Abstract
Objective
Effective post-infarction repair requires timely suppression of innate immune signals to prevent the catastrophic consequences of uncontrolled inflammation on cardiac geometry and function. In macrophages, Interleukin Receptor-Associated Kinase (IRAK)-M acts as a functional decoy preventing uncontrolled TLR/Interleukin-1-mediated responses. Our study investigates the role of IRAK-M as a negative regulator of the post-infarction inflammatory response and as a modulator of cardiac remodeling.
Methods and results
In WT mouse infarcts IRAK-M was upregulated in infiltrating macrophages and fibroblasts exhibiting a biphasic response. When compared to wildtype animals, infarcted IRAK-M −/− mice had enhanced adverse remodeling and worse systolic dysfunction; however, acute infarct size was comparable between groups. Adverse remodeling in IRAK-M −/− animals was associated with enhanced myocardial inflammation and protease activation. The protective actions of IRAK-M involved phenotypic modulation of macrophages and fibroblasts. IRAK-M −/− infarcts showed increased infiltration with pro-inflammatory CD11b+/Ly6Chi monocytes; leukocytes harvested from IRAK-M null infarcts exhibited accentuated cytokine expression. In vitro, IRAK-M expression was upregulated in cytokine-stimulated murine cardiac fibroblasts and suppressed their matrix-degrading properties without affecting their inflammatory activity.
Conclusions
Endogenous IRAK-M attenuates adverse post-infarction remodeling suppressing leukocyte inflammatory activity, while inhibiting fibroblast-mediated matrix degradation.
Keywords: cardiac remodeling, cytokines, macrophages, metalloproteinases, immune system
INTRODUCTION
Tissue necrosis triggers an intense inflammatory reaction that serves to clear the wound from dead cells and matrix debris, while activating reparative pathways. Because of the catastrophic consequences of excessive or prolonged inflammation on tissue architecture and organ function, repair of injured tissues requires timely activation of endogenous inhibitory pathways that restrain and suppress pro-inflammatory signals. Initially believed to be a “passive” process due to dissipation of the injurious stimulus, it is now clear that suppression and resolution of the acute inflammatory response is a biosynthetically active response that requires activation of molecular mediators that inhibit the inflammatory reaction1,2.
Because the mammalian heart has negligible regenerative capacity, repair of the infarcted myocardium is dependent on activation of an inflammatory reaction that ultimately results in formation of a collagen-based scar. Perhaps more so than in any other tissue or type of injury, negative regulation of myocardial inflammation in the ischemic heart is of critical significance for the reparative process 3. Why is the myocardium so vulnerable to the consequences of an accentuated reparative inflammatory reaction? First, the selective pressures responsible for the evolution of the response to injury were likely driven by the potentially catastrophic effects of bacterial contamination in insults associated with traumatic breakdown of epithelial barriers. Thus, mammals may have evolved to interpret necrotic host cells as a sign of infection 1, mounting a robust inflammatory response that may be excessive in a context of ischemic injury and in the absence of microbial contamination. Second, in the myocardium, function is dependent on optimal maintenance of tissue architecture and chamber geometry. Even relatively subtle alterations in myocardial architecture have profound consequences on cardiac geometry and mechanics leading to contractile dysfunction and chamber dilation, a process termed “ventricular remodeling”4,5. In the infarcted heart, an excessive, or prolonged, inflammatory reaction could be catastrophic by enhancing cardiomyocyte hypocontractility and apoptosis, while promoting protease activation and matrix degradation, thus markedly accentuating remodeling. More extensive cardiac remodeling following myocardial infarction is associated with increased mortality and a high incidence of arrhythmias and heart failure 6. Emerging evidence suggests that defects in the molecular signals implicated in suppression and resolution of the inflammatory response may be involved in the pathogenesis of post-infarction cardiac remodeling7,8.
Toll-Like Receptor (TLR) and Interleukin (IL)-1 –mediated pathways are critically involved in the post-infarction inflammatory response and in the pathogenesis of cardiac remodeling 9,10,11. Considering the broad pro-inflammatory actions of TLR/IL-1 activation and the potentially catastrophic effects of uncontrolled inflammation, it is not surprising that several distinct pathways have evolved to restrain TLR and IL-1 responses12. One such endogenous inhibitory signal, Interleukin-1 receptor-associated kinase (IRAK)-M is the only IRAK that lacks endogenous kinase activity, acting as a functional decoy that inhibits TLR and IL-1 responses13,14. IRAK-M is primarily expressed in monocytes and macrophages and plays an important role in restraining inflammatory activation induced by infectious pathogens13,15; however, its potential involvement in regulating the inflammatory and reparative process in ischemic injury has not been studied. We hypothesized that IRAK-M expression may be upregulated in the infarcted heart preventing uncontrolled pro-inflammatory signaling and protecting from the development of adverse remodeling following myocardial infarction. Our findings provide the first evidence for a role of IRAK-M in cardiac remodeling and suggest that, in addition to its effects on macrophage inflammatory activity, IRAK-M is induced in cytokine-stimulated fibroblasts regulating their matrix-degrading properties.
