Abstract
Extracellular matrices in diverse biological systems are crosslinked by dityrosine covalent bonds catalyzed by the peroxidase/oxidase system. We show that the Immunomodulatory Peroxidase (IMPer), an enzyme secreted by the mosquito Anopheles gambiae midgut, and dual oxidase (Duox) form a dityrosine network that decreases gut permeability to immune elicitors and protects the microbiota by preventing activation of epithelial immunity. It also provides a suitable environment for malaria parasites to develop within the midgut lumen without inducing nitric oxide synthase expression. Disruption of this barrier results in strong and effective pathogen-specific immune responses.
Keywords: malaria, A. gambiae, mosquito, Plasmodium, immunomodulatory peroxidase, IMPer, dual oxidase, Duox, epithelial immunity, dityrosine
Insects, like most metazoa, harbor large numbers of commensal bacteria within their guts. Midgut epithelial cells need to protect the host from pathogenic organisms but must do so without mounting immune responses against the normal microbiota. This is especially challenging in blood-feeding insects, because commensal bacteria proliferate extensively during blood digestion (1). In Drosophila, dual oxidase (Duox) has been shown to mediate a microbicidal response that prevents overproliferation of dietary bacteria and yeast (2) (3). Duox is a transmembrane protein that generates hydrogen peroxide, a substrate required by peroxidases (4).
In many hematophagous insects, including the mosquito Anopheles gambiae, the midgut secretes a peritrophic matrix (PM) in response to blood feeding. The PM is an acellular, semipermeable layer of chitin polymers that surrounds the blood meal and prevents blood cells and gut bacteria from coming in direct contact with midgut epithelial cells (5, 6). Mucins are secreted into the ectoperitrophic space (7) between the PM and the midgut epithelium. In this study, we characterize a heme peroxidase, immunomodulatory peroxidase (IMPer) (AGAP013327-PA or HPX15 http://cegg.unige.ch/insecta/immunodb/) that is secreted by An. gambiae midgut epithelial cells in response to blood feeding. We found that IMPer, together with Duox, catalyzes protein crosslinking in the mucin layer, reduces permeability to immune elicitors, and prevents immune responses against bacteria and Plasmodium parasites. We uncovered an unexpected, previously unrecognized function of the peroxidase/Duox system that protects the gut microbiota.
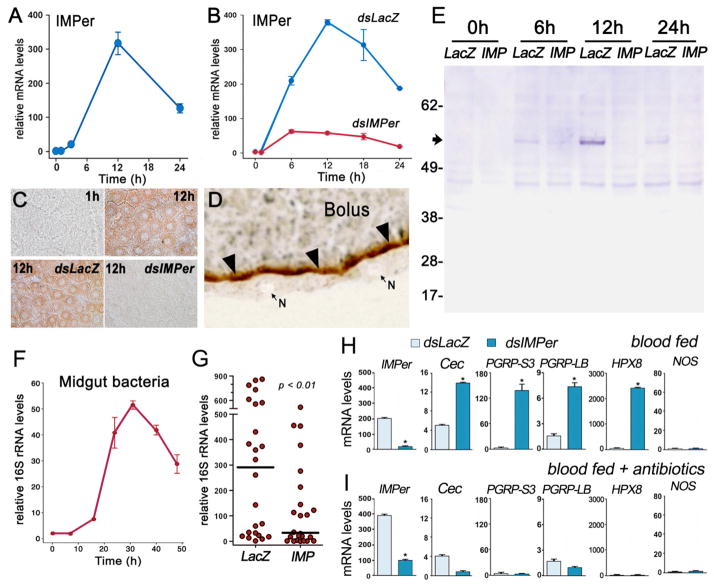
Blood feeding induces IMPer mRNA, protein, and enzymatic activity in the midgut of An. gambiae females that peak around 12 h after feeding and are effectively silenced by systemic injection of IMPer dsRNA (Fig. 1, A–E). IMPer enzymatic activity is localized in the periphery of the blood bolus (Fig. 1D) and is greatly reduced when IMPer is silenced (Fig. S1). Anti-IMPer antibodies recognize two bands (56 and 57 kDa) in midgut homogenates, close to the expected size for IMPer (56.5 kDa) (Fig. 1E).
Fig. 1.
