Abstract
Dark-grown hypocotyls of a starch-deficient mutant (NS458) of tobacco (Nicotiana sylvestris) lack amyloplasts and plastid sedimentation, and have severely reduced gravitropism. However, gravitropism improved dramatically when NS458 seedlings were grown in the light. To determine the extent of this improvement and whether mutant hypocotyls contain sedimented amyloplasts, gravitropic sensitivity (induction time and intermittent stimulation) and plastid size and position in the endodermis were measured in seedlings grown for 8 d in the light. Light-grown NS458 hypocotyls were gravitropic but were less sensitive than the wild type (WT). Starch occupied 10% of the volume of NS458 plastids grown in both the light and the dark, whereas WT plastids were essentially filled with starch in both treatments. Light increased plastid size twice as much in the mutant as in the WT. Plastids in light-grown NS458 were sedimented, presumably because of their larger size and greater total starch content. The induction by light of plastid sedimentation in NS458 provides new evidence for the role of plastid mass and sedimentation in stem gravitropic sensing. Because the mutant is not as sensitive as the WT, NS458 plastids may not have sufficient mass to provide full gravitropic sensitivity.
The availability of starch-deficient mutants has provided new opportunities to test the starch-statolith hypothesis, i.e. the idea that gravity sensing relies on the mass of amyloplasts that sediment. In WT plants amyloplast sedimentation occurs in highly specific tissues such as the central rootcap (columella) and in the starch sheath (endodermis) in stems (Sack, 1987, 1991). In mutants with little or no starch, plastid sedimentation appears to be absent from these locations (Caspar and Pickard, 1989; Kiss and Sack, 1990). Roots and hypocotyls of starchless or starch-deficient mutants of Arabidopsis thaliana are gravitropic, but sensing is reduced, as measured by an increased threshold of stimulation and by a greater variability in organ orientation in response to prolonged stimulation (Caspar and Pickard, 1989; Kiss et al., 1989, 1996). Light-grown roots of a starch-deficient mutant of tobacco (Nicotiana sylvestris), NS458, are also gravitropic and have reduced sensitivity (Kiss and Sack, 1989).
The findings that five different mutants at four loci have no or decreased starch and reduced gravitropism support the conclusions that starch plays a role in sensing when present, and that at least moderate levels of starch are necessary for full gravitropic sensitivity (Poff et al., 1994; Kiss et al., 1996; Sack, 1997). These hypotheses are also supported by many other correlative data, including older experiments with maize plastid mutants and with the depletion of starch by experimental manipulation (Hertel et al., 1969; Sack, 1991, 1997).
Data from NS458 hypocotyls provide striking support for the starch-statolith hypothesis, since the endodermis contains small, unsedimented plastids and these hypocotyls are severely deficient in gravitropism when grown in the dark (Kiss and Sack, 1990). However, preliminary experiments indicated that light-grown NS458 hypocotyls were strongly gravitropic without obvious increases in starch content, data that raised the possibility that light restored gravitropism independent of plastid mass or position. To analyze further the effects of light, we determined whether light-grown NS458 hypocotyls are as sensitive to gravity as WT hypocotyls, and whether light affects the amount of starch or plastid position compared with dark-grown mutant hypocotyls. This analysis shows that light induces plastid sedimentation in the NS458 endodermis, probably through an increase in plastid volume and in total starch content, and that light-grown mutant hypocotyls exhibit significant gravitropism but are less sensitive than the WT.
MATERIALS AND METHODS
Plant Material and Cultivation
Seeds used were WT tobacco (Nicotiana sylvestris Speg. et Comes) and the starch-deficient mutant NS458 (fourth generation with reselection for the phenotype after the first backcross). NS458 is deficient in the activity of plastidic phosphoglucomutase (Hanson and McHale, 1988).
Seedlings were grown in square polystyrene Petri dishes (100 × 15 mm) on 1% (w/v) agar containing nutrients supplemented with 1% (w/v) Suc, as described by Kiss and Sack (1990). The dishes were sealed with Parafilm (American National Can, Greenwich, CT) and placed on the edge so that the surface of the agar was vertical, and were placed under continuous illumination (60–80 μmol m−2 s−1 from 40-W cool-white fluorescent lamps, General Electric). After 8 d of cultivation, the hypocotyls were about 1 to 1.5 mm long. For assessment of plastid sedimentation, dark-grown seedlings were also used and cultivated as described by Kiss and Sack (1990).