METHODS
(detailed description of the methodology is provided in the online supplement)
Animal protocols
C57BL/6, IRAK-M −/− mice13 and IL1RI −/− animals11 in a C57BL/6 background were purchased from Jackson Laboratories. All protocols were approved by the committee on animal research care at Baylor College of Medicine and at Albert Einstein College of Medicine. A total of 150 C57BL6 mice and 156 IRAK-M −/− mice were used in the study. Two to three month-old mice were anesthetized by isoflurane inhalation (isoflurane 2–3% vol/vol). Myocardial infarction was induced using a closed-chest mouse model of reperfused myocardial infarction, as previously described 16. After 6h-28d of reperfusion, the chest was opened and the heart was immediately excised, fixed in zinc-formalin and embedded in paraffin for histological studies, or snap frozen in liquid nitrogen and stored at –80°C for RNA and protein extraction. Sham animals were prepared identically without undergoing coronary occlusion/reperfusion. In additional mice, infarct size was assessed after 24h of reperfusion using triphenyltetrazolium chloride (TTC)/Evans Blue staining, as previously described by our laboratory17.
Echocardiography
Echocardiographic studies were performed before instrumentation and after 7 days and 28 days of reperfusion (WT, n=14; IRAK-M−/−, n = 18) using a 25-MHz probe (Vevo 770; Visualsonics. Toronto ON) as previously described8.
Perfusion Fixation and Assessment of Ventricular Volumes
Systematic morphometric assessment of ventricular dimensions, ventricular volumes and scar size in perfusion-fixed hearts was performed as previously described8.
Immunohistochemistry and Quantitative Histology
Leukocytes were identified in formalin-fixed paraffin-embedded sections using immunohistochemistry with the following primary antibodies: monoclonal anti-neutrophil antibody (Serotec, Raleigh NC) and rat anti-mouse Mac-2 (Cedarlane Burlington, Canada) for macrophages. The collagen network was identified using picrosirius red staining.
RNA Extraction and qPCR assay
Isolated total RNA from the hearts and cultured fibroblasts was reverse transcribed to cDNA using the iScript™ cDNA sythesis kit (Bio-Rad) following the manufacturer’s guidelines. Quantitative PCR was performed using the SYBR green (Bio-Rad) method on the iQ™5 Real- Time PCR Detection System (Bio-Rad).
Zymography
MMP activity in the infarcted myocardium was assessed using gelatin zymography as previously described8.
Preparation of single cell suspensions from infarcted mouse hearts and flow cytometric analysis
Single cell suspensions were obtained from infarcted WT and KO hearts as previously described17.
Fibroblast isolation and stimulation
Fibroblasts were isolated from normal mouse hearts and stimulated as previously described18,17.
Isolation of fibroblasts and macrophages from infarcted hearts
Macrophages and fibroblasts were isolated from control and infarcted hearts for immunofluorescent staining and for RNA extraction.
Immunofluorescent staining of isolated cells and paraffin-embedded sections
Primary cells were seeded in chambers of Culture Slides (BD Falcon) and allowed to attach 24hr to 72hr. After rinsing with PBS, fibroblasts or macrophages were fixed for 10 min in 2% solution of paraformaldehyde (Sigma) in PBS and permeabilized using 0.1% Triton-X (Sigma) in PBS. Paraffin sections were deparaffinized, hydrated and rinsed in distilled water. Antigen retrieval was performed by heating sections in an antigen retrieval solution (Abcam) for 30min at 95°C. The sections were blocked 30 minutes with Dulbecco’s phosphate-buffered saline with Mg2+, Ca2+ (DPBS) containing 10% rabbit serum. Subsequently, slides were double-stained with goat anti-mouse IRAK-M (Santa Cruz, 1:200) and rat anti-mouse Mac2 (Cedarlane Burlington, Canada, 1:200) or mouse anti-α-SMA (Sigma, St. Louis, MO, 1:200). The mouse on mouse (M.O.M) kit (Vector Laboratories) was used for α-SMA staining. Alexa 488-conjugated (Molecular Probes) or Alexa 594-conjugated secondary antibody (Molecular Probes) was used. The immunostained sections were digitally imaged using a Zeiss fluorescence microscope.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using unpaired, 2-tailed Student’s t test using Welch’s correction for unequal variances and 1-way ANOVA with Tukey’s multiple comparison test. Paired t test was used to compare echocardiographic parameters before myocardial infarction and after 7 to 28 days of reperfusion. Statistical analyses were performed using Prism software. P<0.05 was considered to be significant. Mortality was compared using the log rank test.
RESULTS
1. Biphasic upregulation of IRAK-M in reperfused mouse infarcts
qPCR analysis demonstrated significant IRAK-M mRNA upregulation in the infarcted myocardium. The time course of IRAK-M induction showed a biphasic response (Figure 1), characterized by marked early upregulation after 6h of reperfusion, followed by a second peak after 7 days of reperfusion (Figure 1A).
Figure 1.
