Abstract
The neuropilin (Nrp) family are essential multifunctional vertebrate cell surface receptors. Nrps were initially characterized as receptors for class III Semaphorin (Sema3) family members, functioning in axon guidance. Nrps have also been shown to be critical for Vascular Endothelial Growth Factor (VEGF) dependent angiogenesis. Intriguingly, recent data show that Nrp function in these seemingly divergent pathways is critically determined by ligand-mediated cross-talk, which underlies Nrp function in both physiological and pathological processes. In addition to functioning in these two pathways, Nrps have been shown to specifically function in a number of other fundamental signaling pathways as well. Multiple general mechanisms have been found to directly contribute to the pleiotropic function of Nrp. Here we review critical general features of Nrps function as essential receptors integrating multiple molecular cues into diverse cellular signaling.
Nrp Architecture
Nrp was first identified in the optic tectum of Xenopus and referred to as A5-antigen (1, 2). There are two conserved Nrp family members in vertebrates, Nrp1 and Nrp2, which share the same overall domain structure and are 44% identical at the amino acid level (3, 4). Nrp family members are type I transmembrane proteins that possess an N-terminal signal peptide, two calcium-binding C1r/C1s/Uegf/Bmp1 (CUB) domains (a1a2) (5), two coagulation factor V/VIII-like discoidin domains (b1b2) (6, 7), a Meprin/A5-antigen/ptp-Mu (MAM) domain (c), a single transmembrane helix, and a short intracellular domain. The N-terminal four extracellular domains are necessary and sufficient for ligand binding. The a1 domain has been shown to interact with the sema domain of Sema3 ligands (8). The b1 domain contains a specific C-terminal arginine binding pocket and is essential for binding to the C-terminal domain of both VEGF and Sema3 ligands (9–14). Additionally, the b1 and b2 domains physically interact to form an extended patch of basic residues that is involved in binding heparin, a highly sulfated member of the glycosaminoglycan (GAG) family of carbohydrates (10). The c domain is dispensable for ligand binding but essential for ligand-dependent signaling (15). The transmembrane helix possesses a conserved GXXXG repeat and shows strong inherent dimerization potential (16). The intracellular domain of Nrp interacts with PSD-95/Dlg/ZO-1 (PDZ)-domain proteins, such as GAIP Interacting Protein, C-terminus (GIPC), via its C-terminal residues (17). Deletion of the Nrp1 intracellular domain does not result in embryonic lethality, as seen with the whole gene, but instead causes defects in vascular patterning (18).
There are alternative splice forms of Nrp1 and Nrp2 that have been shown to have functional implications. The most commonly identified alternative splice forms of both Nrp1 and Nrp2 result from intron inclusion in the region between b2 and c domains (19, 20). The included intron contains a stop-codon resulting in production of secreted soluble proteins that preserve ligand binding and thus function as endogenous pathway inhibitors. Alternative splicing of Nrp2 also produces two species with divergent C-terminal intracellular domains. While multiple splice forms of both Nrp receptors exist, the functional significance of these divergent sequences is largely unknown. In addition to splice variants, there are a number of post-translational modifications that alter Nrp function. Nrp1 and Nrp2 can be modified by both N- and O-linked glycans. Nrp1 has been shown to be modified by heparan- or chondroitin-sulfate which results in increased ligand binding (21). Thus, this modification may poise or preactivate Nrp for signaling. In contrast, Nrp2 is modified by polysialic acid resulting in enhanced dendritic cell migration (22, 23).
Nrp Function in Semaphorin Dependent Axon Guidance
Nrp was initially identified and functionally characterized as a receptor for Sema3 family ligands in neurons. Neurons function in cooperative neural networks connected by dendrites and axons. The number and type of axonal connections are tightly regulated by guidance cues. One family of essential axon guidance molecules are the Semaphorins. There are seven Sema3 family members (Sema3A-G) that are secreted, diffuse through tissues, and provide guidance cues (24). Indeed, axon guidance cues mediated by Sema3 family members are essential during development for correct neuronal patterning in dorsal root ganglia, facial, vagal, olfactorysensory, cortical, hippocampal, and cerebellar nerves, along with others (25).
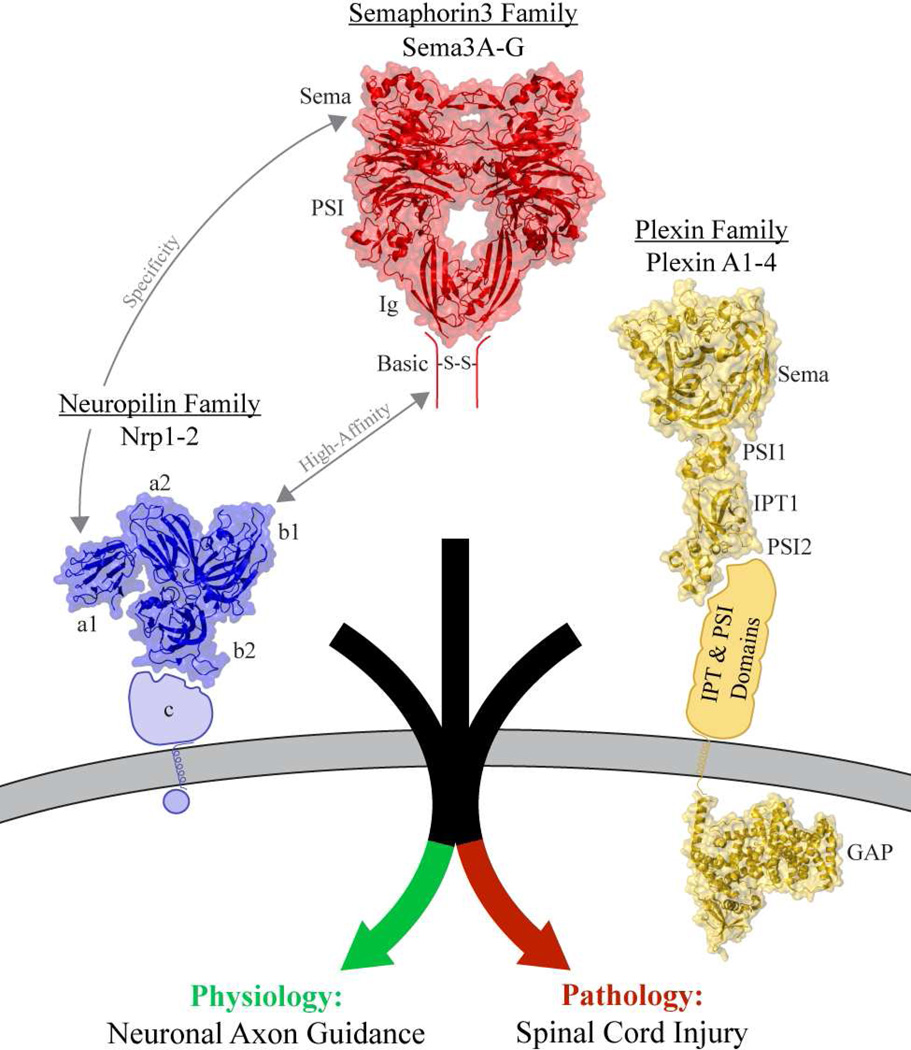
Sema3 family members bind Nrp receptors on the cell surface (3, 26) (Figure 1). The N-terminal a1 domain of Nrp1 and Nrp2 selectively binds the sema domain of different Sema3 family members (27–29). Notably, Sema3A specifically signals via Nrp1 (3, 26) and Sema3F via Nrp2 (30). The Nrp b1 domain allows high-affinity non-selective binding to the Sema3 C-terminal domain (13, 15, 30). Following Sema3 binding by Nrp, Plexin family signaling receptors (PlexinA1-4) are then engaged and activated to directly guide axonal growth (31, 32). Nrp functions by coupling specific high-affinity Sema3 binding to Plexin-dependent regulation of cytoskeleton dynamics in the axonal growth cone (31). Collapse of the actin cytoskeleton results in axon repulsion and this appears to represent the most common modality for Nrp-dependent Sema3 function. However, Sema3 signaling can also be attractive rather than repulsive, such as when cGMP levels are high (33).