Measurement of Gravitropic Curvature and Growth
To measure curvature and growth, light-grown seedlings were photographed and then transferred to the dark and either turned to the horizontal (for measurement of curvature) or kept upright (for measurement of growth). Dark-grown seedlings were intermittently photographed using Kodak T-Max 400 ASA film and illumination with dim-green light (intensity of approximately 0.9 μmol m−2 s−1 at the level of the hypocotyl) provided by an incandescent lamp filtered through two layers of a Roscolux filter (no. 1090, Rosco Laboratories, Port Chester, NY) with a peak transmission of 526 nm and one-half bandwidth of 58 nm. Growth rates were measured during the first 6 h in darkness using digitally scanned photographic negatives and NIH Image program (National Institutes of Health, Bethesda, MD). Gravitropic curvature was measured from photographic prints as the increment over the initial angle of each individual hypocotyl. At the start of the gravitropism experiments hypocotyl angles of both genotypes were within 10° from the vertical, since seedlings were grown for 8 d with the light source directly above, inducing phototropism.
For the measurement of the presentation time, hypocotyls were turned to the horizontal and immediately placed in the dark for 15 to 150 min and then rotated on a clinostat for 2 h. Seedlings were rotated at 1 rpm on a horizontal clinostat, with the axis of rotation parallel to the root-hypocotyl axis. A plot of curvature versus the stimulation time was constructed and regression lines for the WT and NS458 were calculated. The intercept of the extrapolated regression line and the x axis was taken as an estimate of the presentation time.
To measure the perception time by intermittent stimulation, Petri dishes were turned to the horizontal and immediately mounted on a clinostat in the dark. The seedlings were exposed to a total of 12 cycles (10 min each) consisting of gravistimulation (for 0.5–8 min) when the clinostat was stationary and the seedlings were horizontal, followed by rotation for the rest of the cycle. For 0.5-min stimulations, seedlings were rotated for only 9 min in each cycle. After the 12th cycle, the dishes were rotated for an additional 60 min. Curvatures were tested for significance using a paired Student's t test.
Plastid Sedimentation, Size, and Starch Content
Petri dishes containing light-grown seedlings were inverted (placed upside down) and kept in darkness for 1 h. The dishes were then filled with fixative consisting of 1.8% (w/v) formaldehyde, 5% (v/v) acetic acid, and 45% (v/v) ethanol, through a small hole in the top edge of the dish. Seedlings were then briefly vacuum infiltrated and fixed overnight, and were kept in an inverted orientation throughout. The hypocotyls were then washed in 50% ethanol, dehydrated, and embedded in paraffin or Steedman's wax (Vitha et al., 1997). Longitudinal 10-μm sections were dewaxed and stained for starch with an IKI solution (2% [w/v] KI, 1% [w/v] I).
In WT stems the endodermis is obvious because of the presence of large, sedimented amyloplasts. In NS458 the endodermis can still be recognized by its location as the innermost layer of the cortex. Only endodermal cells within the most apical 0.5 mm (light-grown hypocotyls) or 3 mm (dark-grown hypocotyls) were analyzed for sedimentation and plastid size. Sedimentation was evaluated by counting the number of plastids in the top, middle, and bottom thirds of individual endodermal cells visualized in the microscope. Sections from five hypocotyls were assessed for each genotype and light treatment (WT versus NS458; light versus dark).
For measurement of plastid size, the sections were photographed using a Plan 100× oil-immersion lens (model NA 1.3, Zeiss), and negatives were digitally scanned. NIH Image program software was used to trace plastid outlines at a total magnification of 20,000 to determine the minimum and maximum diameters of each plastid. For NS458, individual starch grains within plastids were also measured. Optical sectioning of endodermal plastids in hypocotyls indicated that plastid shape could best be approximated as an ellipsoid in both genotypes. Thus, plastid volume, V, was calculated for an ellipsoid, where V = [4/3 × π × (length/2)] × (width/2)2. The same calculation was used for the volume of starch grains within the NS458 plastids.
Stokes' law was used to calculate the theoretical velocity, v, of plastid sedimentation, where v = 2/9 × (d1 − d2) × g × r2/η, and (d1− d2) is the difference between the densities of the plastid and the cytoplasm, g is the gravitational constant, r is the plastid radius (calculated from plastid volume assuming a sphere), and η is the viscosity of the cytoplasm (here assumed to be 0.3 Pa; Björkman, 1988). The density values used were 1.015 g cm−3 for cytoplasm, 1.42 for amyloplasts, and 1.45 for starch. For NS458 plastids, starch occupied 10% of plastid volume (see Results), and the density of the remaining volume was assumed to be 1.113 g cm−3 (approximated from the density of 30% Glc). The potential energy of the sedimenting particle was calculated as (d1 − d2) × V × g × s, where V is the plastid volume and s is the distance displaced (here set at 10 μm).
