Abstract
Mosquito midgut invasion by ookinetes of the malaria parasite Plasmodium disrupts the barriers that normally prevent the gut microbiota from coming in direct contact with epithelial cells. This triggers a long-lived response characterized by increased abundance of granulocytes, a subpopulation of hemocytes, circulating in the insect’s hemocoel, and enhanced immunity to bacteria that indirectly reduces survival of Plasmodium parasites upon reinfection. In mosquitoes, differentiation of hemocytes was necessary and sufficient to confer innate immune memory.
The innate immune system is thought to be hard-wired and unable to establish immunological memory (1). Insects lack adaptive immunity and rely on the innate immune system to mount defense responses against pathogenic organisms; however, memory-like responses have been described in invertebrates, a phenomena that has been termed immune priming (2). There are multiple examples of nonspecific (3–5) and pathogen-specific (6–11) priming in insects that can be long-lasting. Although these studies challenge the dogma that invertebrates are incapable of adaptive immune responses, a mechanism for innate immune memory has not been established.
An. gambiae mosquitoes are the major vectors of Plasmodium falciparum malaria in Africa. In the present study we found that Plasmodium ookinete invasion of the mosquito midgut, in the presence of gut bacteria, primed a robust long-lived enhanced antibacterial response that also reduced Plasmodium survival upon re-challenge. Immune priming resulted in quantitative and qualitative differentiation of hemocytes, the insect equivalent of white blood cells, that persisted for the lifespan of the mosquito.
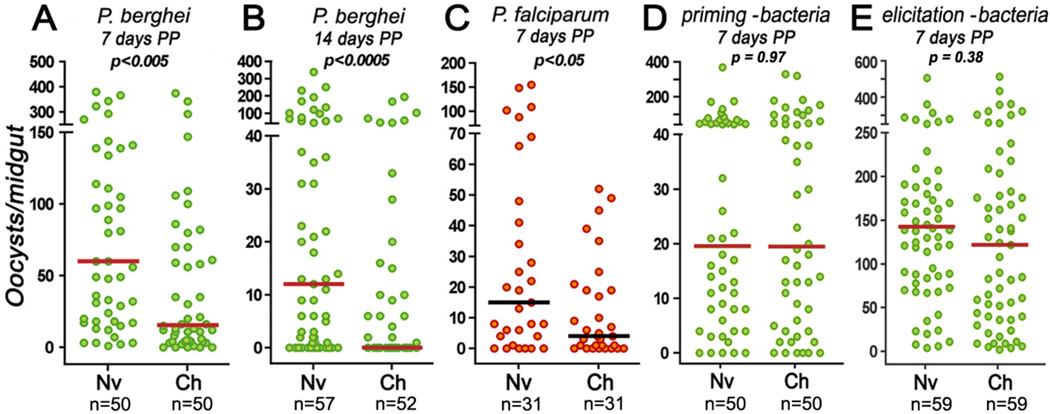
We investigated the effect of pre-exposure of mosquitoes to Plasmodium infection on a subsequent infection (Fig. S1) (12). Two groups of mosquitoes were fed on the same infected mouse. One group was placed at 28°C immediately after blood feeding, a temperature that prevents Plasmodium berghei ookinete formation and mosquito infection (Fig. S2). We refer to these mosquitoes as the naïve group. The challenged group was kept at 21°C for 48 h to allow ookinete formation and midgut invasion, and was then switched to 28°C to reduce oocyst survival (Fig. S2). Seven days post-feeding (dpf), both groups were infected by feeding on a second mouse. Re-exposure to Plasmodium infection greatly reduced the intensity of infection in the challenged group (Fig. 1A) (P < 0.005). This immune enhancement was also observed in females re-challenged 14 d post-priming (dpp) (P < 0.0005) (Fig. 1B). The time between priming and re-challenge was not extended because of age-related mosquito mortality. Pre-exposure of mosquito females to Plasmodium falciparum (NF54 strain) infection had a similar effect, reducing oocyst density when re-challenged with the same parasite (P < 0.05, KS test) (Fig. 1C and S3).
Fig. 1.
