Abstract
Objective
11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) regenerates active cortisol from inert cortisone in adipose tissue. Elevated adipose tissue 11βHSD1 activity is observed in obese humans and rodents where it is linked to obesity and its metabolic consequences. Menopause is also associated with increased abdominal fat accumulation suggesting that estrogen is also important in adipose tissue metabolism. The purpose of this current study was to establish whether estrogen signalling through estrogen receptors-α and -β (ERα and ERβ) can influence 11βHSD1 in premenopausal and postmenopausal adipose tissue.
Methods
19 premenopausal (aged 26±5, BMI 23.6±1.6) and 23 postmenopausal healthy women (aged 63±4, BMI 23.4±1.9) were studied. Subcutaneous adipose tissue biopsies and fasting venous blood samples were taken. Body composition was measured by bio-electrical impedance analysis. Human SGBS adipocyte cells were treated with ERα and ERβ-specific agonists for 24h. Basic anthropometric data, Serum 17β-estradiol and progesterone concentrations, ERα and ERβ mRNA levels and 11βHSD1 mRNA, protein and activity levels were assessed.
Results
ERβ and 11βHSD1, but not ERα mRNA was significantly increased in adipose tissue from postmenopausal women compared to premenopausal women. ERβ had a significant positive correlation with the mRNA level of 11βHSD1 in adipose tissue from pre- and postmenopausal women. This association between ERβ and 11βHSD1 was greatest in adipose tissue from postmenopausal women. In human SGBS adipocytes, diarylpropiolnitrile (DPN), a selective ERβ agonist increased 11βHSD1 mRNA, protein and activity levels.
Conclusions
We conclude that in adipose tissue, ERβ-mediated estrogen-signalling can upregulate 11βHSD1 and that this may be of particular importance in postmenopausal women.
Keywords: menopause, Estrogen receptor β, 11β-Hydroxysteroid Dehydrogenase Type 1, adipose tissue
Introduction
The onset of menopause is associated with an accumulation of adipose tissue1 and this may contribute to the increased metabolic and cardiovascular risk seen in postmenopausal women. Chronic glucocorticoid excess (e.g. in Cushing’s syndrome) also causes obesity and its associated metabolic dysfunction. Whilst plasma cortisol levels are not elevated in obesity2, recent evidence suggests that there is a selective increase in glucocorticoid regeneration in adipose tissue3. Specifically, the microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) which catalyses the intracellular reactivation of cortisol from inert cortisone, is selectively increased in adipose tissue in obese humans and in rodent models of obesity4;5. This appears to be of pathophysiological importance since mice engineered to selectively over-express 11βHSD1 in adipose tissue develop visceral obesity and metabolic syndrome6, whereas mice lacking 11βHSD1 resist glucose intolerance, insulin resistance and hyperlipidemia on high-fat diet7. In humans, 11βHSD1 inhibition has analogous effects8.
Estrogen signalling is predominantly mediated via the two nuclear estrogen receptors α and β (ERα and ERβ), both of which are present in human adipose tissue9. Human studies on sex steroid control of 11βHSD1 in adipose tissue are scarce and inconclusive; reporting both up-regulation and no effect of estrogen, using a variety of doses mainly in vitro10. Here we have explored the possibility of a functional link between ERα/β signalling and 11βHSD1 expression/activity in adipose tissue from premenopausal and postmenopausal women.
Methods
Experiments in Humans
All women gave written, informed consent and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. The study of pre- and postmenopausal women was approved by the Ethical Committee for human research at Umeå University, Umeå, Sweden, approval ID 03-339. 19 premenopausal and 23 postmenopausal, healthy, normal weight women were recruited by advertisements in the local newspapers and within the Umeå University Hospital and campus areas. Exclusion criteria were: diabetes, thyroid dysfunction, hepatic and renal disease, use of tobacco, hormonal contraceptives, systemic gonadal hormone replacement therapy, or oral glucocorticoid medication. None of the postmenopausal women reported menstrual periods within the last 12 months. One premenopausal woman used inhaled steroids for asthma (budesonide, 400 μg/24 h). Three postmenopausal women had well-controlled hypertension treated with β-blockers, diuretics, or calcium antagonist, one took tolterodine for urinary incontinence and bisphosphonates for osteoporosis, and two used topical E2 or estriol treatment. Further details of the participants included in this study have been described previously11.