Figure 1.
Nrp function is essential for Sema3 dependent axon guidance. Nrps specifically bind to Sema3 family members through a bivalent binding mechanism allowing both specific and high-affinity binding. Engagement and activation of Plexin family receptors activates signaling in both physiological axon guidance and pathological spinal cord injury. Structures of proteins or homologues with known structures were utilized including: Semaphorin N-terminus: PDB=1OLZ (140); Neuropilin N-terminal domains: PDB=2QQK (109); Plexin extracellular domain N-terminus: PDB=3OKT (141); Plexin intracellular domain: PDB=3IG3 (142).
Nrp in Spinal Cord Injury
Nrp function in Sema3 signaling is important not only for physiological axon guidance, but also for signaling in spinal cord injury (34, 35) (Figure 1). Following spinal cord injury, a glial scar forms to stabilize and seal the wound. However, the glial scar also serves as a barrier to regenerating axons due to production of axon repulsion molecules. Sema3 expression significantly contributes to the inhibitory nature of the glial scar and so inhibitors of Nrp-Sema3 signaling hold promise as therapeutics for treatment of spinal cord injury (36, 37).
Nrp Function in VEGF Dependent Angiogenesis
Angiogenesis is the process by which new vasculature is formed by branching or splitting from existing vasculature. Physiological angiogenesis is critical during development, for homeostatic maintenance of vasculature, and wound healing. Among the most potent proangiogenic cytokine families is the VEGF family. VEGF-A was the first identified family member (38, 39), which also includes VEGF-B, -C, D, and placental growth factor (PlGF) (40). The different family members have both unique and partially overlapping functions. VEGF-A, - B and PlGF regulate hemangiogenesis by Nrp1 (41–43). VEGF-C and -D regulate lymphangiogenesis by Nrp2 (44). Also, VEGF-B has recently been shown to control lipid transport in endothelial cells by Nrp1 (45). To add to the complexity, VEGF-A, -B, and PlGF all have numerous alternative splice forms with varying physical and functional properties (46, 47) whereas VEGF-C and -D require proteolytic processing from a pre-protein for activation (48). Nrp1 was initially identified as a VEGF-A165 splice-form specific receptor (41). More recent work has demonstrated that Nrp1 can also bind other VEGF-A isoforms (49, 50) yet uniquely and specifically physically engages VEGF-A165 (14).
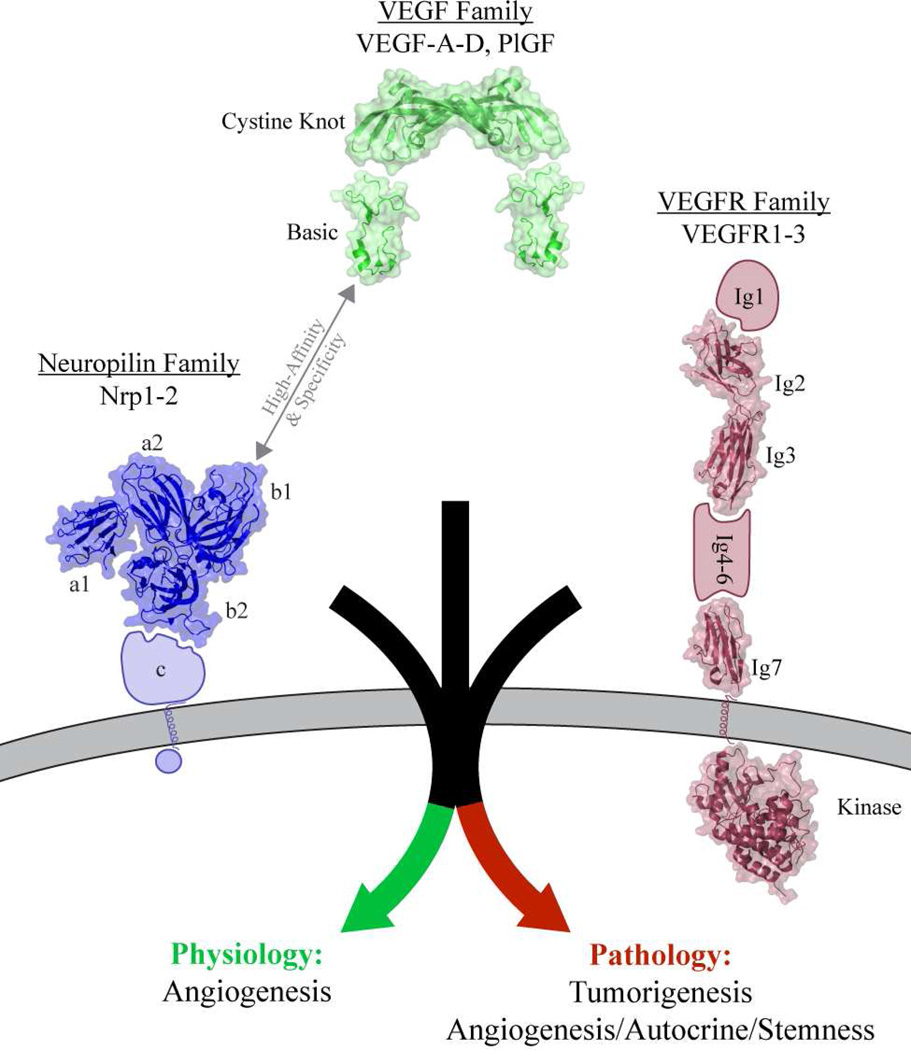
VEGF-A is secreted by an initiating tissue, typically in response to hypoxia, and diffuses to nearby vessels where it binds to two families of essential endothelial cell-surface receptors: VEGFR receptor tyrosine kinases (40) and Nrps (41, 43) (Figure 2). VEGF-A binding causes initiation of the angiogenic cascade, which involves activation of endothelial cells, basement membrane remodeling, proliferation of endothelial cells, and directional guidance leading to the growth of new vasculature towards the initiating tissue. Importantly, angiogenic signaling is only accomplished through the coordinated activity of VEGF, VEGFR, and Nrp (51). While VEGF binding to VEGFR weakly activates its intracellular kinase activity, Nrp is required for strong and sustained kinase activation leading to initiation of the pro-angiogenic cascade (52, 53). Nrp1 null mice die in utero due to defective vasculature formation, thus emphasizing the critical role of Nrp in angiogenesis (54). Importantly, abnormalities associated with loss of Nrp1 are caused by defective endothelial cell migration rather than proliferation (55).