Effect of pre-exposure to Plasmodium infection on the immune response to subsequent infections. (A) 7 days post priming (dpp) or (B) 14 dpp with P. berghei in naïve (Nv) or challenged (Ch) mosquitoes. (C) Effect of pre-challenge with P. falciparum on a second infection at 7 dpp. Plasmodium infections were evaluated 7 days after the second infection. Effect of eliminating the gut microbiota with oral antibiotics before the (D) first or (E) second challenge with P. berghei on a second infection 7 dpp. Each circle represents the number of parasites in an individual midgut, and the line indicates the median.
Large numbers of commensal bacteria are present in the midgut lumen when Plasmodium ookinetes invade epithelial cells. The blood meal is surrounded by a chitinous peritrophic matrix (PM) that normally prevents bacteria from interacting directly with epithelial cells, but ookinetes disrupt this barrier (13) to invade midgut cells (Fig. S4), causing irreversible damage (14). Elimination of the gut microbiota enhances Plasmodium infection (15–17) and activation of some mosquito antibacterial responses have been shown to indirectly kill Plasmodium (17).
Gut bacteria were eliminated by oral administration of antibiotics before the first feeding (Fig. S1, yellow area), which prevented immune priming to Plasmodium (Fig. 1D). Then priming was allowed to occur in the presence of bacteria, but antibiotics were given 2 days prior to the second infection (Fig. S1, beige area). Elimination of the gut microbiota when mosquitoes are re-challenged prevented elicitation of the priming response (Fig. 1E). It was the interaction between bacteria and invaded midgut cells that appeared to elicit the response, which indicates that the decrease in Plasmodium infection in the challenged group (Fig. 1A and B) was not due to persistent immune activation in response to the first plasmodial infection.
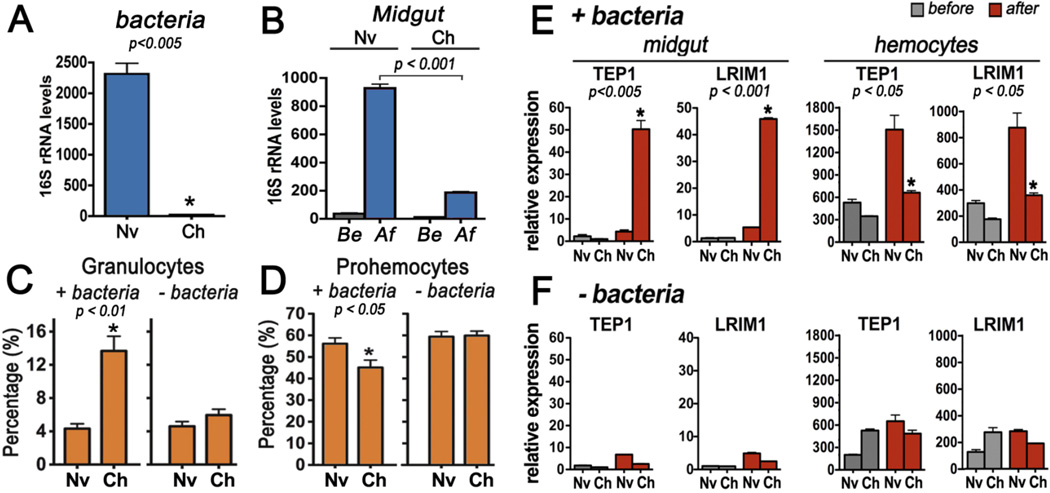
The effect of priming on mosquito commensal bacteria was investigated. Total bacteria in challenged mosquitoes 7 dpp were up to 100-fold lower (P< 0.005) (Fig. 2A). Bacteria levels in the midgut increased when either naïve or challenged mosquitoes receive a second infected meal, but are significantly reduced in the challenged group (P < 0.001) 24 hours post infection (hpi) (Fig. 2B).
Fig. 2.
Effect of immune priming with Plasmodium/bacteria on commensal bacteria, hemocyte populations, and gut-associated hemocyte-specific antiplasmodial mRNAs. (A) Effect of priming on whole-body bacterial levels in naïve (Nv) and challenged (Ch) mosquitoes 7 days post priming (dpp). (B) Total midgut bacteria before (Be) or 24 hours after (Af) a second infectious meal was given 7 dpp. Bacterial 16S rRNA levels were determined using real-time PCR. Effect of immune priming on the relative abundance of (C) granulocytes and (D) prohemocytes 7dpp in the presence or absence of gut microbiota. Effect of immune priming on the relative abundance of TEP1 and LRIM1 mRNAs associated with the midgut or circulating hemocytes in (E) the presence or (F) the absence of gut microbiota determined before (grey bars) or 24 hours after (red bars) a second infectious meal was given 7 dpp. Bars represent the Mean ± SEM.
