Summary
Background
Previous studies have identified effects of age and vascular risk factors on brain injury in elderly individuals. We aimed to establish whether the effects of high blood pressure in the brain are evident as early as the fifth decade of life.
Methods
In an investigation of the third generation of the Framingham Heart Study, we approached all participants in 2009 to ask whether they would be willing to undergo MRI. Consenting patients underwent clinical assessment and cerebral MRI that included T1-weighted and diffusion tensor imaging to obtain estimates of fractional anisotropy, mean diffusivity, and grey-matter volumes. All images were coregistered to a common minimum deformation template for voxel-based linear regressions relating fractional anisotropy, mean diffusivity, and grey-matter volumes to age and systolic blood pressure, with adjustment for potential confounders.
Findings
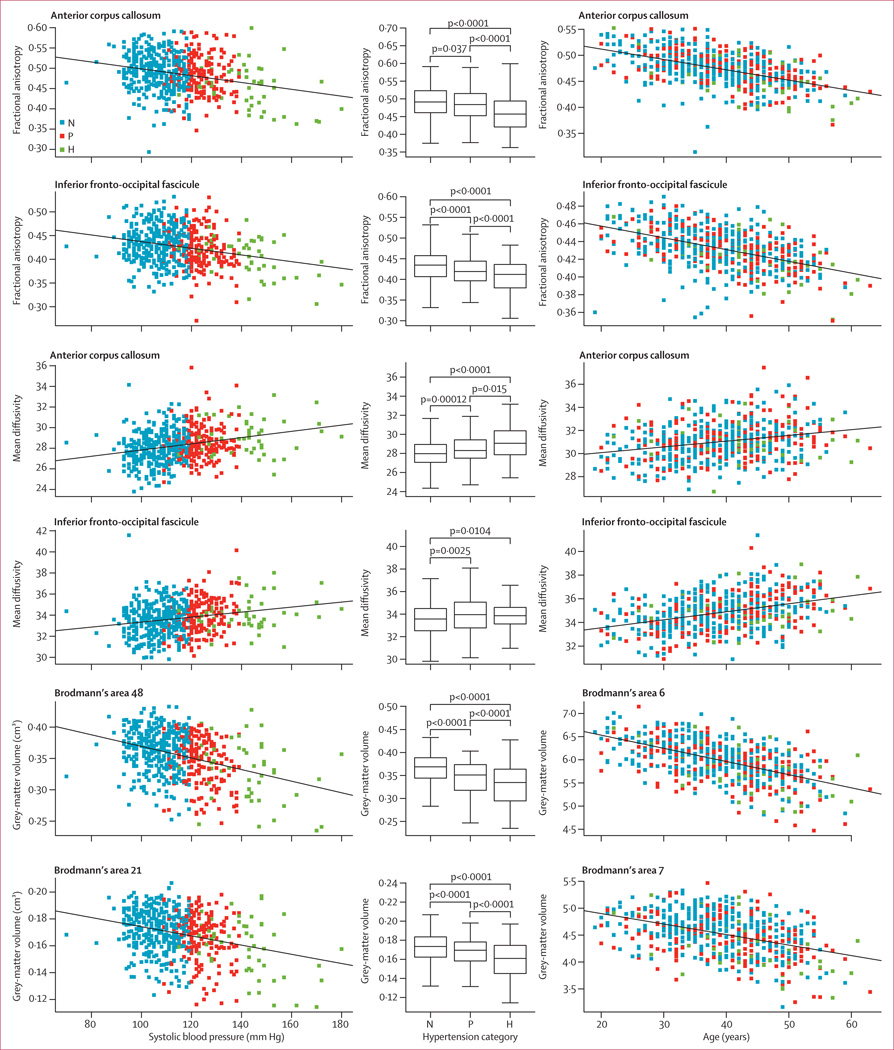
579 (14·1%) of 4095 participants in the third-generation cohort (mean age 39·2 years, SD 8·4) underwent brain MRI between June, 2009 and June, 2010. Age was associated with decreased fractional anisotropy and increased mean diffusivity in almost all cerebral white-matter voxels. Age was also independently associated with reduced grey-matter volumes. Increased systolic blood pressure was linearly associated with decreased regional fractional anisotropy and increased mean diffusivity, especially in the anterior corpus callosum, the inferior fronto-occipital fasciculi, and the fibres that project from the thalamus to the superior frontal gyrus. It was also strongly associated with reduced grey-matter volumes, particularly in Brodmann’s area 48 on the medial surface of the temporal lobe and Brodmann’s area 21 of the middle temporal gyrus.
Interpretation
Our results suggest that subtle vascular brain injury develops insidiously during life, with discernible effects even in young adults. These findings emphasise the need for early and optimum control of blood pressure.