Figure 2.
Nrp function is essential for VEGF dependent angiogenesis. Nrps specifically bind to the C-terminal basic domain of VEGF family members. Cooperative binding of VEGF by VEGFR and Nrp activates the angiogenic cascade necessary for developmental and homeostatic angiogenesis and also pathological signaling associated with tumorigenesis and other types of aberrant signaling. Structures of proteins with known structures were utilized including: VEGF-A cystine knot domain: PDB=2VPF (143); VEGF-A basic domain: PDB=4DEQ (14); Neuropilin N-terminal domains: PDB=2QQK (109); VEGFR2 Ig-like domain 2–3: PDB=2X1X (144); VEGFR2 Ig-like domain 7: PDB=3KVQ (137); VEGFR2 intracellular domain: PDB=1VR2 (145).
Ligand Mediated Cross-Talk
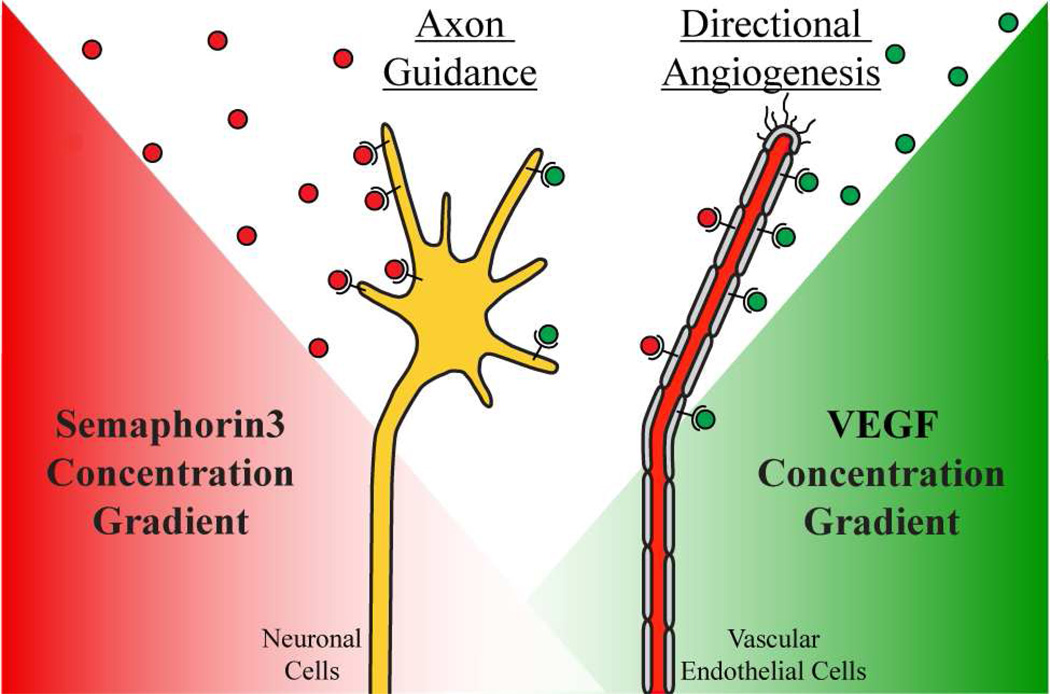
Intriguingly, it has been noted that blood vessels and nerves utilize similar signals and principles for guidance (56–58). Indeed, recent studies have identified an embryonic stem cell-derived population of cells that differentiate towards either vascular or neuronal cell-type depending on the microenvironment (59). Nrp receptors are expressed in nerves and endothelial cells, and both cell types are responsive to secreted ligands of the Sema3 and VEGF families (60–62). Not only are both cell types responsive to each family of ligands, but the different ligands can compete for Nrp binding (Figure 3). As previously discussed, the b1 domain of Nrp is responsible for binding the C-terminal domain of both VEGF and Sema3. Three coagulation-factor loops of the b1 domain form a conserved binding pocket specific for C-terminal arginine containing ligands (10, 14, 63). All VEGF family members possess a C-terminal arginine, which is necessary for binding to Nrp. In contrast, no Sema3 family members possess a C-terminal arginine. However, all Sema3 family members contain furin-like protease RXXR consensus sites in their C-terminal domain (64). Indeed, Sema3F has been identified to possess potent anti-angiogenic activity (65, 66). This anti-angiogenic activity was shown to result from direct competition between Sema3F and VEGF-A for binding to their shared C-terminal arginine binding pocket in the Nrp b1 domain (13). The interplay of VEGF and Sema3 is important for diseases associated with angiogenesis. Aberrant hypo-vascularization was recently demonstrated in the retina where expression of Sema3A prevents revascularization of ischemic neural tissue (67). In contrast, aberrant hyper-vascularization is associated with VEGF upregulation or Sema3 downregulation in pathological angiogenesis of solid tumors (68–70).
Figure 3.
Cross-talk between Nrp ligands allows coordinated regulation of neuronal and vascular tissues. Both neurons and endothelial cells express Nrp which can respond to either Sema3 or VEGF family guidance cues. Regulation of competitive Nrp binding between different ligands allows for an additional level of dominant control of Nrp function.
Nrp in Tumor Angiogenesis
Nrp function is important not only for physiological angiogenesis but also in VEGF-dependent pathological hyper-vascularization in tumor angiogenesis (71). It has long been known that the extent of tumor vasculaturization is correlated to tumor size (72) and that diffusible factors are able to induce neovascularization in solid tumors (73). Anti-angiogenesis therapies (74) are now used against a range of solid tumors. Avastin, a monoclonal antibody targeting VEGF-A, has been among the most successful angiogenesis inhibitors (75). While providing significant benefit it has been noted that when patients relapse, those that received anti-angiogenesis treatment often have more aggressive and metastatic tumors (76, 77). Aberrant Nrp expression and function promotes tumorigenesis and metastasis in vivo in a variety of solid tumors (51, 78–80). Surprisingly, in addition to expression in tumor vasculature, direct Nrp overexpression in malignant cells has been widely reported.
Nrp Tumor Cell Expression and Autocrine Activation
The initial identification of Nrp as a receptor for VEGF noted its expression in both endothelial and tumor cells (41). Since then, aberrant Nrp expression in a wide variety of malignant cells has been observed (69, 81–84). The function of Nrp in tumor cells, and its contribution to tumorigenesis, is the source of considerable interest. Recently, Nrp expression was demonstrated to be critical for autocrine activation of tumor cells, influencing carcinoma survival (85), growth (86), and migration (87, 88). Nrp-dependent autocrine activation provides a basis for understanding why Nrp expression and function directly correlate to both tumor aggressiveness (89) and the metastatic potential of a variety of solid tumors (90, 91). Further, Nrp function in tumor cells has been tied to dedifferentiation and stemness (92). It has recently been shown that Nrp1 directly contributes to the self-renewal of cancer stem cells in skin cancer (93). Indeed, Nrp1 deletion blocked the ability of VEGF to promote cancer stem cell self-renewal. Further, an autocrine activation pathway involving Nrp1 was shown to contribute to stem-like cell viability and tumor growth in glioma (94). The multi-functional role for Nrp in tumor initiation and progression has motivated research focusing on developing novel Nrp inhibitory modalities.