No significant difference in total number of hemocytes or in relative abundance of oenocytoids (Fig. S5) was observed between naïve and challenged mosquitoes 7 dpp; however, the granulocyte population increased by up to 3.2 fold (Fig. 2C) (P < 0.01) and prohemocytes decreased by 10% (Fig. 2D) (P < 0.05) in the challenged group, suggesting that prohemocyte precursors differentiated into granulocytes. This response was still present 14 dpp (Fig. S6) but was no longer observed when priming was prevented by eliminating the gut microbiota (Fig. 2C and D). Hemocytes respond to ookinete invasion by attaching to the basal surface of the midgut, increasing the mRNA levels of hemocyte-specific genes such as thioester-containing protein 1 (TEP1) and leucine-rich repeat immune protein 1 (LRIM1) associated with midguts dissected 24 hpi (18, 19). This response is transient, as higher transcript levels are not detected 48 hpi (19). In our experiments midgut invasion increased TEP1 and LRIM1 mRNA levels in naïve and primed mosquitoes 24 hpi (Fig. 2E), but in challenged mosquitoes the increase in TEP1 and LRIM1 levels were up to 12 and 8 fold higher, respectively (P < 0.005)(Fig. 2E). As expected, a concomitant reduction in TEP1 and LRIM1 levels was observed in the population of circulating hemocytes from the same mosquitoes (P < 0.05) (Fig. 2E), which we speculate indicates that some hemocytes that express TEP1 and LRIM1 disappear from circulation as they attach to the midgut. This response was only observed when primed mosquitoes were re-infected, indicating an enhanced response to re-challenge rather than a persistent long-lasting response following the initial exposure to Plasmodium/bacteria. This enhanced hemocyte response was not observed in mosquitoes in which priming was prevented by eliminating bacteria from the gut microbiota (Fig. 2F).
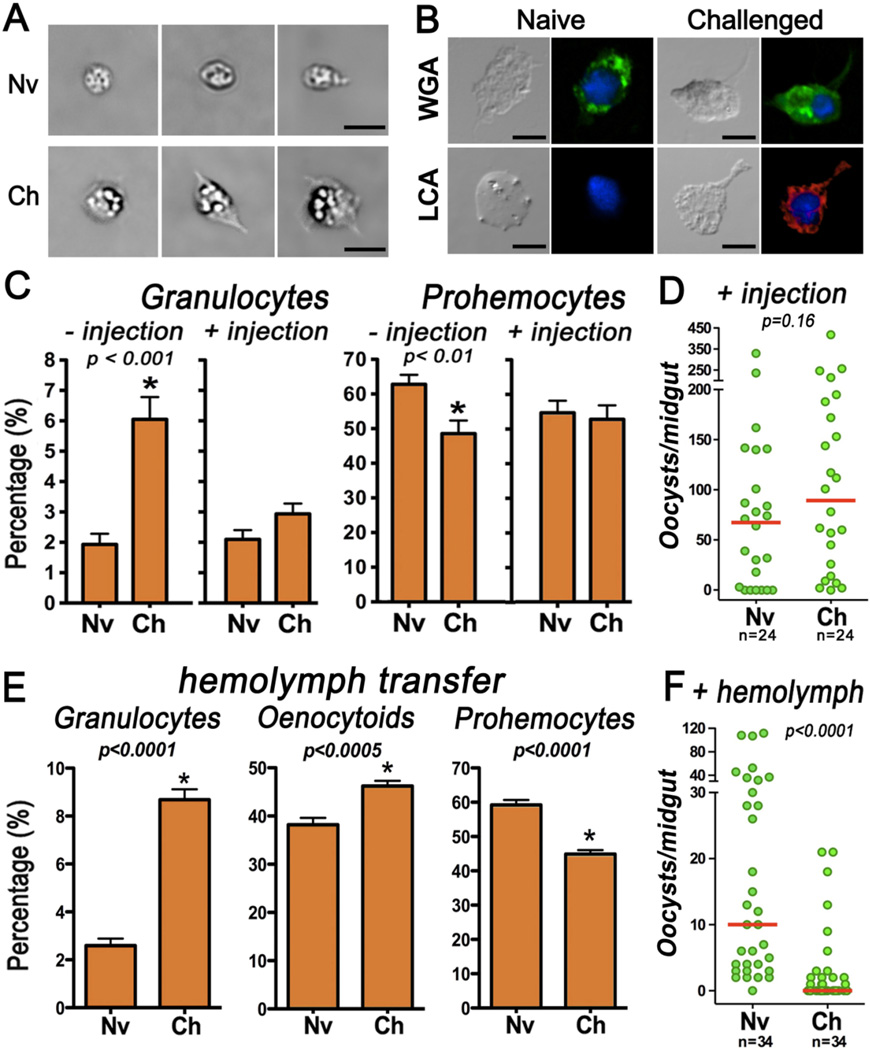
Morphologic differences between granulocytes from naïve and challenged mosquitoes were apparent immediately after hemocytes were placed in plastic Neubauer chambers for counting. The cytoplasm of granulocytes from challenged mosquitoes was larger and more granular, and pseudopodial extensions were observed (Fig. 3A). However, when naïve or challenged granulocytes attached to a glass surface, these morphological differences were no longer apparent (Fig. 3B). Comparison of the lectin-binding properties of naïve and challenged