Funding
National Institutes of Health and National Heart, Lung, and Blood Institute; National Institute on Aging; and National Institute of Neurological Disorders and Stroke.
Introduction
Increased blood pressure is estimated to affect 50 million people in the USA.1 Additionally, it is associated with a 62% population attributable risk for cerebrovascular disease and a 49% attributable risk for cardiovascular disease in individuals older than 50 years, and is the greatest risk factor for mortality.1 Despite increasing awareness, less than 60% of identified individuals receive treatment for their hypertension and adequate control of blood pressure is achieved in less than a third of those treated.1 Systolic blood pressure (SBP) increases with age,2 resulting in a lifetime risk for hypertension of almost 90%, which further attests to the high frequency of this disorder.3
Previous studies of individuals aged 60 years or older suggest that, even in people with well controlled hypertension,4 increased SBP is associated with clinically silent brain injuries such as increased brain atrophy,5,6 grey-matter atrophy,7 white-matter injury8,9 including white-matter hyperintensities (WMH),10 and clinically asymptomatic infarcts.11,12 These differences in brain structure are also associated with cross-sectional5,6,9,13 and longitudinal14,15 reductions in cognitive ability, which could begin as early as age 50 years16 and are associated with an increased likelihood of incident dementia.17
With sensitive measures of white-matter injury from diffusion tensor imaging, studies from the past 5 years also suggest that increased blood pressure is associated with subtle injury to white matter, even in the absence of WMH. For example, altered fractional anisotropy can occur,18 particularly in the anterior corpus callosum,18–20 a region where altered fractional anisotropy is associated with cognitive impairment and dementia.18,21,22
Apart from clinically evident vascular events, midlife hypertension is an important modifiable risk factor for cognitive decline, mild cognitive impairment, and vascular dementia late in life.23 Unfortunately, treatment of increased blood pressure late in life to prevent cognitive impairment has had mixed results.24 Although the usefulness of this approach is not well established, reasonable evidence exists to suggest that in people younger than 50 years, lowering blood pressure can help to prevent cognitive decline and dementia late in life.23
Therefore, establishing whether midlife blood pressure differences are associated with concomitant subtle brain injury could identify the earliest changes in brain health linked to hypertension. These findings would further emphasise the importance of aggressive, early management of hypertension as a preventive strategy against cognitive decline late in life, in addition to the recognised benefits of treatment on cardiovascular and cerebrovascular events.1
Our aim was to explore cross-sectional associations between increased SBP,25 age, measures of white-matter injury (fractional anisotropy and mean diffusivity), and grey-matter atrophy in the third generation of Framingham Heart Study participants. Because of the young age of the cohort, we postulated that increased SBP would be associated with lower fractional anisotropy and higher mean diffusivity values, because these measures are sensitive to white-matter pathology. We also tested whether WMH volumes and cerebral atrophy increased, which would suggest that SBP can lead to vascular brain injury as early as the fifth decade of life.
Methods
Study design and participants
The Framingham Heart Study is a single-site, community-based, prospective cohort study in Framing ham (MA, USA) that was initiated in 1948 to investigate risk factors for cardiovascular disease. It comprises three generations of participants. Study of the 4095 participants in the third-generation cohort (G3) began in 2000, with the recruitment and first assessment occurring between 2002 and 2005. From June 2009, all individuals recruited to the G3 cohort were approached to participate in our MRI study. We excluded participants with prevalent stroke at the MRI assessment or other neurological disorders that might confound the assessment of brain volumes, or because of poor MRI quality (appendix).
The institutional review boards at all participating institutions approved the study. Patients or their legal representatives gave written informed consent.
Procedures
Participants had one MRI at baseline, when they were also monitored with previously described surveillance techniques26,27 for the development of stroke or dementia. Stroke was defined as an acute-onset focal neurological deficit of presumed vascular aetiology lasting 24 h or more. Dementia was diagnosed according to criteria of the Diagnostic and Statistical Manual of Mental Disorders IV.28 We recorded mean SBP and diastolic blood pressure (DBP) at time of MRI, which were calculated with two sets of values measured separately by two physicians when the patient was seated. Present smoking status was recorded in interviews. We recorded any drugs taken by each patient with prescription lists. We used three categorical variables: hypertension categories (normal: SBP <120 mm Hg and DBP <80 mm Hg; prehypertension: SBP 120–139 mm Hg or DBP 80–89 mm Hg; and hypertension: SBP ≥140 mm Hg or DBP ≥100 mm Hg) as described in the seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure;1 whether the individual was being treated for hypertension; and smoking status at time of clinical assessment. We did not include the presence of diabetes mellitus in the models because of its low prevalence in our sample (n=7).
