Abstract
Background
Vitamin D status has been linked to the risk of cardiovascular disease (CVD). However, the optimal 25hydroxy-vitamin D (25(OH)-vitamin D) levels for potential cardiovascular health benefits remain unclear.
Methods and Results
We searched MEDLINE and EMBASE from 1966 through February 2012 for prospective studies that assessed the association of 25(OH)-vitamin D concentrations with CVD risk. A total of 24 articles met our inclusion criteria, from which 19 independent studies with 6,123 CVD cases in 65,994 participants were included for a meta-analysis. Comparing the lowest to the highest 25(OH)-vitamin D categories, the pooled relative risks (RR) was 1.52 (95% CI: 1.30-1.77) for total CVD, 1.42 (95% CI: 1.19-1.71) for CVD mortality, 1.38 (95% CI: 1.21-1.57) for coronary heart disease, and 1.64 (95% CI: 1.27-2.10) for stroke. These associations remained strong and significant when analyses were limited to studies that excluded participants with baseline CVD and had better controlled for season and confounding. We used a fractional polynomial spline regression analysis to assess the linearity of dose-response association between continuous 25(OH)-vitamin D and CVD risk. The CVD risk increased monotonically across decreasing 25(OH)-vitamin D below approximately 60 nmol/L, with a RR of 1.03 (95% CI: 1.00-1.06) per 25 nmol/L decrement in 25(OH)-vitamin D.
Conclusions
This meta-analysis demonstrated a generally linear, inverse association between circulating 25(OH)-vitamin D in the range of 20-60 nmol/L and risk of CVD. Further research is needed to clarify the association of 25(OH)-vitamin D higher than 60 nmol/L with CVD risk and assess causality of the observed associations.
Keywords: 25hydroxy-vitamin D, cardiovascular disease, meta-analysis, prospective study
Introduction
The pivotal role of vitamin D in calcium homeostasis and bone metabolism is well known. More recently, vitamin D insufficiency has been linked to many non-skeletal health problems and chronic diseases.1, 2 Vitamin D has pleiotropic effects that may favorably influence cardiovascular health through multiple mechanisms, including down-regulation of the renin-angiotensin system,3 enhancement in insulin secretion and insulin sensitivity,4, 5 protection against angiogenesis,6 and modulation of inflammatory processes.7 Cross-sectional and retrospective case-control studies have shown deficient (typically defined as <25 nmol/L; to convert 25(OH)-vitamin D in nmol/L to ng/mL, divide by 2.496) or insufficient (25-50 nmol/L) levels of circulating 25hydroxy-vitamin D (25(OH)-vitamin D, the standard biomarker for vitamin D status) in patients with established cardiovascular disease (CVD)8-10. Prospective studies found mixed results; some,11-14 but not all,15, 16 reported an inverse association between baseline circulating 25(OH)-vitamin D and subsequent risk of CVD. Overall, existing evidence supports the hypothesis that low 25(OH)-vitamin D is associated with increased risk of CVD.17, 18 However, a closer investigation of prior studies indicates that the association between 25(OH)-vitamin D and CVD risk may be nonlinear and reach a plateau between 50-75 nmol/L.19 Others have suggested a possible U-shaped relation, with slight increase in CVD risk at both low (<50 nmol/L) and high (>125 nmol/L) levels of 25(OH)-vitamin D.11, 16 The shape of vitamin D and CVD association over the wide spectrum of 25(OH)-vitamin D levels has yet to be determined.
The current dietary recommendations for vitamin D by the Institute of Medicine (IOM)20 are based on vitamin D required for bone health. The IOM determined that the evidence was insufficient to address the amount of vitamin D that may be beneficial for CVD. With widespread attention to a possible role of vitamin D in prevention of CVD, it becomes increasingly important to further evaluate the dose-response association between vitamin D status and CVD and to determine the optimal 25(OH)-vitamin D level that may confer potential cardiovascular benefits. We therefore conducted a systematic review and a meta-analysis to quantitatively assess the association between circulating 25(OH)-vitamin D concentrations and CVD risk in prospective studies, focusing on the dose-response gradient.
Methods
Data Sources and Literature Search Strategy
We identified the pertinent published literature through a systematic search of MEDLINE and EMBASE from 1966 through February 2012. We selected generic as well as specific search terms related to vitamin D and CVD, based on analysis of the Medical Subject Headings and text words from a priori identified key articles. Search terms used for vitamin D included vitamin D, 25hydroxy-vitamin D, 1,25dihydroxy-vitamin D, calcidiol, and calcitriol. Search terms used for CVD included cardiovascular disease, ischemic heart disease, coronary artery disease (CHD), cardiovascular mortality, myocardial infarction, and stroke. The search results using vitamin D related terms and CVD related terms were then combined. We further restricted the search to English-language articles, human studies, and adult subjects aged ≥18 years. The same set of search terms and search strategies were applied to each database. We also manually searched the reference lists of selected articles for additional studies. More details on the literature search strategy are presented in Supplement Method.
Study Selection
The studies eligible for inclusion were prospective studies that examined the association between circulating concentrations of 25(OH)-vitamin D at baseline and risk of CVD events during follow-up. Studies were excluded if they were 1) ecological, cross-sectional, or retrospective case-control studies due to concerns about the directionality of association and the potential bias from reverse causation; 2) review articles, commentaries, editorials, or case reports; 3) studies that did not measure circulating 25(OH)-vitamin D; 4) studies that did not ascertain major clinical CVD events including CVD death, myocardial infarction, or stroke as endpoints; 5) studies that did not compare CVD event rates between different 25(OH)-vitamin D concentrations; and 6) studies of participants selected by confirmed medical conditions (such as diabetes or end-stage kidney disease). Articles that passed the abstract screening were retrieved for a full text review. Two investigators (Wang and Song) independently reviewed all identified articles and assessed each study for adherence to the selection criteria. The inter-rater reliability, calculated as the percentage of agreement between two investigators, is 98%.
Data Extraction
Two investigators (Wang and Song) extracted key data from the selected articles using a standardized form and independently verified the extracted data for completeness and accuracy. Disagreements were reconciled through group discussion. For each article, we extracted data on the authors, year of publication, location of the study, study design, participant characteristics, duration of follow-up, number of cases and noncases for each CVD endpoint, category of circulating 25(OH)-vitamin D level, covariates adjusted for in multivariable analyses, and estimates of association (relative risks (RR) or odds ratios (OR) and 95% confidence intervals (CI)). For studies conducted in different subsamples of the same cohort, we extracted data of each publication separately. When necessary, missing information (including number of cases and noncases in each category of 25(OH)-vitamin D and estimates of association) was obtained by direct contact with the original study authors.