Expression and localization of IMPer and effect of IMPer silencing on bacterial growth and immune activation. (A) IMPer mRNA expression and (B) effect of IMPer silencing on IMPer mRNA levels at different times after blood feeding (mean ± SEM). (C) Effect of IMPer silencing on midgut peroxidase activity detected using 3,3′-diaminobenzidine (DAB) staining at different times after feeding. (D) Localization of inducible peroxidase activity detected using DAB staining (brown) in midgut sections 12 h after feeding. N, nucleus of epithelial cells. (E) Western blot of IMPer protein in midgut homogenates collected at different times after feeding in control (dsLacZ injected) or IMPer-silenced mosquitoes. (F) Bacterial 16S rRNA levels in midguts collected at different times after blood feeding (mean ± SEM). (G) Effect of IMPer silencing on bacterial 16S rRNA of individual midguts 24 h after feeding (line indicates the median) (mean ± SEM). (H) IMPer, Cecropin, PGRP-S3, PGRP-LB, HPX8, and NOS mRNA levels in control (dsLacZ-injected) and IMPer-silenced midguts 24 h after feeding (mean ± SEM). (I) Same as (H) but in antibiotic-fed mosquitoes.
Midgut bacteria proliferation peaks around 30 h after feeding (Fig. 1F). Unexpectedly, IMPer silencing reduces the median level of bacterial 16S rRNA in individual midguts collected 24 h after feeding by 8.8 fold (P < 0.01) (Fig. 1G) and bacterial genome copies by 9 fold (P < 0.01) (Fig. S2). This indicates that IMPer does not mediate a microbicidal response but, on the contrary, it is required for bacterial survival.
The possibility that IMPer modulates midgut antibacterial responses was explored. Gene expression microarray analysis identified several putative midgut immune genes that are induced by a protein meal containing a large dose of heat-killed bacteria, which interact directly with epithelial cells before the PM barrier is formed (Fig. S3 and Table S1). qRT-PCR confirmed the induction of ten markers (Fig. S4). Four of them—the antibacterial peptide Cecropin, Peptidoglycan Recognition Protein–S3 (PGRP-S3), PGRP-LB (a negative regulator of the Imd pathway) (8), and Heme-Peroxidase 8 (HPX8)—are induced when IMPer is silenced, while expression of nitric oxide synthase (NOS) is not affected (Fig. 1H). These markers are no longer induced when midgut bacteria are eliminated by pretreating mosquitoes with oral antibiotics (Fig 1I).
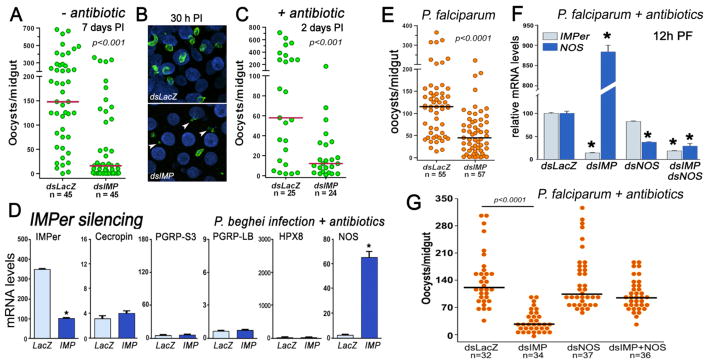
In addition to its direct participation in antimicrobial activity, the Duox system also catalyzes crosslinking of extracellular matrices in very diverse biological systems by forming covalent bonds between tyrosine residues (dityrosine bonds) (9, 10). Our initial findings suggest that IMPer could be required to form a barrier that limits rate of diffusion of immune elicitors and prevents immune activation. Given the negative effect of IMPer silencing on bacterial growth, we next asked whether IMPer also decreased Plasmodium berghei (rodent malaria) survival. IMPer silencing reduces the median number of P. berghei oocysts present 7 days post infection (PI) by 9.2 fold (Fig. 2A). This effect is already observed early in the invasion process (30 h after feeding), when the number of intact ookinetes is greatly reduced (Fig. S5). In IMPer-silenced females, ookinetes invade the midgut but are killed and appear fragmented (Fig. 2B). The drastic reduction of Plasmodium infection in IMPer-silenced mosquitoes is not due to activation of antibacterial responses, as it is also observed in females pretreated with oral antibiotics (Fig. 2C). As expected, antibacterial markers are not induced when antibiotic-treated females are infected with Plasmodium, indicating that IMPer silencing activates these genes only when bacterial elicitors are present in the midgut lumen. Instead, there is a dramatic induction of NOS, an enzyme that generates nitric oxide (a potent antiplasmodial effector molecule) (Fig. 2D). These findings indicate that IMPer is required for Plasmodium parasites to develop in the midgut without activating the immune pathway(s) that regulate NOS expression. We have previously shown that overactivation of the STAT pathway induces high levels of NOS 4 days PI that greatly reduce early oocyst survival (11), but the abnormal induction of NOS in IMPer-silenced mosquitoes is observed earlier.