General Modes of Nrp Action
Nrps are normally expressed in a variety of tissue types including the previously discussed nerves and vascular tissues along with immune and hematopoietic cells (95, 96). In addition to the critical role for Nrp in the Sema3 and VEGF pathways, interactions with a number of other ligands and receptors have been reported. Other ligands identified include members of the fibroblast growth factor (FGF) family, platelet-derived growth factor (PDGF), transforming growth factor β1 (TGF-β1), and hepatocyte growth factor/scatter factor (HGF/SF) functioning via receptors in the FGFR, PDGFR, TβRs and c-Met families, respectively (89, 97–104). In light of the diverse expression profile and functional interactions, determining the general mechanism(s) of Nrp action is critical to further our understanding of physiological and pathological functions of Nrp.
Nrp Functions by Physically Organizing the Full Signaling Complex
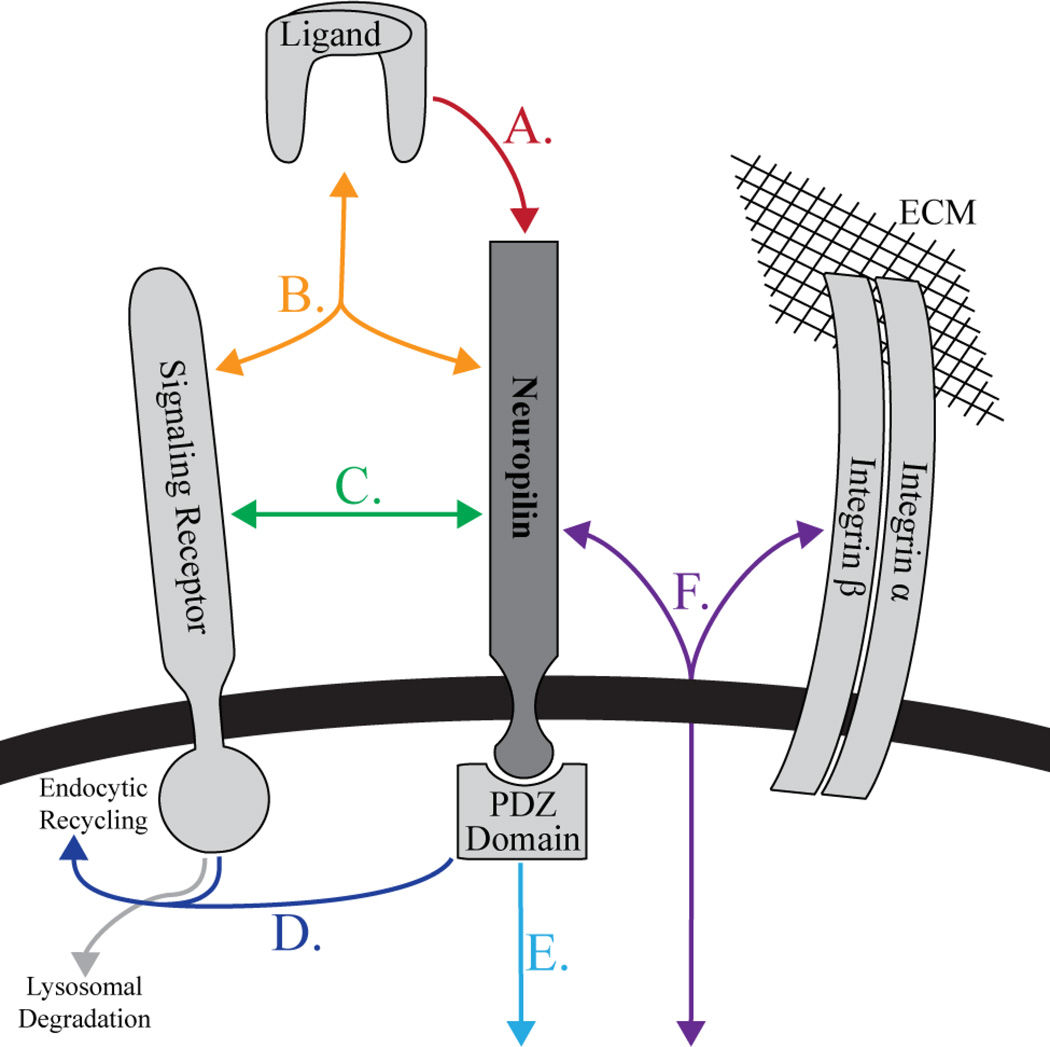
As a primary function, Nrps promote pathway activation by recruiting ligands to the cell surface through high-affinity interactions (Figure 4, A). This is particularly important in Sema3 binding, which is the only diffusible Sema family and requires Nrp for cell-surface binding (34). Additionally, Nrps show selective binding to different members within the VEGF and Sema3 families. The basis for specific Nrp1-VEGF-A interactions in hemangiogenesis has been described biochemically and structurally (14, 105), and other family members are actively being pursued.
Figure 4.
Nrp utilizes common mechanisms to regulate diverse signaling pathways. A) Specific high-affinity ligand binding initiates Nrp mediated signaling. B) Engagement and organization of an active signaling complex is accomplished through specific receptor-ligand and co-receptor contacts. C) Ligand-independent receptor association forms a pre-activated receptor complex poised for signaling. D) Nrp regulates dynamic trafficking of signaling complexes. E) Binding of intracellular PDZ-domain proteins, including GIPC, allow direct coupling between Nrp ligand binding and signaling functions. F) Direct engagement of integrins allows Nrp to couple molecular events with cellular cues.
Ligand binding is followed by assembly of the active signaling complex, where Nrp functions to promote and stabilize the active signaling holoenzyme (31, 52, 106) (Figure 4, B). Assembly of the active signaling complex requires both receptor recruitment and binding associated conformational changes, providing the physical mechanism required for signal transduction. Importantly, Nrp ligands are dimeric, with conserved intermolecular disulfide bonds. Thus, the minimum signaling holocomplex is thought to involve a dimeric ligand bound to two Nrp molecules and two signaling receptors. Energetically, it appears that highly favorable ligand binding drives the formation of specific homo- and hetero-typic receptor contacts that do not occur spontaneously (107, 108). This is consistent with optimal receptor function, with physical mechanisms strongly inhibiting spontaneous ligand-independent activation.
Understanding Nrp function in holocomplex assembly requires knowledge of the structure and interactions of the receptor both before and after ligand binding. The a2b1b2 domains of Nrp have been reported to adopt a stably associated trimeric core with loosely tethered a1 domain (109). The c/MAM domain, which is dispensable for ligand binding but essential for ligand-induced signaling (15), has been reported to form homo- and hetero-dimers between Nrp-1 and Nrp-2 (27). However, experiments with purified protein have reported that there is no self-association or dimerization propensity observable for the Nrp ectodomain, leaving the structure and physical coupling of the MAM domain within Nrp an open question (109).