IRAK-M upregulation in the infarcted mouse heart. A. IRAK-M mRNA expression in the infarcted myocardium showed a biphasic response: significant upregulation was noted after 6h of reperfusion followed by a second peak after 7 days of reperfusion (**p<0.01 versus sham). B–C. Dual immunofluorescence combining IRAK-M staining (red) and Mac-2 immunofluorescence (B, green) to identify macrophages, or α-SMA staining to label myofibroblasts and smooth muscle cells (C, green). Dual immunofluorescent staining localized IRAK-M (red) in Mac2+ macrophages (B- arrows) and spindle-shaped α-smooth muscle actin+ myofibroblasts (C- arrows) infiltrating the infarcted myocardium (1h ischemia/7 days reperfusion). Counterstained with DAPI (blue). D-G. Fibroblasts (D) and macrophages (E) isolated from infarcted hearts 3 days after reperfusion expressed IRAK-M (green, arrows). IRAK-M immunofluorescence in fibroblasts (F) and macrophages (G) isolated from IRAK-M null infarcts served as a negative control. Cells were counterstained with DAPI (blue). H–J. IRAK-M loss did not affect the size of the infarct after 1h ischemia and 24h of reperfusion. TTC/Evans Blue staining was used to measure the area at risk (AAR) and the infarcted area (INF) in the ischemic and reperfused heart. WT and IRAK-M null animals had comparable AAR (H), infarcted area (I) and INF:AAR ratio (J).
2. IRAK-M is localized in infarct macrophages and myofibroblasts
Dual immunofluorescence was used to study IRAK-M localization in the infarcted myocardium. IRAK-M immunoreactivity in the infarcted heart was localized in Mac2+ infarct macrophages and in spindle-shaped, α-smooth muscle actin-positive myofibroblasts (Figure 1B, C). Moreover, infarct myofibroblasts and CD11b+ leukocytes isolated from the infarcted heart after 72 h of reperfusion exhibited IRAK-M expression (Figure 1D–G). In order to study cell-type specific changes in the timing of IRAK-M expression we assessed IRAK-M mRNA levels in cardiac fibroblasts and CD11b+ leukocytes harvested from the infarcted heart. Isolated fibroblasts had a 3-fold increase in IRAK-M mRNA levels after 24h-72h of reperfusion in comparison to control cardiac fibroblasts. When compared to control CD11b+ cells harvested from normal hearts, leukocytes isolated after 6h of reperfusion showed a trend towards increased IRAK-M mRNA expression (supplemental figure I).
3. IRAK-M loss is associated with enhanced adverse remodeling despite the absence of effects on the size of the infarct
IRAK-M null and WT animals had comparable mortality following myocardial infarction (pNS). TTC/Evans Blue staining demonstrated that IRAK-M loss does not affect the size of the infarct after 1h of ischemia and 24h of reperfusion (Figure 1H–J). Two independent techniques, echocardiographic imaging (Figure 2A–G, supplemental table I) and quantitative morphometry (Figure 2H–L), demonstrated that IRAK-M loss was associated with enhanced adverse remodeling following myocardial infarction. Systolic and diastolic chamber dimensions measured through echocardiography (LVEDD, LVESD, LVESV and LVEDV) (Figure 2A–G) and morphometrically-derived LVEDV and LVEDD (Figure 2H–L) were significantly higher in IRAK-M null mice after 7 and 28 days of reperfusion indicating increased chamber dilation. Left ventricular mass was also significantly higher in infarcted IRAK-M null hearts suggesting accentuated hypertrophic remodeling. Increased adverse remodeling in the absence of IRAK-M was associated with reduced fractional shortening reflecting worse systolic dysfunction (Figure 2D). Because acute infarct size was comparable between WT and IRAK-M null mice (Figure 1H–J), accentuated adverse remodeling in IRAK-M null hearts was not due to more extensive cardiomyocyte injury. Moreover, scar size after 7–28 days of reperfusion was comparable between IRAK-M −/− and WT animals (Figure 2I).
Figure 2.
Echocardiography (A–G) and quantitative morphometry (H–L) demonstrate that IRAK-M absence results in accentuated dilative remodeling and worse systolic dysfunction following reperfused myocardial infarction. A. Representative images of long axis B-mode and short axis M-mode echocardiography in WT and IRAK-M KO mice at baseline and after 7–28 days of reperfusion. B–G: IRAK-M null mice had worse systolic dysfunction and accentuated remodeling of the infarcted heart when compared with WT animals. Quantitative analysis of echocardiographic parameters demonstrated that IRAK-M null hearts had increased chamber dilation (indicated by higher LVEDD, LVESD, LVEDV and LVESV; B, C, E, F), worse systolic dysfunction (indicated by lower FS, D) and accentuated hypertrophy (evidenced by higher LV mass, G) after 7–28 days of reperfusion (**p<0.01, *p<0.05 vs. corresponding WT). H. Quantitative morphometric analysis demonstrates that IRAK-M disruption is associated with increased post-infarction remodeling. Hearts were perfusion-fixed and systematic morphometric analysis of the geometry of the left ventricle was performed through assessment of sections cut at 250µm intervals from base to apex. Representative images show cross-sections of WT and IRAK-M null sham and infarcted hearts (after 7 and 28 days of reperfusion). I-L: Quantitative analysis demonstrated that, although scar size was comparable between IRAK-M null and WT animals after 7–28 days of reperfusion (B), IRAK-M −/− mice had increased LVEDD and LVEDV and higher LV mass when compared with WT animals (**p<0.01, *p<0.05 vs. corresponding WT mice).