hemocytes to fluorescence-conjugated lectins revealed that wheat germ agglutinin (WGA) stained all hemocytes from naïve and challenged mosquitoes, including granulocytes (Fig. 3B). In contrast, only granulocytes from challenged mosquitoes stained with Lens culinaris agglutinin (LCA) (Fig. 3B and Table S1), while most (96%) of naïve granulocytes were negative and 4% stained weakly (P < 0.0001; χ2).
Fig. 3.
Effect of immune priming on granulocyte morphology and binding properties, and of water injection on or hemolymph transfer on hemocyte populations and Plasmodium infection. (A) Morphology of naïve (Nv) and challenged (Ch) granulocytes in a plastic Neubauer chamber (bar = 7.5 µm). (B) Effect of priming on lectin-binding properties of granulocytes. WGA, wheat germ agglutinin; LCA, Lens culinaris agglutinin (bar = 5 µm). Effect of water injection (69 nl) on circulating (C) granulocytes and prohemocytes. (D) Effect of water injection 7 days post priming on Plasmodium infection. Effect of cell-free hemolymph transfer from Nv or Ch mosquito on (E) circulating granulocytes, oenocytoids, and prohemocytes and (F) on Plasmodium infection 4 days post transfer. Each circle represents the number of parasites in an individual midgut, and the line indicates the median. Bars represent the Mean ± SEM.
We disrupted granulocytes by injecting Sephadex beads resuspended in water or PBS into the hemocele to test whether they were necessary to elicit the priming response. A control group was injected with water alone. Unexpectedly, when challenged mosquitoes were injected with a small volume of water (69 nl), the proportion of circulating granulocytes decreased to levels similar to those of naïve mosquitoes, and differences in the proportion of prohemocytes between naïve and challenged mosquitoes were no longer observed (Fig. 3C). This 2-fold reduction in circulating granulocytes in the challenged group is highly significant (P<0.0001) and abolished priming, suggesting that these cells are necessary to elicit the priming response (Fig. 3D). Priming is also disrupted when challenged mosquitoes are subjected to aseptic injury or are injected with PBS or Sephadex beads in water (Fig. S7). We speculate that primed granulocytes are probably recruited to the injury site, disappear from circulation and are no longer available to mediate an enhanced immune response when mosquitoes are re-infected with Plasmodium.
