Abstract
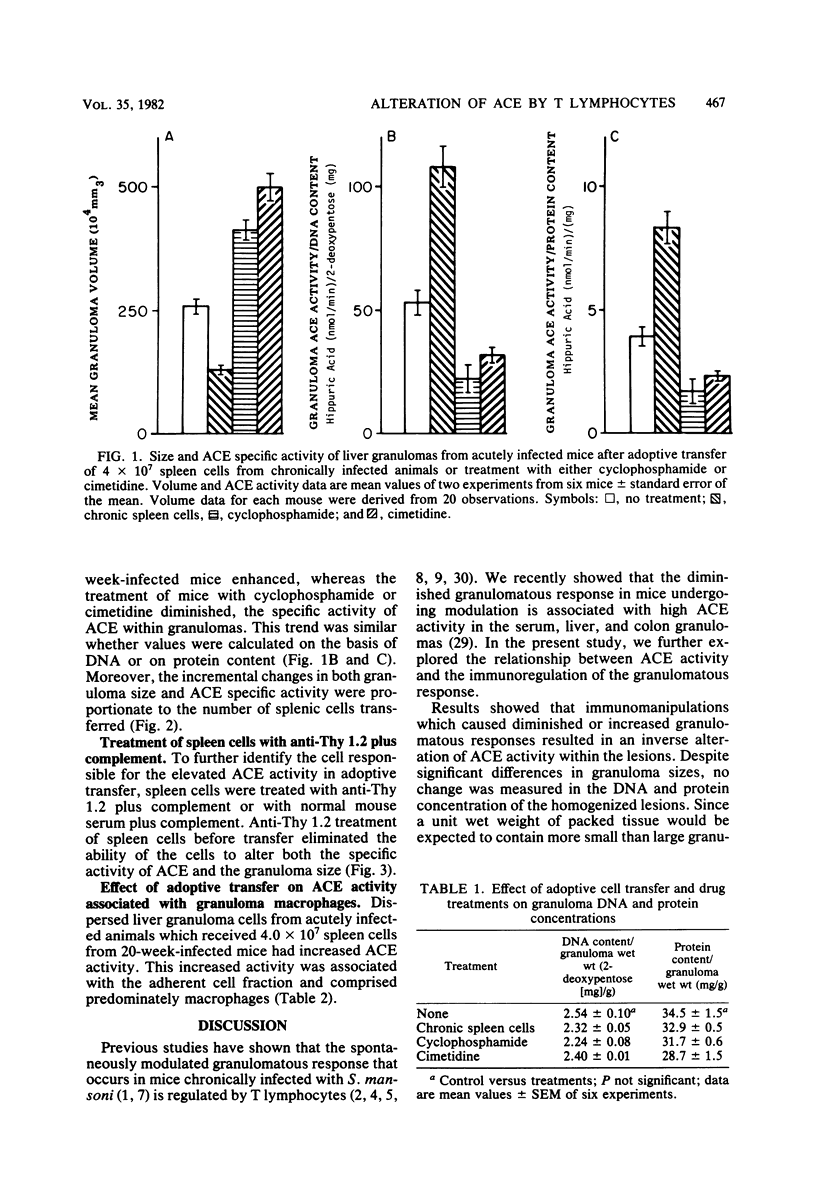
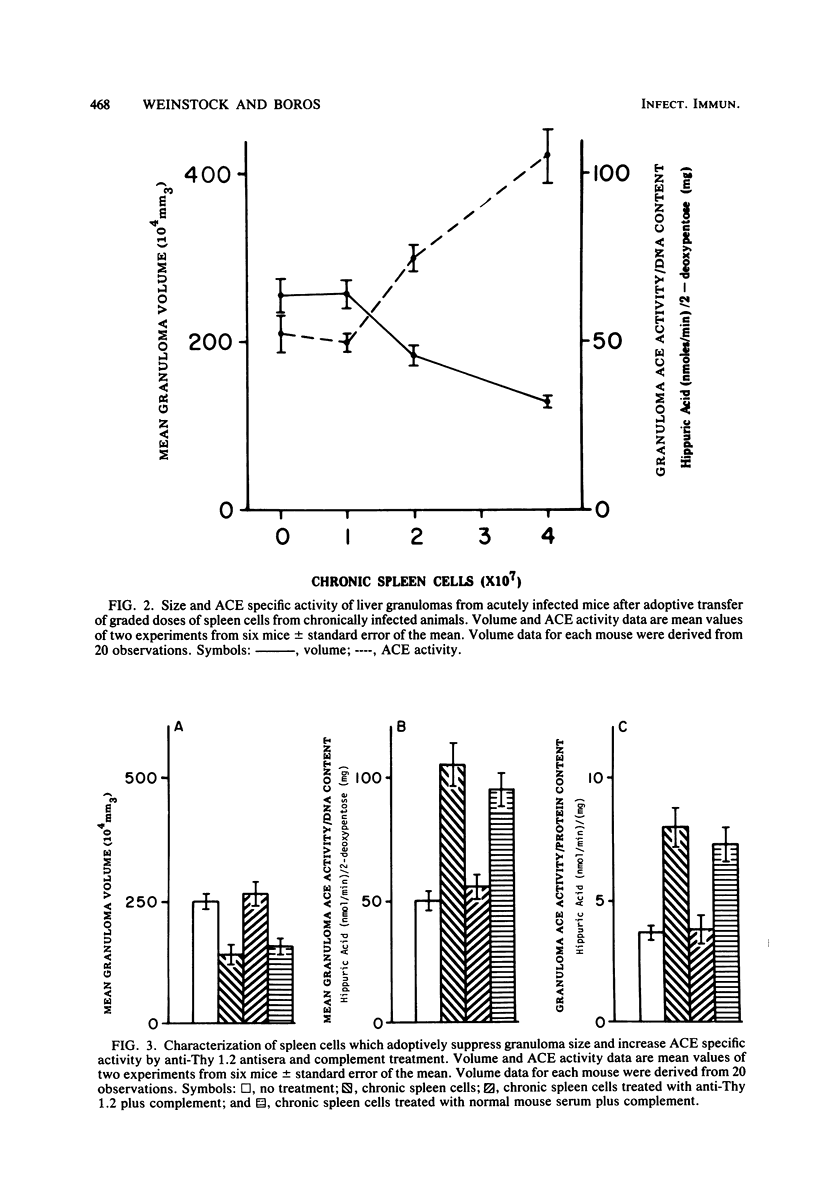
Granulomatous inflammatory lesions in murine schistosomiasis mansoni undergo a spontaneous diminution at the chronic phase of the disease concurrent with an increase in angiotensin I-converting enzyme (ACE) activity. The objective of this investigation was to determine whether T cells influence ACE activity within the granulomas. Cyclophosphamide and cimetidine treatments of mice, which augment delayed-type hypersensitivity reactions, enhanced liver granuloma size and decreased granuloma ACE activity. The suppression of liver granuloma by the adoptive transfer of spleen cells from chronically infected, immunomodulated mice into recipients with acute infection increased ACE activity of granulomas and granuloma macrophages. The increased ACE activity was dependent upon the transfer of Thy 1.2+ splenic lymphocytes. The level of the ACE activity was proportionate to the number of administered spleen cells. Thus, the level of ACE activity within the egg-induced granuloma is dependent upon T lymphocytes present in the spleen and granulomas of infected animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979 Mar;28(2):291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Todd C. W. Adoptive suppression of granuloma formation by T lymphocytes and by lymphoid cells sensitive to cyclophosphamide. Cell Immunol. 1979 Aug;46(1):192–200. doi: 10.1016/0008-8749(79)90258-2. [DOI] [PubMed] [Google Scholar]
- Dezsö B., Fóris G. Effect of angiotensin II on the Fc receptor activity of rat macrophages. Immunology. 1981 Feb;42(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L., Fukuyama K., Danno K., Kwan-Wong E. Granulomatous inflammation in normal and athymic mice infected with schistosoma mansoni: an ultrastructural study. J Pathol. 1979 Apr;127(4):207–215. doi: 10.1002/path.1711270408. [DOI] [PubMed] [Google Scholar]