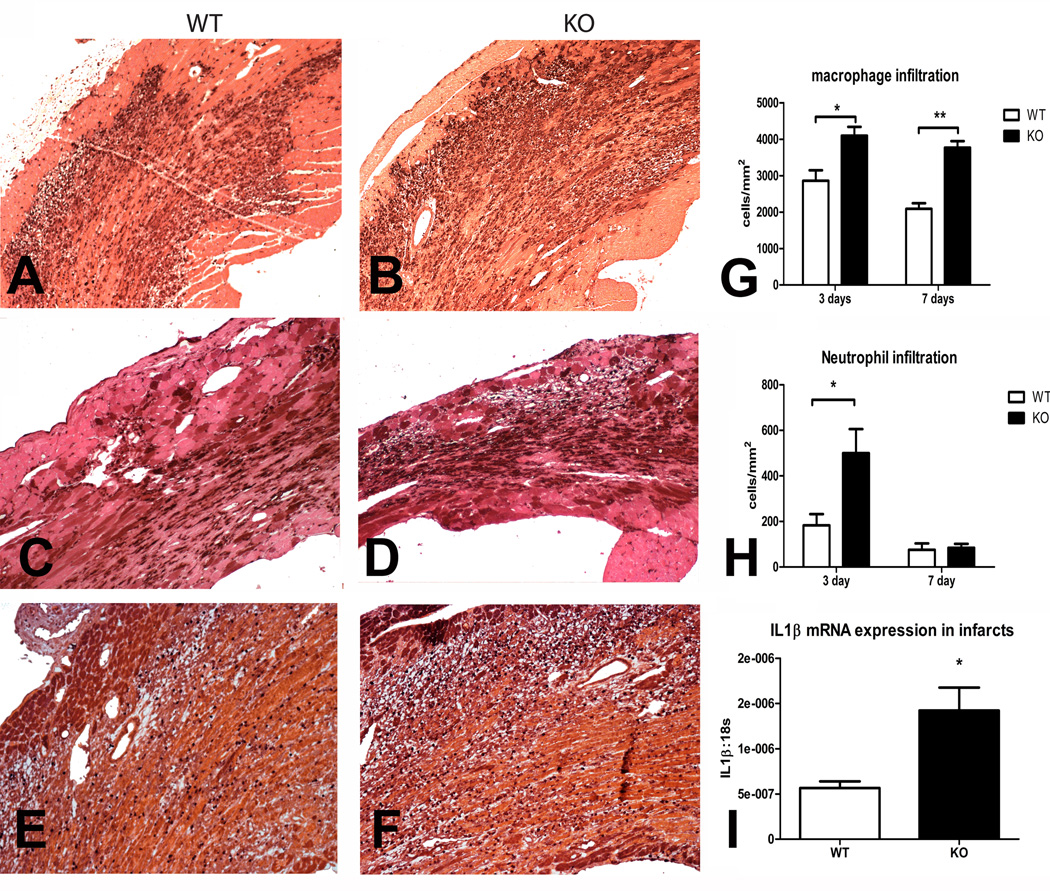
5. IRAK-M −/− mice have enhanced post-infarction inflammation exhibiting increased myocardial cytokine mRNA expression and accentuated macrophage and neutrophil infiltration
Increased chamber dilation in infarcted IRAK-M null hearts was associated with an accentuated inflammatory response. Immunohistochemical staining demonstrated that IRAK-M null infarcts had enhanced macrophage and neutrophil infiltration after 3–7 days of reperfusion (Figure 3). Moreover, myocardial IL-1β mRNA upregulation was markedly accentuated in IRAK-M null infarcts (Figure 3I).
Figure 3.
IRAK-M null mice exhibit an accentuated inflammatory response following myocardial infarction. A–D. Representative images show identification of macrophages in the infarcted myocardium using Mac-2 immunohistochemistry after 72h (A–B) and 7 days of reperfusion (C–D) E–F: Representative images show immunohistochemical staining with an anti-neutrophil antibody after 72h of reperfusion. G: Quantitative analysis showed that macrophage density was significantly higher in IRAK-M null infarcts (**p<0.01, *p<0.05 vs. corresponding WT). H: Neutrophil density was markedly higher in IRAK-M null infarcts after 72h of reperfusion; however, resolution of the neutrophil infiltrate occurred in a timely manner in both groups. I: IL-1β mRNA levels were markedly higher in IRAK-M null infarcts suggesting accentuation of the inflammatory response. All sections were counterstained with eosin.
6. IRAK-M −/− mice have increased MMP expression and activity in the infarcted heart associated with reduced collagen deposition in the healing scar
Adverse dilative remodeling in IRAK-M deficient mice was accompanied by enhanced MMP expression and activity. qPCR analysis demonstrated that myocardial MMP-3, MMP-8 and MMP-9 mRNA levels were significantly higher in IRAK-M null infarcts when compared with infarcted WT hearts after 24h of reperfusion (Figure 4A–D). MMP-2 mRNA expression levels were also 50% higher in IRAK-M null infarcts; however, the difference in comparison to WT infarcts did not reach statistical significance (p=0.28). Myocardial TIMP-1 and -2 mRNA expression was also higher in infarcted IRAK-M null animals (Figure 4E–F). Gelatin zymography demonstrated that IRAK-M null infarcts had significantly higher latent and active MMP-2 levels when compared with WT animals after 72h of reperfusion (Figure 4H). A trend towards increased active MMP-9 levels was noted in IRAK-M null animals (p=0.19). Accentuated activation of MMPs in IRAK-M −/− infarcts resulted in a marked reduction in collagen deposition in the healing scar (Figure 4I–J).