Clinical protocol
Premenopausal women were evaluated during the follicular phase of the menstrual cycle to negate the possible effects of hormonal fluctuations. Menstrual phase or postmenopausal status was confirmed by measuring serum 17β-estradiol and progesterone levels (described below).
Anthropometric measurements and adipose tissue biopsies were performed on separate days. Weight to the nearest 0.1 kg (with subjects wearing light clothes) and height and waist circumference to the nearest 0.5 cm was measured. Body composition was measured by bio-electrical impedance analysis using a Bodystat 1500 (Bodystat Ltd., Isle of Man, British Isles). Approximately 2 g of periumbilical superficial subcutaneous adipose tissue was excised under local anaesthesia with lidocaine (Xylocaine® without adrenaline, AstraZeneca, Sweden) after an overnight fast. Tissue was snap frozen in liquid nitrogen within 5 minutes after removal, and stored at −80°C until further analyses.
Venous blood samples for routine laboratory tests were drawn at the time of anthropometric measurements. Venous blood samples for serum analyses (described below) were drawn in the mornings of the adipose tissue biopsies after at least eight hours of fasting.
Laboratory methods
Serum Analyses
17β-Estradiol, progesterone and cortisol were measured in samples drawn at the time of the biopsy. Serum cortisol and progesterone were analyzed by electrochemiluminescence immunoassays, on a Modular Analytics E170 (all from Roche AB, Stockholm, Sweden). 17β-Estradiol was measured using an ultra sensitive radioimmunoassay (ESTR-US-CT, CIS bio international, Gif-sur-Yvette, Cedex, France) (intra- and interassay coefficients of variation (CV); 2.8–18.1% and 5.8–17.6%, respectively).
Cell culture
Human SGBS (Simpson-Golabi-Behmel Syndrome) adipocyte cells which have previously been shown to express both ERα and ERβ12 were maintained and differentiated into adipocytes, as previously described13. For experimental manipulations, cells were serum-starved overnight in phenol-red-free medium containing 0.1% BSA. After serum starvation, cells were treated with diarylpropiolnitrile (DPN) or propyl-pyrazole triol (PPT), at the concentrations indicated for 24 h. All experiments were performed in triplicate and repeated three times.
Quantification of mRNA
Total RNA was extracted from snap-frozen adipose tissue or SGBS adipocytes according to the manufacturer’s protocol using the RNeasy® lipid tissue midi kit (Qiagen Nordic, Qiagen House, West Sussex, UK). RNA concentrations were measured on a ND-1000 Spectrophotometer (NanoDrop Technologies, Bancroft Building, Wilmington, DE, USA) and integrity was evaluated on a 1% agarose electrophoretic gel and visualized with ethidium bromide under UV-light.
One microgram of RNA was reverse transcribed into cDNA using TaqMan® Reverse Transcription Reagents (Roche Molecular Systems, Inc., Branchburg, NJ, USA). cDNA was incubated in triplicate with 1x gene-specific assay mix (Applied Biosystems, Warrington, UK) in 1x Universal PCR Master Mix (Roche). PCR cycling and detection of fluorescent signal was carried out on an ABI Prism® 7000 Sequence Detection System (Applied Biosystems). Triplicates were deemed acceptable if the standard deviation of the crossing point was < 0.5 cycles. A standard curve (y axis crossing point, x axis log concentration) for each gene was generated by serial dilution of cDNAs pooled from different samples and fitted with a straight line and deemed acceptable if reaction efficiency was between 1.7 and 2.1. For SGBS cells, results were corrected for with TATA box binding protein (TBP) and for human adipose tissue, results were corrected for cyclophilin A which had the lowest coefficients of variation and the best stability value, based on the Normfinder algorithm (http://www.mdl.dk/publicationsnormfinder.htm) out of three tested endogenous controls.