Endodermal cell length was measured using an ocular micrometer from 4-μm sections of hypocotyls that were fixed in glutaraldehyde and embedded in Spurr's resin as described by Kiss and Sack (1990). Cell-length measurements were made only in regions also used to measure plastid size and sedimentation.
RESULTS
Light Significantly Improves NS458 Hypocotyl Gravitropism
NS458 hypocotyls grown in the dark for an extended period appeared almost agravitropic (Fig. 1E), whereas WT hypocotyls under similar conditions were upright (Fig. 1A). Light substantially improved the gravitropism of NS458 hypocotyls compared with those grown in the dark. (Note that gravitropism was allowed to develop in the dark, regardless of whether the plants had previously been grown in the light or the dark.) Whereas gravitropic curvature was about 10° (after 70 h horizontal) for dark-grown NS458 hypocotyls, it was almost 50° (after 50 h) for light-grown NS458 hypocotyls, a value close to that of the light-grown WT hypocotyls (Fig. 2). In addition, the onset of gravitropic curvature was much earlier and the rate of curvature was much greater in light- versus dark-grown NS458 hypocotyls (Fig. 2). This difference was not the result of growth rate, because dark-grown hypocotyls elongated twice as fast as light-grown hypocotyls (Table I). Also, within the same light treatment, WT and NS458 hypocotyls had the same growth rates (Table I). Thus, the 50% slower rate of gravitropic curvature of light-grown NS458 compared with WT hypocotyls (Fig. 2B) also cannot be explained by a difference in growth rate. Cultivation in the light had much less of an effect on the gravitropism of the WT hypocotyls (Fig. 2).
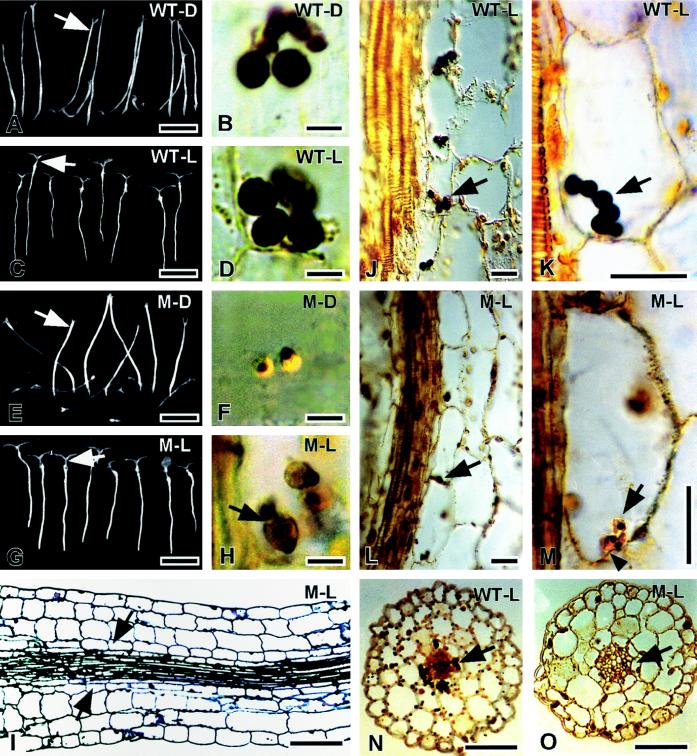
Figure 1.
Gravitropism and endodermal plastid sedimentation. Dark-grown WT plants (WT-D) are gravitropic (A) and contain large, sedimented amyloplasts (B) located approximately at the arrow in A. Dark-grown NS458 hypocotyls (M-D) are severely disoriented (E) and the endodermis (at the arrow in E) contains small plastids with small starch grains (F). Light-grown WT seedlings (WT-L, C) contain large amyloplasts that are sedimented in the endodermis (D, arrows in J and K). Light-grown NS458 seedlings (M-L, G) contain plastids that are larger and contain more starch (H) (compared with dark-grown mutants, F) that are sedimented in the endodermis (arrows in L and M). The position of the lower cell wall is indicated by an arrowhead in M. The endodermis in light-grown NS458 hypocotyls (arrows in I, L, M, and O) can be identified based on its position as the innermost layer of the cortex adjacent to the stele, even though it has less starch than the WT (N). All sections come from tissues that were fixed after being inverted for 1 h (see Methods) except for the section in I, which was kept horizontal for 1 h before fixation. All sections are longitudinal except for those in N and O, which are cross-sections. The gravity vector is toward the bottom of all figures, except for the cross-sections. All sections are of Steedman's wax stained with IKI to localize starch (blue-black) except for the section in I, which is of Spurr's resin stained with toluidine blue. Scale bars = 5 μm (B, D, F, and H), 50 μm (J–M), 100 μm (I, N, and O), or 10 mm (A, C, E, and G).