Figure 4.
Adverse remodeling in infarcted IRAK-M−/− hearts is associated with increased MMP mRNA expression and enhanced protease activity. A–F: qPCR demonstrated increased expression of MMPs (A, MMP-2; B, MMP-3; C, MMP-8; D, MMP-9) and TIMPs (E, TIMP-1; F, TIMP-2) in IRAK-M null infarcts after 24h of reperfusion. G: Zymography was used to quantitatively assess MMP activity in the infarcted myocardium after 72h of reperfusion. H: Quantitative analysis demonstrated that levels of active MMP-2 were markedly higher in IRAK-M null infarcts (**p<0.01 vs. WT). A trend towards increased MMP-9 activity was also noted (p=0.19). I-J: Picrosirius red staining was used to assess collagen deposition in the scar (1hI /7 days R). Increased MMP activity in IRAK-M −/− mice was associated with less collagen deposition. (**p<0.01, *p<0.05 vs. corresponding WT).
7. Cytokine-stimulated IRAK-M expression in cardiac fibroblasts
Because myofibroblasts are an important source of IRAK-M in the healing infarct (Figure 1), we explored the mechanisms of IRAK-M regulation in mouse cardiac fibroblasts. Stimulation with IL-1β, TNF-α and PDGF-BB, cytokines and growth factors released in the infarcted myocardium, upregulated IRAK-M synthesis in WT mouse cardiac fibroblasts (Figure 5A). Moreover, the TLR agonist lipopolysaccharide (LPS) induced marked IRAK-M upregulation in cardiac fibroblasts. IRAK-M null cells were used as a negative control and exhibited negligible IRAK-M mRNA expression.
Figure 5.
A. IRAK-M regulation in stimulated cardiac fibroblasts. IL-1β, the TLR agonist LPS, TNF-α, and PDGF-BB upregulated IRAK-M mRNA expression in cardiac fibroblasts (**p<0.01, *p<0.05 vs. control). mRNA isolated from IRAK-M −/− fibroblasts was used as a negative control. B–C. IRAK-M loss enhances cytokine-stimulated MMP expression, but does not affect inflammatory cytokine synthesis in isolated cardiac fibroblasts. B. IRAK-M −/− fibroblasts exhibited increased MMP-2 and MMP-8 expression upon stimulation with IL-1β, when compared with WT fibroblasts. In contrast, MMP-3 and TIMP-1 expression was comparable between groups. C. IL-1β-mediated upregulation of TNF-α and MCP-1 was comparable between WT and IRAK-M null fibroblasts. IRAK-M null fibroblasts had reduced IL-6 upregulation upon IL-1β stimulation.
8. IRAK-M expression limits the matrix-degrading potential, but not the pro-inflammatory capacity, of IL-1β-stimulated cardiac fibroblasts
In order to examine whether the effects of IRAK-M loss on cardiac remodeling are due to defective regulation of fibroblast inflammatory and matrix-degrading capacity, we compared baseline and IL-1-β-stimulated expression of MMPs and inflammatory mediators between IRAK-M null and WT cardiac fibroblasts (Figure 5). Upon IL-1β stimulation MMP-2 and MMP-8 mRNA expression was significantly higher in IRAK-M null cardiac fibroblasts, when compared with WT cells (Figure 5B). Moreover, the supernatant collected from IRAK-M null cells exhibited accentuated baseline MMP-2 activity in comparison to the supernatant obtained from WT cells (supplemental figure II). In contrast, IRAK-M loss was not associated with accentuation of fibroblast inflammatory gene synthesis. Baseline and IL-1β-stimulated TNF-α and MCP-1 mRNA expression was comparable between IRAK-M null and WT fibroblasts, whereas IL-1-induced IL-6 mRNA expression was significantly lower in IRAK-M null cells (Figure 5C). Because IRAK-M may act by stabilizing MAPK Phosphatase (MKP)-1, thus negatively regulating TLR2-induced p38 MAPK phosphorylation 19, we compared the effects of IRAK-M loss on p38 MAPK phosphorylation in stimulated mouse cardiac fibroblasts. IL-1β and LPS-stimulated cardiac fibroblasts exhibited comparable activation of p38 MAPK signaling in response to IL-1β and LPS (supplemental figure III).
9. IRAK-M absence is associated with increased leukocyte inflammatory activity and enhanced recruitment of pro-inflammatory Ly6Chi monocytes
In order to examine whether accentuated inflammation in IRAK-M null hearts is due to uncontrolled inflammatory activity in macrophages we used flow cytometry to assess phenotype and cytokine expression in monocytic cells harvested from the infarcted myocardium. After 72h of reperfusion, the absolute number of CD45+ leukocytes and of CD45+/CD11b+ monocytes per weight of infarcted myocardium was markedly higher in IRAK-M null animals when compared to WT mice (Figure 6). Moreover, the number of IL-1β+ cells, and the number of CD45+/CD11b+ monocytes expressing IL-1β in the infarcted heart was markedly higher in IRAK-M null mice when compared to WT animals. IRAK-M loss was associated with selectively increased infiltration of the infarcted myocardium with pro-inflammatory Ly6C+ monocytes; in contrast, the number of reparative Ly6Clo monocytes was comparable between IRAK-M −/− and WT infarcts (Figure 6, supplemental table II).