Fig. 2.
Effect of IMPer silencing on Plasmodium infection and midgut immune activation. Effect of IMPer silencing on P. berghei infection (A) 7 days post infection (PI), (B) 30 h PI, and (C) 2 days PI in antibiotic-fed mosquitoes. (D) IMPer, Cecropin, PGRP-S3, PGRP-LB, HPX8, and NOS mRNA levels in control (dsLacZ-injected) and IMPer-silenced midguts of antibiotic-treated mosquitoes 24 h PI (mean ± SEM). (E) Effect of IMPer silencing on P. falciparum infection 8 days PI. (F) Effect of silencing IMPer, NOS, or co-silencing IMPer and NOS, on IMPer and NOS mRNA expression 12 h PI in antibiotic-fed mosquitoes infected with P. falciparum (mean ± SEM) and (G) P. falciparum infection 7 days PI. Asterisks indicate significant differences relative to the dsLacZ control. Each circle represents the number of parasites in an individual midgut, and the line indicates the median.
Plasmodium falciparum infection is also greatly reduced in IMPer-silenced An. gambiae (Fig. 2, E and G) and An. stephensi (Fig. S6) mosquitoes 7 days PI. Furthermore, IMPer silencing in antibiotic-treated An. gambiae mosquitoes infected with P. falciparum also triggers a strong induction of midgut NOS expression 12 h after feeding (Fig. 2F) and reduces infection (P < 0.0001) (Fig. 2G). At this time, immature ookinetes are still developing in the blood bolus, within the PM matrix. When IMPer is not present, epithelial cells can detect Plasmodium immune elicitors, possibly glycosylphosphatidylinositols (GPIs), which activate NOS expression. Previous studies have shown that oral administration of Plasmodium GPIs activates midgut NOS expression through the Akt/protein kinase B pathway (12). To determine whether high levels of NOS mediate parasite killing, both IMPer and NOS were silenced. NOS mRNA levels are 30-fold less in the double-silenced mosquitoes than when only IMPer is silenced (Fig. 2F). Lower NOS levels prevent the deleterious effect of IMPer silencing on Plasmodium infection (Fig. 2G), indicating that the antiplasmodial effect of IMPer silencing is mediated by NOS, probably by increasing the rate of nitration when parasites invade gut epithelial cells (13).
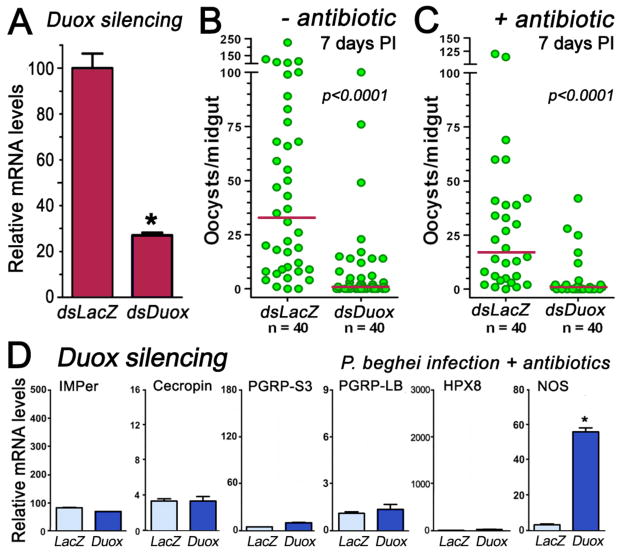
Duox generates hydrogen peroxide, a substrate required for IMPer to be active, on the luminal surface of epithelial cells. We investigated whether Duox is also required to prevent activation of antiplasmodial responses. dsDuox silencing reduces midgut Duox mRNA levels by 77% (Fig. 3A) and drastically reduces Plasmodium infection in the presence (Fig. 3B) or absence (Fig. 3C) of bacteria. This phenotype is very similar to that observed when IMPer is silenced (Fig. 2A). In antibiotic-treated females infected with Plasmodium, Duox silencing does not induce expression of the antibacterial markers (Fig. 3E); however, NOS expression is highly induced. Together, our data support the hypothesis that IMPer and Duox are both required to prevent activation of midgut responses to Plasmodium immune elicitors.