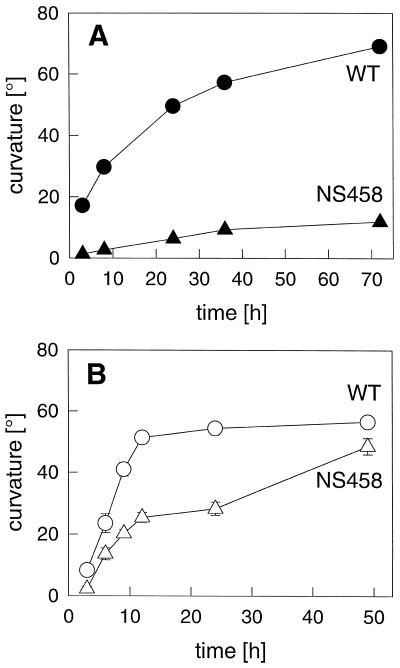
Figure 2.
Time course of gravitropic curvature after rotating dishes 90°. A, Dark-grown plants (data redrawn from Kiss and Sack, 1990). B, Seedlings grown for 8 d in the light were placed in the dark and immediately reoriented to the horizontal. Compared with dark-grown NS458 hypocotyls that exhibit almost no gravitropism, light-grown NS458 hypocotyls eventually curve almost as much as the WT. se bars are shown when greater than the size of the symbol. The experiment was repeated three times with similar results.
Table I.
Growth rates (mean ± se) of vertically grown hypocotyls
| Genotype | Growth Rate
|
|
|---|---|---|
| Light | Darka | |
| μm h−1 | ||
| WT | 15.0 ± 2.9 | 28.5 ± 1.3 |
| NS458 | 12.5 ± 2.9 | 25.8 ± 1.6 |
Growth was measured during a 6-h period in the dark, regardless of whether the plants were previously grown in the light or in the dark for 8 d; n = 37 to 83 plants.
Data from Kiss and Sack (1990).
Light-Grown WT Hypocotyls Are More Sensitive
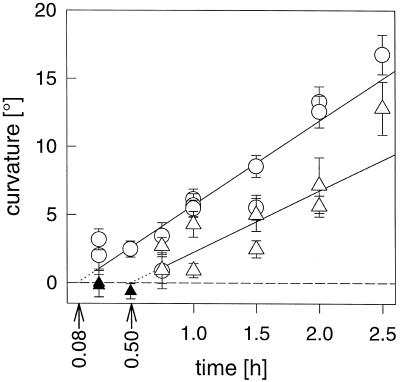
One measure of gravitropic sensitivity is the presentation time (also known as the induction time), which is the shortest single dose of gravistimulation that is a threshold to curvature. Regression lines were calculated from plots of stimulation time versus curvature (Fig. 3). The regression lines shown in Figure 3 were calculated from all stimulation times, which included at least one value (from different experiments) that was statistically different (P < 0.05) from zero curvature. This criterion excluded the 0.25- and 0.5-h values (Fig. 3, ▴) for NS458 from the calculations. For the regression lines shown in Figure 3, the presentation times were 4.8 and 30 min for the WT and for NS458, respectively. Varying combinations of inclusion of the zero-curvature values and exclusion of values for longer stimulation times (2 or 2.5 h) produced a range of values for the presentation times from −14.8 to 16.3 min for the WT and 15.8 to 33.1 min for NS458 (regression lines not shown). Whereas these variations indicate the difficulty of obtaining a precise value for this threshold, they do show that WT hypocotyls are more sensitive to single, short periods of reorientation than are hypocotyls of NS458.
Figure 3.
Presentation time of light-grown hypocotyls. Gravitropic curvature in response to single horizontal doses from 0.25 to 2.5 h. The intercept of regression lines (r = 0.95 for the WT [○] and 0.61 for NS458 [▵, ▴]) with the x axis yields estimated presentation times of 0.08 and 0.50 h for the WT and NS458, respectively. Only time points with at least one nonzero value for curvature were included (open symbols) in the calculation of the regression. Thus, the values indicated by the filled triangles for NS458 were not used for the regression shown in this figure. se bars are shown when greater than the size of the symbol. Each symbol represents one experiment with 30 to 44 seedlings.