Figure 6.
IRAK-M loss is associated with infiltration of the infarcted myocardium with pro-inflammatory monocytes. Flow cytometric analysis of cell suspensions harvested from the infarcted heart after 72h of reperfusion demonstrated marked increases in the number of infiltrating mononuclear cells and IL1β-positive cells. Cell suspensions from infarcted hearts of C57BL/6 and IRAK-M null mice were stained with LIVE/DEAD® Fixable Dead Cell Stain, -CD45, -CD11b, -Ly6C, -F4/80 and -IL1β mAbs. A–B: Representative dot plots show significantly higher number of live CD45+ hematopoietic cells (A), CD11b+/F4/80- monocytes (gate 1) and CD11b+F4/80+ macrophages/dendritic cells (gate 2) (B) in IRAK-M−/− infarcts after 72hr of reperfusion. C: Representative dot plots show the percentage of IL1β positive cells in Ly6Chi and Ly6Clo monocytes. D: IL1β expression in Ly6Clo and Ly6Chi subgroups of monocytic cells is shown in representative histograms comparing findings from WT (blue) and IRAK-M null infarcts (red). E–L: Quantitative analysis of flow cytometric findings showed that IRAK-M −/− infarcts had markedly higher absolute number of CD45+ hematopoietic cells (E), CD45+/CD11b+ monocytic cells (F), Ly6Chi/CD45+/CD11b+ pro-inflammatory monocytes (G) and F4/80+ macrophages (H) per mg of weight of the infarcted heart. Monocytes and macrophage subpopulations harvested from IRAK-M −/− infarcts had increased expression of the pro-inflammatory cytokine IL-1β reflecting accentuated inflammatory activity. The number of IL-1β+/CD45+ hematopoietic cells (I), IL-1β+/CD45+/CD11b+ monocytic cells (J), IL-1β+/CD11b+/Ly6Chi pro-inflammatory monocytes (K), and IL-1β+/F4/80+ macrophages (L) was markedly higher in IRAK-M null infarcts (**p<0.01, *p<0.05 vs. corresponding WT animals).
DISCUSSION
Our study demonstrates for the first time the critical role of endogenous negative regulation of the innate immune response in preventing uncontrolled inflammation following ischemic injury and in protecting the infarcted myocardium from adverse remodeling. We found that upregulation of the endogenous suppressor of TLR/IL-1 signaling IRAK-M in macrophages and fibroblasts infiltrating the infarcted heart is an important inhibitory mechanism that limits chamber dilation protecting the heart from uncontrolled inflammation and excessive matrix degradation. Beyond the established role of IRAK-M in suppression of macrophage-derived inflammatory responses, our study reveals novel cellular actions of IRAK-M in inhibition of the matrix-degrading capacity of cytokine-stimulated fibroblasts.
TLR signaling is critically involved in the response to injury following myocardial infarction20. Generation of danger-associated molecular patterns (DAMPs)21, such as heat shock proteins22 and fibronectin fragments23 stimulates TLR signaling in the infarcted heart. TLR-2 activation in infarct leukocytes accentuates inflammatory injury following myocardial infarction9 and promotes cardiac fibrosis and adverse remodeling24, whereas TLR-4 signaling exerts pro-inflammatory actions stimulating lymphocyte infiltration and enhancing chamber dilation10. Activation of cell surface TLRs recruits the adaptor molecule myeloid differentiation factor 88 (MyD88)10; subsequent binding of members of the IRAK family induces pro-inflammatory pathways in the infarcted heart. Studies using genetically targeted animals suggested an important role for IRAK-4 in mediating the post-infarction inflammatory response25. Moreover, rapid activation of IRAK-1 in the ischemic26 and infarcted heart27 plays an important role in mediating inflammatory and apoptotic responses. Although the innate immune response is important for recognition of ischemic injury and for subsequent recruitment of inflammatory leukocytes in the infarcted myocardium, tight regulation of the TLR cascade is needed to prevent uncontrolled inflammation and excessive matrix degradation.
Both in vivo and in vitro studies support the role of IRAK-M in negative regulation of TLR-dependent responses. In contrast to IRAK-1 and IRAK-4, IRAK-M lacks kinase activity, but functions as a decoy, inhibiting TLR/IL-1-driven pro-inflammatory signaling. IRAK-M null mice exhibit accentuated inflammatory responses following bacterial and viral infections13,15,28. In vitro, IRAK-M deficient macrophages display enhanced activation of IL-1/TLR signaling14,28. Several distinct mechanisms have been suggested to explain the inhibitory effects of IRAK-M on the TLR response. First, IRAK-M is thought to bind MyD88/IRAK-4 inhibiting IRAK-4 phosphorylation of IRAK-129. These interactions prevent IRAK-1-mediated activation of IκB kinase and Mitogen Activated Protein Kinase (MAPK) inhibiting NF-κB and AP-1 signaling13. Second, IRAK-M may act by preventing IRAK-1 from dissociating from the MyD88 complex, thus inhibiting NF-κB activation. Third, IRAK-M may exert IRAK-1-independent actions by stabilizing MAPK Phosphatase (MKP)-1, thus negatively regulating TLR2-induced p38 phosphorylation19.