In addition to analysis of WMH on MRI—the burden of which is usually low in young adults—we analysed diffusion tensor imaging measures of fractional anisotropy and mean diffusivity, which are more sensitive measures of white-matter integrity than is WMH. Briefly, fractional anisotropy and mean diffusivity characterise the microstructural properties and integrity of white matter. Fractional anisotropy quantifies the amount of anisotropy of water diffusion and mean diffusivity quantifies mean water diffusion within white matter.22
We segmented WMH with fluid attenuated inversion recovery and grey matter with T1-weighted images by semiautomated procedures, as previously described.29,30 All images, including fractional anisotropy, mean diffusivity, grey-matter volume, and WMH maps, were coregistered to a minimum deformation template22,31 for group statistical analyses. Each pixel (also known as a voxel) in these coregistered volumetric images corresponds to the same location in the brain across all individuals. This correspondence allows corresponding imaging measures (fractional anisotropy or mean diffusivity measures, grey-matter density, or WMH occurrence) to be related to various independent variables. We quantified total cranial volume on the basis of fluid attenuated inversion recovery with a previously reported analysis protocol29 and corrected for differences in head size. WMH volumes were log transformed to normalise population variance.
Statistical analyses
The primary goal of the statistical analysis was to establish if, at the voxel level, increased SBP and age were associated with increased brain injury as indicated by decreased fractional anisotropy, raised mean diffusivity, or grey-matter atrophy. To achieve this goal, we used a linear regression with measures of fractional anisotropy, mean diffusivity, or grey-matter volumes as the dependent variable and continuous SBP and age as independent variables, adjusting for smoking status, hypertension treatment, total cranial volume, and sex. The T maps obtained for both independent variables (SBP and age) were assessed for significance with threshold-free cluster enhancement with an α of 0·05; we corrected for multiple comparisons with permutation-based correction (n=1000).32
We then overlaid the thresholded T maps with the Johns Hopkins University probabilistic fibre map atlases33 and a template of Brodmann’s areas, which were warped to the minimum deformation template space, to provide a post-hoc description of significant voxels in terms of the white-matter tracts or grey-matter regions to which they most probably belonged.
A second objective of our study was to estimate a global measure of brain health in individuals with prehypertension and hypertension as compared with normotensive individuals. To achieve this goal, we used the mean fractional anisotropy and mean diffusivity and the volume of grey matter in regions that had been identified in the voxel-based analysis as significantly associated with increasing SBP. We then used a principal component analysis to decompose the data into a linear combination of orthogonal, with a singular value de composition implementation.34 The objective of this decomposition was to generate a composite measure of brain health.
The resulting first principal component (PC1) explained the greatest amount of variance in the dataset and was then used as the dependent variable in a linear regression with hypertension category and age as independent variables, adjusting for smoking status, hypertension treatment, total cranial volume, and sex. Because the PC1 measure seemed to be quadratically related to age on visual inspection, we also created a model that included a quadratic effect of age. We assessed increase in quality of quadratic fit compared with the linear fit of age with a likelihood ratio test. The interactions of SBP with age and the quadratic effect of age were tested but were not significant; therefore, we removed them from the model. Because hypertension is associated with accelerated brain ageing,4,6 we then compared group differences in this summary measure to establish estimates of the number of years of age necessary to achieve the same mean value for the two hypertensive groups.
Finally, we explored whether WMH in young adults could provide further information about white-matter injury associated with hypertension in these participants. We did a linear regression with WMH volume as the dependent variable and SBP as the independent variable, adjusting for age, smoking status, hypertension treatment, total cranial volume, and sex.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. PM, AB, JJH, and CDC had full access to all the data and final responsibility for the decision to submit for publication.
Results
Our study includes 579 (14·1%) of 4095 participants in G335 who underwent brain MRI between June, 2009, and June, 2010. Compared with the rest of G3, individuals included in our study were significantly younger, had significantly lower SBP and DBP, and were less likely to be receiving treatment for hypertension (table 1). Additionally, fewer were male and they were less likely to smoke or have diabetes than were the individuals in the rest of G3 (table 1). Participants were aged 19–63 years (median 39, IQR 33–45). As expected in this sample of young adults, mean WMH volume was low (table 1).
Table 1.