Data Synthesis and Analysis
We first reported original data of the selected articles in summary table. To maximize statistical power for hypothesis testing, we performed meta-analyses to combine estimates of association across all studies, using DerSimonian and Laird’s random-effects model in which each study is weighted by the inverse of the sum of within-study plus between-study variance.21 Because ORs in nested case-control studies were close estimates for RRs, we refer to all association estimates as RR. The pooled RR of total CVD with 95% CI was calculated comparing the lowest with the highest categories of 25(OH)-vitamin D level as well as per standard unit decrease in continuous 25(OH)-vitamin D when data are available. We also examined the associations of 25(OH)-vitamin D with death due to CVD, CHD, and stroke separately.
Heterogeneity across studies was assessed by Cochran’s Q statistic, the I2 and H statistics. The percentages of I2 around 25% (I2=25), 50% (I2=50), and 75% (I2=75) indicate low, medium, and high heterogeneity, respectively. An H statistics <1.2 indicates little heterogeneity and an H >1.5 raises caution regarding notable heterogeneity. We assessed publication bias using visual inspection of Begg’s modified funnel plots, in which the RR was plotted on a logarithmic scale (log[RR]) against its standard error from each study, and Begg’s adjusted rank correlation test and Egger’s regression asymmetry test.
We assessed the dose-response association between 25(OH)-vitamin D and risk of CVD using a fractional polynomial spline regression analysis. We also used the method described by Greenland and Longnecker to test the linear trend from the correlated RRs and 95% CIs across multiple categories of continuous variable.22 The median level of 25(OH)-vitamin D in each category reported in the original study was assigned to the corresponding RR. If not reported, the values assigned were the mean or the midpoint of the lower and upper bounds in each category. For the extreme open-ended categories, half of the width of adjacent category was subtracted (for the lowest category) or added (for the uppermost category) to obtain the midpoint. These methods to assign exposure level for analysis of dose-response relation have been reported previously.23
Analyses were further stratified to examine the difference in pooled RRs by baseline age of participants, duration of follow-up, number of CVD cases, 25(OH)-vitamin D assay methods, exclusion of baseline CVD, and control for key confounders including age, sex, season of blood collection, body mass index (BMI), and physical activity. Sensitivity analyses were conducted to evaluate the robustness of the pooled estimates. All analyses were performed using the STATA software (version 10.1, STATA Corp., College Station, Texas). Statistical significance was defined as two-tailed <0.05.
Results
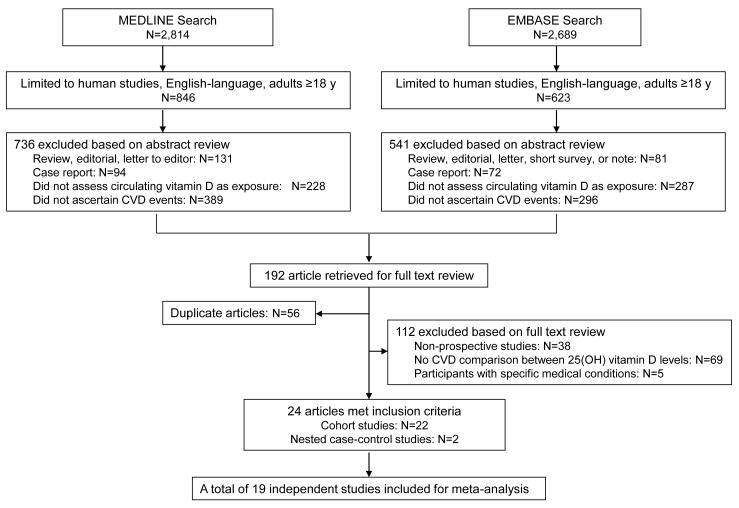
A total of 1,469 articles were identified through literature search, of which 24 articles met our inclusion criteria (Figure 1). The characteristics of these studies, including 22 cohort11, 13-16, 24-40 and 2 nested case-control studies,12, 41 are shown in Supplement Table. All studies provided the RRs of CVD according to categories of 25(OH)-vitamin D concentration, and 10 studies provided the RRs in association with continuous 25(OH)-vitamin D.11, 12, 25, 27, 32, 33, 35, 37, 38, 41 One study examined CHD and stroke separately15 and one study examined smokers and non-smokers separately,34 these results were considered as independent studies in pooled analysis. When multiple studies were conducted in the same cohort, the study with larger sample size and/or more complete data16, 25, 32, 38 was included for pooled analysis. As a result, the final meta-analysis included 19 individual studies that reported data on categorical 25(OH)-vitamin D, with 10 studies also reporting data on continuous 25(OH)-vitamin D. Table 1 shows the results of each study included in the meta-analysis.
Figure 1.
Study Search and Selection Flow Diagram. Search terms for vitamin D included vitamin D, 25hydroxy-vitamin D, 1,25dihydroxy-vitamin D, calcidiol, and calcitriol. Search terms for cardiovascular disease (CVD) included cardiovascular disease, ischemic heart disease, coronary artery disease, cardiovascular mortality, myocardial infarction, and stroke. Initial search results were further limited to English-language articles, human studies, and studies of adults 18 years. In each box, the sum of studies in all categories may exceed the total number because of overlapping classification.
Table 1.