Finally, we explored whether transfer of cell-free hemolymph or hemolymph containing hemocytes from challenged mosquitoes could confer enhanced immunity in recipient mosquitoes that had only fed on sugar. Transfer of cell-free hemolymph from challenged mosquitoes by systemic injection increased the relative abundance of circulating granulocytes and oenocytoids (Fig. 3E), decreased prohemocytes (Fig. 3E), and greatly reduced both the intensity (Fig. 3F) (P < 0.0001) and the prevalence of Plasmodium infection (from 92% to 51%; P < 0.001; χ2) in the recipients, relative to mosquitoes that received hemolymph from naïve donors. When hemolymph containing hemocytes from challenged mosquitoes was transferred, granulocytes increased and prohemocytes were reduced in the recipients, but oenocytoids were not affected, and Plasmodium infection was also reduced significantly (P < 0.0001) (Fig. S8). Transfer of cell-free hemolymph did not affect the total number of hemocytes in the recipients, but a significant reduction in circulating hemocytes (P<0.005) was observed in mosquitoes that receive challenged hemocytes (Fig. S9). Transfer of these cells may reduce the viability, proliferation and/or adherence of endogenous hemocytes.
A strong priming response was established when ookinetes breached the gut barriers and bacteria came in contact with injured epithelial cells. The mosquito immune system was stimulated to a systemic state of enhanced immune surveillance. Upon re-exposure to a similar insult, mosquitoes mounted a more effective antibacterial response that indirectly harmed Plasmodium parasites. We conclude that the mosquito immune system can adapt by modulating the abundance and responsiveness of different hemocyte populations. Exposure to Plasmodium/bacteria infection increases the proportion of circulating granulocytes and triggers changes in the morphology and binding properties of these cells. Circulating granulocytes mediate responses that enhance antiplasmodial immunity in challenged mosquitoes. Possibly, a soluble hemocyte differentiation factor(s) is released into the hemolymph of challenged mosquitoes that, when transferred, induces hemocyte differentiation in the recipients and confers enhanced antiplasmodial immunity. We propose that long-term functional changes in the immune system that allow an insect to mount a more effective immune response upon reencountering a microbe is a form of innate immune memory. Future studies will be required to define the characteristics of this type of response and to establish how it differs from immune memory in vertebrates. Understanding the molecular mechanism(s) mediating insect immune responses such as induced hemocyte differentiation, may also provide insights into the evolution of immune memory.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research, NIAID, National Institutes of Health. We thank André Laughinghouse and Kevin Lee for insectary support; Corrie Ortega for experimental support; Lily Koo for confocal assistance; José Ribeiro and Jesus Valenzuela for their comments and insight; and Brenda Rae Marshall for editorial assistance. F.B. and L. A. were funded by the CNPq and CAPES Brazilian government agencies, respectively.
Footnotes
Supporting Online Material
Materials and Methods
Table S1
Figs. S1 to S8
References and Notes
- 1.Hoffmann JA. Nature. 2003;426:33. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel P. Annu. Rev. Entomol. 2005;50:529. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- 3.Moret Y, Siva-Jothy MT. Proc. Biol. Sci. 2003;270:2475. doi: 10.1098/rspb.2003.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MJ, Moret Y, Schmid-Hempel P. Parasitology. 2003;126:253. doi: 10.1017/s0031182002002755. [DOI] [PubMed] [Google Scholar]
- 5.Eleftherianos I, et al. Insect Biochem. Mol. Biol. 2006;36:517. doi: 10.1016/j.ibmb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Sadd BM, Schmid-Hempel P. Curr. Biol. 2006;16:1206. doi: 10.1016/j.cub.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Proc. Biol. Sci. 2009;276:145. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. Curr. Biol. 2003;13:489. doi: 10.1016/s0960-9822(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 10.Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Biol. Lett. 2005;1:386. doi: 10.1098/rsbl.2005.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth O, et al. J. Anim. Ecol. 2009 [Google Scholar]
- 12.See the supporting material on Science Online.
- 13.Huber M, Cabib E, Miller LH. Pro.c Natl. Acad. Sci. U. S. A. 1991;88:2807. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. EMBO J. 2000;19:6030. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beier MS, Pumpuni CB, Beier JC, Davis JR. J. Med. Entomol. 1994;31:561. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Manfredini F, Dimopoulos G. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meister S, et al. PLoS Pathog. 2009;5:e1000542. doi: 10.1371/journal.ppat.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blandin S, et al. Cell. 2004;116:661. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 19.Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC. Curr. Biol. 2005;15:1185. doi: 10.1016/j.cub.2005.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.