Another measure of sensitivity was derived from intermittent stimulation experiments, which indicate the shortest repeated stimulation times that can be integrated to produce statistically significant curvature. WT hypocotyls showed significant curvature if they were repeatedly horizontal (stationary) for 1 min or longer, whereas at least 2-min periods were necessary to induce significant curvature in NS458 hypocotyls (Table II). Thus, by the measure of intermittent stimulation, light-grown NS458 hypocotyls are only half as sensitive as WT hypocotyls.
Table II.
Gravitropic curvature after intermittent stimulation
| Genotype | Cycle (minutes stationary:minutes
rotating)
|
|||||
|---|---|---|---|---|---|---|
| 0.5:9 | 1:9 | 2:8 | 5:5 | 6:4 | 8:2 | |
| WT | 0.6 | 3.2a | 2.1a | 4.7a | 5.2a | 6.8a |
| 1.7a | 1.1b | 2.9a | 3.9a | 5.5a | ||
| 0.9 | 1.7a | 6.2a | 1.8a | |||
| 2.1a | 4.8a | |||||
| 0.7 | 2.7a | |||||
| 3.1a | 0.2 | |||||
| NS458 | 0.0 | 5.9a | 1.4b | 3.3a | 6.3a | 5.8a |
| 0.7 | 0.2 | 0.0 | 4.3a | 4.5a | ||
| 1.2 | 0.5 | 2.0b | −0.4 | |||
| 0.7 | 0.8 | |||||
| 1.0 | 2.0b | |||||
| 0.3 | 1.3b | |||||
The seedlings were intermittently stimulated for 2 h (12 10-min cycles with 0.5–8 min of stationary, horizontal stimulation for each cycle) and then rotated continuously on a clinostat for 1 additional hour. The curvatures (degrees) were tested by paired Student's t tests to determine whether the values were significantly different from 0. Each number represents the mean from one experiment with 33 to 50 seedlings.
Curvature is significantly greater than 0° (α = 0.01).
Curvature is significantly greater than 0° (α = 0.05).
Light Increases Mutant Plastid Size but Not the Volume Fraction of Starch
Light increased the plastid size in the endodermis of both genotypes (Table III). Regardless of light treatment, WT plastids were larger than NS458 plastids (Fig. 1, B, D, F, and H). The volume of WT plastids consisted almost entirely of starch, whereas only about 10% of plastid volume was occupied by starch in both light- and dark-grown NS458 plastids (Table III). Thus, although plastids of the light-grown NS458 have more starch on an absolute basis than in the dark-grown mutant, the volume fraction of starch is the same in both the light and in the dark.
Table III.
Plastid and starch volumes in endodermal cells of hypocotyls
| Genotype Treatment | Plastid Volume | Volume of Starch in Plastid |
|---|---|---|
| μm3 | %a | |
| WT, light | 59.1 ± 2.9 | NDb |
| WT, dark | 38.6 ± 3.7 | ND |
| NS458, light | 32.0 ± 2.9 | 9.3 ± 0.8 |
| NS458, dark | 11.3 ± 0.5 | 9.9 ± 0.5 |
Mean ± se; n = 33 to 100 plastids.
ND, Not determined.
Light Induces Mutant Plastid Sedimentation
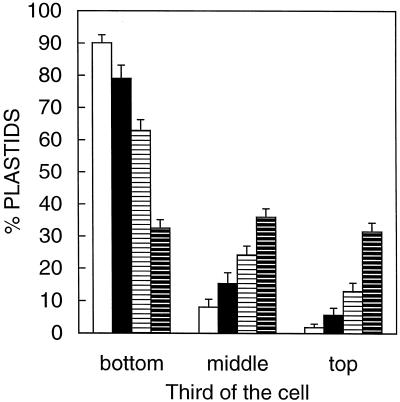
In both dark- and light-grown WT hypocotyls, the plastids in the endodermis (starch sheath) were amyloplasts that were filled with starch and consistently sedimented (Fig. 1, B, D, J, and K). Previous qualitative observations have shown that dark-grown NS458 hypocotyls lack obvious plastid sedimentation (Kiss and Sack, 1990). Quantification of plastid position confirms this conclusion because plastids were found with equal frequency in the apical, middle, and basal thirds of endodermal cells (Fig. 4). However, plastid sedimentation did occur in the endodermis of light-grown NS458 hypocotyls (Fig. 1, L, and M). Endodermal cells can be identified based on their position outside of the stele and inside of the other cortical layers, even in the absence of an obvious starch sheath (Fig. 1, I, L, and O). Quantification of plastid position in light-grown NS458 hypocotyls confirmed the presence of plastid sedimentation, even though it was not as complete as in the WT (Fig. 4). NS458 endodermal cells were shorter than those of the WT in light-grown hypocotyls (Table IV), a difference that might overestimate the extent of sedimentation in NS458 relative to the wild type because sedimentation was measured by dividing the cell into thirds rather than into cell segments of fixed lengths. Endodermal cell length was essentially unaffected by light treatment in the wild type, whereas these cells were much longer in dark- versus light-grown NS458 hypocotyls (Table IV).