Estimates of association between circulating 25(OH)-vitamin D concentrations and risk of cardiovascular disease in 19 prospective studies included in the meta-analysis
| Source | CVD endpoint |
Estimates of association |
Variables adjusted in model | ||||
|---|---|---|---|---|---|---|---|
| Event | Case, N |
Noncase N |
Categories of 25(OH)- vitamin D in nmol/L |
RR (95% CI) | RR (95% CI) per 25 nmol/L decline in continuous 25(OH)- vitamin D |
||
| Marniemi et al,15 2005 |
Incident MI | 130 | 559 | Tertiles Middle vs. Lowest Highest vs. Lowest |
0.99 (0.64-1.53) 0.77 (0.47-1.27) |
Age, sex, smoking, and functional capacity |
|
| Incident stroke |
70 | 590 |
Middle vs. Lowest Highest vs. Lowest |
1.13 (0.62-2.05) 1.00 (0.51-1.94) |
|||
|
| |||||||
| Wang et al,11 2008 |
Incident CVD |
120 | 1619 | Arbitrary cutpoints 25.0-<37.4 vs. ≥37.4 <25.0 vs. ≥37.4 |
1.53 (1.00-2.36) 1.80 (1.05-3.08) |
1.67 (1.15-2.42) among hypertensives |
Age, sex, systolic BP, antihypertensive treatment, diabetes, serum creatinine, total-to-HDL cholesterole ratio, smoking, and BMI |
|
| |||||||
| Giovannucci et al,12 2008 |
Incident MI | 454 | 900 | Arbitrary cutpoints 56.4-74.6 vs. ≥74.9 37.7-56.2 vs. ≥74.9 ≤37.44 vs. ≥74.9 |
1.60 (1.10-2.22) 1.43 (0.96-2.13) 2.09 (1.24-3.54) |
1.24 (1.02, 1.50) | Age, month of blood collection, smoking, family history of MI, diabetes, hypertension, alcohol intake, BMI, physical activity, region, race, multivitamin use, ra3 fat intake, fasting status, LDL and HDL-cholesterol and triglyceride |
|
| |||||||
| Pilz et al,25 2008 |
CVD mortality |
479 | 2796 | Quartiles 38.9-57.2 vs. >57.4 25.2-38.7 vs. >57.4 4.74-25.0 vs. >57.4 |
1.31 (0.95-1.80) 1.43 (1.04-1.95) 2.46 (1.82-3.32) |
1.50 (1.32-1.71) | Age, sex, BMI, physical activity, smoking, diabetes, BP, LDL and HDL- cholesterol, triglyceride, albumin level, cystatin C level, N-terminal pro-brain natriuretic peptide level, and use of CVD medications |
| CHD mortality |
115 | 3160 |
38.9-57.2 vs. >57.4 25.2-38.7 vs. >57.4 4.74-25.0 vs. >57.4 |
0.94 (0.51-1.74) 1.15 (0.64-2.07) 1.60 (0.90-2.83) |
1.23 (0.96-1.57) | ||
| Stroke mortality |
41 | 3234 |
38.9-57.2 vs. >57.4 25.2-38.7 vs. >57.4 4.74-25.0 vs. >57.4 |
1.12 (0.35-3.57) 1.65 (0.56-4.82) 2.50 (0.87-7.13) |
1.59 (0.99-2.57) | ||
|
| |||||||
| Melamed et al,16 2008 |
CVD mortality |
777 | 12554 | Quartiles 60.9-80.1 vs. >80.1 44.4-60.7 vs. >80.1 <44.4 vs. >80.1 |
0.83 (0.65-1.07) 0.88 (0.69-1.14) 1.20 (0.87-1.64) |
Age, sex, race, season, smoking, BMI, physical activity, hypertension, history of CVD, diabetes, total cholesterol, HDL cholesterol, use of cholesterol lowering medications, estimated glomerular filtration rate, serum albumin, log albumin to creatinine ratio, log C-reactive protein, use of vitamin D supplement, and low socioeconomic status |
|
|
| |||||||
| Pilz et al,14 2009 |
CVD mortality |
20 | 594 | Quartiles Lowest (30.6±6.9) vs. upper three |
5.02 (1.88-13.42) | Age, sex, diabetes, smoking, hypertension, HDL cholesterol, glomerular filtration rate, waist-to-hip ratio, exercise |
|
|
| |||||||
| Kilkkinen, et al,27 2009 |
CVD mortality |
933 | 5286 | Quintiles 48-61 vs. 62-180 (m) & 44-55 vs. 56-151 (w) 38-47 vs. 62-180 (m) & 34-43 vs. 56-151 (w) 29-37 vs. 62-180 (m) & 26-33 vs. 56-151 (w) 5-28 vs. 62-180 (m) & 4-25 vs. 56-151 (w) |
1.13 (0.91-1.41) 1.07 (0.85-1.35) 1.38 (1.10-1.73) 1.32 (1.05-1.65) |
1.11 (1.01-1.22) | Age, sex, marital status, education, BMI, alcohol consumption, smoking, physical activity, and season of baseline examination |
| CHD mortality |
640 | 5579 |
48-61 vs. 62-180 (m) & 44-55 vs. 56-151 (w) 38-47 vs. 62-180 (m) & 34-43 vs. 56-151 (w) 29-37 vs. 62-180 (m) & 26-33 vs. 56-151 (w) 5-28 vs. 62-180 (m) & 4-25 vs. 56-151 (w) |
1.05 (0.81-1.36) 0.80 (0.61-1.06) 1.29 (0.99-1.69) 1.10 (0.84-1.44) |
1.05 (0.94-1.18) | ||
| Stroke mortality |
293 | 5926 |
48-61 vs. 62-180 (m) & 44-55 vs. 56-151 (w) 38-47 vs. 62-180 (m) & 34-43 vs. 56-151 (w) 29-37 vs. 62-180 (m) & 26-33 vs. 56-151 (w) 5-28 vs. 62-180 (m) & 4-25 vs. 56-151 (w) |
1.43 (0.91-2.24) 2.01 (1.31-3.08) 1.66 (1.05-2.63) 2.07 (1.34-3.20) |
1.27 (1.06-1.52) | ||
|
| |||||||
| Bolland et al,28 2010 |
Total CVD | 110 | 1361 | Arbitrary cutpoints <50 vs. ≥50 |
1.2 (0.8-1.8) | Treatment allocation, baseline age, body weight, smoking status, systolic BP, history of CHD, stroke, or transient ischemic attack, dyslipidemia, and diabetes |
|
| MI Stroke |
52 59 |
1419 1412 |
<50 vs. ≥50 <50 vs. ≥50 |
1.2 (0.7-2.2) 1.4 (0.8-2.5) |
|||
| CVD mortality |
63 | 1408 | <50 vs. ≥50 | 0.9 (0.5-1.6) | |||
|
| |||||||
| Semba, et al,29 2010 |
CVD mortality |
107 | 899 | Quartiles 40.2-63.9 vs. >63.9 26.2-39.9 vs. >63.9 <26.2 vs. >63.9 |
2.28 (1.09-4.79) 1.76 (0.80-3.89) 2.57 (1.12-5.91) |