GAG binding also plays a critical role in Nrp-dependent signaling. GAGs are a diverse family of naturally occurring sulfated polysaccharides that orchestrate numerous processes including the function of endothelial cells in angiogenesis (110). GAG binding dramatically enhances Nrp-ligand interactions and drives dimerization of Nrp (10, 107). Thus, GAG binding likely plays a dual role by bridging and stabilizing the dimer holocomplex assembly.
Intriguingly, a number of receptors have been shown to associate with Nrp in a ligand independent fashion. A direct and specific interaction has been demonstrated between Nrp1 and VEGFR1 (107). Nrp and Plexin family receptors have also been shown to associate in a ligand independent manner (111). In these cases, rather than having sequential receptor binding, the pre-associated receptor complex is poised to bind and signal and only requires conformational changes (Fig 4, C).
Nrp Trafficking
Following ligand binding and assembly of the active signaling complex, Nrp has been demonstrated to have a critical function in receptor trafficking (Figure 4, D). Intriguingly, it was shown that Sema3 and VEGF induce Nrp1 endocytosis via distinct pathways (112). Further, Nrp promotes the partitioning of VEGFR into vesicles that are recycled back to the cell surface, whereas in the absence of Nrp, VEGFR2 is targeted for degradation instead of recycling (113). The intracellular GIPC-binding domain of Nrp was demonstrated to be essential for the observed VEGFR recycling, suggesting the involvement of a Nrp-associated cytoplasmic protein.
The Nrp Intracellular Domain
The discovery of the Nrp-binding PDZ-protein GIPC provided a mechanism by which Nrp may directly couple to intracellular adaptors and pathways (17) (Figure 4E). The critical portion of the Nrp intracellular domain is the three C-terminal amino acids, conserved in Nrp1 and Nrp2, that constitute a PDZ-binding motif and allow binding to PDZ-domain proteins. Increasing evidence implicates the intracellular domain of Nrp in a variety of biological processes. Mice expressing a mutant Nrp receptor with deleted cytoplasmic domain were generated (18). While capable of binding ligand, these Nrp receptors are decoupled from signal transduction via its PDZ-binding motif and GIPC and result in vascular abnormalities distinct from those of full Nrp1 knockout. Angiogenesis was unaffected but vascular patterning was disrupted as evidenced by frequent crossing of arteries and veins in the retina. A similar approach was applied in zebrafish, demonstrating that knockdown of Nrp1 does disrupt angiogenesis and only full length Nrp, not a Nrp1 construct lacking the three C-terminal amino acids, is able to rescue disrupted blood vessel growth (114). Further, deletion of the PDZ-binding C-terminal amino acids of Nrp1 disrupted stable association of the full angiogenic signaling complex with VEGFR-2 (115). There is also growing evidence for Nrp function in the absence of other recognized receptor families. Specifically, Nrp is capable of mediating adhesiveness (116) and migration (117) of endothelial cells in the absence of VEGFR2, functions that require the C-terminal PDZ-binding motif of the Nrp intracellular domain.
Interaction with a common intracellular adaptor, such as GIPC, suggests that Nrp function in physiologically diverse pathways may be due to coupling to basic cellular signaling pathways. Intriguingly, the integrin subunits α6 and α5 have been reported to interact with GIPC via their C-terminus (118), (119).
Nrp Coupling with Integrins
Integrins mediate interactions with the extracellular matrix and as a result are important for cellular adhesion and migration. Indeed, the biological function first associated with Nrp1 was its ability to mediate heterophilic cellular adhesion (120). A variety of studies have now demonstrated that both Nrp1 and Nrp2 physically and functionally associate with integrins (121–123) (Figure 4, F).
Exogenous Sema3 was able to inhibit the adhesion of endothelial cells to the integrin ligands fibronectin and vitronectin, while VEGF enhanced the adhesive strength of endothelial cells (121). Furthermore, a recent study demonstrated that Nrp1 was necessary for endothelial cell adhesion to fibronectin (124). Surprisingly, rescue of fibronectin binding following Nrp1 knockdown was only seen using vectors carrying full-length Nrp but not Nrp constructs lacking the C-terminal PDZ-binding motif, suggesting coupling between integrin and PDZ-domain proteins. αvβ3 integrin has been shown to interact with Nrp1 in a VEGF-dependent fashion and this serves to sequester Nrp1 from the active VEGF/VEGFR2 signaling complex thereby limiting angiogenesis (125). The function of integrins in controlling cellular migration led to investigation of the interplay between Nrp1 and integrin β1, demonstrating that blockade of either Nrp1 or β1 resulted in reduced invasiveness and adhesion in a pancreatic cancer cell line (122). Recently, an important specific role for Nrp2 in integrin-mediated adhesion has also been defined. Nrp2 makes specific interactions with integrin α6β1 at focal adhesion sites. Depleting Nrp2 expression in breast carcinoma cells inhibited focal adhesion assembly of these cells and regulated the downstream targets of α6β1 integrin that ultimately modulate laminin adhesion (123). These data demonstrate that Nrp and integrin receptors function cooperatively to regulate cellular adhesion and there is considerable interest in understanding the precise nature of the physical coupling and extent of biological function.
Inhibitory Modalities Targeting Nrp
In light of Nrp function in diverse diseases, significant effort has been devoted to developing potent specific Nrp inhibitors for use either alone or in combination with other inhibitory modalities, such as Avastin.
Inhibition of Sema3 signaling has been actively pursued for regenerative therapy following spinal cord injury (126). A selective inhibitor of Sema3A binding to Nrp1 (127) showed significant benefit in recovery from spinal cord injury (128). However, the entire Sema3 family is upregulated in the glial scar (37) indicating that broad-spectrum inhibition of Sema3 signaling is desired. Initial work has demonstrated that Nrp2 inhibition allows penetration of axons into a model glial scar (129). Currently, efforts are focusing on developing potent, broad-spectrum, Nrp inhibitory modalities suitable for use in the central nervous system.
Inhibitory modalities targeting Nrp-dependent VEGF induced angiogenesis have been extensively explored. A soluble Nrp splice form, containing only the ligand binding region of the extracellular domain of Nrp has been employed and found to inhibit tumorigenesis (19). A number of peptides and a synthetic peptidomimetic inhibitor of Nrp have been described (9, 13, 130, 131). Methods to overexpress inhibitory molecules, for example Sema3A, have been reported with promising activity in tumor angiogenesis (132). Finally, antibodies targeting both Nrp1 and Nrp2 have been developed that show promising activity in animal models with observed devascularization of solid tumors and decreases in metastasis (133, 134). These anti- Nrp antibodies are currently being tested in clinical trials. Surprisingly, one of the observed side effects of administration of the Nrp1 antibody, MNRP1685A, was platelet depletion (135). This was shown to be due to specific binding of MNRP1685A to platelet cell surface Nrp1, modest platelet activation, aggregation, and clearance.