Characteristics of participant
| DTI sample* (n=579) | Rest of G3 (n=3516) | p value | |
|---|---|---|---|
| Age (years) | 39·2 (8·4) | 40·3 (8·9) | 0·005 |
| Systolic blood pressure (mm Hg) | 115 (14) | 117 (14) | 0·001 |
| Diastolic blood pressure (mm Hg) | 74 (10) | 76 (10) | 0·001 |
| Women | 345 (59·6%) | 1838 (52·5%) | 0·001 |
| Current smoking | 62 (10·7%) | 573 (16·3%) | 0·001 |
| Hypertension treatment | 41 (7·1%) | 393 (11·2%) | 0·003 |
| Diabetes | 7 (1·2%) | 117 (3·3%) | 0·006 |
| Prevalent cardiovascular disease | 4 (0·7%) | 62 (1.8%) | 0·003 |
| Atrial fibrillation | 2 (0·4%) | 17 (0·5%) | 0·65 |
| White-matter hyperintensities (cm3) | 1·7 (1·0) | NA | NA |
| Total intracranial volume (cm3) | 1232·8 (147·5) | NA | NA |
Data are mean (SD) or n (%), unless otherwise stated. DTI=diffusion tensor imaging. NA=not applicable.
Individuals in our study who underwent an MRI examination including DTI acquisition.
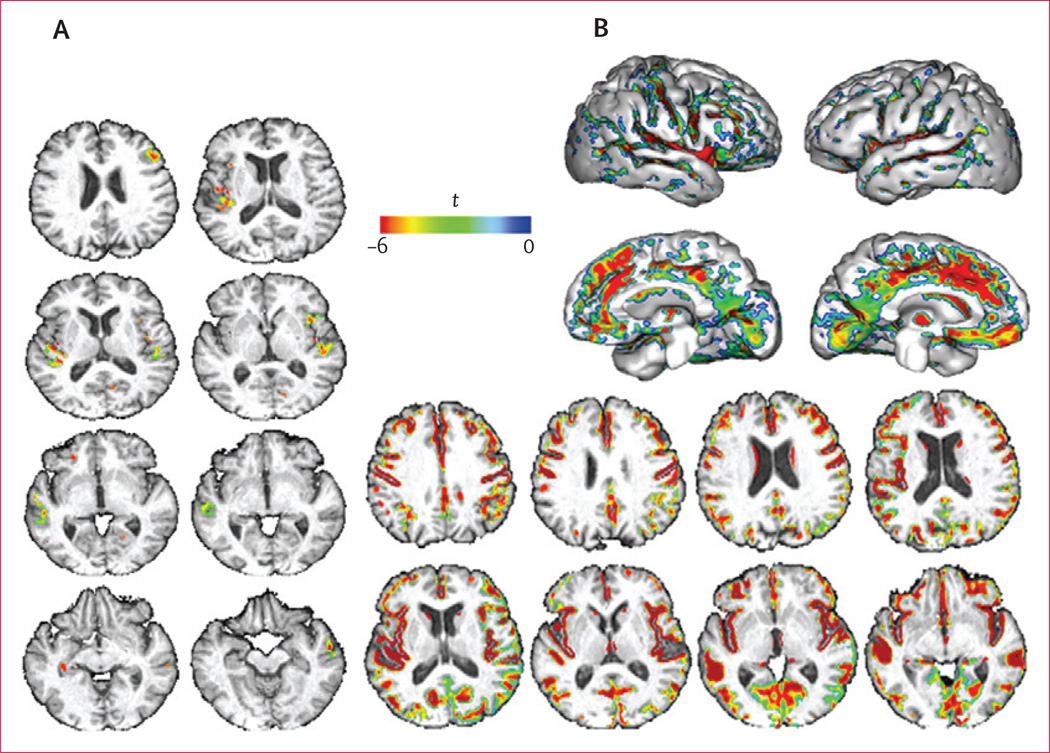
Age was independently associated with decreased fractional anisotropy within voxels that covered 49·4 cm3 of the white matter and SBP was associated in voxels covering 2·9 cm3. Additionally, age was associated with increased mean diffusivity within voxels that covered 87·6 cm3 and SBP in voxels covering 10·5 cm3 of the white matter. High SBP was strongly associated with decreased fractional anisotropy and increased mean diffusivity within white-matter tracts such as the anterior corpus callosum, the inferior fronto-occipital fasciculi, and the fibres that project from the thalamus to the superior frontal gyrus (figures 1, 2; tables 2, 3). Age was associated with decreased fractional anisotropy and increased mean diffusivity in almost all cerebral white-matter voxels (figure 1). The anterior corpus callosum, the inferior fronto-occipital fasciculi, the inferior longitudinal fasciculus, the cingulum bundle, and the fibres that project from the thalamus to the superior frontal gyrus (table 3) had a large number of voxels that showed significant associations between age and decreased fractional anisotropy or increased mean diffusivity (figure 2; tables 2, 3).
Figure 1. Regions of the cerebral white matter in which systolic blood pressure (A, B) and age (C, D) are associated with decreased fractional anisotropy and increased mean diffusivity.