Age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, and Mini-Mental Exam score |
|
|
| |||||||
| Anderson et al,31 2010 |
Composite CVD |
1301 | 18768 | Arbitrary cutpoints 39.9-74.9 vs. >74.9 ≤37.4 vs. >74.9 |
1.22 (1.08-1.38) 1.79 (1.53-2.10) |
Age, sex, hypertension, hyperlipidemia, diabetes, and peripheral vascular disease, prior fracture, renal failure, prior pulmonary embolism, depression, skeletal disorder, hypothyroidism, infection, and headache |
|
| CVD mortality |
1193 | 28493 |
39.9-74.9 vs. >74.9 ≤37.4 vs. >74.9 |
1.20 (1.05-1.38) 1.77 (1.51-2.07) |
|||
| Incident MI | 765 | 21116 |
39.9-74.9 vs. >74.9 ≤37.4 vs. >74.9 |
1.15 (0.98-1.35) 1.45 (1.18-1.78) |
|||
| Incident stroke |
208 | 25890 |
39.9-74.9 vs. >74.9 ≤37.4 vs. >74.9 |
1.31 (0.95-1.81) 1.78 (1.20-2.66) |
|||
|
| |||||||
| Cawthon et al.32 2010 |
CVD mortality |
110 | 1380 | Quartiles 62.9-<74.9 vs. ≥74.9 49.9-<62.9 vs. ≥74.9 <49.9 vs. ≥74.9 |
1.12 (0.61-2.06) 1.21 (0.65-2.28) 1.52 (0.83-2.80) |
1.30 (1.00-1.69) (base- adjusted) |
Age, clinic, season of blood collection, serum calcium and phosphate, glomerular filtration rate, percentage body fat, weight, race, health status, presence of at least one medical condition, alcohol use, education, activity level, marital status, and presence of a functional or mobility limitation |
|
| |||||||
| Michaelsson et al,33 2010 |
: CVD mortality |
259 | 935 | Percentiles <46 vs. >93 46-93 vs. >93 |
1.57 (0.91-2.70) 1.06 (0.69-1.63) |
1.05 (0.89-1.24) | Age, weight, height, calcium intake, alcohol intake, season of blood collection, social class, smoking, physical activity, self-perceived health, diabetes, other chronic disease, vitamin D intake, fish intake, multiple plasma biomarkers including cholesterol, triglycerides, and insulin, systolic and diastolic BP, lipid- lowering treatment and antihypertensive treatment |
| CHD mortality |
121 | 1073 |
<46 vs. >93 46-93 vs. >93 |
2.09 (0.90-4.87) 1.40 (0.70-2.81) |
1.17 (0.91-1.50) | ||
| Stroke mortality |
33 | 1161 |
<46 vs. >93 46-93 vs. >93 |
0.76 (0.20-2.97) 0.51 (0.19-1.37) |
1.00 (0.62-1.62) | ||
|
| |||||||
| Hutchinson et al,34 2010 |
CVD mortality |
325 | 4426 | Quartiles among non- smokers: 3rd (56.2±6.4) vs. highest (72.3±13.2) |
0.71 (0.51-1.01) 0.84 (0.61-1.15) |
Age, sex, BMI, physical activity, diabetes, hypertension, creatinine, prior CVD, prior cancer |
|
| 2nd (46.7±6.0) vs. highest 1st (33.8±7.6) vs. highest |
1.08 (0.79-1.48) | ||||||
| CVD mortality |
188 | 2222 | Quartiles among smokers: 3rd (76.4±6.5) vs. highest (97.5±14.9) 2nd (64.7±6.0) vs. highest 1st (49.2±9.1) vs. highest |
1.04 (0.67-1.60) 1.10 (0.73-1.67) 0.93 (0.61-1.44) |
|||
|
| |||||||
| Jassal et al,35 2010 |
CVD mortality |
111 | 962 | Quartiles 102-117 vs. 120-362 87-100 vs. 120-362 10-85 vs. 120-362 |
0.90 (0.42-1.94) 1.07 (0.55-2.09) 1.11 (0.54-2.26) |
0.96 (0.82-1.12) | Age, gender, BMI, systolic BP, LDL- cholesterol, fasting glucose, physical activity, log(urine albumin/creatinine ratio), glomerular filtration rate, prevalent CVD, season, use of diuretics, calcium channel blockers, beta-blockers, and angiotensin-converting enzyme inhibitors |
| CHD mortality |
37 | 1036 | Quartiles 102-117 vs. 120-362 87-100 vs. 120-362 10-85 vs. 120-362 |
1.54 (0.34-6.91) 1.20 (0.35-4.07) 1.50 (0.40-5.61) |
1.15 (0.81-1.63) | ||
| Stroke mortality |
22 | 1051 | 0.95 (0.57-1.58) | ||||
|
| |||||||
| Hosseinpanah et al,41 2011 |
Incident CVD |
251 | 251 | Arbitrary cutpoints 25.0-37.4 vs. ≥37.4 <25.0 vs. ≥37.4 |
1.46 (0.83-2.56) 2.90 (1.67-5.12) |
1.11 (1.00-1.22) | BMI, fasting plasma glucose, systolic and diastolic BP, triglyceride, HDL cholesterol, smoking, physical activities, and premature CVD family history |
|
| |||||||
| Eaton et al,37 2011 |
CVD mortality |
79 | 2350 | Quartiles 50.0-<65.4 vs. 65.4-146.7 36.5-<50.0 vs. 65.4-146.7 3.25-<36.5 vs. 65.4-146.7 |
1.16 (0.75-1.80) 1.14 (0.74-1.78) 1.27 (0.81-1.99) |
1.00 (0.78-1.28) | Age, ethnicity, trial indicator, smoking, hypertension, systolic BP, diabetes, CVD, fracture at ≥55 y, cancer, waist circumference, BMI, physical activity, and alcohol consumption |
|
| |||||||
| Kestenbaum et al,38 2011 |
Incident MI | 299 | 2013 | Arbitrary cutpoints 37.4-74.9 vs. >74.9 <37.4 vs. >74.9 |
1.20 (0.90-1.59) 1.40 (0.93-2.12) |
1.25 (1.08-1.44) | Age, race, sex, season, clinic site, diabetes, antihypertensive medications, smoking, education, physical activity, BMI, systolic BP, C-reactive protein, total and HDL cholesterol, calcium, phosphorus, glomerular filtration rate |
| CVD mortality |
389 | 1923 |
37.4-74.9 vs. >74.9 <37.4 vs. >74.9 |
1.01 (0.78-1.30) 1.17 (0.83-1.67) |
1.06 (0.94-1.19) | ||
Abbreviations: CVD, cardiovascular disease; MI, myocardial infarction; BMI, body mass index; BP, blood pressure.