Future Directions of Nrp Research
Because of the complex interplay of Nrp with other molecules, a number of critical areas regarding the mechanism of Nrp function remain to be explored. Of particular interest is the specific roles for Nrp in distinct signaling cascades and tissue types. Connected to this is the extent to which Nrp functions by the same general mechanism in highly diverse pathways or if there are distinct mechanisms employed in different signaling cascades.
The basis for ligand binding specificity has begun to come into focus and continues to be explored. In particular, the contribution of physical mechanisms governing ligand binding selectivity versus tissue specific expression remains to be determined. The physical basis for divalent Sema3 binding to Nrp remains an important unanswered question. Specifically, the contribution and coupling between the a1 and b1 interaction sites and the effect of posttranslational modification by furin to binding and signaling remain outstanding questions.
The nature of Nrp coupling to signaling receptors to form a functional signaling holocomplex represents a major area of research. In particular, unique heterophilic receptor/receptor contacts are likely critical to the formation and stability of the complex. Although necessary for signaling, the role of the Nrp MAM domain is largely unknown. Thus, the MAM domain might physically couple to cognate receptors. Indeed, the membrane proximal seventh Ig-like domain of VEGFR-2 has been shown to form a dimer required for activation (136, 137), which could be coupled to MAM domain function in the holo-complex. Further, the specific function of the transmembrane domain of Nrp may be to directly couple to signaling receptors or to allow precise physical arrangement of the intracellular domain. The transmembrane domain of VEGFR-2 has been demonstrated to facilitate correct orientation of homo-dimers to couple ligand binding to receptor activation (138). Nrp may require similar specific alignment of the transmembrane domain and may, in fact, directly couple to the transmembrane domain of cognate signaling receptors. Indeed, it is possible that the extracellular juxtamembrane, transmembrane, and intracellular juxtamembrane domains may coordinately function to physically couple ligand binding to receptor activation.
The function of the intracellular domain of Nrp and the extent to which Nrp can function independently of a canonical signaling receptor, for example VEGFR or Plexin, remains to be determined. Of particular interest are the molecules that can form direct interactions or indirectly bridge with Nrp. While GIPC is clearly a critical Nrp adaptor protein, other PDZ-domain proteins have also been suggested to function with Nrp. In particular, Nrp was recently identified as a positive regulator of hedgehog signaling (139) and the PDZ-domain protein required for this activity is currently being pursued. Connected to this is the extent to which autocrine signaling represents a fundamental mode of Nrp activation in disease, with particular interest in the connection to cancer stem cell maintenance. Continued development of novel inhibitory modalities targeting the different fundamental mechanisms of Nrp action will be both mechanistically informative and have direct relevance to human health.
In summary, Nrp family receptors utilize multiple general mechanisms by which they serve pleiotropic functions integrating distinct fundamental pathways essential to physiological and pathological function.
ACKNOWLEDGEMENTS
We thank colleagues past and present for valuable advice and helpful discussions.
Abbreviations
- Nrp
Neuropilin
- Sema3
class III Semaphorin family
- VEGF
Vascular Endothelial Growth Factor
- MAM
Meprin/A5-antigen/ptp-Mu
- PDZ
PSD-95/Dlg/ZO-1
- GIPC
GAIP Interacting Protein C-terminus
- GAG
glycosaminoglycan
- VEGFR
Vascular Endothelial Growth Factor Receptor
- PlGF
placental growth factor
Footnotes
This work was supported by NIH grants R01GM094155 (C.W.V.K) and T32HL072743 (M.W.P.).
REFERENCES
- 1.Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev Biol. 1987;122:90–100. doi: 10.1016/0012-1606(87)90335-6. [DOI] [PubMed] [Google Scholar]
- 2.Takagi S, Hirata T, Agata K, Mochii M, Eguchi G, Fujisawa H. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7:295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 5.Gaboriaud C, Gregory-Pauron L, Teillet F, Thielens NM, Bally I, Arlaud GJ. Structure and properties of the Ca(2+)-binding CUB domain, a widespread ligand-recognition unit involved in major biological functions. Biochem J. 2011;439:185–193. doi: 10.1042/BJ20111027. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes-Prior P, Fujikawa K, Pratt KP. New insights into binding interfaces of coagulation factors V and VIII and their homologues lessons from high resolution crystal structures. Curr Protein Pept Sci. 2002;3:313–339. doi: 10.2174/1389203023380639. [DOI] [PubMed] [Google Scholar]
- 7.Kiedzierska A, Smietana K, Czepczynska H, Otlewski J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta. 2007;1774:1069–1078. doi: 10.1016/j.bbapap.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069–18076. doi: 10.1074/jbc.M201681200. [DOI] [PubMed] [Google Scholar]
- 9.von Wronski MA, Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, Ramalingam K, Arunachalam T, Eaton S, Linder KE, Yan F, Pochon S, Tweedle MF, Nunn AD. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- 10.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci U S A. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzec A, Ladam P, Vassy R, Badache S, Bouchemal N, Navaza A, du Penhoat CH, Perret GY. Structure-function analysis of the antiangiogenic ATWLPPR peptide inhibiting VEGF(165) binding to neuropilin-1 and molecular dynamics simulations of the ATWLPPR/neuropilin-1 complex. Peptides. 2007;28:2397–2402. doi: 10.1016/j.peptides.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 2010;49:4068–4075. doi: 10.1021/bi100327r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker MW, Xu P, Li X, Vander Kooi CW. Structural Basis for Selective Vascular Endothelial Growth Factor-A (VEGF-A) Binding to Neuropilin-1. J Biol Chem. 2012;287:11082–11089. doi: 10.1074/jbc.M111.331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 16.Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Cremel G, Hubert P, Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646–654. doi: 10.1091/mbc.E07-06-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development. 2011;138:4185–4191. doi: 10.1242/dev.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci U S A. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211–222. doi: 10.1006/geno.2000.6381. [DOI] [PubMed] [Google Scholar]
- 21.Shintani Y, Takashima S, Asano Y, Kato H, Liao Y, Yamazaki S, Tsukamoto O, Seguchi O, Yamamoto H, Fukushima T, Sugahara K, Kitakaze M, Hori M. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. Embo J. 2006;25:3045–3055. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- 23.Rey-Gallardo A, Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL, Gerardy-Schahn R, Rutishauser U, Corbi AL, Vega MA. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology. 2010;20:1139–1146. doi: 10.1093/glycob/cwq078. [DOI] [PubMed] [Google Scholar]
- 24.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 25.Koncina E, Roth L, Gonthier B, Bagnard D. Role of semaphorins during axon growth and guidance. Adv Exp Med Biol. 2007;621:50–64. doi: 10.1007/978-0-387-76715-4_4. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 28.Koppel AM, Feiner L, Kobayashi H, Raper JA. A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron. 1997;19:531–537. doi: 10.1016/s0896-6273(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 29.Merte J, Wang Q, Vander Kooi CW, Sarsfield S, Leahy DJ, Kolodkin AL, Ginty DD. A forward genetic screen in mice identifies Sema3A(K108N), which binds to neuropilin-1 but cannot signal. J Neurosci. 2010;30:5767–5775. doi: 10.1523/JNEUROSCI.5061-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 32.Puschel AW. The function of neuropilin/plexin complexes. Adv Exp Med Biol. 2002;515:71–80. doi: 10.1007/978-1-4615-0119-0_6. [DOI] [PubMed] [Google Scholar]