Consistent with a critical role for IRAK-M in regulation of the inflammatory response, IRAK-M loss was associated with increased cytokine expression in the infarcted heart, leading to enhanced infiltration of the infarcted myocardium with inflammatory leukocytes (Figure 3) and accentuated MMP expression and activity (Figure 4). Defective negative regulation of the inflammatory response did not affect the extent of cardiomyocyte injury following reperfused infarction. However, enhanced inflammation and protease activity in IRAK-M null infarcts resulted in worse geometric remodeling of the infarcted ventricle and increased systolic dysfunction. Endogenous IRAK-M upregulation functions as a key molecular signal in cardiac repair protecting the infarcted heart from uncontrolled inflammation and excessive matrix degradation.
Although traditionally viewed as a macrophage specific product, IRAK-M expression is increasingly identified in other cell types. In patients with early-onset persistent asthma high level IRAK-M expression has been demonstrated in pulmonary epithelial cells30. In mice the pattern of IRAK-M expression seems broader, as IRAK-M synthesis has been demonstrated in murine neutrophils31, NIH 3T3 fibroblasts32, B cells33, dendritic cells34 and various types of epithelial cells35. However, in vivo biological actions of IRAK-M appear to involve predominantly inhibitory effects on monocytes/macrophages. Our study demonstrates for the first time that effects of IRAK-M in the infarcted heart involve actions in both macrophages and cardiac fibroblasts.
Cardiac fibroblasts expressed significant amounts of IRAK-M both in vivo and in vitro (Figures 1, 5). A wide range of stimuli, including the TLR agonist lipopolysaccharide, the pro-inflammatory cytokine IL-1β and the fibrogenic growth factors TGF-β and PDGF enhanced IRAK-M expression in cardiac fibroblasts (Figure 5). IRAK-M loss did not affect the inflammatory potential of cardiac fibroblasts (Figure 5C). However, IRAK-M expression played an important role in restraining the matrix-degrading potential of cardiac fibroblasts; in its absence cytokine-induced fibroblast MMP synthesis was markedly accentuated (Figure 5B). Thus, our observations suggest a broader role for IRAK-M in matrix remodeling and tissue repair.
In addition to its effects on cardiac fibroblasts, IRAK-M modulated mononuclear cell phenotype and function. Using flow cytometric analysis of cells harvested from the infarcted heart we found that IRAK-M deficiency had profound effects on the phenotype and inflammatory activity of monocytes infiltrating the infarcted myocardium. IRAK-M null infarcts exhibited a markedly higher number of IL-1β-expressing CD45+/CD11b+ leukocytes. Enhanced IL-1β expression by IRAK-M null infarct monocytes likely reflects their increased capacity to synthesize pro-inflammatory cytokines upon stimulation with TLR ligands in comparison to wildtype cells13. Moreover, IRAK-M −/− infarcts exhibited alterations in the profile of monocyte subsets recruited in the infarcted myocardium 36. IRAK-M absence was associated with an abundance of pro-inflammatory Ly6Chi monocytes in the infarct (Table 2, Figure 6). The increased proportion of inflammatory monocytes in IRAK-M null infarcts may simply reflect a global accentuation of inflammatory activity leading to increased activation of chemokine-mediated pathways responsible for recruitment of pro-inflammatory cells 37. In addition, because TLR signaling plays an important role in inflammatory monopoiesis 38, IRAK-M expression may selectively inhibit generation and release of pro-inflammatory Ly6Chi cells.
Our findings have important therapeutic implications. More than twenty years ago, extensive experimental evidence suggested that inflammatory mediators and infiltrating leukocytes may induce death of surviving cardiomyocytes in the infarcted myocardium extending ischemic myocardial injury 39. Unfortunately, the effectiveness of anti-inflammatory strategies in reducing infarct size in large animal models of reperfused infarction did not translate into clinical success; both anti-integrin approaches and complement inhibition failed to reduce acute myocardial injury in patients with acute myocardial infarction 40. Our experimental findings are consistent with these clinical observations suggesting limited effects of the inflammatory reaction on cardiomyocyte survival. IRAK-M absence resulted in accentuated inflammation without affecting the size of the infarct (Figure 1). Moreover, marked attenuation of the post-infarction inflammatory response due to disruption of IL-1 signaling 11, or loss of adhesion molecule-mediated interactions 41, had no effect on the size of the infarct. If inhibition of inflammation does not protect ischemic cardiomyocytes, is there a future for strategies modulating the inflammatory reaction following myocardial infarction? Our findings underscore the importance of negative regulators of the innate immune response in protection of the infarcted ventricle from adverse remodeling. Enhanced, or prolonged, innate immune signaling in the infarcted myocardium may not acutely increase the extent of ischemic injury, but is associated with defective suppression of the inflammatory response and uncontrolled activation of proteases. Excessive protease-mediated matrix degradation induces chamber dilation, promoting systolic dysfunction. In patients surviving an acute myocardial infarction the extent of dilative remodeling may reflect, at least in part, their ability to control the inflammatory reaction. Genetic defects, or pathologic conditions, that disrupt key endogenous regulatory signals (such as IRAK-M) may be responsible for worse remodeling leading to rapid development of heart failure. In this subpopulation of patients, anti-inflammatory strategies may attenuate chamber dilation, protecting from progression of heart failure.