The prevalidated assays used were: human ERα, Hs00174860_m1; human ERβ, Hs00230957_m1; human 11βHSD1, Hs01547870_m1; and the endogenous controls cyclophilin A (PPIA), Hs99999904_m1 and TBP Hs00427620_m1; (all from Applied Biosystems).
Western blot
SGBS cells were washed in ice-cold PBS and lysates prepared as described previously13. 50μg of protein were diluted in sample buffer containing DTT, denatured and run on 12% polyacrylamide gels, and transferred to nitrocellulose for Western blotting. Membranes were probed with 11βHSD1 antibody (The Binding Site Group Ltd, Birmingham, UK). Protein loaded was corrected for using a monoclonal antibody against β-tubulin (Millipore, Watford, UK). Proteins were visualised with an Alexa Fluor® secondary antibody (Li-cor Biosciences, Cambridge, UK) and band intensities were quantified using the Odyssey infrared imaging system (Li-cor Biosciences).
Enzymology
11βHSD1 activity was measured in the 11β-reductase direction in intact differentiated SGBS adipocytes. 3 × 105 cells/ well in 6-well plates were differentiated as described above. Each well was incubated at 37°C in 2ml of serum - and phenol-red-free medium containing 1pM [3H]-cortisone (GE Healthcare; Aylesbury, UK) and 1μM cortisone (Sigma-Aldrich Company Ltd, Dorset, UK). All incubations were performed in triplicate for 24h. Assay conditions were optimized to ensure first-order kinetics. After incubation steroids were extracted from 2ml of medium with Sep-pak columns (C18, 0.3g, Waters, Milford, MA, USA). The organic phase was evaporated under nitrogen and extracts were re-suspended in mobile phase (60% water, 10% acetonitrile, 30% methanol). Percentage recovery was 90 ± 2.3% for cortisone and 92 ± 3.1% for cortisol. Steroids were separated by HPLC (Waters) and compared to known tritiated standards (GE Healthcare) using a C18 Reverse-phase column (Sunfire C18,15cm,id 2.6mm, pore size 5μm; Waters) and were quantified by on-line liquid scintillation counting.
Statistical analyses
Data are presented as mean ± SEM, unless stated otherwise, and were natural log-transformed when necessary to achieve parametric distributions. Unpaired Student’s t-tests were used to compare means between groups. Associations between parameters were assessed using Pearson correlation (r). Partial correlation analysis was used in all groups to adjust for obesity measures. Statistical calculations were performed using the SPSS software (release 14.0.1, SPSS Inc., 233 S. Wacker Drive, Chicago, IL). P<0.05 was considered statistically significant.
Results
Participant characteristics
The percentage of body fat and waist-hip-ratio was significantly higher in postmenopausal women compared to premenopausal women (Table 1). Serum E2 and progesterone levels were significantly lower in postmenopausal vs. premenopausal women regardless of menstrual phase (Table 1). Serum cortisol levels were not different between pre- and postmenopausal women. Notably, there was a high variability regarding ERβ and 11βHSD1 expression in the postmenopausal study group.
TABLE 1.
Participant characteristics
| Premenopausal (n = 19) | Postmenopausal (n = 23) | |
|---|---|---|
| Age, y | 26 ± 5 | 63 ± 4a |
| Body fat, % | 27.4 ± 3.4 | 36.0 ± 5.3a |
| Body mass index, kg/m2 | 23.6 ± 1.6 | 23.4 ± 1.9 |
| Waist circumference, cm | 80.1 ± 6.8 | 82.8 ± 5.5 |
| Waist-hip ratio | 0.80 ± 0.06 | 0.84 ± 0.05b |
| Cortisol, nM | 476 ± 184 | 472 ± 141 |
| Estradiol, pM | 201 ± 142 | 19.6 ± 6.3a |
| Progesterone, nM | 2.30 ± 0.79 | 1.32 ± 0.47a |
Data are expressed as mean ± SD.