Units for 25(OH)-vitamin D were presented in nmol/L. To convert 25(OH)-vitamin D in nmol/L to ng/mL, divide by 2.496.
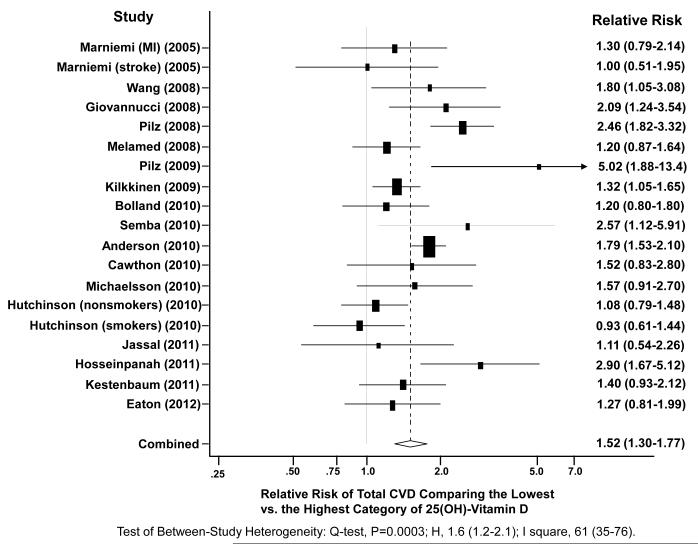
We used multivariable RRs that adjusted for conventional CVD risk factors including hypertension, diabetes, and hypercholesterolemia reported in each study for the pooled analysis. The primary analysis included a total of 6,123 CVD cases from 65,994 participants. Figure 2 shows the study-specific and the pooled RRs for total CVD comparing the lowest versus the highest categories of 25(OH)-vitamin D level (pooled RR=1.52, 95% CI: 1.30-1.77). The P value for heterogeneity from the Cochran’s Q test (Q=45.9, df=18) was 0.0003, suggesting that the variations between studies were not solely attributable to sampling variation. The I2 (61, 95% CI: 35-76) and the H statistic (1.6, 95% CI: 1.2-2.1) also suggested high between-study heterogeneity. The funnel plot was symmetrical and the Begg test (p=0.16) and the Egger test (p=0.11) indicated no publication bias. When the RR of CVD in association with continuous 25(OH)-vitamin D in 10 studies were combined, the pooled RR per 25 nmol/L decrease in 25(OH)-vitamin D was 1.18 (95% CI: 1.07-1.29).
Figure 2.
A Random-Effect Meta-Analysis of 19 Independent Studies. Adjusted relative risk (RR) and 95% confidence interval (CI) of total cardiovascular events (including myocardial infarction, stroke, and cardiovascular death) was estimated comparing the lowest category versus the highest category of baseline circulating 25(OH)-vitamin D concentration. Squares indicate RR in each study. The size of the square is proportional to the precision of the RR (inverse of its variance). Horizontal line represents the 95% CI. The pooled RR and 95% CI are indicated by the unshaded diamond.
Based on available data for specific CVD events, the random-effects pooled RRs were 1.42 (95% CI: 1.19-1.71) for CVD mortality, 1.38 (95% CI: 1.21-1.57) for CHD, and 1.64 (95% CI: 1.27-2.10) for stroke, comparing the lowest with the highest category of 25(OH)-vitamin D. In stratified analyses to assess potential sources of heterogeneity between studies, the inverse association between 25(OH)-vitamin D and risk of CVD was consistent regardless of the age of participants, number of CVD cases, 25(OH)-vitamin D assay methods, exclusion of baseline CVD, and control for key confounders in the individual studies (Table 2). Associations appeared stronger in the studies that followed participants for <10 years, and attenuated when follow-up time was longer (p-interaction: 0.005).
Table 2.
Stratified meta-analyses of risk of cardiovascular disease comparing the lowest category with the highest category of 25(OH)-vitamin D concentration
| Study Characteristics | N of individual studies |
N of CVD cases |
Pooled RR (95% CI) |
P for interaction |
Test of between-study heterogeneity | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Q-Test | H | I2 (%) | |||||
| All studies | 19 | 6123 | 1.52 (1.30-1.77) | 0.0003 | 1.6 (1.2-2.1) | 61 (35-76) | |
| Average baseline age | 0.57 | ||||||
| <65 y11, 12, 14, 16, 25, 27, 34, 41 | 9 | 3547 | 1.66 (1.26-2.19) | <0.0001 | 2.1 (1.5-2.9) | 78 (57-88) | |
| ≥65 y15, 28, 29, 31-33, 35, 37, 38 | 10 | 2576 | 1.51 (1.32-1.74) | 0.35 | 1.1 (1.0-1.4) | 10 (0-51) | |
| Duration of follow-up | 0.005 | ||||||
| <10 y11, 14, 16, 25, 28, 29, 31, 32, 41 | 9 | 3275 | 1.86 (1.47-2.34) | 0.005 | 1.7 (1.2-2.4) | 64 (26-82) | |
| ≥10 y12, 15, 27, 33-35, 37, 38 | 10 | 2848 | 1.27 (1.11-1.44) | 0.53 | 1.0 (1.0-1.6) | 0 (0-62) | |
| Number of cases | 0.72 | ||||||
| <20011, 14, 15, 28, 29, 32, 35, 37 | 9 | 857 | 1.46 (1.15-1.85) | 0.17 | 1.2 (1.0-1.8) | 31 (0-68) | |
| ≥20012, 16, 25, 27, 31, 33, 34, 38, 41 | 10 | 5266 | 1.54 (1.26-1.89) | <0.0001 | 1.9 (1.4-2.6) | 73 (49-86) | |
| 25(OH)-vitamin D assay method | 0.97 | ||||||
| Radioimmunoassay11, 12, 15, 16, 25, 27-29 | 9 | 3180 | 1.54 (1.23-1.92) | 0.01 | 1.6 (1.1-2.3) | 60 (17-81) | |
| Other immunoassays14, 31, 34, 35, 37, 41* | 7 | 2275 | 1.53 (1.11-2.11) | <0.0001 | 2.1 (1.4-3.0) | 77 (51-89) | |
| Mass spectrometry32, 33, 38 | 3 | 668 | 1.47 (1.10-1.97) | 0.94 | 1.0 (1.0-3.1) | 0 (0-90) | |
| Exclusion of baseline cardiovascular disease | 0.53 | ||||||
| No14, 16, 25, 28, 29, 32-35, 37† | 11 | 2565 | 1.46 (1.14-1.87) | 0.001 | 1.7 (1.3-2.4) | 67 (38-83) | |
| Yes11, 12, 15, 27, 31, 33, 38, 41† | 9 | 3735 | 1.61 (1.36-1.90) | 0.11 | 1.3 (1.0-1.9) | 39 (0-72) | |
| Control for key confounding ‡ | 0.98 | ||||||
| No11, 14, 15, 25, 28, 31, 34 | 9 | 2743 | 1.50 (1.16-1.94) | 0.0001 | 2.0 (1.4-2.8) | 75 (51-87) | |
| Yes12, 16, 27, 29, 32, 33, 35, 37, 38, 41 | 10 | 3380 | 1.49 (1.26-1.77) | 0.18 | 1.2 (1.0-1.7) | 29 (0-66) | |
Methods include competitive protein-binding assay, chemiluminescence immunoassay, immunometry, and enzyme immunoassay.