Fig. 3.
Effect of Duox silencing on Plasmodium infection and midgut immune activation. (A) Midgut Duox silencing 4 days post injection of dsDoux (B) Effect of Duox silencing on P. berghei infection 7 days post infection (PI). (C) Same as (B), but in antibiotic-fed females. (D) Effect of Duox silencing on Cecropin, PGRP-S3, PGRP-LB, heme-peroxidase 8, and NOS expression in midguts of antibiotic-treated mosquitoes collected 24 h PI (mean ± SEM).
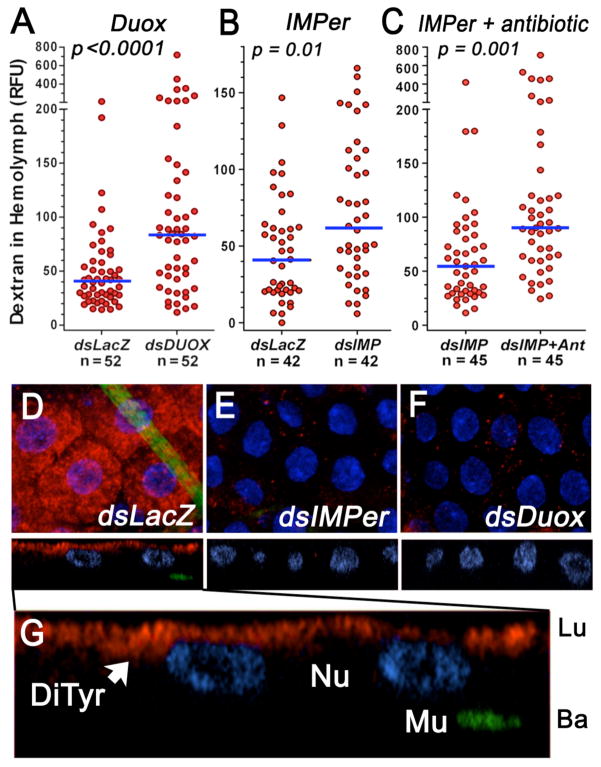
We propose a model (Fig. S7) in which the IMPer/Duox system mediates protein crosslinking by forming dityrosine bonds. This network of covalently linked proteins decreases the rate of diffusion of immune elicitors, decreasing their interaction with pathogen recognition receptors on the surface of midgut cells. In agreement with this model, silencing Duox (Fig. 4A) or IMPer (Fig. 4B) or reducing immune elicitors with oral antibiotics (Fig. S8) significantly increases the rate of absorption of a fluorescent dextran administered in the blood meal, indicating that the dextran has increased access to the gut surface. Decreasing immune elicitors in IMPer-silenced mosquitoes further enhances gut permeability (Fig. 4C). The dityrosine network is dynamic and probably transient, as IMPer is expressed 6–18 h after feeding, a time when bacteria proliferate but blood digestion is not fully active.
Fig. 4.
Gut permeability and dityrosine network. Effect of (A) Duox or (B) IMPer silencing without or (C) with oral antibiotics (Ant.) on midgut permeability to fluorescent dextran (4 kDa). Each circle represents fluorescence in the hemolymph of an individual mosquito 18–20 h after feeding (line indicates the median). (D–G) Immunofluorescence staining of midguts 14 h after feeding. Dityrosine bonds (red) and muscle actin (green) in mosquitoes injected with (D) dsLacZ, (E) dsIMPer, or (F) dsDuox. (G) Enlargement of (D). DiTyr, dityrosine staining; Lu, lumen; Ba, basal; Mu, muscle; Nu, nuclei.