- 33.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 37.De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- 38.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 41.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, Kumar A, Rissanen TT, Wang B, Nagai N, Fons P, Fariss R, Zhang Y, Wawrousek E, Tansey G, Raber J, Fong GH, Ding H, Greenberg DA, Becker KG, Herbert JM, Nash A, Yla-Herttuala S, Cao Y, Watts RJ, Li X. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 44.Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- 45.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 46.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 47.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Wronski MA, Tweedle MF, Nunn AD. Binding of the C-terminal amino acids of VEGF121 directly with neuropilin-1 should be considered. FASEB J. 2007;21:1292. doi: 10.1096/fj.07-0504ufm. author reply 1293. [DOI] [PubMed] [Google Scholar]
- 50.Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- 51.Klagsbrun M, Takashima S, Mamluk R. The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- 52.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 53.Becker PM, Waltenberger J, Yachechko R, Mirzapoiazova T, Sham JS, Lee CG, Elias JA, Verin AD. Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circ Res. 2005;96:1257–1265. doi: 10.1161/01.RES.0000171756.13554.49. [DOI] [PubMed] [Google Scholar]
- 54.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 55.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 56.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 57.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arese M, Serini G, Bussolino F. Nervous vascular parallels: axon guidance and beyond. Int J Dev Biol. 2011;55:439–445. doi: 10.1387/ijdb.103242ma. [DOI] [PubMed] [Google Scholar]
- 59.Gualandris A, Noghero A, Geuna M, Arese M, Valdembri D, Serini G, Bussolino F. Microenvironment drives the endothelial or neural fate of differentiating embryonic stem cells coexpressing neuropilin-1 and Flk-1. FASEB J. 2009;23:68–78. doi: 10.1096/fj.08-112847. [DOI] [PubMed] [Google Scholar]
- 60.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CC, Kreusch A, McMullan D, Ng K, Spraggon G. Crystal structure of the human neuropilin-1 b1 domain. Structure. 2003;11:99–108. doi: 10.1016/s0969-2126(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 64.Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 66.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honore JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beausejour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–760. doi: 10.2741/1569. [DOI] [PubMed] [Google Scholar]
- 69.Vacca A, Scavelli C, Serini G, Di Pietro G, Cirulli T, Merchionne F, Ribatti D, Bussolino F, Guidolin D, Piaggio G, Bacigalupo A, Dammacco F. Loss of inhibitory semaphorin 3A (SEMA3A) autocrine loops in bone marrow endothelial cells of patients with multiple myeloma. Blood. 2006;108:1661–1667. doi: 10.1182/blood-2006-04-014563. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai A, Doci CL, Gutkind JS. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 2012;22:23–32. doi: 10.1038/cr.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res. 2009;15:1860–1864. doi: 10.1158/1078-0432.CCR-08-0563. [DOI] [PubMed] [Google Scholar]
- 72.Algire GH, HW C. VASCULAR REACTIONS OF NORMAL AND MALIGNANT TISSUES INVIVO .1. VASCULAR REACTIONS OF MICE TO WOUNDS AND TO NORMAL AND NEOPLASTIC TRANSPLANTS. J Natl Cancer Inst. 1945;6:73–85. [Google Scholar]
- 73.Greenblatt M, Shubi P. Tumor angiogenesis: transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst. 1968;41:111–124. [PubMed] [Google Scholar]
- 74.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 75.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 76.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231:1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 79.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 80.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 81.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 82.Banerjee SK, Zoubine MN, Tran TM, Weston AP, Campbell DR. Overexpression of vascular endothelial growth factor164 and its co-receptor neuropilin-1 in estrogen-induced rat pituitary tumors and GH3 rat pituitary tumor cells. Int J Oncol. 2000;16:253–260. doi: 10.3892/ijo.16.2.253. [DOI] [PubMed] [Google Scholar]
- 83.Stephenson JM, Banerjee S, Saxena NK, Cherian R, Banerjee SK. Neuropilin-1 is differentially expressed in myoepithelial cells and vascular smooth muscle cells in preneoplastic and neoplastic human breast: a possible marker for the progression of breast cancer. Int J Cancer. 2002;101:409–414. doi: 10.1002/ijc.10611. [DOI] [PubMed] [Google Scholar]
- 84.Kreuter M, Woelke K, Bieker R, Schliemann C, Steins M, Buechner T, Berdel WE, Mesters RM. Correlation of neuropilin-1 overexpression to survival in acute myeloid leukemia. Leukemia. 2006 doi: 10.1038/sj.leu.2404384. [DOI] [PubMed] [Google Scholar]
- 85.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 86.Cao Y, E G, Wang E, Pal K, Dutta SK, Bar-Sagi D, Mukhopadhyay D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72:3912–3918. doi: 10.1158/0008-5472.CAN-11-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 88.Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene. 2009;28:3537–3550. doi: 10.1038/onc.2009.204. [DOI] [PubMed] [Google Scholar]
- 89.Goel HL, Chang C, Pursell B, Leav I, Lyle S, Xi HS, Hsieh CC, Adisetiyo H, Roy-Burman P, Coleman IM, Nelson PS, Vessella RL, Davis RJ, Plymate SR, Mercurio AM. VEGF/Neuropilin-2 Regulation of Bmi-1 and Consequent Repression of IGF-IR Define a Novel Mechanism of Aggressive Prostate Cancer. Cancer Discov. 2012;2:906–921. doi: 10.1158/2159-8290.CD-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O, Lidereau R. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–171. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 91.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 92.Cao Y, Wang L, Nandy D, Zhang Y, Basu A, Radisky D, Mukhopadhyay D. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008;68:8667–8672. doi: 10.1158/0008-5472.CAN-08-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, Blanpain C. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 94.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, Rich JN, Bartek J. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Romeo PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- 96.Yamada Y, Oike Y, Ogawa H, Ito Y, Fujisawa H, Suda T, Takakura N. Neuropilin-1 on hematopoietic cells as a source of vascular development. Blood. 2003;101:1801–1809. doi: 10.1182/blood-2002-01-0119. [DOI] [PubMed] [Google Scholar]
- 97.West DC, Rees CG, Duchesne L, Patey SJ, Terry CJ, Turnbull JE, Delehedde M, Heegaard CW, Allain F, Vanpouille C, Ron D, Fernig DG. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem. 2005;280:13457–13464. doi: 10.1074/jbc.M410924200. [DOI] [PubMed] [Google Scholar]
- 98.Holmes DI, Zachary IC. Vascular endothelial growth factor regulates stanniocalcin-1 expression via neuropilin-1-dependent regulation of KDR and synergism with fibroblast growth factor-2. Cell Signal. 2008;20:569–579. doi: 10.1016/j.cellsig.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 99.Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R, Cheng SY. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26:5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67:10309–10316. doi: 10.1158/0008-5472.CAN-07-3256. [DOI] [PubMed] [Google Scholar]