The voxel-based regression included diffusion tensor imaging measures (fractional anisotropy or mean diffusivity) as the dependent variable, and age and systolic blood pressure as independent variables. Number of cigarettes smoked per day, hypertension treatment (presence or absence), intracranial volume, and sex were covariates.
Figure 2. Regression analyses for brain areas in which associations between variables were significant and box plots showing comparisons between hypertension categories after adjustment for age.
N=normotensive. P=prehypertensive. H=hypertensive.
Table 2.
Associations between decreasing fractional anisotropy and increasing systolic blood pressure and age by fibre label
| Total number of voxels |
Number of voxels associated with increasing systolic blood pressure |
Number of voxels associated with age |
|
|---|---|---|---|
| Corpus callosum* | |||
| Medial occipital gyrus | 2454 | 0 | 526 |
| Post-central gyrus | 1707 | 18 | 417 |
| Pre-central gyrus | 2159 | 89 | 747 |
| Rectus | 537 | 0 | 382 |
| Superior frontal gyrus | 15 550 | 1220 | 9117 |
| Superior occipital gyrus | 4336 | 0 | 1288 |
| Superior parietal gyrus | 4959 | 0 | 951 |
| Cingulum | 5873 | 150 | 2622 |
| Corticospinal tract | 5788 | 0 | 842 |
| Inferior fronto-occipital tract | 9121 | 304 | 4124 |
| Inferior longitudinal fasciculus | 7896 | 20 | 2417 |
| Short association fibres | |||
| Inferior occipital gyrus | 2021 | 0 | 539 |
| Superior frontal gyrus and supramarginal gyrus | 5013 | 14 | 1070 |
| Superior parietal gyrus and angular gyrus | 4038 | 0 | 391 |
| Superior longitudinal fasciculus | |||
| Frontal and parietal | 4815 | 0 | 706 |
| Frontal and temporal | 2682 | 0 | 442 |
| Parietal and temporal | 5341 | 0 | 1268 |
| Thalamic radiations† | |||
| Middle frontal gyrus | 1638 | 46 | 460 |
| Superior frontal gyrus | 7374 | 142 | 1494 |
| Uncinate | 3444 | 42 | 1509 |
Data are n. Regions of interest listed here only when the number of voxels associated with systolic blood pressure or age made up at least 1% of the total number of associated voxels. Fibre labels according to the Johns Hopkins University probabilistic fibre map atlases.33
Labels refer to fibres connecting the cortical areas in both hemispheres through the corpus callosum.
Labels indicate fibres that project from the thalamus.
Table 3.
Associations between increasing mean diffusivity and increasing systolic blood pressure and age by fibre label
| Total number of voxels |
Number of voxels associated with increasing systolic blood pressure |
Number of voxels associated with increasing age |
|
|---|---|---|---|
| Corpus callosum* | |||
| Medial occipital gyrus | 2454 | 282 | 1758 |
| Post-central gyrus | 1707 | 0 | 909 |
| Pre-central gyrus | 2159 | 0 | 1193 |
| Rectus | 537 | 96 | 201 |
| Superior frontal gyrus | 15 550 | 1051 | 4644 |
| Superior occipital gyrus | 4336 | 391 | 3035 |
| Superior parietal gyrus | 4959 | 34 | 3081 |
| Cingulum | 5873 | 14 | 3253 |
| Corticospinal tract | 5788 | 62 | 2763 |
| Inferior fronto-occipital tract | 9121 | 1828 | 5102 |
| Inferior longitudinal fasciculus | 7896 | 263 | 4358 |
| Short association fibres | |||
| Inferior occipital gyrus | 2021 | 0 | 710 |
| Superior frontal gyrus and supramarginal gyrus | 3904 | 0 | 1269 |
| Postcentral gyrus | 1758 | 4 | 873 |
| Precentral gyrus | 3326 | 0 | 1166 |
| Superior parietal gyrus and angular gyrus | 4038 | 37 | 1811 |
| Superior parietal and middle occipital gyrus | 857 | 19 | 666 |
| Superior longitudinal fasciculus | |||
| Frontal and parietal | 4815 | 181 | 3143 |
| Frontal and temporal | 2682 | 441 | 2101 |
| Parietal and temporal | 5341 | 383 | 3253 |
| Thalamic radiations† | |||
| Inferior frontal | 401 | 121 | 175 |
| Middle frontal gyrus | 1638 | 404 | 1024 |
| Post-central gyrus | 1408 | 34 | 1113 |
| Pre-central gyrus | 1992 | 120 | 1650 |
| Superior frontal gyrus | 7374 | 978 | 5201 |
| Superior occipital gyrus | 989 | 76 | 518 |
| Superior parietal gyrus | 1722 | 77 | 1401 |
| Uncinate | 3444 | 425 | 620 |
Data are n. Regions of interest listed here only when the number of voxels associated with systolic blood pressure or age made up at least 1% of the total number of associated voxels. Fibre labels according to the Johns Hopkins University probabilistic fibre map atlases.33
Labels refer to fibres connecting the cortical areas in both hemispheres through the corpus callosum.