One study provided results that excluded and did not exclude participants with baseline cardiovascular disease separately.
Control for a minimum of confounders including age, sex, season of blood collection, BMI, and physical activity.
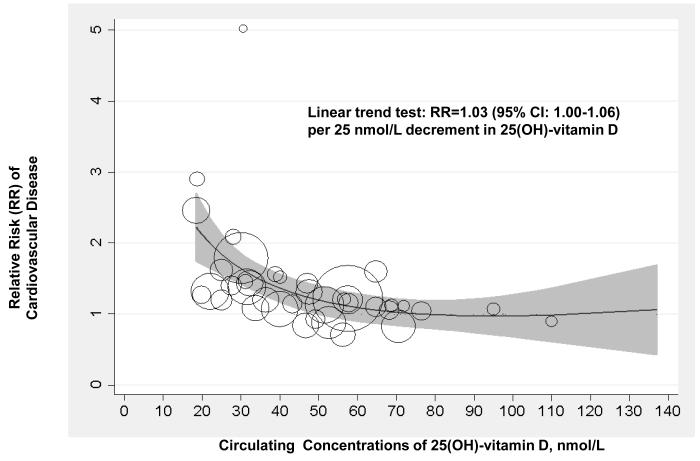
Figure 3 shows the dose-response association between circulating 25(OH)-vitamin D and risk of CVD, based on RRs of CVD according to categories of 25(OH)-vitamin D in 16 studies that provided complete data. Overall, the test for a linear relation across the range of 25(OH)-vitamin D from 20 up to 140 nmol/L was marginally significant (p=0.06). The CVD risk increased monotonically across decreasing 25(OH)-vitamin D concentration below approximately 60 nmol/L, with a pooled RR of 1.03 (95% CI: 1.00-1.06) per 25 nmol/L decrement in 25(OH)-vitamin D. There was no clear increase or decrease in CVD risk with 25(OH)-vitamin D over 60 nmol/L, based on the few data points. When the analysis was restricted to those studies that excluded participants with baseline CVD and had better controlled for confounding,12, 27, 33, 38, 41 the pooled RR for CVD per 25 nmol/L decrement in 25(OH)-vitamin D was 1.07 (95% CI: 1.03-1.12).
Figure 3.
Dose-response Association between Circulating 25(OH)-vitamin D and Risk of Cardiovascular Disease in 16 Prospective Studies. The analysis was conducted using a fractional polynomial spline regression. Circles indicate relative risk (RR) in each study. The size of the circle is proportional to the precision of the RR (inverse of its variance). The grey shaded region shows the 95% CIs around the regression line. The method described by Greenland and Longnecker for the dose-response analysis22 was used to compute the linear trend from the correlated RRs and 95% CIs across categories of 25(OH)-vitamin D.
In sensitivity analyses, we first evaluated the influence of individual studies on the pooled RRs of CVD comparing the lowest versus highest categories of 25(OH)-vitamin D. We omitted 2 small studies reporting very strong associations,14, 41 one study25 that was not population-based, and one34 using an assay found to overestimate 25(OH)-vitamin D.42 Subsequently, we repeated tests for the dose-response association between continuous 25(OH)-vitamin D and risk of CVD by assigning different exposure values to the open-ended categories of 25(OH)-vitamin D. The results did not change materially in these sensitivity analyses (data not shown).
Discussion
Our meta-analysis of prospective observational studies showed an inverse association between baseline circulating 25(OH)-vitamin D and risk of CVD with considerable heterogeneity between studies. Comparing the lowest versus the highest categories of 25(OH)-vitamin D concentration, the pooled RR for total CVD was 1.52 (95% CI: 1.30-1.77). The increment of CVD risk with decreasing 25(OH)-vitamin D was generally linear over the range of 25(OH)-vitamin D from 20 to 60 nmol/L, with a modest and marginally significant pooled RR of 1.03 (95% CI: 1.00-1.06) per 25 nmol/L decrement in 25(OH)-vitamin D. These associations remained significant when the analyses were restricted to the studies that excluded participants with baseline CVD and had better controlled for confounding.
A large body of evidence from laboratory studies suggest that vitamin D potentially has beneficial effects on cardiovascular risk factor profiles and development of CVD.3-7 Population studies have shown favorable associations of circulating vitamin D with CVD risk factors, particularly hypertension,43 impaired glucose tolerance or type 2 diabetes,44 and inflammation.45 In clinical studies, circulating 25(OH)-vitamin D was low in patients with myocardial infarction,8 stroke9, and peripheral arterial disease.10 Despite of the promising data from cross-sectional and case-control studies, results of prospective studies on the association between baseline 25(OH)-vitamin D and subsequent incidence of CVD events remain inconsistent. Our systematic review of literature, in line with earlier reviews,17, 18 found that the majority, albeit not all, prospective studies support an association between low circulating 25(OH)-vitamin D and increased risk of CVD events. The pooled RRs demonstrated a strong, highly significant, inverse association. Although there is considerable heterogeneity between individual studies, stratified analysis found that the inverse association was generally consistent in various subgroups except for the duration of follow-up. A stronger association was found in studies with <10 years follow-up than those with longer follow-up. This finding may reflect greater changes in 25(OH)-vitamin D over longer periods of time, or the competing risks for fatal and non-fatal diseases in older populations.