- 101.Glinka Y, Prud'homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, Charlton M, Watts RJ, Mukhopadhyay D, Shah VH. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379–2394. doi: 10.1172/JCI41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao Y, Szabolcs A, Dutta SK, Yaqoob U, Jagavelu K, Wang L, Leof EB, Urrutia RA, Shah VH, Mukhopadhyay D. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J Biol Chem. 2010;285:31840–31848. doi: 10.1074/jbc.M110.151696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banerjee S, Sengupta K, Dhar K, Mehta S, D'Amore PA, Dhar G, Banerjee SK. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol Carcinog. 2006;45:871–880. doi: 10.1002/mc.20248. [DOI] [PubMed] [Google Scholar]
- 105.Geretti E, Shimizu A, Kurschat P, Klagsbrun M. Site-directed mutagenesis in the B-neuropilin-2 domain selectively enhances its affinity to VEGF165, but not to semaphorin 3F. J Biol Chem. 2007;282:25698–25707. doi: 10.1074/jbc.M702942200. [DOI] [PubMed] [Google Scholar]
- 106.Shraga-Heled N, Kessler O, Prahst C, Kroll J, Augustin H, Neufeld G. Neuropilin-1 and neuropilin-2 enhance VEGF121 stimulated signal transduction by the VEGFR-2 receptor. FASEB J. 2007;21:915–926. doi: 10.1096/fj.06-6277com. [DOI] [PubMed] [Google Scholar]
- 107.Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 108.Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppanen VM, Alitalo K, Winkler FK, Ballmer-Hofer K. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood. 2012;119:1781–1788. doi: 10.1182/blood-2011-11-390922. [DOI] [PubMed] [Google Scholar]
- 109.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. Embo J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stringer SE. The role of heparan sulphate proteoglycans in angiogenesis. Biochem Soc Trans. 2006;34:451–453. doi: 10.1042/BST0340451. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 112.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, Mukhopadhyay D, Horowitz A. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103:e71–e79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood. 2011;118:816–826. doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- 114.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 115.Prahst C, Heroult M, Lanahan AA, Uziel N, Kessler O, Shraga-Heled N, Simons M, Neufeld G, Augustin HG. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J Biol Chem. 2008;283:25110–25114. doi: 10.1074/jbc.C800137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murga M, Fernandez-Capetillo O, Tosato G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 2005;105:1992–1999. doi: 10.1182/blood-2004-07-2598. [DOI] [PubMed] [Google Scholar]
- 117.Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 118.Tani TT, Mercurio AM. PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J Biol Chem. 2001;276:36535–36542. doi: 10.1074/jbc.M105785200. [DOI] [PubMed] [Google Scholar]
- 119.El Mourabit H, Poinat P, Koster J, Sondermann H, Wixler V, Wegener E, Laplantine E, Geerts D, Georges-Labouesse E, Sonnenberg A, Aumailley M. The PDZ domain of TIP-2/GIPC interacts with the C-terminus of the integrin alpha5 and alpha6 subunits. Matrix Biol. 2002;21:207–214. doi: 10.1016/s0945-053x(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 120.Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev Biol. 1995;170:207–222. doi: 10.1006/dbio.1995.1208. [DOI] [PubMed] [Google Scholar]
- 121.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 122.Fukasawa M, Matsushita A, Korc M. Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther. 2007;6:1173–1180. doi: 10.4161/cbt.6.8.4363. [DOI] [PubMed] [Google Scholar]
- 123.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates alpha6beta1 integrin in the formation of focal adhesions and signaling. J Cell Sci. 2012;125:497–506. doi: 10.1242/jcs.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem. 2009;284:33966–33981. doi: 10.1074/jbc.M109.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 127.Kikuchi K, Kishino A, Konishi O, Kumagai K, Hosotani N, Saji I, Nakayama C, Kimura T. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J Biol Chem. 2003;278:42985–42991. doi: 10.1074/jbc.M302395200. [DOI] [PubMed] [Google Scholar]
- 128.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 129.Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, Verhaagen J, Fawcett JW. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci. 2003;24:913–925. doi: 10.1016/j.mcn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 130.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N, Ellard K, Aqil R, Lynch R, Chapman C, Hartzoulakis B, Nally J, Stewart M, Cheng L, Menon M, Tickner M, Djordjevic S, Driscoll PC, Zachary I, Selwood DL. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem. 2010;53:2215–2226. doi: 10.1021/jm901755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, Squadrito ML, Venneri MA, Mazzone M, Larsson E, Carmeliet P, De Palma M, Naldini L, Tamagnone L, Rolny C. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol. 2011;31:741–749. doi: 10.1161/ATVBAHA.110.211920. [DOI] [PubMed] [Google Scholar]
- 133.Liang WC, Dennis MS, Stawicki S, Chanthery Y, Pan Q, Chen Y, Eigenbrot C, Yin J, Koch AW, Wu X, Ferrara N, Bagri A, Tessier-Lavigne M, Watts RJ, Wu Y. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007;366:815–829. doi: 10.1016/j.jmb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 134.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, Berry L, Kasman I, Zlot C, Cheng Z, Le Couter J, Filvaroff EH, Plowman G, Peale F, French D, Carano R, Koch AW, Wu Y, Watts RJ, Tessier-Lavigne M, Bagri A. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 135.Darbonne WC, Du X, Dhawan P, Hartley D, Tarrant J, Taylor H, Cain G, Shih LM, Brachmann RK, Phung Q, Weekes CD, LoRusso P, Patnaik A, Xiang H, Ramakrishnan V. Mechanism for platelet reduction in anti-neuropilin-1 (MNRP1685A)–treated phase I patients. J Clin Oncol. 2011;29:e13598. [Google Scholar]
- 136.Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007;14:249–250. doi: 10.1038/nsmb1202. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y, Xie P, Opatowsky Y, Schlessinger J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc Natl Acad Sci U S A. 2010;107:1906–1911. doi: 10.1073/pnas.0914052107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dosch DD, Ballmer-Hofer K. Transmembrane domain-mediated orientation of receptor monomers in active VEGFR-2 dimers. FASEB J. 2010;24:32–38. doi: 10.1096/fj.09-132670. [DOI] [PubMed] [Google Scholar]
- 139.Hillman RT, Feng BY, Ni J, Woo WM, Milenkovic L, Hayden Gephart MG, Teruel MN, Oro AE, Chen JK, Scott MP. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333–2346. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 141.Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci U S A. 2009;106:15610–15615. doi: 10.1073/pnas.0906923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muller YA, Christinger HW, Keyt BA, de Vos AM. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 A resolution: multiple copy flexibility and receptor binding. Structure. 1997;5:1325–1338. doi: 10.1016/s0969-2126(97)00284-0. [DOI] [PubMed] [Google Scholar]
- 144.Leppanen VM, Prota AE, Jeltsch M, Anisimov A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K, Alitalo K. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci U S A. 2010;107:2425–2430. doi: 10.1073/pnas.0914318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McTigue MA, Wickersham JA, Pinko C, Showalter RE, Parast CV, Tempczyk-Russell A, Gehring MR, Mroczkowski B, Kan CC, Villafranca JE, Appelt K. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure. 1999;7:319–330. doi: 10.1016/s0969-2126(99)80042-2. [DOI] [PubMed] [Google Scholar]