Labels indicate fibres that project from the thalamus.
Age was independently associated with decreased grey-matter volumes within voxels that covered 171·9 cm3, as was SBP in voxels covering 2·9 cm3 of grey matter (appendix). Increased SBP was strongly associated with reduced grey-matter volumes, particularly in Brodmann’s area 48 on the medial surface of the temporal lobe and Brodmann’s area 21 of the middle temporal gyrus (figures 2, 3). Age was diffusely associated (ie, in almost all grey-matter voxels) with reduced grey-matter volumes (appendix).
Figure 3. Cerebral regions in which decreasing grey-matter volumes were significantly associated with increasing systolic blood pressure (A) and age (B).
The voxel-based regression included grey-matter probability as the dependent variable and age and systolic blood pressure as independent variables. Number of cigarettes smoked per day, hypertension treatment (treated or not), intracranial volume, and sex were covariates.
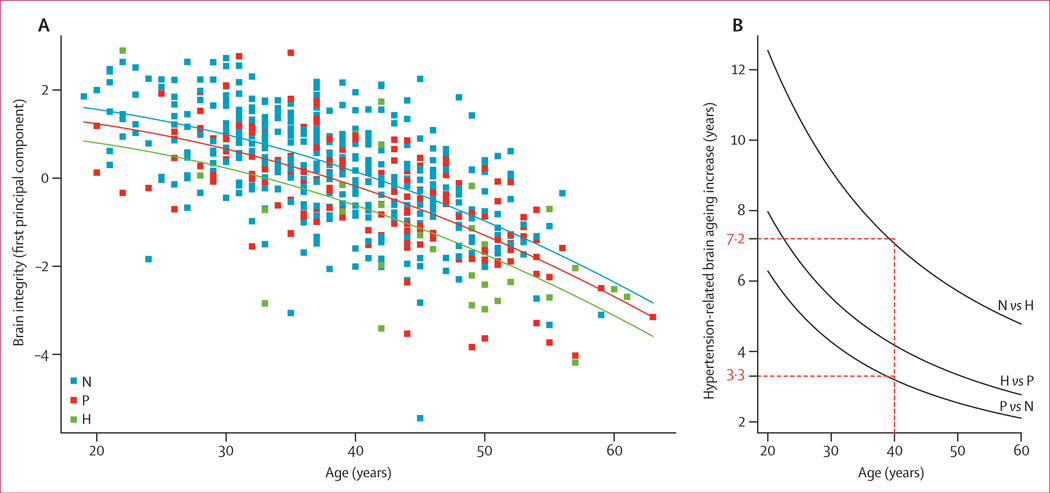
The PC1 of the principal components analysis accounted for 57% of variance. Small values of the PC1 coefficient indicate low mean fractional anisotropy and grey-matter volume, and high mean mean diffusivity (ie, greater severity of brain injury). The contributions of fractional anisotropy (principal component loading 0·62), grey matter (−0·51), and mean diffusivity (0·60) to PC1 were roughly equal.
Linear regression suggested that reduced brain integrity as expressed by PC1 was significantly associated with hypertension category and increasing age (figure 4). With the addition of a quadratic effect of age, the goodness of fit of the model rose, as indicated by the likelihood ratio test (p=0·0047). The linear (R2=0·43) and quadratic (R2=0·44) models explained substantial variation in the principal component. Brain integrity was usually higher in normotensive individuals (β=1·88, 95% CI 0·46–3·3) than in prehypertensive (β=1·56, 95% CI 1·37–1·75; p=0·00089) and hypertensive participants (β=1·12, 95% CI 0·74–1·51; p=0·00011). It linearly (β=–0·096, 95% CI −0·09 to 0·07; p<0·0001) and quadratically decreased (β=−0·0014, 95% CI −0·0023 to −0·00042; p=0·0047) with increasing age. Brain integrity differed between hypertension categories as a function of age (figure 4).
Figure 4. Regression curves relating brain integrity as expressed by the first principal component as a function of the hypertension category and age of the individual (A) and the difference in brain ageing increase between hypertension categories according to age (B).
Red dotted lines indicate brain integrity of a 40-year-old by hypertensive status; brain integrity of a 40-year-old prehypertensive individual corresponded to that of a normotensive individual 3·3 years older, but this difference was 7·2 years for hypertensive individuals. N=normotensive. P=prehypertensive. H=hypertensive.