Figure 4.
Plastid sedimentation in light- and dark-grown hypocotyls. Shown is the percentage of plastids present in each third of endodermal cells of seedlings that were inverted for 1 h, fixed in this orientation, embedded in wax, sectioned, and stained. Plastids are sedimented, except in dark-grown NS458 hypocotyls. Bars = +se. A total of 39 to 61 endodermal cells from five different hypocotyls were scored for plastid sedimentation for each genotype and condition. Open bars, Light-grown WT; black bars, dark-grown WT; white bars with horizontal lines, light-grown NS458; and black bars with horizontal white lines, dark-grown NS458.
Table IV.
Length of endodermal cells of WT and NS458 hypocotyls
| Genotype | Light | Dark |
|---|---|---|
| μm | ||
| WT | 97 ± 2.9 | 89 ± 5.0 |
| NS458 | 66 ± 2.1 | 126 ± 6.6 |
Cell length (mean ± se) from the apical part of the hypocotyl (see text). n = 40 to 85 cells. All values are different (α < 0.01; analysis of variance, Newman-Keuls multiple comparisons).
DISCUSSION
The light promotion of hypocotyl gravitropism in NS458 correlates with the onset of plastid sedimentation, a finding consistent with the hypothesis that gravitropic sensing is plastid based. Whereas gravitropism in dark-grown NS458 hypocotyls is severely impaired (Fig. 2) (Kiss and Sack, 1990), mutant hypocotyls from seedlings grown in the light are strongly gravitropic. This light promotion cannot be attributed to effects on growth, because the growth rates of WT and NS458 hypocotyls are equal and because the growth of both genotypes is twice as fast in the dark as in the light.
Light, especially red light, has been shown to promote gravitropism in stems in other genera (Britz and Galston, 1982; Woitzik and Mohr, 1988), and light and phytochrome modulate stem gravitropism (Poppe et al., 1996; Robson and Smith, 1996). We did not investigate either the wavelength(s) or the duration of irradiation necessary for the light promotion of gravitropism in NS458 hypocotyls. But unlike in the studies cited above, we examined the effects of light on gravitropic sensitivity and on the size and sedimentation of endodermal plastids.
The finding that endodermal plastid sedimentation is absent in dark-grown NS458 hypocotyls but is largely present in light-grown mutant hypocotyls suggests that this sedimentation is responsible for the significant promotion by light of gravitropism. This is supported by data for the WT in which the extent of both amyloplast sedimentation and gravitropism are comparable in the light and in the dark. However, we cannot rule out the possibility that light promotes gravitropism independently of an effect on plastid size, e.g. via signal transduction.
Plastid sedimentation in light-grown NS458 hypocotyls was seen only in the endodermis close to the shoot apex. This is the same location for amyloplast sedimentation in the stems of WT plants of most genera (Perbal and Rivière, 1980; Sack, 1987, 1991; Caspar and Pickard, 1989; Volkmann et al., 1993). Of course, the concept that plastid sedimentation functions in gravitropic sensing is a century old and is supported by numerous correlational data (Sack, 1991, 1997). This hypothesis is also strongly supported by the recent finding that hypocotyls of the scarecrow mutant of Arabidopsis are agravitropic and lack an endodermis and amyloplast sedimentation (Fukaki et al., 1997). Our data for NS458 hypocotyls provide one more correlation that plastid mass and sedimentation in the endodermis are necessary for substantial gravitropism in stems.
Plastid sedimentation in light-grown NS458 probably results from an increase in plastid mass. Although the NS458 mutant has been described as starchless (Hanson and McHale, 1988; Sicher and Kremer, 1996), its plastids do have small amounts (approximately 10% by volume) of starch (Kiss and Sack, 1990; this study). In endodermal cells the percent of plastid volume occupied by starch is no different in light- or in dark-grown NS458 hypocotyls (Table III). This may indicate that light does not alter the already defective enzyme activity of plastidic phosphoglucomutase or the possible metabolic feedback mechanisms that affect the volume fraction of starch. Nevertheless, light increases plastid volume overall, both in the WT and in NS458, and this results in a greater absolute amount of starch, which may be sufficient to induce endodermal plastid sedimentation in the mutant. However, it is possible that sedimentation also results from additional effects of light, such as a decrease in cytoplasmic viscosity or from an increase in the density of the plastid through the accumulation of soluble sugars or of other compounds.