P < 0.001 versus premenopausal women.
ERβ and 11βHSD1 mRNA levels are increased in adipose tissue from postmenopausal women
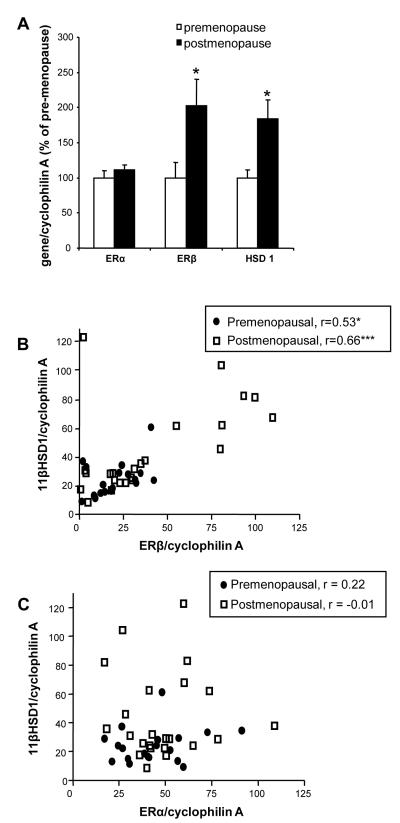
There was no difference in the level of ERα mRNA between pre- and postmenopausal women, (Figure 1A). The expression level of ERβ and 11βHSD1 mRNA was two-fold higher in postmenopausal women compared to premenopausal women in follicular phase (Figure 1A).
Figure 1. A strong positive correlation between 11βHSD1 and ERβ in adipose tissue.
(A) ERα, ERβ and 11βHSD1 mRNA expression in SAT from premenopausal women in follicular phase of the menstrual cycle compared to postmenopausal women. Data are mean ± standard error, n=19 for premenopausal women and n=23 for postmenopausal women. *P<0.05 vs premenopause. (B) Correlations between ERβ and 11βHSD1 in SAT from pre- and postmenopausal women (n=19 for premenopausal women and n=23 for postmenopausal women). Closed circles represent premenopausal women and open squares represent postmenopausal women where r = 0.53, P <0.05 for premenopausal women and r = 0.66, P<0.0001 for postmenopaisal women. (C) Correlations between ERα and 11βHSD1 in SAT from pre- and postmenopausal women (n=19 for premenopausal women and n=23 for postmenopausal women). Closed circles represent premenopausal women and open squares represent postmenopausal women where r = 0.22 for premenopausal women and r = −0.01 for postmenopaisal women. SAT, subcutaneous adipose tissue; ER, estrogen receptor ; r , Pearson correlation coefficient; 11βHSD1, 11β-Hydroxysteroid dehydrogenase type 1;
11βHSD1 mRNA is correlated with ERβ but not ERα mRNA in adipose tissue
A strong positive correlation between 11βHSD1 and ERβ mRNA levels was observed in subcutaneous adipose tissue irrespective of menopausal status (Figure 1B). These associations remained after adjustments for obesity measures, (BMI, waist and fat percentage). In contrast, no association between ERα and 11βHSD1 expression was found (Figure 1C).