Linear regression indicated that WMH volume was not associated with SBP (β=−0·008, 95% CI −0·09 to 0·07; p=0·84). Figure 5 shows the spatial distribution of WMH in the sample. Although the proportion of treated hypertensive individuals in our sample was low (7%), we repeated all analyses after removal of this subgroup. The results did not differ significantly from those of previous analyses (data not shown).
Figure 5. Number of patients with white-matter hyperintensities at a voxel location.
Discussion
Results from our study indicate that SBP is associated with injury to white-matter microstructure and regional grey matter in healthy young adults. Injury worsened continuously as SBP increased and was significantly different between normotensive and prehypertensive individuals in some regions. Moreover, the anatomical locations of these differences at fairly young ages are similar to the distribution of WMH associated with SBP late in life36 and are associated with decreased cognitive performance, albeit in the normal range.13
Previous work from the Framingham Heart Study8,10,13,37 and other studies of elderly individuals have identified cross-sectional and longitudinal associations between midlife vascular risk factors, brain atrophy, WMH, and cognitive ability late in life (panel). Our study extends these findings by showing that raised SBP has a subtle negative effect on white-matter integrity and grey-matter volume at even young adult ages, with preferential susceptibility to specific anterior white-matter tracts and grey-matter regions even before evidence of increased WMH volumes.
Evidence of even subtle brain injury at young ages should affect how physicians think about hypertension diagnoses and treatment for two important reasons. First, our evidence strongly suggests that the effect of SBP on the brain is continuous with increasing pressure, suggesting that what might be the optimum SBP will have to be reconsidered.38 Second, these data strongly suggest that substantial brain injury could precede clinically manifest disease, indicating that prevention of stroke or cognitive impairment due to hypertensive vascular disease might require treatment at younger ages than presently thought.37 Although present medical treatment recommendations have had a clear effect on mean blood pressure in the population41 and mortality from hypertension-associated vascular disease,1 the incidence and prevalence of common hypertension-associated disorders such as myocardial infarction42 have not been greatly changed, indicating further need for early diagnosis and individual treatment.
Although our data clearly show that brain injury occurs at fairly young ages, the cause is unclear. The biological mechanisms triggered by increased SBP are complex43 and cannot be addressed with these data. With ageing, artery stiffening causes SBP to increase, which con tributes to loading of stiff components of the atrial wall and subsequently increases arterial stiffness.43 This process also affects small arteries and wave reflection. One hypothesis posits that vascular dysauto-regulation due to arterial remodelling causes transient reductions in blood flow to white-matter watershed areas of vascular supply,44 resulting in transient hyporaemia, hypoxaemia, and subtle myelin injury.45 This hypothetical process, however, explains neither the specific vulnerability of anterior white-matter tracts to increases in SBP nor the diffuse grey-matter atrophy reported in our study and others.4–6,18
Understanding of the regional specificity of this association is especially important in light of a growing body of scientific literature suggesting that reduced fractional anisotropy in these regions is associated with decreased cognitive performance in individuals aged 60 years or older46,47 and similar negative associations between cognition and brain atrophy in people with raised SBP.5,6 Because arterial stiffness has been associated with cognitive impairment in elderly individuals,48 studies investigating the effect of pulse wave velocity—the gold standard measure for clinical assessment of arterial stiffness in the cerebral microcirculation—are needed, as are interventional studies with cognitive deterioration as a predefined outcome to elucidate the chronology and factors linking arterial ageing, microvascular damage, and cognitive decline.
However, we recorded no effect of SBP on WMH. As previously suggested in elderly individuals, WMH could be the final stage of broadly distributed and progressive white-matter degeneration,49 which is a process that might not be sufficiently advanced in younger individuals and would therefore be a less sensitive measure than is diffusion tensor imaging of cerebrovascular disease in this otherwise healthy young cohort.50 This view is supported by the absence of a cross-sectional association between concurrent SBP and WMH in young individuals. Midlife SBP is strongly associated with WMH burden late in life when vascular brain injury processes have progressed to an advanced stage.5,51,52 Further substantiation of this hypothesis will necessitate longitudinal study of how diffusion tensor imaging measures and WMH coevolve spatially and temporally from midlife onward.
Notably, we focused on measures of only SBP as a marker of vascular health for two reasons. First, in this fairly young cohort, DBP and SBP measures were highly correlated. Second, increased SBP is the most common form of hypertension and is a more robust risk factor for cardiovascular disease than is DBP, particularly after 50 years of age.25 Additionally, we did not specifically investigate the effects of low blood pressure on brain integrity. Although evidence suggests that reduced SBP is associated with cerebral atrophy in elderly individuals,53 this finding has not been investigated in young adults and is worthy of further research. However, our results showed an inverse monotonic relation between SBP and brain atrophy.