Some previous studies have suggested a possible non-linear association between 25(OH)-vitamin D and risk of CVD, with a threshold effect or even a U-shaped relation.11, 16 Due to the limited data and the heterogeneity between studies, especially with respect to categories of 25(OH)-vitamin D used, specific CVD outcomes, and confounders adjusted for, the optimal levels of 25(OH)-vitamin D for cardiovascular health has yet to be determined. To resolve this uncertainty, we examined the dose-response association and found that to be generally linear for 25(OH)-vitamin D ranging from 20 to approximately 60 nmol/L. The magnitude of linear association over the broad spectrum of 25(OH)-vitamin D was only modest: the pooled RR for CVD per 25 nmol/L decrement in 25(OH)-vitamin D was 1.03 when all studies were included, and 1.07 when limited to the studies that excluded participants with baseline CVD and had better controlled for confounding. The linear association appeared relatively stronger when combining data from 10 studies that reported the RR of CVD in association with continuous 25(OH)-vitamin D, presumably due to difference in data source, levels of 25(OH)-vitamin D, and modeling strategy. When we specifically evaluated the individual studies with extreme circulating 25(OH)-vitamin D levels, we found that the lowest category of 25(OH)-vitamin D studied was <25 nmol/L; in 3 studies11, 25, 41 that reported the results, all showed significant associations between low 25(OH)-vitamin D and increased risk of CVD. On the other hand, 8 studies12, 16, 31-35, 38 included 25(OH)-vitamin D >65 nmol/L in the comparison group versus a higher reference, with one study examining multiple categories well above 85 nmol/L;35 six found no significant change in the risk of CVD, suggesting a possible threshold effect.
Since our meta-analysis is based on observational study data, the result is subject to potential bias in all observational studies. Although potential confounders such as age, BMI, and physical activity have been adjusted for in individual studies, residual confounding cannot be ruled out. Causality underlying the observed association and the effect of vitamin D supplement on cardiovascular health cannot be addressed in our analysis and remain to be determined in future studies. Preliminary findings from clinical trials have suggested that vitamin D supplementation may reduce cardiovascular mortality46 or CVD event rate,47 supporting a possible role of vitamin D in CVD prevention. Nevertheless, existing trial data are insufficient and inconclusive. There is suggestive evidence that a moderate to high vitamin D exposure may be needed for prevention of CVD.48 It should be noted that the Women’s Health Initiative (WHI) Calcium-Vitamin D trial, in which 36,282 postmenopausal women were assigned to take calcium (1000 mg/d) plus vitamin D3 (400 IU/d) or placebo for 7 years, found no reduction in CHD or stroke incidence with combined calcium and vitamin D supplement.49 One of the most plausible reasons is that the vitamin D dose used in the WHI was too low that it did not change circulating vitamin D levels significantly.49 Although randomized trials are important to establish the causality of vitamin D-CVD relation, their inferences are commonly linked with a single dose of vitamin D that yields a narrow change rather than a full spectrum of 25(OH)-vitamin D. Our meta-analysis of prospective observational studies allows an evaluation on the dose-response association between vitamin D and CVD risk over a broad range of 25(OH)-vitamin D level, which will not only complement findings from ongoing randomized trials but also help to inform future trials that test the effect of vitamin D supplements on CVD regarding the optimal doses.
This meta-analysis has several potential limitations. First, because our analyses were based on published studies, publication bias is a concern. However, a visual inspection of plot and formal tests indicated no evidence of substantial publication bias. Second, some individual studies that reported very strong associations may have influenced the meta-analysis results. Nevertheless, our sensitivity analyses showed that the pooled RR was similar after removing the studies with high RRs. Third, the optimal 25(OH)-vitamin D for cardiovascular health may differ by race/ethnicity. However, since virtually all existing studies were conducted among predominantly Caucasian cohorts, we were unable to assess race/ethnicity specific associations between vitamin D and CVD, even after combining data in this meta-analysis. Finally, the potential bias in each individual study and high heterogeneity between studies preclude definitive conclusions.
Despite of the controversy on causation, improving vitamin D status has been proposed as one promising strategy for CVD prevention. Given the high prevalence of vitamin D insufficiency in the general U.S. population, the potential public health impact of vitamin D improvement would be substantial. Current dietary guidelines for vitamin D by IOM20 that recommend 600 IU/day for persons 1 to 70 years of age and 800 IU/day for persons over 70 years, corresponding to a circulating 25(OH)-vitamin D of 50 nmol/L or more assuming minimal sun exposure, are solely based on the beneficial effect of vitamin D on bone health. The IOM report concluded that evidence was insufficient to demonstrate that vitamin D protects against CVD and called for additional research to elucidate this association. Our meta-analysis based on existing literature showed that low levels of circulating 25(OH)-vitamin D are associated with an increased risk of CVD. The dose-response curve between 25(OH)-vitamin D and CVD risk indicated that the association was generally linear across the range of 25(OH)-vitamin D from 20 to 60 nmol/L with a marginally significant trend. More prospective studies and randomized clinical trials are needed to further clarify the association between 25(OH)-vitamin D higher than 60 nmol/L and CVD risk and to assess the causality of observed associations.
Supplementary Material
What is Known.
-
-
Vitamin D has pleiotropic effects that may favorably influence cardiovascular health.
-
-
Cross-sectional and retrospective case-control studies found deficient or insufficient levels of circulating 25hydroxy-vitamin D in patients with established cardiovascular disease.
What this Article Adds.
-
-
Baseline circulating 25hydroxy-vitamin D was inversely associated with risk of cardiovascular disease in majority, but not all, of prospective studies.
-
-
The inverse association was consistent in various subgroup analysis stratified by study characteristics.