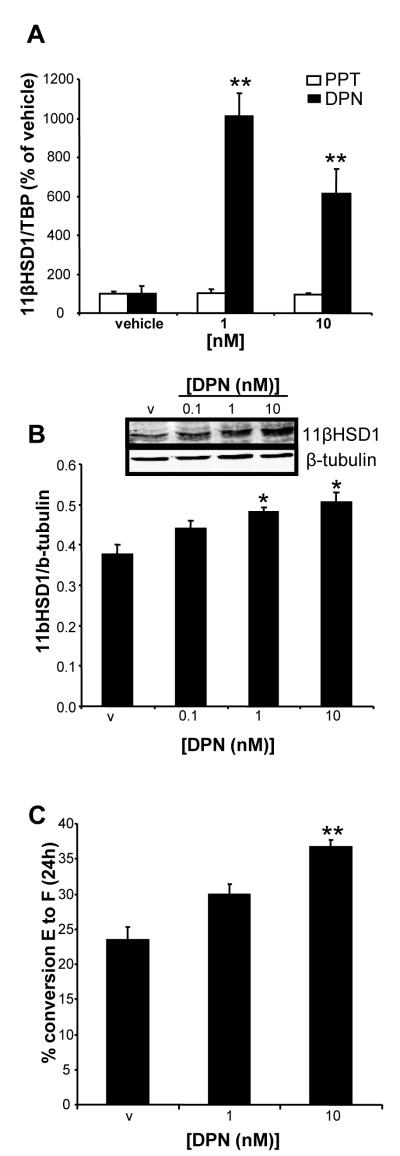
Adipocyte 11βHSD1 expression and activity is up-regulated by an ERβ-agonist in vitro
The ERβ-specific agonist, DPN, up-regulated 11βHSD1 mRNA in human SGBS adipocytes (Figure 2A). In contrast, the ERα-specific agonist PPT did not have any effect on 11βHSD1 mRNA levels (Figure 2A). 11βHSD1 protein expression (Figure 2B) and activity (Figure 2C) were also increased by DPN.
Figure 2. 11βHSD1 expression and activity is up-regulated by an ERβ-specific agonist in vitro.
(A) 11βHSD1 gene expression in SGBS cells after incubation with the ERβ-specific agonist DPN. Data are mean ± standard error, n=3 triplicates. **P<0.01 vs vehicle. (B) Representative Western blot of 11βHSD1 protein expression normalized against β-tubulin, Data are mean ± standard error, n=3 triplicates. *P<0.05 vs vehicle (v) (C) 11βHSD1 enzyme activity in intact cells after DPN stimulation measured as % conversion of cortisone (E) to cortisol (F) after 24h incubation. Data are mean ± standard error. n=3 triplicates. **P<0.01 vs vehicle (v). ER, estrogen receptor ; 11βHSD1, 11β-Hydroxysteroid dehydrogenase type 1; DPN, diarylpropionitrile; SGBS, Simpson-Golabi-Behmel Syndrome.
Discussion
These results identify a potential mechanism by which estrogens acting via ERβ may enhance local glucocorticoid action in adipose tissue of postmenopausal women, involving up-regulation of adipose 11βHSD1 expression. This association between ERβ and 11βHSD1 was greatest in adipose tissue from postmenopausal women. Crucially, a direct effect of selectively activating ERβ on adipocyte 11βHSD1 expression and activity was demonstrated in vitro.
In postmenopausal women, where ovarian steroid biosynthesis has ceased, the major source of estrogens is local aromatase expression in the adipose tissue, the function of which increases with age14. We previously reported a positive association between aromatase and 11βHSD1 gene expression in adipose tissue suggesting an up-regulatory effect of local estrogen production on 11βHSD1 expression in adipose tissue11. Estrogen signalling is predominantly mediated via the two nuclear receptors ERα and ERβ, both of which are present in human adipose tissue9. Here, we have shown that ERβ mRNA levels are two-fold higher in subcutaneous adipose tissue from postmenopausal women compared to premenopausal women and that expression of this receptor is strongly correlated with adipose expression of 11βHSD1. Consequently, in postmenopausal women, adipose 11βHSD1 may be increased by local estrogens acting via ERβ, resulting in increased levels of active glucocorticoids in adipose tissue. Since increased glucocorticoid activity in adipose tissue is associated with obesity4, this mechanism may contribute to the increase in body fat observed in our cohort of postmenopausal women. Furthermore, aromatase promoter activity is driven by glucocorticoids hence increased 11βHSD1 activity in adipose tissue will further amplify this process. The reasons for the high variability in ERβ and 11βHSD1 gene expression among postmenopausal women is not clear and needs further studies regarding putative regulatory factors including a possible interaction with aromatase activity.
