiol. Biotechnol. 36:1073–1083 [DOI] [PubMed] [Google Scholar]
- 22. Perez-Redondo R, et al. 2012. ArgR of Streptomyces coelicolor is a versatile regulator. PLoS One 7:e32697 doi:10.1371/journal.pone.0032697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullan ST, Chandra G, Bibb MJ, Merrick M. 2011. Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics 12:175 doi:10.1186/1471-2164-12-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rigali S, et al. 2006. The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol. Microbiol. 61:1237–1251 [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez-Garcia A, Ludovice M, Martin JF, Liras P. 1997. Arginine boxes and the argR gene in Streptomyces clavuligerus: evidence for a clear regulation of the arginine pathway. Mol. Microbiol. 25:219–228 [DOI] [PubMed] [Google Scholar]
- 26. Santamarta I, et al. 2007. Connecting primary and secondary metabolism: AreB, an IclR-like protein, binds the ARE(ccaR) sequence of S. clavuligerus and modulates leucine biosynthesis and cephamycin C and clavulanic acid production. Mol. Microbiol. 66:511–524 [DOI] [PubMed] [Google Scholar]
- 27. Stirrett K, Denoya C, Westpheling J. 2009. Branched-chain amino acid catabolism provides precursors for the Type II polyketide antibiotic, actinorhodin, via pathways that are nutrient dependent. J. Ind. Microbiol. Biotechnol. 36:129–137 [DOI] [PubMed] [Google Scholar]
- 28. Strauch E, Takano E, Baylis HA, Bibb MJ. 1991. The stringent response in Streptomyces coelicolor A3(2). Mol. Microbiol. 5:289–298 [DOI] [PubMed] [Google Scholar]
- 29. Tang L, Zhang YX, Hutchinson CR. 1994. Amino acid catabolism and antibiotic synthesis: valine is a source of precursors for macrolide biosynthesis in Streptomyces ambofaciens and Streptomyces fradiae. J. Bacteriol. 176:6107–6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vohradsky J, Thompson CJ. 2006. Systems level analysis of protein synthesis patterns associated with bacterial growth and metabolic transitions. Proteomics 6:785–793 [DOI] [PubMed] [Google Scholar]
- 31. Yang YH, et al. 2009. NdgR, an IclR-like regulator involved in amino-acid-dependent growth, quorum sensing, and antibiotic production in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 82:501–511 [DOI] [PubMed] [Google Scholar]
- 32. Yang YH, et al. 2012. Characterization of a new ScbR-like gamma-butyrolactone binding regulator (SlbR) in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 96:113–121 [DOI] [PubMed] [Google Scholar]
- 33. Zhang J, et al. 2012. PEAKS DB: De Novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteomics 11:M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]