Monoclonal antibodies were used to detect dityrosine bonds on the midgut surface. A network of dityrosine-linked proteins is observed on the luminal surface of epithelial cells of blood-fed control mosquitoes injected with dsLacZ (Fig. 4, D and G) but is absent when either IMPer (Fig. 4E) or Duox (Fig. 4F) is silenced, in agreement with the proposed model.
In conclusion, An. gambiae midgut epithelial cells have the ability to activate pathogen-specific responses to bacteria and Plasmodium and to modulate the permeability of the mucus layer to soluble molecules present in the blood bolus. The dityrosine network formed by the IMPer/Duox system allows bacteria to proliferate without activating epithelial immunity but also makes mosquitoes more susceptible to Plasmodium infection, as parasites can develop within the midgut lumen without being detected. In Drosophila, silencing of a secreted peroxidase results in high mortality when flies are fed live or dead bacteria, but not when they are fed sterile food (14). Furthermore, antioxidants can rescue this mortality, indicating that high levels of reactive oxygen species (ROS) are mediating death (14). Our studies suggest that this enzyme may also be involved in the formation of a dityrosine network in Drosophila and that disruption of this barrier could result in chronic immune activation and ROS generation. In mosquitoes, IMPer or Duox silencing does not affect mosquito survival (Fig. S9), probably because they are “batch” feeders and gut bacteria and blood-meal remnants are expelled 2–3 days after feeding.
The Duox system appears to protect epithelial cells from potential pathogens using a dynamic two-prong strategy. Bacterial elicitors are known to activate Duox activity quickly through the phospholipase C-beta signaling pathway (3) (15). This would generate hydrogen peroxide and activate IMPer, forming the dityrosine network and decreasing the permeability of the mucus layer to immune elicitors. Expression of microbicidal effector genes would be induced when this initial response cannot prevent contact of immune elicitors with pathogen recognition receptors on the surface of epithelial cells; for example, when pathogenic bacteria or Plasmodium parasites breach the PM and the mucus barriers. This two complementary mechanisms would allow an effective immune response while minimizing the deleterious effects that chronic activation of potent effector molecules could have on commensal bacteria and on the host.
Supplementary Material
Summary.
A dityrosine network forms a permeability barrier that prevents immune activation of mosquito midgut epithelial cells.
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank André Laughinghouse, Kevin Lee, Tovi Lehman, and Robert Gwadz for insectary support; Juraj Kabat for assistance with confocal imaging; José Ribeiro and Jesus Valenzuela for their comments and insight; and Brenda Marshall for editorial assistance.
Footnotes
References and Notes
- 1.Dong Y, Manfredini F, Dimopoulos G. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha EM, Oh CT, Bae YS, Lee WJ. Science. 2005;310:847. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 3.Ha EM, et al. Dev Cell. 2009;16:386. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Meitzler JL, Ortiz de Montellano PR. J Biol Chem. 2009;284:18634. doi: 10.1074/jbc.M109.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devenport M, Fujioka H, Jacobs-Lorena M. Insect Mol Biol. 2004;13:349. doi: 10.1111/j.0962-1075.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 6.Shen Z, Jacobs-Lorena M. J Biol Chem. 1998;273:17665. doi: 10.1074/jbc.273.28.17665. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z, Dimopoulos G, Kafatos FC, Jacobs-Lorena M. Proc Natl Acad Sci U S A. 1999;96:5610. doi: 10.1073/pnas.96.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidman-Remy A, et al. Immunity. 2006;24:463. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Edens WA, et al. J Cell Biol. 2001;154:879. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong JL, Creton R, Wessel GM. Dev Cell. 2004;7:801. doi: 10.1016/j.devcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Gupta L, et al. Cell Host Microbe. 2009;5:498. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J, Gowda DC, Krishnegowda G, Luckhart S. Infect Immun. 2005;73:2778. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Gupta L, Han YS, Barillas-Mury C. J Biol Chem. 2004;279:53475. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 14.Ha EM, et al. Dev Cell. 2005;8:125. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Ha EM, et al. Nat Immunol. 2009;10:949. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.