Although light-grown NS458 plastids have sufficient mass to sediment, they do so with much less consistency than heavier, WT amyloplasts. We calculate from Stokes' law that the theoretical velocity of sedimentation of light-grown NS458 plastids (approximately 2 μm min−1) is about twice that of dark-grown mutant plastids, but much less than that of WT amyloplasts (7.8 and 10.3 μm min−1 from dark- and light-grown hypocotyls, respectively). According to Björkman (1988), the potential energy of the sedimenting particle must be much higher than thermal noise (Brownian motion; 2 × 10−21 J) to trigger gravitropic sensing. It is estimated that dark-grown NS458 plastids possess a potential energy (approximately 1 × 10−19 J) that is only 1 to 2 orders of magnitude higher than thermal noise, and that is only 25% of the potential energy of light-grown mutant plastids (4 × 10−19 J). The potential energies of WT amyloplasts from dark- and light-grown plants are estimated to be 1.5 × 10−18 J and 2.3 × 10−18 J, respectively.
How then does a relatively small increase in mutant plastid mass or potential energy provide an orientational signal sufficient to restore significant gravitropism to light-grown NS458 hypocotyls? Spaceflight experiments have demonstrated that gravitropic sensing can occur at thresholds as low as 0.1g (Brown et al., 1995). This suggests that the gravitropic sensing apparatus is “overbuilt” and may be capable of discriminating a signal from noise at some threshold greater than thermal noise (Sack, 1997). If all gravitropic sensing is plastid based, then the potential energy in the plastids of dark-grown NS458 may be sufficient to barely trigger enough gravitropic sensing to produce the residual levels of gravitropism observed in these hypocotyls (Kiss and Sack, 1990). The estimated 4-fold greater potential energy of light-grown (compared with dark-grown) mutant plastids may be sufficiently higher than threshold levels to dramatically increase the orientational signal and thus gravitropism. The increased mass may also be important in inducing sedimentation, which places these plastids in a part of the endodermal cell that may contain or be enriched in mechanosensitive receptors that transduce plastid mass.
This reasoning might also explain why light-grown WT hypocotyls are at least twice as sensitive as NS458 hypocotyls, as estimated by the presentation time and by intermittent stimulation. The reduced sensitivity of the light-grown mutant compared with the WT might result both from the lower plastid mass and from the less-consistent sedimentation of mutant plastids. Based on data from roots of starch-deficient mutants of Arabidopsis, Kiss et al. (1996, 1997) estimated that mutants, the plastids of which contain ≥60% of the starch of the WT, exhibit full WT levels of gravitropic sensitivity. The starch content of NS458 plastids is significantly below this level.
The relative importance of plastid sedimentation to gravitropic sensitivity may differ in stems and roots. As in hypocotyls, light-grown roots of NS458 are strongly gravitropic but less sensitive than the WT, but mutant roots apparently lack sedimentation in columella cells (Kiss and Sack, 1989). These data show that plastid sedimentation is not necessary for significant gravitropism in roots, whereas there is a strong correlation for hypocotyls of the same mutant. This difference might be related to the much smaller size of columella versus endodermal cells.
Although light promotes gravitropism in NS458 hypocotyls, comparable data are not available for an Arabidopsis mutant that is also deficient in plastidic phosphoglucomutase, the pgm1 locus represented by two alleles, TC7 and ACG21 (Caspar and Pickard, 1989; Kiss et al., 1996). pgm1 appears to be completely devoid of starch (Caspar, 1994). Dark-grown hypocotyls are severely disoriented, although they are capable of weak gravitropism (Caspar and Pickard, 1989; Kiss et al., 1997). Depending on the report, light-grown pgm1 hypocotyls either show WT levels of curvature (fig. 7 in Caspar and Pickard, 1989) or less gravitropic curvature than in mutant hypocotyls grown in the dark (figs. 5 and 6 in Kiss et al., 1997). Neither sensitivity nor plastid size and position were measured in these studies. Based on our results with NS458, we predict that light-grown pgm1 hypocotyls should be slightly more sensitive than dark-grown mutant hypocotyls; whereas light might enlarge pgm1 plastids, the increase in plastid mass should be much less than in NS458 because, unlike NS458 plastids, pgm1 plastids entirely lack starch.