Despite a significant increase in percentage fat mass and waist-hip ratio in the postmenopausal women the BMI of both groups of women were in the normal range probably reflecting a decrease in lean mass and bone mineral density which is commonly associated with menopause15. 11βHSD1 activity has been shown to be increased in adipose tissue of even moderately obese women4 On the other hand it has been reported that there is no significant correlation between the respective levels of ERα or ERβ in subcutaneous or omental adipose tissue with obesity16. We have had an opportunity to assess this correlation in subcutaneous and visceral adipose tissue of obese premenopausal women and there remains a strong association between ERβ and 11βHSD1 in both depots (unpublished results). Therefore, we would predict that the correlation would still remain between ERβ and 11βHSD1 in obese women. Unfortunately we did not have the opportunity to assess whether there is an association between ERβ and 11βHSD1 expression in visceral adipose tissue in this cohort of women. This would have been interesting since redistribution of adipose tissue to abdominal visceral depots is commonly observed with menopause. Several human studies have shown higher expression of 11βHSD1 in the subcutaneous depot than other depots. Furthermore, the subcutaneous depot appears to be more predictive for obesity and metabolic parameters17. Importantly, subcutaneous 11βHSD1 correlates with measures of central fat accumulation and is an independent predictor for central fat accumulation17, emphasizing the important role of the 11βHSD1 enzyme in subcutaneous adipose tissue.
This observation of a pro-adipogenic role for ERβ is supported by studies in knock-out mice. ERα knock-out (αERKO) and double knock-out (α/βERKO) mice show marked obesity18;19 whereas ERβ knock-out (βERKO) mice are normal weight. However, αERKO mice also have elevated 17β-estradiol levels thus increasing ERβ signalling20 and when ERβ signalling is removed in αERKO by ovariectomy, the fat-pad weights and adipocyte size decrease20, suggesting adipogenic effects of ERβ signalling. Furthermore, in humans, Shin and colleagues reported that subjects with more ERβ than ERα in omental adipose tissue had a greater degree of adiposity16.
In vitro, the ERβ agonist, DPN had a maximal effect on 11βHSD1 at 1-10nM. Since DPN has its highest specificity for ERβ at these concentrations21, these results support our conclusion that activation of ERβ can directly up-regulate 11βHSD1 in adipocytes. These findings are also supported by previous data showing that 17β-estradiol can activate the 11βHSD1 promoter in transfected adipocytes22.
Conclusion
In conclusion, this study implies a link between adipose ERβ signalling and local glucocorticoid metabolism that may promote adipogenesis. This is particularly relevant in the postmenopausal setting where the beneficial effects of activating ERα are diminished due to a decrease in circulating 17β-estradiol levels and may contribute to the increase in obesity observed in postmenopausal women.
Acknowledgements
This work was supported by grants from Diabetes UK. We thank Drs Cecilia Nordensson, Anders Kristoffersson and Magnus Strand, Umeå University Hospital, for performing the adipose tissue biopsies, research nurses Veronica Sjögren, Britt-Inger Norberg and Inger Arnesjö for all the practical work with recruiting subjects. Professor M. Wabitsch, Ulm University, Germany for kindly providing us with the SGBS cells. Dr Jonas Burén, Umeå University, for valuable scientific discussions.