Supplementary Material
ACKNOWLEDGMENTS
SOURCES OF FUNDING: Supported by National Institutes of Health grants R01 HL76246 and HL85440 and the Wilf Family Cardiovascular Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None.
REFERENCES
- 1.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Pfeffer JM. Ventricular enlargement and reduced survival after myocardial infarction. Circulation. 1987;75:IV93–IV97. [PubMed] [Google Scholar]
- 5.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 6.St John Sutton M, Lee D, Rouleau JL, Goldman S, Plappert T, Braunwald E, Pfeffer MA. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003;107:2577–2582. doi: 10.1161/01.CIR.0000070420.51787.A8. [DOI] [PubMed] [Google Scholar]
- 7.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 8.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 10.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D, Pauschinger M, Schultheiss HP, Tschope C. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–6961. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 11.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 14.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, Kunkel SL, Standiford TJ. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J Immunol. 2010;184:1410–1418. doi: 10.4049/jimmunol.0901709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 19.Su J, Xie Q, Wilson I, Li L. Differential regulation and role of interleukin-1 receptor associated kinase-M in innate immunity signaling. Cell Signal. 2007;19:1596–1601. doi: 10.1016/j.cellsig.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Gardner SE, Clarke MC. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–2786. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105:1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 24.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 25.Maekawa Y, Mizue N, Chan A, Shi Y, Liu Y, Dawood S, Chen M, Dawood F, de Couto G, Li GH, Suzuki N, Yeh WC, Gramolini A, Medin JA, Liu PP. Survival and cardiac remodeling after myocardial infarction are critically dependent on the host innate immune interleukin-1 receptor-associated kinase-4 signaling: a regulator of bone marrow-derived dendritic cells. Circulation. 2009;120:1401–1414. doi: 10.1161/CIRCULATIONAHA.109.865956. [DOI] [PubMed] [Google Scholar]
- 26.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem. 2005;280:21997–22005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial Ischemia Activates an Injurious Innate Immune Signaling via Cardiac Heat Shock Protein 60 and Toll-like Receptor 4. J Biol Chem. 2011;286:31308–31319. doi: 10.1074/jbc.M111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard LL, Moore BB. IRAK-M regulation and function in host defense and immune homeostasis. Infect Dis Rep. 2010;2:pii, e9. doi: 10.4081/idr.2010.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D, Uda M, Pilia S, Sanna S, Masala M, Crisponi L, Fattori M, Devoto M, Doratiotto S, Rassu S, Mereu S, Giua E, Cadeddu NG, Atzeni R, Pelosi U, Corrias A, Perra R, Torrazza PL, Pirina P, Ginesu F, Marcias S, Schintu MG, Del Giacco GS, Manconi PE, Malerba G, Bisognin A, Trabetti E, Boner A, Pescollderungg L, Pignatti PF, Schlessinger D, Cao A, Pilia G. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007;80:1103–1114. doi: 10.1086/518259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard LL, Ballinger MN, Thomas PE, Wilke CA, Standiford TJ, Kobayashi KS, Flavell RA, Moore BB. A role for IL-1 receptor-associated kinase-M in prostaglandin E2-induced immunosuppression post-bone marrow transplantation. J Immunol. 2010;184:6299–6308. doi: 10.4049/jimmunol.0902828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosati O, Martin MU. Identification and characterization of murine IRAK-M. Biochem Biophys Res Commun. 2002;293:1472–1477. doi: 10.1016/S0006-291X(02)00411-4. [DOI] [PubMed] [Google Scholar]
- 33.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnis ME, Song XT, Bear A, Foster AE, Gottschalk S, Brenner MK, Chen SY, Rooney CM. IRAK-M removal counteracts dendritic cell vaccine deficits in migration and longevity. J Immunol. 2010;185:4223–4232. doi: 10.4049/jimmunol.0903507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshima N, Ishihara S, Rumi MA, Aziz MM, Mishima Y, Kadota C, Moriyama I, Ishimura N, Amano Y, Kinoshita Y. A20 is an early responding negative regulator of Toll-like receptor 5 signalling in intestinal epithelial cells during inflammation. Clin Exp Immunol. 2010;159:185–198. doi: 10.1111/j.1365-2249.2009.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 38.Serbina NV, Hohl TM, Cherny M, Pamer EG. Selective expansion of the monocytic lineage directed by bacterial infection. J Immunol. 2009;183:1900–1910. doi: 10.4049/jimmunol.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilgore KS, Lucchesi BR. Reperfusion injury after myocardial infarction: the role of free radicals and the inflammatory response. Clin Biochem. 1993;26:359–370. doi: 10.1016/0009-9120(93)90112-j. [DOI] [PubMed] [Google Scholar]
- 40.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 41.Briaud SA, Ding ZM, Michael LH, Entman ML, Daniel S, Ballantyne CM. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol. 2001;280:H60–H67. doi: 10.1152/ajpheart.2001.280.1.H60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.