In summary, our data are consistent with the conclusions that the light promotion of gravitropism in NS458 results from an increase in plastid mass that induces plastid sedimentation and greatly increases gravitropic sensitivity compared with the dark-grown mutant. These data support the importance of both plastid mass and plastid sedimentation in gravitropic sensing.
ACKNOWLEDGMENTS
We are thankful to Roger Hangarter for helpful discussions and to Shawn Davis and Liming Zhao for technical assistance.
Abbreviation:
- WT
wild type
Footnotes
This research was supported by the National Aeronautics and Space Administration (grant no. NAGW-4472).
LITERATURE CITED
- Björkman T. Perception of gravity by plants. Adv Bot Res. 1988;15:1–41. doi: 10.1016/0273-1177(92)90283-4. [DOI] [PubMed] [Google Scholar]
- Britz SJ, Galston AW. Light-enhanced perception of gravity in stems of intact pea seedlings. Planta. 1982;154:189–192. doi: 10.1007/BF00387915. [DOI] [PubMed] [Google Scholar]
- Brown AH, Chapman DK, Johnsson A, Heathcote D. Gravitropic responses of the Avena coleoptile in space and on clino-stats. I. Gravitropic response thresholds. Physiol Plant. 1995;95:27–33. [PubMed] [Google Scholar]
- Caspar T. Genetic dissection of the biosynthesis, degradation, and biological functions of starch. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 913–936. [Google Scholar]
- Caspar T, Pickard BG. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Benfey PN, Liu Y-G, Shibata D, Fujisawa H, Tasaka M (1997) The SGR1/SCR and SGR7/SHR genes are required for the formation of the endodermis/starch sheath which is essential for shoot gravitropism. Presented at the 8th International Meeting on Arabidopsis Research, Madison, WI
- Hanson KR, McHale NA. A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol. 1988;88:838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel R, de la Fuente RK, Leopold AC. Geotropism and the lateral transport of auxin in the corn mutant amylomaize. Planta. 1969;88:204–214. doi: 10.1007/BF00385063. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Kiss JZ, Sack FD. Reduced gravitropic sensitivity in roots of a starch-deficient mutant of Nicotiana sylvestris. Planta. 1989;180:123–130. [PubMed] [Google Scholar]
- Kiss JZ, Sack FD. Severely reduced gravitropism in dark-grown hypocotyls of a starch-deficient mutant of Nicotiana sylvestris. Plant Physiol. 1990;94:1867–1873. doi: 10.1104/pp.94.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Perbal G, Rivière S. Perception et réaction géotropiques de l'épicotyle d'Asparagus officinalis L. Physiol Plant. 1980;48:51–58. [Google Scholar]
- Poff KL, Janoudi A-K, Rosen ES, Orbović, Konjević R, Fortin M-C, Scott TK. The physiology of tropisms. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 639–664. [Google Scholar]
- Poppe C, Hangarter R, Sharrock RA, Nagy P, Schäfer E. The light-induced reduction of the gravitropic growth-orientation of seedlings of Arabidopsis thaliana (L.) Heynh. is a photomorphogenic response mediated synergistically by the far-red-absorbing forms of phytochromes A and B. Planta. 1996;199:511–514. doi: 10.1007/BF00195180. [DOI] [PubMed] [Google Scholar]
- Robson PRH, Smith H. Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol. 1996;110:211–216. doi: 10.1104/pp.110.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. The structure of the stem endodermis in etiolated pea seedlings. Can J Bot. 1987;65:1514–1519. doi: 10.1139/b87-209. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Sicher RC, Kremer DF. Rubisco activity is altered in a starchless mutant of Nicotiana sylvestris grown in elevated carbon dioxide. Environ Exp Bot. 1996;36:385–391. [Google Scholar]
- Vitha S, Baluška F, Mews M, Volkmann D. Immunofluorescence detection of F-actin on low melting point wax sections from plant tissues. J Histochem Cytochem. 1997;45:89–95. doi: 10.1177/002215549704500112. [DOI] [PubMed] [Google Scholar]
- Volkmann DV, Winn-Börner U, Waberzeck K. Graviresponsiveness of cress seedlings and structural status of presumptive statocytes from the hypocotyl. J Plant Physiol. 1993;142:710–716. [Google Scholar]
- Woitzik F, Mohr H. Control of hypocotyl gravitropism by phytochrome in a dicotyledonous seedling (Sesamum indicum L.) Plant Cell Environ. 1988;11:663–668. [Google Scholar]