Our study has some limitations. First, the Framingham Heart Study cohort is mostly of white descent and therefore does not fully represent the general population of the USA.35 Second, voluntary participation in MRI studies is often biased, with the healthiest individuals being more likely to participate, particularly in samples of older adults.54 This second concern is probably mitigated by the fairly good health and young age of our study cohort. Third, our study is cross-sectional. Further longitudinal studies are needed to investigate whether duration of increased SBP has cumulative effects on white-matter integrity in young adults.
Despite these limitations, the public health implications of our results are clear. Our findings support the need for early and optimum control of blood pressure, which is presently neither routinely achieved39 nor subject to testing in many randomised controlled clinical trials.40 Whether structural brain differences associated with chronic hypertension are preventable or if they can be reversed—as in a study reporting encouraging benefits of memory training on microstructure of white matter55—is unclear. In view of evidence that both the degree and duration of hypertension are associated with continuous adverse effects on late-life brain injury and cognition,5,6 early and aggressive treatment could be necessary for success.
Panel: Research in context.
Systematic review
We searched PubMed on Jan 15, 2012, for reports published in English of the effects of blood pressure on white-matter integrity in healthy adults and older individuals with the terms "blood pressure" and "DTI". Previous work from the Framingham Heart Study has identified cross-sectional and longitudinal associations between midlife vascular risk factors and late-life cognitive ability, strokes, brain atrophy, and white-matter hyperintensities that represent severe white-matter injury.8,14,37 With sensitive measures of white-matter injury from diffusion tensor imaging, studies from the past 4 years18–20 have suggested that increased blood pressure is associated with subtle injury to white matter in the anterior corpus callosum in elderly individuals, and greater injury in this region has been linked to cognitive impairment and dementia.21,22
Interpretation
We have shown that systolic blood pressure is associated with injury to white-matter microstructure and regional grey matter in healthy young adults. Our findings extend those of earlier studies37,38 by indicating that white-matter injury begins before the age of 50 years. Our results emphasise the need for early and optimum control of blood pressure, which is neither routinely achieved39 nor subject to testing in many randomised controlled clinical trials.40
Acknowledgments
This study was funded by the National Institutes of Health and National Heart, Lung, and Blood Institute (contract N01-HC-25195); the National Institute on Aging (R01 AG033040, AG16495, AG08122, AG030514, and P30-AG10129); and the National Institute of Neurological Disorders and Stroke (R01-NS17950).
Footnotes
See Online for appendix
Contributors
All authors participated in study conceptualisation and design. PM drafted the report. PM, SS, OC, and CDC analysed and interpreted data. SS, AB, JJH, RA, EF, OC, PAW, and CDC revised the report.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 4.Salerno JA, Murphy DG, Horwitz B, et al. Brain atrophy in hypertension: a volumetric magnetic resonance imaging study. Hypertension. 1992;20:340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 5.Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 7.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 10.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 11.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Wright CB, Yoshita M, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 14.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Carmelli D. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54:2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- 16.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Debette S, Beiser A, Decarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leritz EC, Salat DH, Milberg WP, et al. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010;24:199–208. doi: 10.1037/a0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salat DH, Williams VJ, Leritz EC, et al. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DY, Fletcher E, Martinez O, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;4:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 26.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease: the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 27.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID-I), clinical version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 29.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via em algorithm. J R Stat Soc B Met. 1977;39:1–38. [Google Scholar]
- 31.Kochunov P, Lancaster JL, Thompson P, et al. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr. 2001;25:805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang J, Oishi K, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venables WN, Ripley B. Modern applied statistics with S. Berlin: Springer; 2002. [Google Scholar]
- 35.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 36.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med. 2001;161:2343–2350. doi: 10.1001/archinte.161.19.2343. [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 39.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 40.Franklin SS. Blood pressure and cardiovascular disease: what remains to be achieved? J Hypertens Suppl. 2001;19:S3–S8. [PubMed] [Google Scholar]
- 41.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 42.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 43.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 44.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 45.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YZ, Chang C, Wei XE, Fu JL, Li WB. Comparison of diffusion tensor image study in association fiber tracts among normal, amnestic mild cognitive impairment, and Alzheimer’s patients. Neurol India. 2011;59:168–173. doi: 10.4103/0028-3886.79129. [DOI] [PubMed] [Google Scholar]
- 47.Teipel SJ, Meindl T, Wagner M, et al. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- 48.Hanon O, Haulon S, Lenoir H, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- 49.Maillard P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 51.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 52.van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 53.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes: the SMART-MR study. J Hypertens. 2010;28:1498–1505. doi: 10.1097/HJH.0b013e32833951ef. [DOI] [PubMed] [Google Scholar]
- 54.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Engvig A, Fjell AM, Westlye LT, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]