- Kellermeyer R. W., Warren K. S. The role of chemical mediators in the inflammatory response induced by foreign bodies: comparison with the schistosome egg granuloma. J Exp Med. 1970 Jan 1;131(1):21–39. doi: 10.1084/jem.131.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Nosal A., Schlessner A., Sastre-Foken A. Serum angiotensin-converting enzyme for diagnosis and therapeutic evaluation of sarcoidosis. Am Rev Respir Dis. 1979 Aug;120(2):329–335. doi: 10.1164/arrd.1979.120.2.329. [DOI] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Properties of a subpopulation of T cells bearing histamine receptors. J Clin Invest. 1975 Apr;55(4):856–874. doi: 10.1172/JCI107997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D. K., Melmon K. L. Histamine-induced suppressor factor (HSF): further studies on the nature of the stimulus and the cell which produces it. Cell Immunol. 1979 May;44(2):404–415. doi: 10.1016/0008-8749(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Histamine-induced suppressor factor (HSF): effect on migration inhibitory factor (MIF) production and proliferation. J Immunol. 1977 May;118(5):1734–1738. [PubMed] [Google Scholar]
- Ryan J. W., Chung A., Ammons C., Carlton M. L. A simple radioassay for angiotensin-converting enzyme. Biochem J. 1977 Nov 1;167(2):501–504. doi: 10.1042/bj1670501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Regulation of delayed-type hypersensitivity reactions by cyclophosphamide-sensitive T cells. J Immunol. 1978 Oct;121(4):1573–1577. [PubMed] [Google Scholar]