-
-
The increment of cardiovascular disease risk with decreasing 25hydroxy-vitamin D was generally linear over the range of 25hydroxy-vitamin D from 20 to 60 nmol/L (8 to 24 ng/mL).
Acknowledgement
We thank Drs. Michal Melamed (NHANES III), Robin Fullman and Peggy M. Cawthon (MsOS) for kindly contributing additional data from their studies to our data extraction and meta-analysis. Drs. Wang and Song had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding: Dr. Wang is supported by a grant R00-HL095649 from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), Bethesda, MD. Dr. Song is funded by a grant R01-DK088078 from the NIH/National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Dr. JoAnn Manson is funded by grants HL34594 and CA138962 from NIH. Dr. Zhang is supported by the Intramural Research Program of the Eunice Kennedy Shriver NIH/National Institute of Child Health & Human Development (NICHD). These funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Michos ED, Melamed ML. Vitamin d and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin d deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin d: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Rammos G, Tseke P, Ziakka S. Vitamin d, the renin-angiotensin system, and insulin resistance. Int Urol Nephrol. 2008 doi: 10.1007/s11255-007-9244-4. [DOI] [PubMed] [Google Scholar]
- 5.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin d deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 6.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-dihydroxyvitamin d3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 7.Deluca HF, Cantorna MT. Vitamin d: Its role and uses in immunology. Faseb J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 8.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin d3 levels: A community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 9.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduced vitamin d in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin d levels and the prevalence of peripheral arterial disease: Results from nhanes 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin d deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin d and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 14.Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM. Vitamin d and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–672. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- 15.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15:188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin d levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokol SI, Tsang P, Aggarwal V, Melamed ML, Srinivas VS. Vitamin d status and risk of cardiovascular events: Lessons learned via systematic review and meta-analysis. Cardiol Rev. 2011;19:192–201. doi: 10.1097/CRD.0b013e31821da9a5. [DOI] [PubMed] [Google Scholar]
- 18.Grandi NC, Breitling LP, Brenner H. Vitamin d and cardiovascular disease: Systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee to review dietary reference intakes for vitamin d and calcium, institute of medicine . Dietary reference intakes for calcium and vitamin d. National academy press; Washington, dc: 2011. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose- response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 23.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, Marz W. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–2613. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 25.Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin d deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 26.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25- hydroxyvitamin d level, cardiovascular disease mortality, and all-cause mortality in older u.S. Adults. J Am Geriatr Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 27.Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliovaara M, Impivaara O, Reunanen A. Vitamin d status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 28.Bolland MJ, Bacon CJ, Horne AM, Mason BH, Ames RW, Wang TK, Grey AB, Gamble GD, Reid IR. Vitamin d insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr. 2010;91:82–89. doi: 10.3945/ajcn.2009.28424. [DOI] [PubMed] [Google Scholar]
- 29.Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR, Guralnik JM, Ferrucci L. Relationship of 25-hydroxyvitamin d with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr. 2010;64:203–209. doi: 10.1038/ejcn.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiscella K, Franks P. Vitamin d, race, and cardiovascular mortality: Findings from a national us sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappe DL, Muhlestein JB. Relation of vitamin d deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Cawthon PM, Parimi N, Barrett-Connor E, Laughlin GA, Ensrud KE, Hoffman AR, Shikany JM, Cauley JA, Lane NE, Bauer DC, Orwoll ES, Cummings SR. Serum 25- hydroxyvitamin d, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010;95:4625–4634. doi: 10.1210/jc.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstrom J, Berglund L, Arnlov J, Hellman P, Blomhoff R, Wolk A, Garmo H, Holmberg L, Melhus H. Plasma vitamin d and mortality in older men: A community-based prospective cohort study. Am J Clin Nutr. 2010;92:841–848. doi: 10.3945/ajcn.2010.29749. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25- hydroxyvitamin d levels are associated with increased all-cause mortality risk in a general population: The tromso study. Eur J Endocrinol. 2010;162:935–942. doi: 10.1530/EJE-09-1041. [DOI] [PubMed] [Google Scholar]
- 35.Jassal SK, Chonchol M, von Muhlen D, Smits G, Barrett-Connor E. Vitamin d, parathyroid hormone, and cardiovascular mortality in older adults: The rancho bernardo study. Am J Med. 2010;123:1114–1120. doi: 10.1016/j.amjmed.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messenger W, Nielson CM, Li H, Beer T, Barrett-Connor E, Stone K, Shannon J. Serum and dietary vitamin d and cardiovascular disease risk in elderly men: A prospective cohort study. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton CB, Young A, Allison MA, Robinson J, Martin LW, Kuller LH, Johnson KC, Curb JD, Van Horn L, McTiernan A, Liu S, Manson JE. Prospective association of vitamin d concentrations with mortality in postmenopausal women: Results from the women’s health initiative (whi) Am J Clin Nutr. 2012;94:1471–1478. doi: 10.3945/ajcn.111.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin d, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58:1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin d, parathyroid hormone, and sudden cardiac death: Results from the cardiovascular health study. Hypertension. 2011;58:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, Sharrett AR, Melamed ML. 25-hydroxyvitamin d deficiency is associated with fatal stroke among whites but not blacks: The nhanes-iii linked mortality files. Nutrition. 2012;28:367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosseinpanah F, Yarjanli M, Sheikholeslami F, Heibatollahi M, Eskandary PS, Azizi F. Associations between vitamin d and cardiovascular outcomes; tehran lipid and glucose study. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Grimnes G, Almaas B, Eggen AE, Emaus N, Figenschau Y, Hopstock LA, Hutchinson MS, Methlie P, Mihailova A, Sneve M, Torjesen P, Wilsgaard T, Jorde R. Effect of smoking on the serum levels of 25-hydroxyvitamin d depends on the assay employed. Eur J Endocrinol. 2010;163:339–348. doi: 10.1530/EJE-10-0150. [DOI] [PubMed] [Google Scholar]
- 43.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 44.Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, Reunanen A. Serum 25-hydroxyvitamin d concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–2570. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 45.Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB, Sr., Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, Keaney JF, Jr., Vasan RS, Benjamin EJ. Vitamin k and vitamin d status: Associations with inflammatory markers in the framingham offspring study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin d3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. Bmj. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168:103–108. doi: 10.1001/archinternmed.2007.31. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin d and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 49.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M. Calcium/vitamin d supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.