Financial Support: This work was supported by grants from Diabetes UK
Footnotes
Conflict of interest/financial disclosure: Jonathan R Seckl has a financial relationship for consultancy with Boeringer-Ingelheim, Sanofi Avenits and Astellas
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, Allison DB, Heymsfield SB. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutrition & Metabolism. 2009;16:6. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. Journal of Clinical Endocrinology & Metabolism. 2001;86(3):1418–21. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 3.Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. Journal of Internal Medicine. 2000;247(2):198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 4.Rask E, Walker BR, Soderberg S, Livingstone DEW, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: Increased adipose 11 beta-hydroxysteroid dehydrogenase type 1 activity. Journal of Clinical Endocrinology & Metabolism. 2002;87(7):3330–6. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- 5.Livingstone DEW, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ, Walker BR. Understanding the role of glucocorticoids in obesity: Tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology. 2000;141(2):560–3. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- 6.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;7;294(5549):2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 7.Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53(4):931–8. doi: 10.2337/diabetes.53.4.931. [DOI] [PubMed] [Google Scholar]
- 8.Hughes KA, Webster SP, Walker BR. 11-Beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1) inhibitors in Type 2 diabetes mellitus and obesity. Expert Opinion on Investigational Drugs. 2008;17(4):481–96. doi: 10.1517/13543784.17.4.481. [DOI] [PubMed] [Google Scholar]
- 9.Anwar A, McTernan PG, Anderson LA, Askaa J, Moody CG, Barnett AH, Eggo MC, Kumar S. Site-specific regulation of oestrogen receptor-alpha and -beta by oestradiol in human adipose tissue. Diabetes Obesity & Metabolism. 2001;3(5):338–49. doi: 10.1046/j.1463-1326.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 10.Dieudonne MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Sex steroids and leptin regulate 11 beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. Journal of Steroid Biochemistry and Molecular Biology. 2006;99(4-5):189–96. doi: 10.1016/j.jsbmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Andersson T, Simonyte K, Andrew R, Strand M, Buren J, Walker BR, Mattsson C, Olsson T. Tissue-Specific Increases in 11 beta-Hydroxysteroid Dehydrogenase Type 1 in Normal Weight Postmenopausal Women. Plos One. 2009;29:4(12) doi: 10.1371/journal.pone.0008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horenburg S, Fischer-Posovszky P, Debatin KM, Wabitsch M. Influence of Sex Hormones on Adiponectin Expression in Human Adipocytes. Hormone and Metabolic Research. 2008;40(11):779–86. doi: 10.1055/s-0028-1083780. [DOI] [PubMed] [Google Scholar]
- 13.McInnes KJ, Brown KA, Knower KC, Chand AL, Clyne CD, Simpson ER. Characterisation of aromatase expression in the human adipocyte cell line SGBS. Breast Cancer Res Treat. 2008;112(3):429–35. doi: 10.1007/s10549-007-9883-2. [DOI] [PubMed] [Google Scholar]
- 14.Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause-the Journal of the North American Menopause Society. 2005;12(2):210–5. doi: 10.1097/00042192-200512020-00016. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obesity Research. 2001;9(10):622–6. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- 16.Shin JH, Hur JY, Seo HS, Jeong YA, Lee JK, Oh MJ, Kim T, Saw HS, Kim SH. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids. 2007;72(6-7):592–9. doi: 10.1016/j.steroids.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Simonyte K, Rask E, Naslund I, Angelhed JE, Lonn L, Olsson T, Mattsson C. Obesity Is Accompanied by Disturbances in Peripheral Glucocorticoid Metabolism and Changes in FA Recycling. Obesity. 2009;17(11):1982–7. doi: 10.1038/oby.2009.99. [DOI] [PubMed] [Google Scholar]
- 18.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochemical and Biophysical Research Communications. 2000;30;278(3):640–5. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 19.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;7;97(23):12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ER alpha): a potential role for estrogen receptor beta (ER beta) Hormone and Metabolic Research. 2002;34(11-12):758–63. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 21.Sierens JE, Scobie GA, Wilson J, Saunders PTK. Cloning of oestrogen receptor beta from Old and New World primates: identification of splice variants and functional analysis. Journal of Molecular Endocrinology. 2004;32(3):703–18. doi: 10.1677/jme.0.0320703. [DOI] [PubMed] [Google Scholar]
- 22.Tagawa N, Yuda R, Kubota S, Wakabayashi M, Yamaguchi Y, Kiyonaga D, Mori N, Minamitani E, Masuzaki H, Kobayashi Y. 17 beta-Estradiol inhibits 11 beta-hydroxysteroid dehydrogenase type 1 activity in rodent adipocytes. Journal of Endocrinology. 2009;202(1):131–9. doi: 10.1677/JOE-09-0021. [DOI] [PubMed] [Google Scholar]