- Shibouta Y., Inada Y., Terashita Z., Nishikawa K., Kikuchi S., Shimamoto K. Angiotensin-II-stimulated release of thromboxane A2 and prostacyclin (PGI2) in isolated, perfused kidneys of spontaneously hypertensive rats. Biochem Pharmacol. 1979 Dec 15;28(24):3601–3609. doi: 10.1016/0006-2952(79)90406-4. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Friedland J., Lyons H. A., Gourin A. Elevation of angiotensin-converting enzyme in granulomatous lymph nodes and serum in sarcoidosis: clinical and possible pathogenic significance. Ann N Y Acad Sci. 1976;278:498–513. doi: 10.1111/j.1749-6632.1976.tb47062.x. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Friedland J., Setton C. Angiotensin converting enzyme: induction in rabbit alveolar macrophages and human monocytes in culture. Adv Exp Med Biol. 1979;121(A):149–156. doi: 10.1007/978-1-4684-3593-1_13. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem. 1976;45:73–94. doi: 10.1146/annurev.bi.45.070176.000445. [DOI] [PubMed] [Google Scholar]
- Studdy P., Bird R., James D. G. Serum angiotensin-converting enzyme (SACE) in sarcoidosis and other granulomatous disorders. Lancet. 1978 Dec 23;2(8104-5):1331–1334. doi: 10.1016/s0140-6736(78)91972-4. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Claman H. N. Immune suppression with supraoptimal doses of antigen in contact sensitivity. I. Demonstration of suppressor cells and their sensitivity to cyclophosphamide. J Immunol. 1977 Jul;119(1):240–244. [PubMed] [Google Scholar]
- Van Dijk H., Rapis M., Jacobse-Geels H. E., Willers J. M. Histamine 2 receptor-mediated immunomodulation in the mouse. I. Immunomodulation by the H2 agonist tolazoline. Clin Exp Immunol. 1979 Mar;35(3):470–477. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. The relevance of schistosomiasis. N Engl J Med. 1980 Jul 24;303(4):203–206. doi: 10.1056/NEJM198007243030408. [DOI] [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L., Gee J. B. Enhanced granuloma angiotensin I converting enzyme activity associated with modulation in murine schistosomiasis. Gastroenterology. 1981 Jul;81(1):48–53. [PubMed] [Google Scholar]



